Thrombotic Risk and Coagulation Imbalance in Cirrhosis and Hepatocellular Carcinoma: Clinical Implications and Management
Simple Summary
Abstract
1. Background
2. Incidence and Risk Factors
2.1. Portal Vein Thrombosis
2.2. Venous Thromboembolism
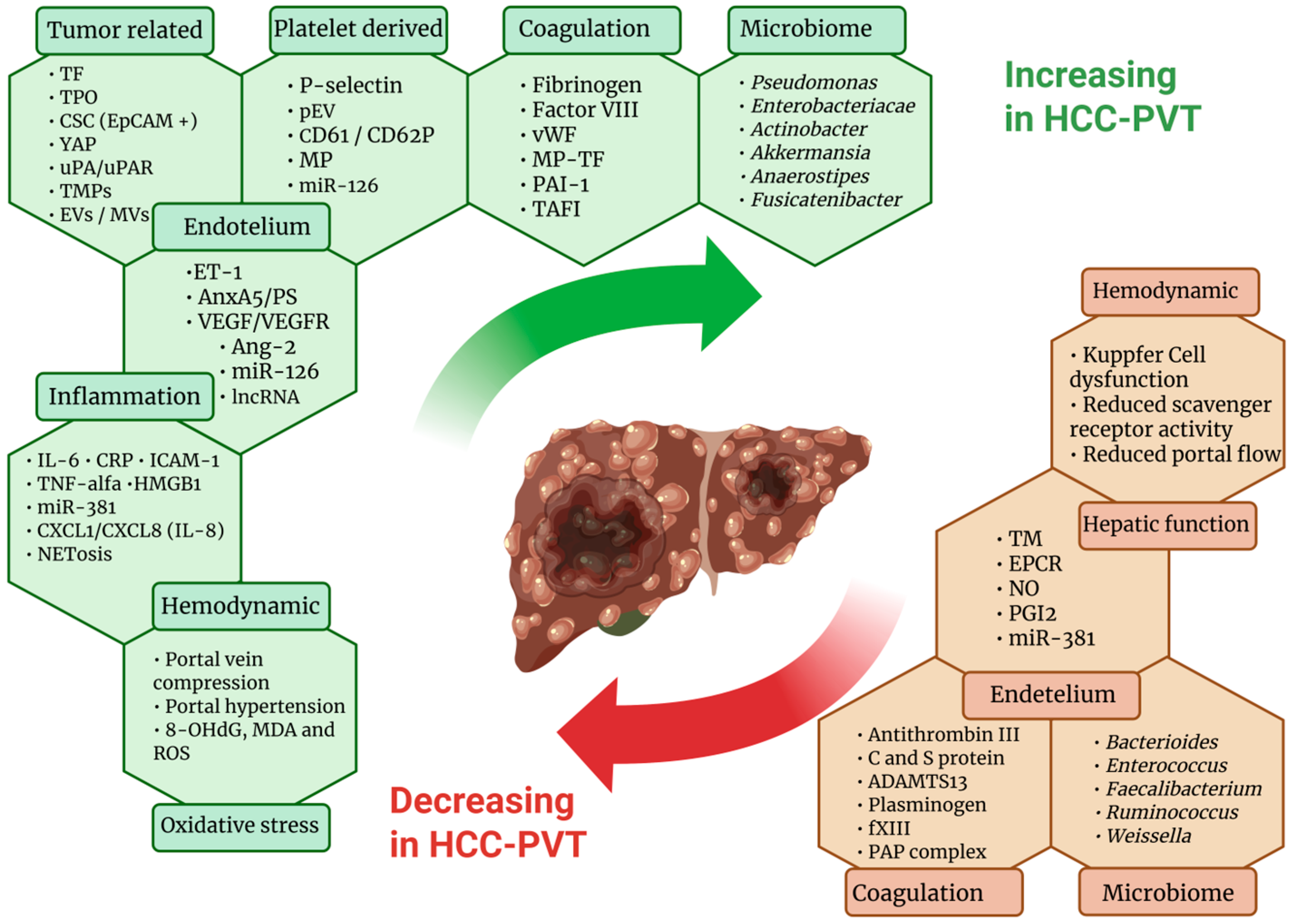
3. Mechanisms of Hemostatic Alterations
3.1. Cirrhosis-Related Alterations
3.2. Tumor-Related Factors
3.3. Platelet-Related Factors
3.4. Coagulation and Fibrinolytic Factors
3.5. Hemodynamic Factors and Endothelial Factors
3.6. Inflammatory and Oxidative Stress Factors
3.7. Gut Microbiome
4. New Diagnostic Tools and Risk Stratification Methods
5. Anticoagulant Treatment and Prophylaxis in HCC Patients
5.1. Anticoagulant Treatment
5.2. Primary Prophylaxis
5.3. Secondary Prophylaxis
| Study | Design | Year | Thrombosis Site | Population | HCC Treatment | AC Treatment or Prophylaxis | Outcome | Safety |
|---|---|---|---|---|---|---|---|---|
| AC primary prophylaxis | ||||||||
| Vivarelli et al. [121] | Retrospective | 2010 | VTE | 229 patients with cirrhosis and HCC | Surgery | 157 (68.5%) in LMWH in primary prophylaxis. | Incidence of VTE 0.63% in AC vs. 1.38% in control group (p = 0.38). | Postoperative hemorrhage 3.18% in AC vs. 1.38% in control group. Esophageal varices were linked to an increased risk of bleeding. |
| Kim BJ et al. [123] | Prospective | 2017 | VTE | 124 patients undergoing liver surgery for malignancy (10, 8.1% HCC) | Surgery | LMWH extended primary prophylaxis (up to 28 days) | Extended pharmacologic thromboprophylaxis resulted in 0 cases of VTE (DVT or PE) during 90-day follow-up. | Only 2 patients (1.6%) had minor bleeding, with no transfusion or reoperation required. |
| P. Serrano et al. [120] | Prospective | 2018 | VTE | 284 patients, 97 (34.2%) patients undergoing laparotomy for hepatobiliary cancer | Surgical, unknown number of HCC patients | LMWH, primary prophylaxis | VTE incidence 0.35% at 1, 2.5% at 3, and 7.2% at 6 months. | Mortality 6.6%. No bleeding analysis. |
| Zhang et al. [122] | Prospective | 2025 | VTE | 140 patients undergoing surgery for HCC | Surgical | LMWH, primary prophylaxis | Incidence of VTE was 4.8% (3/62) vs. 14.9% (11/74) (p = 0.091, OR = 0.31, 95% CI: 0.08–1.16) in the group with reduced prophylaxis vs. no prophylaxis. | Reduced prophylaxis will not increase risk of hemorrhagic complications (OR = 0.34, 95% CI: 0.07–1.69, p = 0.186). |
| AC treatment and secondary prophylaxis | ||||||||
| Rajani et al. [129] | Retrospective | 2010 | PVT | 173 patients with PVT, 40% (80) with cirrhosis, 27% (47) with malignancy | Unknown treatment | 65% of patients in AC treatment with LMWH or VKA | No improvement in survival with anticoagulation therapy. | 2 patients who had not received AC died of AVB. |
