P-POSSUM Falls Short: Predicting Morbidity in Ovarian Cancer (OC) Cytoreductive Surgery
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Ethics
3. Results
3.1. Summary of Our Cohort
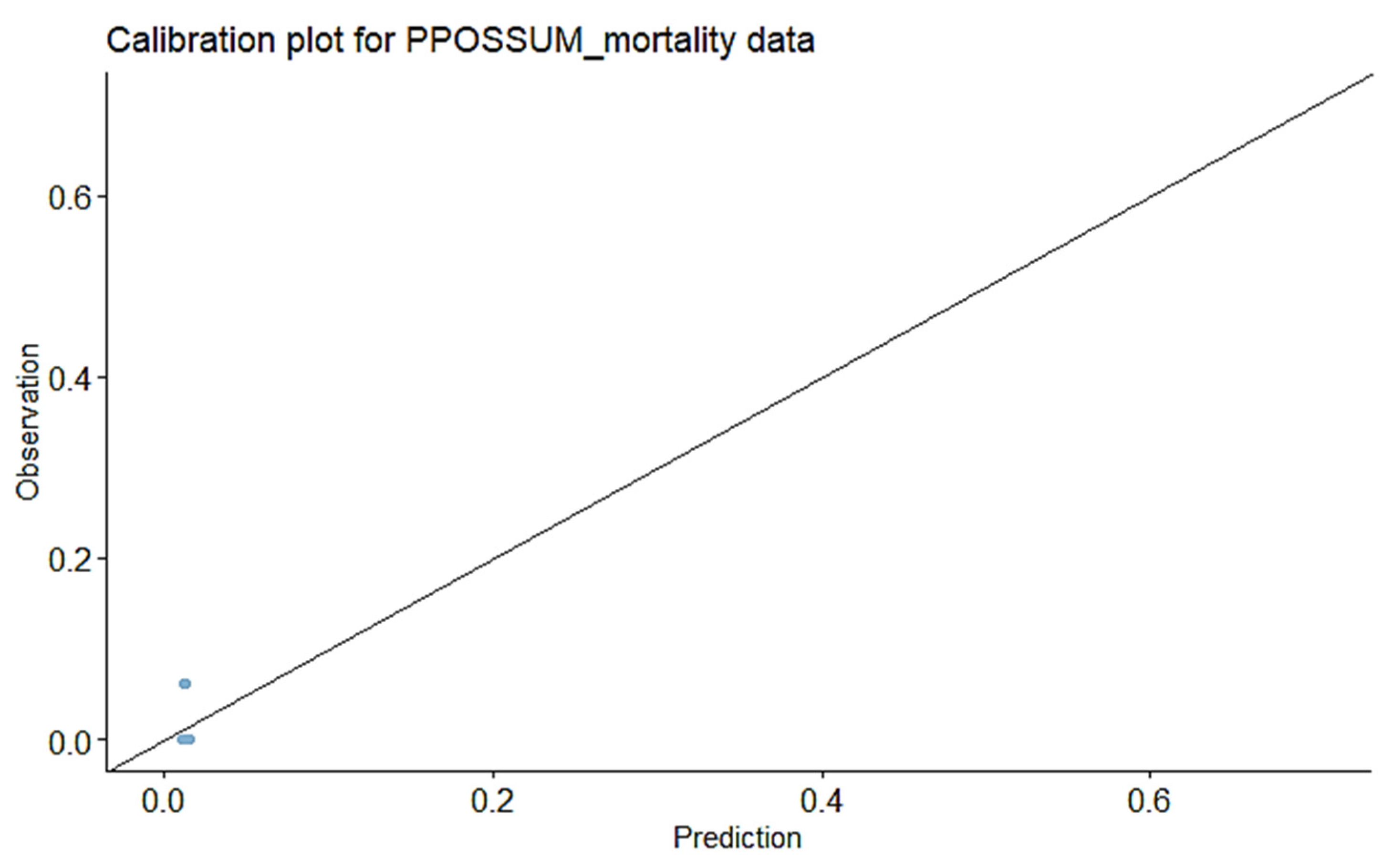
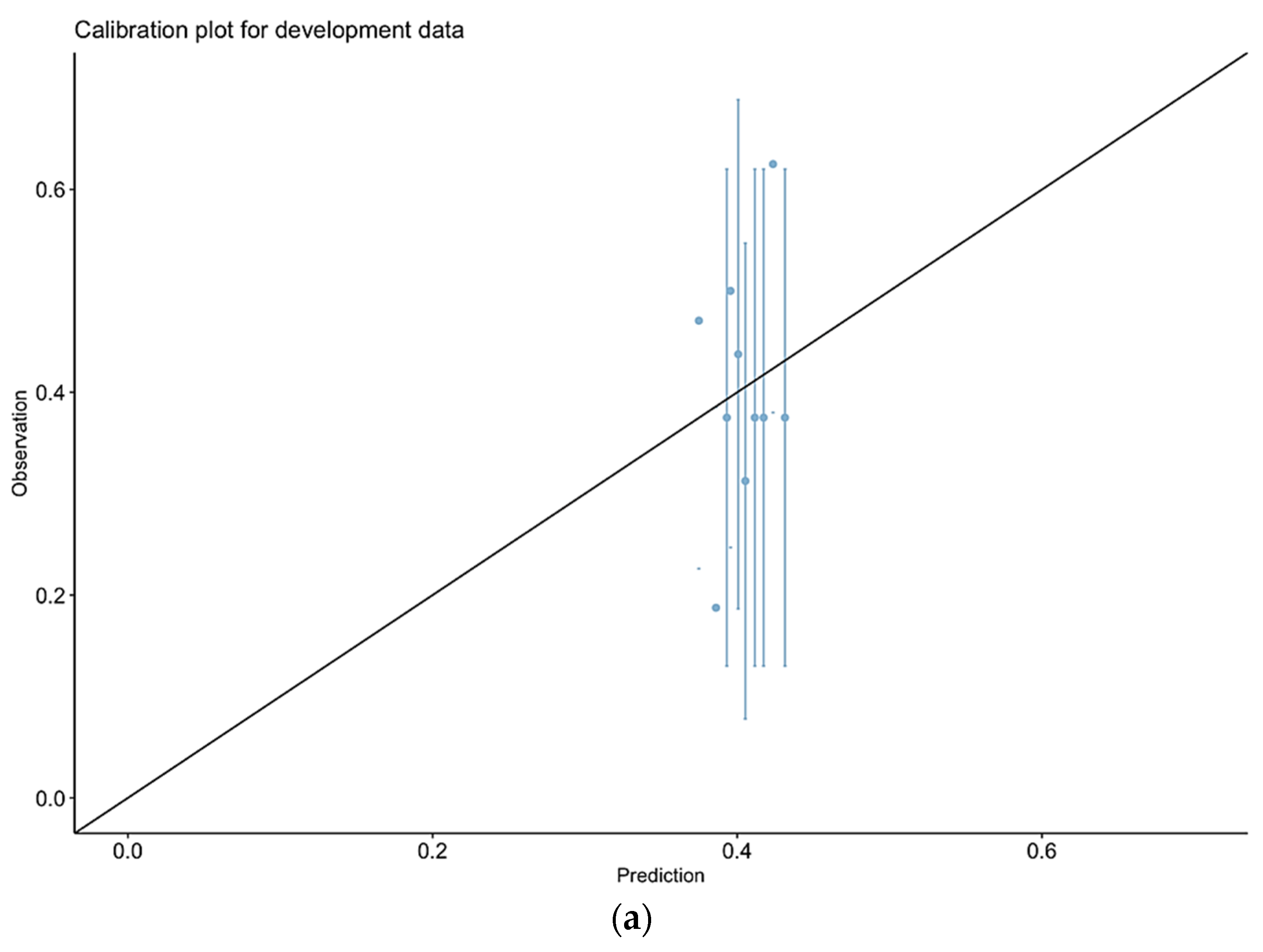
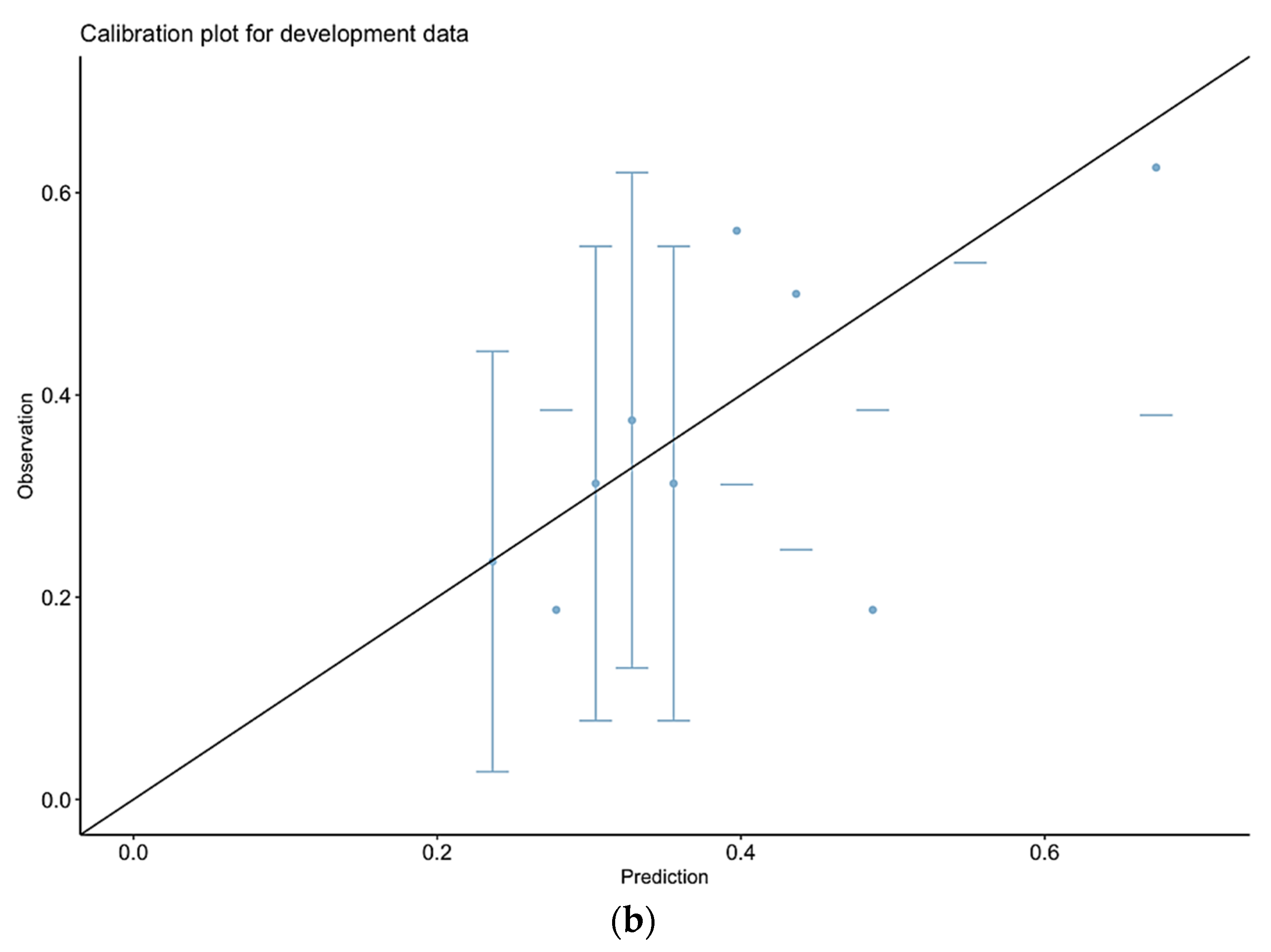
3.2. Calibration of Observed vs. Predicted Morbidity for P-POSSUM
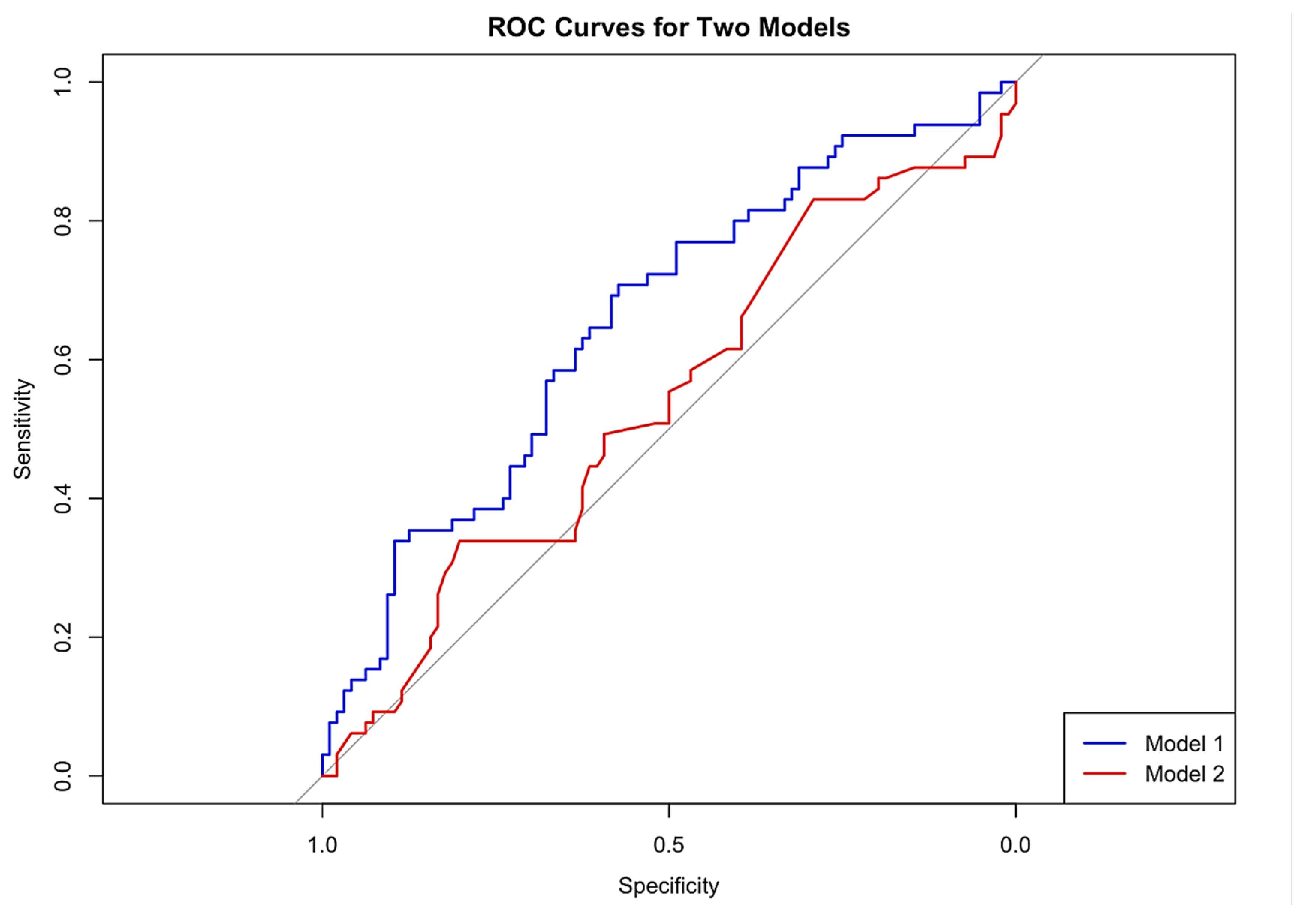
3.3. P-POSSUM Performance in Predicting Post-Operative Morbidity
3.4. Exploratory Stepwise Regression Analysis
3.5. Bootstrapping Stepwise Selection
3.6. Comparisons of Final Model vs. P-POSSUM Alone for Predicting Morbidity
3.7. Calibration of Observed vs. Predicted Morbidity for Our Final Model
3.8. Prediction of Mortality Using P-POSSUM and SORT Scales
4. Discussion
4.1. Summary of Main Results
4.2. Results in the Context of Published Literature
4.3. Strengths and Weaknesses
4.4. Implications for Practice and Future Research
4.5. Sample Size Considerations and Validation of a Future Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Search Strategy, Table Summary of Selected Studies
| PMID | Author, Year, Country | Population (Sample Size) | Setting (Abdominal Procedures, e.g., GO) | Intervention = Scale Assessed, i.e., P-POSSUM/ POSSUM Morbidity/Mortality | AUC If Available (for Each Outcome) | Structured Summary (Conclusions) |
|---|---|---|---|---|---|---|
| 38202180 | Burtin et al., 2023 [26], Germany | 485 patients | Colorectal cancer surgery | POSSUM, P-POSSUM, and CR-POSSUM morbidity and mortality | The P-POSSUM model demonstrated accurate mortality predictions in the 60–70% (O 1.01) and 80–90% (O 1.19) risk ranges but significantly overestimated mortality in other categories. Similarly, the CR-POSSUM model consistently overestimated mortality across all risk levels, with its highest accuracy observed in the 50–60% risk range, yielding an O ratio of 0.89. | POSSUM overpredicted postoperative morbidity. All three scoring systems considerably overpredicted in-hospital mortality. POSSUM score identified