The Prognostic Role of Para-Aortic Lymph Node Metastasis in Patients with Resected Pancreatic Adenocarcinoma
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Surgical and Postoperative Procedures
2.2. Statistical Analysis
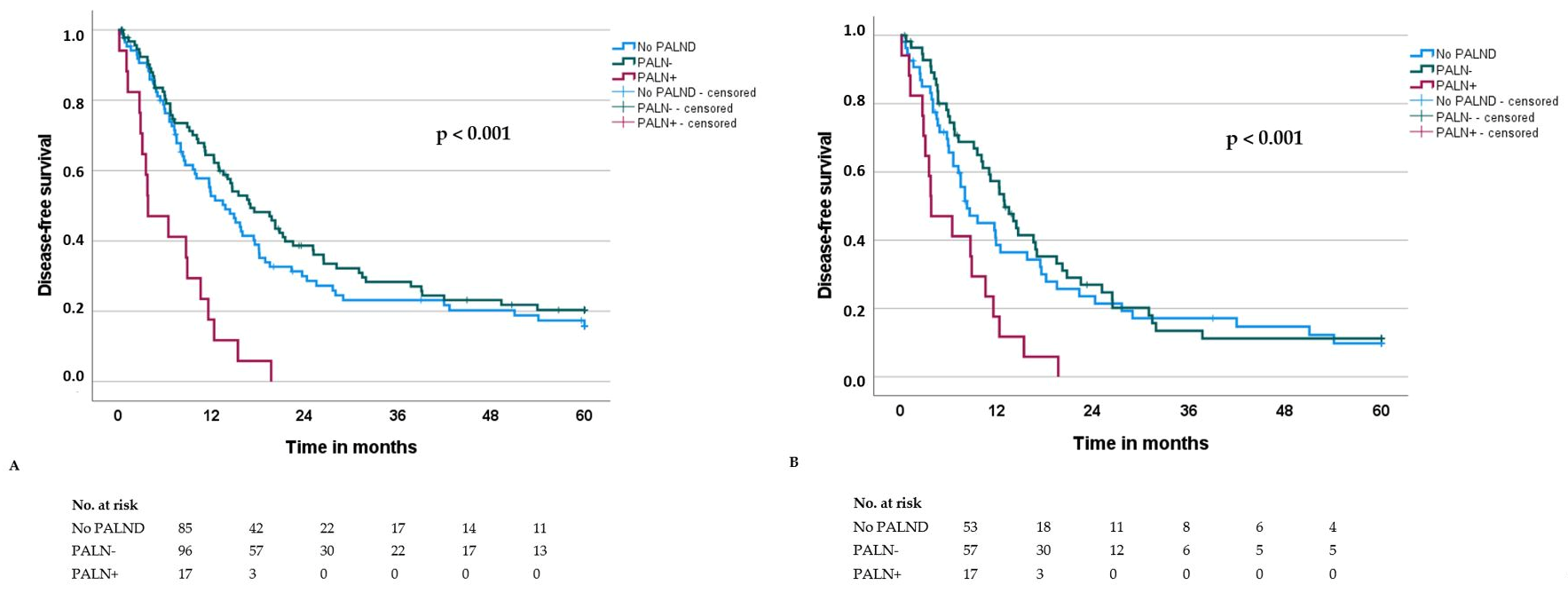
3. Results
3.1. Patient Characteristics
3.2. Outcome Parameters
3.3. Prognostic Factors for Overall Survival After Surgery (OS) and Disease-Free Survival (DFS) in the Whole Patient Cohort (n = 199)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 934–947, Erratum in: Lancet Gastroenterol. Hepatol. 2020, 5, e2. https://doi.org/10.1016/S2468-1253(20)30018-2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Groot, V.P.; Rezaee, N.; Wu, W.; Cameron, J.L.; Fishman, E.K.; Hruban, R.H.; Weiss, M.J.; Zheng, L.; Wolfgang, C.L.; He, J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2018, 267, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Kolbeinsson, H.; Hoppe, A.; Bayat, A.; Kogelschatz, B.; Mbanugo, C.; Chung, M.; Wolf, A.; Assifi, M.M.; Wright, G.P. Recurrence patterns and postrecurrence survival after curative intent resection for pancreatic ductal adenocarcinoma. Surgery 2021, 169, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Tol, J.A.; Gouma, D.J.; Bassi, C.; Dervenis, C.; Montorsi, M.; Adham, M.; Andrén-Sandberg, A.; Asbun, H.J.; Bockhorn, M.; Büchler, M.W.; et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: A consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014, 156, 591–600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petrova, E.; Mazzella, E.; Trojan, J.; Koch, C.; Schulze, F.; Bechstein, W.O.; Schnitzbauer, A.A. Prognostic value of paraaortic lymph node metastases in patients with ductal adenocarcinoma of the pancreatic head. Eur. J. Surg. Oncol. 2023, 49, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Safi, S.A.; Rehders, A.; Haeberle, L.; Fung, S.; Lehwald, N.; Esposito, I.; Ziayee, F.; Krieg, A.; Knoefel, W.T.; Fluegen, G. Para-aortic lymph nodes and ductal adenocarcinoma of the pancreas: Distant neighbors? Surgery 2021, 170, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Sillesen, M.; Hansen, C.P.; Burgdorf, S.K.; Dencker, E.E.; Krohn, P.S.; Gisela Kollbeck, S.L.; Stender, M.T.; Storkholm, J.H. Impact of para aortic lymph node removal on survival following resection for pancreatic adenocarcinoma. BMC Surg. 2023, 23, 214. [Google Scholar] [CrossRef]