| Senzolo et al. [25] | Prosective | 2018 | SVT | 149 patients with liver cirrhosis and SVT, 39 (26.2%) HCC | Unknown treatment | Treatment with LMWH or fondaparinux | Anticoagulation led to 61.9% recanalization at 6 months. One-year survival was higher with recanalization (58.8% vs. 20%, p = 0.03). No thrombotic recurrence or progression occurred. | Major bleeding was rare (4.7%) |
| Davis KA et al. [125] | Retrospective | 2019 | VTE | 82 cirrhotic patients with VTE in VKA (26.8% with malignancy) and 27 in DOAC (25.9% with malignancy) | Unknown treatment | VKA vs. DOAC, treatment or secondary prophylaxis | Recurrent VTE occurred in 10 (12.2%) patients receiving treatment with warfarin and 3 (11.1%) of patients treated with DOAC therapy. | Eleven (13.4%) patients in the warfarin group experienced a major bleed compared to 2 (7.4%) of patients receiving DOAC therapy (p = 0.51) |
| Bikdeli et al. [126] | Prospective (RIETE registry) | 2019 | VTE | Among 43,611 patients with VTE,187 patients with cirrhosis, and 75 patients (40.1%) with active cancer | Unknown treatment | 98.4% on AC (LMWH for most patients) for a median of 109 days | VTE recurrence higher in patients with cirrhosis (HR 2.08). Fatal PE rate was comparable (0.5% in both groups). | Overall bleeding comparable, fatal bleeding higher in patients with cirrhosis (2.1% vs. 0.2%). |
| Mahmoudi T et al. [112] | Retrospective | 2019 | PVT | 51 patients with HCC and PVT | Surgery, LRTs or Systemic treatment | 12 (23.5%) on AC vs. 39 (76.5%) without AC. VKA or LMWH treatment. | PVT progression: 50% with AC, 49%. AC had no impact (HR 1.32, 95% CI 0.41–4.19). | AC therapy carried a bleeding risk, suggesting the need for individualized treatment decisions. |
| Chen et al. [34] | Retrospective | 2021 | DVT | 355 patients with HCC, 66 (18.6%) with DVT | Surgery | 66 (100%) on AC treatment (unspecified) | DVT disappeared within 2 months in 63 (95.5%) cases, with chronic DVT remaining in 3 (4.5%) cases. | No PE or major bleeding occurred during the treatment. |
| Semmler et al. [116] | Retrospective | 2021 | VTE | 33 patients with cirrhosis and HCC | 48.5% on TKI treatment | All on DOACs | Thrombotic events and outcomes not specifically reported. | Bleeding occurred in 31.7%, major in 17.3%; associated with CPS B/C (aSHR: 4.12, p < 0.001), not with HCC. |
| Candeloro et al. [127] | Meta-analysis | 2022 | SVT (mostly PVT) | 1635 patients with SVT, 278 (17%) with liver cirrhosis, 523 (32%) with solid cancer | Unknown treatment | None vs. AC (Heparin, VKA, or DOAC) | Anticoagulation reduced SVT (HR 0.42), bleeding (HR 0.47), and mortality (HR 0.23). In cancer patients, mortality dropped from 23.3 to 5.7 per 100 p-y (HR 8.68). | Among cirrhotic patients, bleeding risk was higher (HR 1.92) but outweighed by benefits. |