patients at risk of anastomotic leakage, sepsis, and return to theatre. The three scoring systems were deemed too imprecise for the estimation of perioperative complications and mortality of patients undergoing colorectal surgery. A revision of the scoring systems could increase their reliability in the clinical setting. |
| 35706623 | Mukherjee et al., 2022 [30], India | 143 patients | Adults undergoing gastrointestinal and gynaecological cancer surgeries who postoperatively required admission to an intensive care unit or high dependency unit. | P-POSSUM morbidity | The correlation coefficient between the predicted morbidity and observed complication was 0.24. | P-POSSUM was not a reliable predictor of postoperative morbidity for patients undergoing major gynaecological and gastrointestinal surgeries for cancer in this institution. There was a significant incidence of major complications with P-POSSUM morbidity prediction score ≥ 60%, leading to the need for more stringent assessment and monitoring in that subgroup. |
| 35042495 | Valenzuela et al., 2022 [37], Colombia | 350 patients. 89.1% of patients had no neoplastic diagnosis | Abdominal surgery | POSSUM and PPOSSUM morbidity and mortality | POSSUM scoring overestimated the risk of morbidity and mortality in patients with high-moderate risk, while the P-POSSUM score was a more accurate predictor of mortality risk. | |
| 37588588 | Torlot et al., 2022 [29], Australia | 31,153 patients | General surgery | 30-day mortality using SORT, NZRISK, P-POSSUM; POSSUM) |
AUC:
SORT = 0.922 NZRISK = 0.909 P-POSSUM = 0.893 POSSUM = 0.881 | All four risk scores showed high discrimination for 30-day mortality but consistently overpredicted risk. SORT was the best performing risk score. Categorising patients based on SORT into low, medium (80–90th percentile), and high risk (90–100th percentile) might guide future allocation of perioperative resources. No tools were sufficiently calibrated to support shared decision making based on absolute predictions of risk. |