- Sperti, C.; Gruppo, M.; Blandamura, S.; Valmasoni, M.; Pozza, G.; Passuello, N.; Beltrame, V.; Moletta, L. Para-aortic node involvement is not an independent predictor of survival after resection for pancreatic cancer. World J. Gastroenterol. 2017, 23, 4399–4406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sho, M.; Murakami, Y.; Motoi, F.; Satoi, S.; Matsumoto, I.; Kawai, M.; Honda, G.; Uemura, K.; Yanagimoto, H.; Kurata, M.; et al. Postoperative prognosis of pancreatic cancer with para-aortic lymph node metastasis: A multicenter study on 822 patients. J. Gastroenterol. 2015, 50, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Paiella, S.; Sandini, M.; Gianotti, L.; Butturini, G.; Salvia, R.; Bassi, C. The prognostic impact of para-aortic lymph node metastasis in pancreatic cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2016, 42, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, L.; Lupinacci, R.M.; Svrcek, M.; Lesurtel, M.; Bubenheim, M.; Vuarnesson, H.; Balladur, P.; Paye, F. Para-aortic lymph node sampling in pancreatic head adenocarcinoma. Br. J. Surg. 2014, 101, 530–538. [Google Scholar] [CrossRef]
- UICC (International Union of Cancer). TNM Classification of Malignant Tumours, 8th ed.; Brierley, J.D., Gospodarowicz, M.K., Wittekind, C., Eds.; Wiley Blackwell: Oxford, UK, 2017. [Google Scholar]
- Ishida, M.; Fujii, T.; Kishiwada, M.; Shibuya, K.; Satoi, S.; Ueno, M.; Nakata, K.; Takano, S.; Uchida, K.; Ohike, N.; et al. Japanese classification of pancreatic carcinoma by the Japan Pancreas Society: Eighth edition. J. Hepato-Biliary-Pancreat. Sci. 2024, 31, 755–768. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hempel, S.; Plodeck, V.; Mierke, F.; Distler, M.; Aust, D.E.; Saeger, H.D.; Weitz, J.; Welsch, T. Para-aortic lymph node metastases in pancreatic cancer should not be considered a watershed for curative resection. Sci. Rep. 2017, 7, 7688. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brunner, M.; Krautz, C.; Maak, M.; Weber, G.F.; Grützmann, R. Pyloruserhaltende partielle Pankreatoduodenektomie mit segmentaler Pfortaderresektion [Pylorus-Preserving Pancreaticoduodenectomy (PPPD) with Segmental Portal Vein Resection]. Zentralbl. Chir. 2022, 147, 233–241. (In German) [Google Scholar] [CrossRef] [PubMed]
- Belfiori, G.; Crippa, S.; Pagnanelli, M.; Gasparini, G.; Aleotti, F.; Camisa, P.R.; Partelli, S.; Pecorelli, N.; De Stefano, F.; Schiavo Lena, M.; et al. Very Early Recurrence After Curative Resection for Pancreatic Ductal Adenocarcinoma: Proof of Concept for a “Biological R2 Definition”. Ann. Surg. Oncol. 2024, 31, 4084–4095. [Google Scholar] [CrossRef] [PubMed]