| Benevento et al. [110] | Retrospective | 2023 | PVT | 162 patients with cirrhosis and PVT, 30 with HCC | Unknown treatment | LMWH (30.9%), VKA (6.2%), fondaparinux (5.5%); no DOACs used | Recanalization in 50.7% of treated patients; similar rates between HCC (46.1%) and non-HCC (51.9%); median time to recanalization: 4.5 months | Bleeding events: 13.3% in HCC vs. 25% in non-HCC (p = 0.230); treatment discontinuation due to bleeding: ~3% in both groups; no significant safety concerns. |
| Kais et al. [114] | Retrospective | 2023 | PVT | 122 cirrhotic patients with HCC | Unknown treatment | 54% anticoagulant (apixaban 56%, LMWH 21%, others 23%) | No survival benefit: median OS 6 months in both groups (HR 0.91, p = 0.72). Recanalization: not reported. | 25% bleeding complications in the AC group. |
| Senzolo et al. [111] | Retrospective | 2024 | PVT | 88 cirrhotic patients with HCC and PVT | Mixed, but mostly LPS-ablation | 36.5% (22/83) PVT patients received anticoagulation (type/dose not specified) | PVT is associated with lower OS; anticoagulation significantly improved PVT outcomes (50% vs. 6.6% improvement; 9.1% vs. 62.3% progression). | No bleeding events or treatment discontinuations occurred. |
| Balcar et al. [113] | Retrospective | 2024 | PVT | 124 cirrhotic patients with HCC, 47 (38%) with PVTT and 49 individuals (40%) with non-tumorous PVT | 94 patients (76%) were treated with effective systemic therapies | 24 individuals (19%) received therapeutic anticoagulation | AC did not affect malignant thrombosis. Systemic therapy (aHR 0.26) but no-AC was independently associated with reduced all-cause mortality. | Non-selective beta-blockers were associated with reduced risk of variceal bleeding or death from any cause (aHR 0.69). |
6. Combination Therapies: Anticoagulants and Immunotherapy
| Study | Design | Year | Thrombosis Site | Population | Anticoagulant Treatment or Prophylaxis | Safety | Risk Factors for Bleeding |
|---|---|---|---|---|---|---|---|
| Larrey et al. [137] | Prospective | 2022 | PVT + DVT/PE | 43 patients with HCC undergoing AtezoBev | 48.8% on curative AC (unspecified) | AVB incidence was higher with atezolizumab–bevacizumab than sorafenib (21% vs. 5% at 1 year, p = 0.02). No bleeding-related death. | - History of AVB (HR: 10.58, p = 0.03). |
| Moriguchi M. et al. [135] | Retrospective | 2023 | PVT + DVT/PE | 185 patients (IMBRAVE150 in: 157; IMBRAVE150 out: 28) | Curative anticoagulants (unspecified) or antiplatelets | 14 had grade ≥ 3 hemorrhage complications: 11 in the anticoagulant group (7.9%) and 3 in the other group (10.7%); no deaths attributable to bleeding events. | - High ALBI score. No significant PFS/OS difference. |
| Ben Khaled et al. [103] | Retrospective | 2024 | PVT + DVT/PE | 325 patients with HCC undergoing AtezoBev vs. 139 undergoing Lenvatinib | 95 (29%) patients in AC (60 LMWH, 3 VKA, 30 DOACs) | 3 months bleeding in 18% of patients on AtezoBev and 11% on Lenv, variceal hemorrhage in 3% for both, and VTE events in 6% vs. 4%, not significant. | For GI bleeding: - Spleen size (OR: 1.1, p = 0.03) - History of variceal bleeding (OR: 3.0, p = 0.04). For non-GI bleeding: - anticoagulation use (OR: 2.2, p = 0.04). |