| 23435569 | Chen et al., 2013 [16], China | Meta-analysis of 16 studies | Hepatobiliary surgery | P-POSSUM morbidity and mortality | Morbidity analysis: POSSUM O/E ratio of 0.78 [95%CI 0.68–0.88]. Mortality analysis: POSSUM 0.35 (95%CI 0.17–0.54) P-POSSUM 0.95 (95%CI 0.65–1.25). | POSSUM overpredicted postoperative morbidity after hepato-biliary-pancreatic surgery. Compared with POSSUM, P-POSSUM was more accurate for predicting postoperative mortality. Modifications to POSSUM and P-POSSUM are needed for audit in hepato-biliary-pancreatic surgery. |
| 18025331 | Horzic et al., 2007 [27], Croatia | 120 patients | Colorectal cancer surgery | P-POSSUM and Cr-POSSUM mortality | AUC for P-POSSUM was 0.70 and for CR-POSSUM was 0.59. | The P-POSSUM system underpredicted mortality by 25%, with no significant difference between the predicted and observed values (p = 0.96). The observed to expected ratio for Cr-POSSUM was 1.11, with no significant difference between the observed and predicted values (p = 0.19). Both P-POSSUM and Cr-POSSUM perform well in predicting mortality after colorectal cancer surgery, but Cr-POSSUM is more accurate. There is a constant need for re-evaluation of existing and any new scoring systems outside original development and validation populations. |
| 16914285 | Das et al., 2006 [31], United Kingdom | 468 patients | Gynaecological oncology patients | P-POSSUM morbidity and mortality | The P-POSSUM algorithm overestimates the risk of mortality for gynaecological oncology patients undergoing surgery. The P-POSSUM algorithm will require further adjustments prior to adoption for gynaecological cancer surgery as a risk adjusted surgical audit tool. | |
| 17103102 | Tez et al., 2006 [28], Turkey | 321 patients | Colorectal surgery | P-POSSUM and CR-POSSUM mortality | Overall, 22 deaths were observed. CR-POSSUM predicted 25 deaths (chi2 = 12.20, p = 0.13), and P-POSSUM predicted 29 deaths (chi2 =18.85, p = 0.002). ROC curve analysis revealed that CR-POSSUM has reasonable discriminatory power for mortality. These data suggest that CR-POSSUM may provide a better estimate of the risk of mortality for patients undergoing colorectal resection. | |