- Pranger, B.K.; Tseng, D.S.J.; Ubels, S.; van Santvoort, H.C.; Nieuwenhuijs, V.B.; de Jong, K.P.; Patijn, G.; Molenaar, I.Q.; Erdmann, J.I.; de Meijer, V.E. How to Approach Para-Aortic Lymph Node Metastases During Exploration for Suspected Periampullary Carcinoma: Resection or Bypass? Ann. Surg. Oncol. 2020, 27, 2949–2958. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.S.; Hwang, H.K.; Lee, W.J.; Kang, C.M. Unexpected Para-aortic Lymph Node Metastasis in Pancreatic Ductal Adenocarcinoma: A Contraindication to Resection? J. Gastrointest. Surg. 2020, 24, 2789–2799. [Google Scholar] [CrossRef] [PubMed]
- Shrikhande, S.V.; Kleeff, J.; Reiser, C.; Weitz, J.; Hinz, U.; Esposito, I.; Schmidt, J.; Friess, H.; Büchler, M.W. Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 2007, 14, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Huan, L.; Yu, F.; Cao, D.; Zhou, H.; Qin, M.; Cao, Y. Comparison of neoadjuvant treatment and surgery first for resectable or borderline resectable pancreatic carcinoma: A systematic review and network meta-analysis of randomized controlled trials. PLoS ONE 2024, 19, e0295983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Pancreatic Head Resection Without PALND | Pancreatic Head Resection with PALND | p-Value (No PALND vs. PALND) | Pancreatic Head Resection with PALND and PALN− | Pancreatic Head Resection with PALND and PALN+ | p-Value (PALN− vs. PALN+) | |
|---|---|---|---|---|---|---|
| Number | 85 | 113 | - | 96 | 17 | - |
| Age (years), median (IQR) | 71 (12) | 72 (15) | 0.620 | 70 (16) | 75 (9) | 0.178 |
| Sex, n (%) Female Male | 44 (52) 41 (48) | 45 (40) 68 (60) | 0.113 | 40 (42) 56 (58) | 5 (29) 12 (71) | 0.426 |
| ASA, n (%) I II III Unknown | 4 (5) 40 (47) 38 (45) 3 (3) | 6 (5) 60 (53) 45 (40) 2 (2) | 0.786 | 6 (6) 50 (52) 38 (40) 2 (2) | 0 (0) 10 (59) 7 (41) 0 (0) | 0.817 |
| pT category, n (%) pT1 pT2 pT3 | 13 (15) 45 (53) 27 (32) | 16 (14) 71 (63) 26 (23) | 0.336 | 16 (17) 58 (60) 22 (23) | 0 (0) 13 (77) 4 (24) | 0.203 |
| pN category, n (%) pN0 pN+ | 32 (38) 53 (62) | 39 (35) 74 (65) | 0.657 | 39 (41) 57 (59) | 0 (0) 17 (100) | 0.002 |
| R classification, n (%) R0 R1 | 76 (89) 9 (11) | 109 (96) 4 (4) | 0.079 | 93 (97) 3 (3) | 16 (94) 1 (6) | 1.000 |