| 16421662 | Ramkumar et al., 2006 [38], UK | 347 patients | Colorectal surgery | POSSUM, P-POSSUM, and CR-POSSUM morbidity and mortality |
POSSUM AUC 0.752.
PPOSSUM AUC 0.749. CR-POSSUM AUC 0.781. | Colorectal POSSUM provides comparable prediction of mortality risk after colorectal resection compared with POSSUM and P-POSSUM. |
| 15048745 | Lam et al., 2004 [39], China | 259 patients | Hepatectomies | POSSUM and P-POSSUM mortality | POSSUM system overpredicted mortality risk in patients who had major hepatectomy for hepatocellular carcinoma. P-POSSUM significantly predicted outcome. A modified disease-specific equation was derived which requires prospective testing. | |
| 15048756 | Mohil et al., 2004 [40], India | 120 patients | Emergency laparotomy | P-POSSUM morbidity and mortality | Observed-expected ratios: Linear regression POSSUM morbidity 0.68 POSSUM mortality 0.39 P-POSSUM mortality 0.66 Exponential analysis POSSUM morbidity 0.91 POSSUM mortality 0.62 P-POSSUM mortality 0.88. | If analysed exponentially, POSSUM is a good predictor of morbidity and mortality in patients undergoing emergency laparotomy. P-POSSUM predicts mortality equally well. Both equations may be used for risk-adjusted surgical audit of patients undergoing emergency laparotomy. |
Appendix B. StepAIC and Bootstrapping Parameter Selection Process for Regression Model
StepAIC Start: AIC=215.3 Outcome=Complication(Y/N): Model parameters: EFS + PPOSSUM_MORBIDITY + Pre-op Albumin + BMI Df Deviance AIC - Pre-Op Albumin 1 205.38 213.38 - PPOSSUM_MORBIDITY 1 205.40 213.40 <none> 205.31 215.31 - BMI 1 207.39 215.39 - EFS 1 215.84 223.84 Step: AIC=213.38 Outcome=Complication(Y/N): Model parameters: EFS + PPOSSUM_MORBIDITY +BMI Df Deviance AIC - PPOSSUM_MORBIDITY 1 205.44 211.44 <none> 205.38 213.38 - BMI 1 207.44 213.44 - Edmonton_Frailty_Score 1 216.26 222.26 Step: AIC=211.44 Outcome=Complication(Y/N): Model parameters: EFS + BMI Df Deviance AIC <none> 205.44 211.44 - BMI 1 207.50 211.50 - Edmonton_Frailty_Score 1 216.48 220.48 Call: glm(formula = NumberComplicationBinomia ~ Edmonton_Frailty_Score + BMI, family = “binomial”, data = dppossum) Coefficients: (Intercept) Edmonton_Frailty_Score BMI -0.06565 0.22262 -0.03965 Degrees of Freedom: 160 Total (i.e. Null); 158 Residual Null Deviance: 217.2 Residual Deviance: 205.4 AIC: 211.4 Summary of Bootstrapping the ‘stepAIC()’ procedure for Call: glm(formula = NumberComplicationBinomia ~ Edmonton_Frailty_Score + PPOSSUM_MORBIDITY + Pre_Op_Albumin + BMI, family = “binomial”, data = dppossum) Bootstrap samples: 2000 Direction: backward Penalty: 2 * df Covariates selected (%) Edmonton_Frailty_Score 96.25 BMI 54.20 PPOSSUM_MORBIDITY 17.95 Pre_Op_Albumin 16.15 Null 1.60 Coefficients Sign + (%) - (%) Edmonton_Frailty_Score 100.00 0.00 Pre_Op_Albumin 31.27 68.73 PPOSSUM_MORBIDITY 25.63 74.37 BMI 0.65 99.35 Stat Significance (%) Edmonton_Frailty_Score 93.19 BMI 52.12 PPOSSUM_MORBIDITY 35.93 Pre_Op_Albumin 31.89 The stepAIC() for the original data-set gave Call: glm(formula = NumberComplicationBinomia ~ Edmonton_Frailty_Score + BMI, family = “binomial”, data = dppossum) Coefficients: (Intercept) Edmonton_Frailty_Score BMI -0.06565 0.22262 -0.03965 Degrees of Freedom: 160 Total (i.e. Null); 158 Residual Null Deviance: 217.2 Residual Deviance: 205.4 AIC: 211.4 Stepwise Model Path Analysis of Deviance Table Initial Model: NumberComplicationBinomia ~ Edmonton_Frailty_Score + PPOSSUM_MORBIDITY + Pre_Op_Albumin + BMI Final Model: NumberComplicationBinomia ~ Edmonton_Frailty_Score + BMI Step Df Deviance Resid. Df Resid. Dev AIC 1 156 205.3046 215.3046 2 - Pre_Op_Albumin 1 0.07089549 157 205.3755 213.3755 3 - PPOSSUM_MORBIDITY 1 0.06897021 158 205.4444 211.4444
Appendix C. ROC Curves and Calibration Plots for P-POSSUM and SORT Morbidity Scales
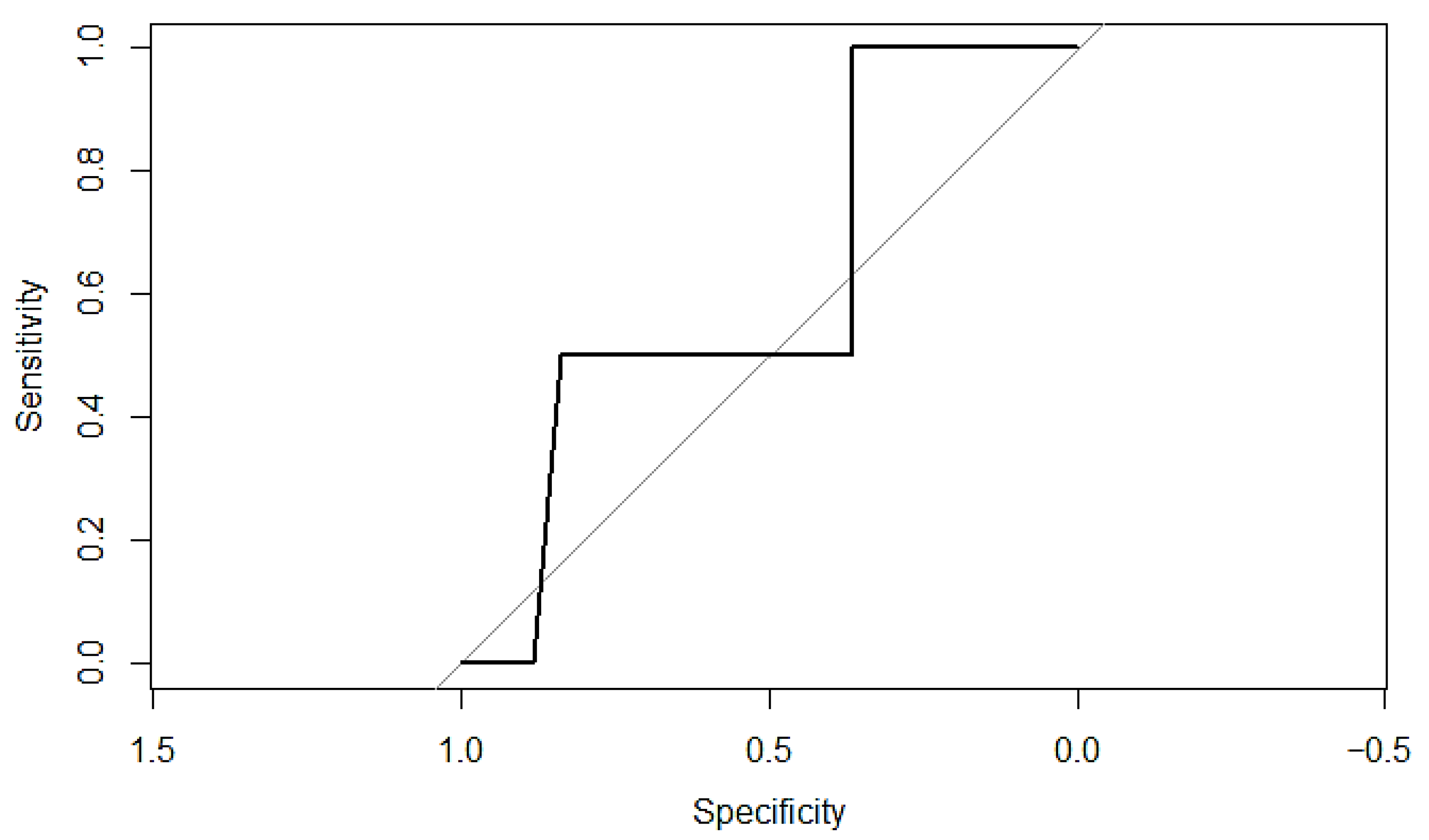
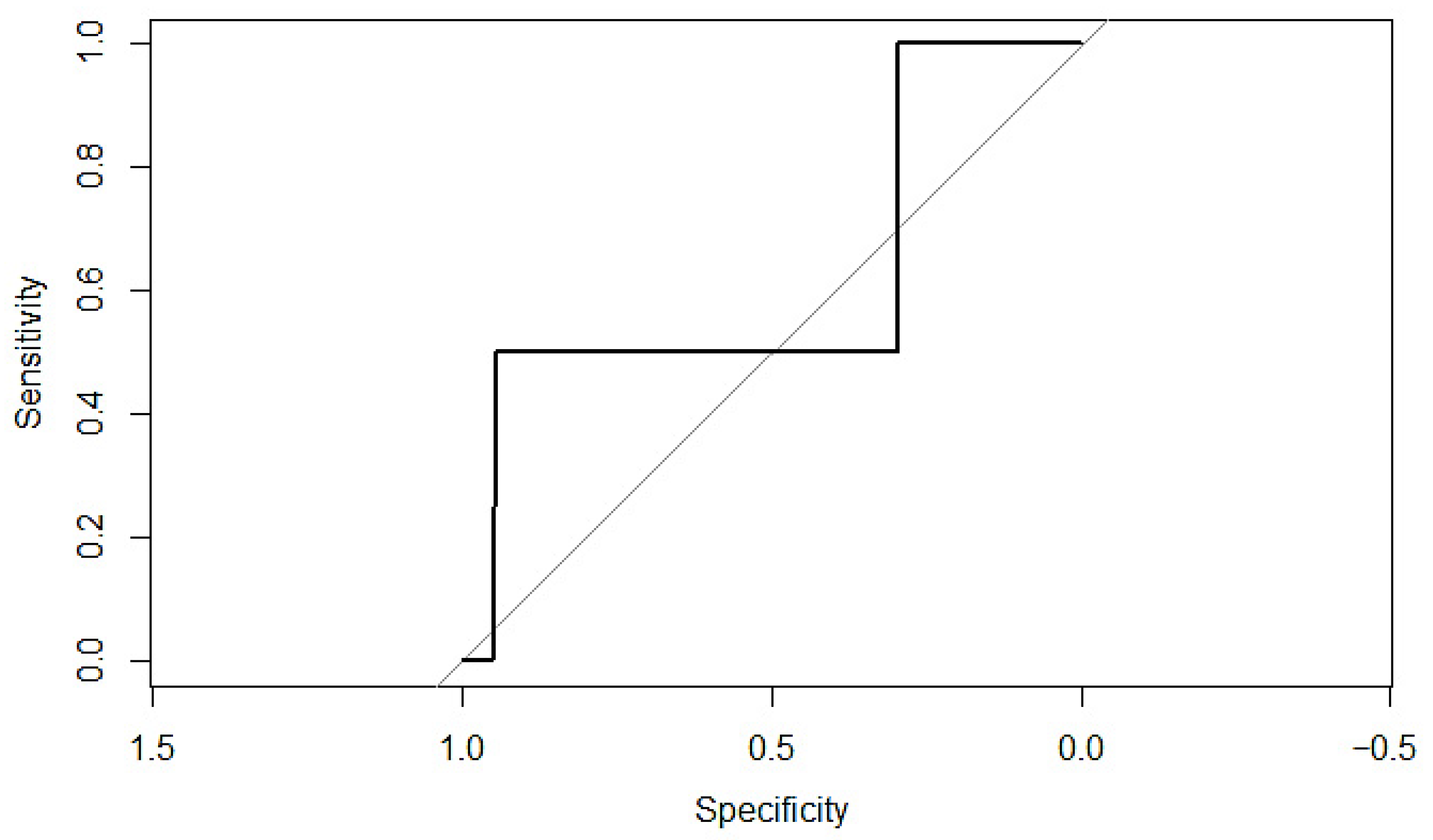

Appendix D. Sample Size Considerations and Validation for Future Model
Model Development