| Grading, n (%) G1/2 G3 | 25 (29) 60 (71) | 35 (31) 78 (69) | 0.876 | 33 (34) 63 (66) | 2 (12) 15 (88) | 0.087 |
| Perineural invasion (Pn), n (%) 0 1 | 22 (26) 63 (74) | 27 (24) 86 (76) | 0.868 | 25 (26) 71 (74) | 2 (12) 15 (88) | 0.239 |
| Lymphangiosis (L), n (%) 0 1 | 60 (71) 25 (29) | 73 (65) 40 (35) | 0.445 | 69 (72) 27 (28) | 4 (24) 13 (76) | <0.001 |
| Vascular invasion (V), n (%) 0 1 | 72 (84) 14 (16) | 100 (88) 13 (12) | 0.403 | 88 (92) 8 (8) | 12 (71) 5 (29) | 0.026 |
| Major morbidity, n (%) | 19 (22) | 21 (19) | 0.592 | 18 (19) | 3 (18) | 1.000 |
| Adjuvant chemotherapy, n (%) | 50 (58) | 70 (62) | 0.663 | 59 (62) | 11 (65) | 1.000 |
| Pancreatic Head Resection Without PALND (n = 85) | Pancreatic Head Resection with PALND (n = 113) | p-Value (No PALND vs. PALND) | Pancreatic Head Resection with PALND and PALN− (n = 96) | Pancreatic Head Resection with PALND and PALN+ (n = 17) | p-Value (PALN− vs. PALN+) | |
|---|---|---|---|---|---|---|
| Overall survival (months), median (SD) | 19.5 (3.2) | 26.4 (2.9) | 0.696 | 29.3 (4.0) | 8.7 (2.5) | <0.001 |
| Recurrence, n (%) | 48 (57) | 64 (57) | 1.000 | 53 (55) | 11 (65) | 0.598 |
| Recurrence pattern, n (%) Metastatic Locoregional Both | 26 (54) 7 (15) 15 (31) | 42 (66) 5 (8) 17 (27) | 0.400 | 34 (64) 5 (9) 14 (26) | 8 (73) 0 (0) 3 (27) | 0.685 |
| Disease-free survival (months), median (SD) | 13.8 (1.9) | 14.4 (1.8) | 0.883 | 17.0 (2.5) | 3.8 (2.0) | <0.001 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| n | Median OS | p | HR | 95% CI | p-Value | |
| Age ≤70 years >70 years | 94 104 | 32.1 17.4 | <0.001 | 1.9 | 1.3–2.8 | 0.002 |
| Sex Female Male | 89 109 | 23.8 23.1 | 0.946 | - | - | - |
| ASA (n = 194) * I/II III | 111 83 | 26.6 17.0 | 0.014 | 1.3 | 0.9–1.8 | 0.232 |
| pT category pT1/pT2 pT3 | 145 53 | 26.6 15.1 | 0.016 | 1.3 | 0.8–2.0 | 0.260 |
| pN category pN0 pN+ | 71 127 | 44.1 17.5 | <0.001 | 2.6 | 1.7–4.2 | <0.001 |
| R classification R0 R1 | 185 13 | 23.7 10.3 | 0.031 | 2.8 | 1.4–5.5 | 0.003 |
| Grading G1/G2 G3 | 60 138 | 37.7 18.3 | <0.001 | 1.7 | 1.0–2.7 | 0.035 |
| Perineural invasion Pn0 Pn1 | 49 149 | 41.4 19.7 | 0.006 | 1.0 | 0.6–1.6 | 0.979 |
| Lymphangiosis L0 L1 | 133 65 | 27.4 17.0 | <0.001 | 1.1 | 0.7–1.7 | 0.751 |
| Vascular invasion V0 V1 | 172 26 | 24.4 12.3 | 0.007 | 2.6 | 1.5–4.4 | <0.001 |
| PALN(D) No PALND PALN− PALN+ | 85 96 17 | 19.5 29.4 8.7 | <0.001 | 1.0 0.8 1.9 | 0.6–1.2 1.0–3.6 | 0.035 |