| Prevalence of Outcome | ||||
| 0.5 | 0.2 | 0.02 | ||
| AUC | 0.7 | 1737 | 2690 | 21,720 |
| 0.8 | 731 | 1109 | 8430 | |
| Prevalence of Outcome | ||||
| 0.5 | 0.2 | 0.02 | ||
| AUC | 0.7 | 416 | 1538 | 18,839 |
| 0.8 | 385 | 1538 | 18,839 | |
References
- National Institute for Health and Care Excellence. Maximal Cytoreductive Surgery for Advanced Ovarian Cancer—IPG757. 2023. Available online: https://www.nice.org.uk/guidance/IPG757 (accessed on 19 September 2025).
- Cummins, C.; Kumar, S.; Long, J.; Balega, J.; Broadhead, T.; Duncan, T.; Edmondson, R.J.; Fotopoulou, C.; Glasspool, R.M.; Kolomainen, D.; et al. Investigating the Impact of Ultra-Radical Surgery on Survival in Advanced Ovarian Cancer Using Population-Based Data in a Multicentre UK Study. Cancers 2022, 14, 4362. [Google Scholar] [CrossRef]
- Harter, P.; Sehouli, J.; Vergote, I.; Ferron, G.; Reuss, A.; Meier, W.; Greggi, S.; Mosgaard, B.J.; Selle, F.; Guyon, F.; et al. Randomized Trial of Cytoreductive Surgery for Relapsed Ovarian Cancer. N. Engl. J. Med. 2021, 385, 2123–2131. [Google Scholar] [CrossRef]
- Iyer, R.; Gentry-Maharaj, A.; Nordin, A.; Burnell, M.; Liston, R.; Manchanda, R.; Das, N.; Desai, R.; Gornall, R.; Beardmore-Gray, A.; et al. Predictors of complications in gynaecological oncological surgery: A prospective multicentre study (UKGOSOC-UK gynaecological oncology surgical outcomes and complications). Br. J. Cancer 2015, 112, 475–484. [Google Scholar] [CrossRef]
- Sekse, R.J.T.; Dunberger, G.; Olesen, M.L.; Osterbye, M.; Seibaek, L. Lived experiences and quality of life after gynaecological cancer-An integrative review. J. Clin. Nurs. 2019, 28, 1393–1421. [Google Scholar] [CrossRef]
- BGCS. Governance Models to Support Patient Safety When Undergoing Maximal Effort Cytoreductive Surgery for Advanced Ovarian/Fallopian Tube/Primary Peritoneal Cancer—A Joint Statement of ACPGBI, ASGBI, AUGIS and BGCS. Available online: https://www.bgcs.org.uk/wp-content/uploads/2021/12/Joint-statement-Version-1.9_NJW_final.pdf (accessed on 19 September 2025).
- Meara, J.G.; Leather, A.J.; Hagander, L.; Alkire, B.C.; Alonso, N.; Ameh, E.A.; Bickler, S.W.; Conteh, L.; Dare, A.J.; Davies, J.; et al. Global Surgery 2030: Evidence and solutions for achieving health, welfare, and economic development. Int. J. Obstet. Anesth. 2016, 25, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Ghaferi, A.A.; Birkmeyer, J.D.; Dimick, J.B. Variation in hospital mortality associated with inpatient surgery. N. Engl. J. Med. 2009, 361, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- GlobalSurg Collaborative and National Institute for Health Research Global Health Research Unit on Global Surgery. Global variation in postoperative mortality and complications after cancer surgery: A multicentre, prospective cohort study in 82 countries. Lancet 2021, 397, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Inci, M.G.; Anders, L.; Woopen, H.; Richter, R.; Guzel, D.; Armbrust, R.; Sehouli, J. Frailty Index for prediction of surgical outcome in ovarian cancer: Results of a prospective study. Gynecol. Oncol. 2021, 161, 396–401. [Google Scholar] [CrossRef]
- Ramos, S.Z.; Kulkarni, A.; Oliver, M.; Danilack, V.A.; Mathews, C. Frailty as a predictor of delayed initiation of adjuvant chemotherapy in patients with ovarian cancer. Int. J. Gynecol. Cancer 2023, 33, 57–65. [Google Scholar] [CrossRef]
- Barnett, G.; Swart, M. Shared decision making for high-risk surgery. BJA Educ. 2021, 21, 300–306. [Google Scholar] [CrossRef]
- Copeland, G.P.; Jones, D.; Walters, M. POSSUM: A scoring system for surgical audit. Br. J. Surg. 1991, 78, 355–360. [Google Scholar] [CrossRef]
- Prytherch, D.R.; Whiteley, M.S.; Higgins, B.; Weaver, P.C.; Prout, W.G.; Powell, S.J. POSSUM and Portsmouth POSSUM for predicting mortality. Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity. Br. J. Surg. 1998, 85, 1217–1220. [Google Scholar] [CrossRef]
- Bare, M.; Alcantara, M.J.; Gil, M.J.; Collera, P.; Pont, M.; Escobar, A.; Sarasqueta, C.; Redondo, M.; Briones, E.; Dujovne, P.; et al. Validity of the CR-POSSUM model in surgery for colorectal cancer in Spain (CCR-CARESS study) and comparison with other models to predict operative mortality. BMC Health Serv. Res. 2018, 18, 49. [Google Scholar] [CrossRef]
- Chen, T.; Wang, H.; Wang, H.; Song, Y.; Li, X.; Wang, J. POSSUM and P-POSSUM as predictors of postoperative morbidity and mortality in patients undergoing hepato-biliary-pancreatic surgery: A meta-analysis. Ann. Surg. Oncol. 2013, 20, 2501–2510. [Google Scholar] [CrossRef] [PubMed]
- Dutton, J.; Zardab, M.; De Braal, V.J.F.; Hariharan, D.; MacDonald, N.; Hallworth, S.; Hutchins, R.; Bhattacharya, S.; Abraham, A.; Kocher, H.M.; et al. The accuracy of pre-operative (P)-POSSUM scoring and cardiopulmonary exercise testing in predicting morbidity and mortality after pancreatic and liver surgery: A systematic review. Ann. Med. Surg 2021, 62, 1–9. [Google Scholar] [CrossRef]
- Tang, T.Y.; Walsh, S.R.; Prytherch, D.R.; Wijewardena, C.; Gaunt, M.E.; Varty, K.; Boyle, J.R. POSSUM models in open abdominal aortic aneurysm surgery. Eur. J. Vasc. Endovasc. Surg. 2007, 34, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Rolfson, D.B.; Majumdar, S.R.; Tsuyuki, R.T.; Tahir, A.; Rockwood, K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006, 35, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Xue, Q.L. The frailty syndrome: Definition and natural history. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef]
- Inci, M.G.; Rasch, J.; Woopen, H.; Mueller, K.; Richter, R.; Sehouli, J. ECOG and BMI as preoperative risk factors for severe postoperative complications in ovarian cancer patients: Results of a prospective study (RISC-GYN-trial). Arch. Gynecol. Obstet. 2021, 304, 1323–1333. [Google Scholar] [CrossRef]
- Smits, A.; Lopes, A.; Das, N.; Kumar, A.; Cliby, W.; Smits, E.; Bekkers, R.; Massuger, L.; Galaal, K. Surgical morbidity and clinical outcomes in ovarian cancer—The role of obesity. BJOG 2016, 123, 300–308. [Google Scholar] [CrossRef]
- Schwarz, S.; Prokopchuk, O.; Esefeld, K.; Groschel, S.; Bachmann, J.; Lorenzen, S.; Friess, H.; Halle, M.; Martignoni, M.E. The clinical picture of cachexia: A mosaic of different parameters (experience of 503 patients). BMC Cancer 2017, 17, 130. [Google Scholar] [CrossRef]
- Ubachs, J.; Ziemons, J.; Minis-Rutten, I.J.G.; Kruitwagen, R.; Kleijnen, J.; Lambrechts, S.; Olde Damink, S.W.M.; Rensen, S.S.; Van Gorp, T. Sarcopenia and ovarian cancer survival: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 1165–1174. [Google Scholar] [CrossRef]
- Burtin, F.; Ludwig, T.; Leuchter, M.; Hendricks, A.; Schafmayer, C.; Philipp, M. More than 30 Years of POSSUM: Are Scoring Systems Still Relevant Today for Colorectal Surgery? J. Clin. Med. 2023, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Horzic, M.; Kopljar, M.; Cupurdija, K.; Bielen, D.V.; Vergles, D.; Lackovic, Z. Comparison of P-POSSUM and Cr-POSSUM scores in patients undergoing colorectal cancer resection. Arch. Surg. 2007, 142, 1043–1048. [Google Scholar] [CrossRef][Green Version]
- Tez, M.; Yoldas, O.; Gocmen, E.; Kulah, B.; Koc, M. Evaluation of P-POSSUM and CR-POSSUM scores in patients with colorectal cancer undergoing resection. World J. Surg. 2006, 30, 2266–2269. [Google Scholar] [CrossRef] [PubMed]
- Torlot, F.; Yew, C.Y.; Reilly, J.R.; Phillips, M.; Weber, D.G.; Corcoran, T.B.; Ho, K.M.; Toner, A.J. External validity of four risk scores predicting 30-day mortality after surgery. BJA Open 2022, 3, 100018. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kedia, A.; Goswami, J.; Chakraborty, A. Validity of P-POSSUM in adult cancer surgery (PACS). J. Anaesthesiol. Clin. Pharmacol. 2022, 38, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Talaat, A.S.; Naik, R.; Lopes, A.D.; Godfrey, K.A.; Hatem, M.H.; Edmondson, R.J. Risk adjusted surgical audit in gynaecological oncology: P-POSSUM does not predict outcome. Eur. J. Surg. Oncol. 2006, 32, 1135–1138. [Google Scholar] [CrossRef]