| Major morbidity Yes No | 40 158 | 15.1 26.6 | <0.001 | 1.0 | 0.6–1.6 | 0.979 |
| Adjuvant chemotherapy Yes No | 120 78 | 26.6 17.0 | 0.012 | 2.5 | 1.6–3.8 | <0.001 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| n | Median OS | p | HR | 95% CI | p-Value | |
| Age ≤70 years >70 years | 94 104 | 18.2 10.1 | 0.001 | 1.6 | 1.1–2.2 | 0.018 |
| Sex Female Male | 89 109 | 13.8 14.4 | 0.759 | - | - | - |
| ASA (n = 194) * I/II III | 111 83 | 14.9 13.0 | 0.063 | - | - | - |
| pT category pT1/pT2 pT3 | 145 53 | 16.0 9.0 | 0.023 | 1.3 | 0.9–2.0 | 0.130 |
| pN category pN0 pN+ | 71 127 | 21.5 10.6 | <0.001 | 1.9 | 1.3–3.0 | 0.002 |
| R classification R0 R1 | 185 13 | 14.6 10.1 | 0.120 | - | - | - |
| Grading G1/G2 G3 | 60 138 | 21.2 11.7 | <0.001 | 1.6 | 1.0–2.4 | 0.034 |
| Perineural invasion Pn0 Pn1 | 49 149 | 23.7 12.3 | 0.003 | 1.2 | 0.8–1.9 | 0.434 |
| Lymphangiosis L0 L1 | 133 65 | 16.6 9.1 | <0.001 | 1.3 | 0.8–1.9 | 0.252 |
| Vascular invasion V0 V1 | 172 26 | 14.7 8.7 | 0.038 | 1.8 | 1.1–3.0 | 0.020 |
| PALN No PALND PALN− PALN+ | 85 96 17 | 13.8 17.0 3.8 | <0.001 | 1.0 0.8 2.2 | 0.6–1.2 1.2–4.0 | 0.006 |
| Major morbidity Yes No | 40 158 | 7.0 15.5 | 0.003 | 1.1 | 0.7–1.8 | 0.600 |
| Adjuvant chemotherapy Yes No | 120 78 | 15.5 8.0 | 0.015 | 2.1 | 1.4–3.1 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunner, M.; Kitzke, L.; Mittelstädt, A.; Merkel, S.; Weber, G.F.; Grützmann, R.; Krautz, C. The Prognostic Role of Para-Aortic Lymph Node Metastasis in Patients with Resected Pancreatic Adenocarcinoma. Cancers 2025, 17, 3418. https://doi.org/10.3390/cancers17213418
Brunner M, Kitzke L, Mittelstädt A, Merkel S, Weber GF, Grützmann R, Krautz C. The Prognostic Role of Para-Aortic Lymph Node Metastasis in Patients with Resected Pancreatic Adenocarcinoma. Cancers. 2025; 17(21):3418. https://doi.org/10.3390/cancers17213418
Chicago/Turabian StyleBrunner, Maximilian, Lena Kitzke, Anke Mittelstädt, Susanne Merkel, Georg F. Weber, Robert Grützmann, and Christian Krautz. 2025. "The Prognostic Role of Para-Aortic Lymph Node Metastasis in Patients with Resected Pancreatic Adenocarcinoma" Cancers 17, no. 21: 3418. https://doi.org/10.3390/cancers17213418
APA StyleBrunner, M., Kitzke, L., Mittelstädt, A., Merkel, S., Weber, G. F., Grützmann, R., & Krautz, C. (2025). The Prognostic Role of Para-Aortic Lymph Node Metastasis in Patients with Resected Pancreatic Adenocarcinoma. Cancers, 17(21), 3418. https://doi.org/10.3390/cancers17213418