- Wong, G.T.C.; Ang, W.C.; Wong, T.C.L.; Choi, S.W. Surgical Outcome Risk Tool (SORT) validation in hepatectomy. Anaesthesia 2017, 72, 1287–1289. [Google Scholar] [CrossRef]
- Oakland, K.; Cosentino, D.; Cross, T.; Bucknall, C.; Dorudi, S.; Walker, D. External validation of the Surgical Outcome Risk Tool (SORT) in 3305 abdominal surgery patients in the independent sector in the UK. Perioper. Med. 2021, 10, 4. [Google Scholar] [CrossRef]
- Chiva, L.; Ordás, P.; Martin-Calvo, N.; Aramendia, J.M.; Sanchez Lorenzo, L.; Gallego Martínez, A.; Vizcay, Á.; Minguez, J.A.; Manzour, N.; Vázquez-Vicente, D.; et al. An international worldwide retrospective cohort observational study comparing primary cytoreductive surgery with neoadjuvant chemotherapy and interval cytoreductive surgery in patients with carcinoma of the ovary, fallopian tubes, and peritoneum (SUROVA trial). Int. J. Gynecol. Cancer 2024, 34, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.D.; Ensor, J.; Snell, K.I.E.; Debray, T.P.A.; Altman, D.G.; Moons, K.G.M.; Collins, G.S. Calculating the sample size required for developing a clinical prediction model. BMJ 2020, 368, m441. [Google Scholar] [CrossRef]
- Riley, R.D.; Snell, K.I.E.; Ensor, J.; Burke, D.L.; Harrell, F.E., Jr.; Moons, K.G.M.; Collins, G.S. Calculating the sample size required for external validation of a multivariable prediction model. Stat. Med. 2021, 40, 1115–1136. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, S.; Niño, L.; Conde, D.; Girón, F.; Rodríguez, L.; Venegas, D.; Rey, C.; Nassar, R.; Vanegas, M.; Jiménez, D. Morbimortality Assessment in Abdominal Surgery: Are We Predicting or Overreacting? BMC Surg. 2022, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, T.; Ng, V.; Fowler, L.; Farouk, R. A Comparison of POSSUM, P-POSSUM and Colorectal POSSUM for the Prediction of Postoperative Mortality in Patients Undergoing Colorectal Resection. Dis. Colon Rectum 2006, 49, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.-M.; Fan, S.-T.; Yuen, A.W.-C.; Law, W.-L.; Poon, K. Validation of POSSUM Scoring Systems for Audit of Major Hepatectomy. Br. J. Surg. 2004, 91, 450–454. [Google Scholar] [CrossRef]
- Mohil, R.S.; Bhatnagar, D.; Bahadur, L.; Rajneesh; Dev, D.K.; Magan, M. POSSUM and P-POSSUM for Risk-Adjusted Audit of Patients Undergoing Emergency Laparotomy. Br. J. Surg. 2004, 91, 500–503. [Google Scholar] [CrossRef]
| Mean age (range), SD (years) | 66.50 (27.00–88.00), SD = 11.3 | |||
| Mean BMI (range), SD (kg/m2) | 27.93 (16.00–55.26), SD = 6.10 | |||
| Mean pre-op albumin (range), SD (g/dL) | 40.48 (12.00–51.00), SD = 6.56 | |||
| Stage at diagnosis N (%) | Stage 1 | Stage 2 | Stage 3 | Stage 4 |
| 23 (14.3%) | 19 (11.8%) | 54 (33.5%) | 65 (40.4%) | |
| ASA grade N (%) | ASA 1 | ASA 2 | ASA 3 | ASA 4 |
| 7 (4.3%) | 77 (47.8%) | 74 (46.0%) | 3 (1.9%) | |
| Histology N (%) | HGSOC | Clear Cell | Endometrioid | Mucinous |
| 109 (67.7%) | 12 (7.5%) | 11 (5.8%) | 10 (6.2%) | |
| Low grade serous | Sarcoma | Mesonephric/Steroid | Granulosa | |
| 9 (5.6%) | 6 (3.7%) | 2 (1.2%) | 2 (1.2%) | |
| Surgical outcomes | ||||
| Type of surgery N (%) | PDS 95 (59%) | IDS 45 (28%) | DDS 21 (13%) | |
| Duration of surgery (mean, range, SD) (minutes) | 223.71 (51.00–631.00), SD = 112.66 | |||
| Overall length of stay (mean, range, SD) (days) | 9.22 (3.00–135.00), SD = 11.43 | |||
| HDU length of stay (mean, range, SD) (days) | 3.82 (0.00–135.00), SD = 10.81 | |||
| Morbidity and mortality scales | ||||
| P-POSSUM morbidity risk mean (range), SD (%) | 59.50 (8.80–98.10), SD = 17.84 | |||
| P-POSSUM mortality risk mean (range), SD (%) | 5.87 (0.40–49.60), SD = 5.40 | |||
| SORT mortality risk mean (range), SD (%) | 3.41 (00.18–26.00), SD = 3.32 | |||
| Frailty assessment | ||||
| Edmonton Frail Scale mean (range), SD (/max score 17) | 3.44 (0.00–15.00), SD = 2.58 | |||
| Morbidity/mortality | ||||
| Highest level of postoperative Clavien–Dindo complication N (%) | CD1 | CD2 | CD3 | CD4 |
| 11 (6.8%) | 38(23.6%) | 10(6.2%) | 4 (2.5%) | |
| Total complications per patient N (%) | 0 | 1 | 2 | 3 |
| 96 (59.6%) | 26 (16.1%) | 22 (13.7%) | 14 (8.7%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sideris, M.; Brincat, M.R.; Blyuss, O.; Oxley, S.G.; Sia, J.; Kalra, A.; Wei, X.; Fierheller, C.T.; Ganesan, S.; Miller, R.E.; et al. P-POSSUM Falls Short: Predicting Morbidity in Ovarian Cancer (OC) Cytoreductive Surgery. Cancers 2025, 17, 3421. https://doi.org/10.3390/cancers17213421
Sideris M, Brincat MR, Blyuss O, Oxley SG, Sia J, Kalra A, Wei X, Fierheller CT, Ganesan S, Miller RE, et al. P-POSSUM Falls Short: Predicting Morbidity in Ovarian Cancer (OC) Cytoreductive Surgery. Cancers. 2025; 17(21):3421. https://doi.org/10.3390/cancers17213421
Chicago/Turabian StyleSideris, Michail, Mark R. Brincat, Oleg Blyuss, Samuel George Oxley, Jacqueline Sia, Ashwin Kalra, Xia Wei, Caitlin T. Fierheller, Subhasheenee Ganesan, Rowan E. Miller, and et al. 2025. "P-POSSUM Falls Short: Predicting Morbidity in Ovarian Cancer (OC) Cytoreductive Surgery" Cancers 17, no. 21: 3421. https://doi.org/10.3390/cancers17213421
APA StyleSideris, M., Brincat, M. R., Blyuss, O., Oxley, S. G., Sia, J., Kalra, A., Wei, X., Fierheller, C. T., Ganesan, S., Miller, R. E., El-Khouly, F., Gooneratne, M., Abbott, T., Pang, C. L., Verma, P., Shah, S., Lawrence, A., Jeyarajah, A., Brockbank, E., ... Manchanda, R. (2025). P-POSSUM Falls Short: Predicting Morbidity in Ovarian Cancer (OC) Cytoreductive Surgery. Cancers, 17(21), 3421. https://doi.org/10.3390/cancers17213421