| Stefanini et al. [32] | Prospective | 2024 | PVT + DVT/PE | 397 patients with HCC undergoing AtezoBev | 9 patients on AC (unknown drugs) | Variceal bleeding (3.5%), digestive non-variceal bleeding (2.5%). | - PS > 0 - PVTT- AFP > 400 ng/mL, - ALBI grade > 1, - N/L ratio > 3. |
| Allaire et al. [134] | Prospective | 2024 | PVT + DVT/PE | 200 patients with HCC undergoing AtezoBev on AVB prophylaxis | 20% on curative AC | AVB incidence: 12% at 12 months; AC not associated with mortality (aHR: 0.75) or AVB (aHR: 1.34). | - PVTT (aHR: 3.25) - history of AVB < 6 months (aHR: 4.32) - varices of any size (aHR: 3.22). |
| Nakabori et al. [136] | Retrospective | 2024 | PVT + DVT/PE | 59 patients with HCC undergoing AtezoBev | 5 (8.5%) patients on DOAC | Bleeding rates did not differ between DOAC and no-AC groups; DOAC use was not associated with bleeding (HR: 1.357, p = 0.770). | - Low albumin (HR: 0.298, p = 0.023) - high ALBI score (HR: 9.083, p = 0.039). |
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Anticoagulation |
| ADAMTS13 | A Disintegrin And Metalloproteinase with Thrombospondin Motifs 13 |
| AFP | Alpha-Fetoprotein |
| ALBI | Albumin Bilirubin |
| Ang-2 | Angiopoietin 2 |
| AnxA5/PS | Annexin A5/Phosphatidylserine |
| AtezoBev | Atezolizumab–Bevacizumab treatment |
| AVB | Acute Variceal Bleeding |
| CD61/CD62P | Cluster of Differentiation 61/62P |
| CI | Confidence Interval |
| CPS | Child–Pugh Score |
| CRP | C-Reactive Protein |
| CSC (EpCAM+) | Cancer Stem Cells (Epithelial Cell Adhesion Molecule positive) |
| CSPH | Clinically Significant Portal Hypertension |
| CTP | Child Turcotte Pugh |
| CXCL | C-X-C Motif Chemokine |
| DOAC | Direct Oral Anticoagulant |
| DVT | Deep Vein Thrombosis |
| ECOG PS | Eastern Cooperative Oncology Group Performance Status |
| EPCR | Endothelial Protein C Receptor |
| ET-1 | Endothelin 1 |
| EVs/MVs | Extracellular Vesicles/Microvesicles |
| HCC | Hepatocellular Carcinoma |
| HMGB1 | High-Mobility Group Box 1 |
| HR | Hazard Ratio |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| IL-6 | Interleukin 6 |
| Lenv | Lenvatinib |
| LMWH | Low-Molecular-Weight Heparin |
| lncRNA | Long Non-Coding RNA |
| LPS | Laparoscopic Surgery |
| LRT | Locoregional Therapy |
| MCF | Maximum Clot Firmness |
| MA | Maximum Amplitude |
| miR | MicroRNA |
| MP | Microparticles |
| MVI | Macrovascular Invasion |
| MWA | Microwave Ablation |
| NETosis | Neutrophil Extracellular Trap Formation |
| NO | Nitric Oxide |
| OLT | Orthotopic Liver Transplantation |
| OR | Odds Ratio |
| OS | Overall Survival |
| PAI-1 | Plasminogen Activator Inhibitor 1 |
| PAP | Plasmin Antiplasmin Complex |
| PE | Pulmonary Embolism |
| pEV | Platelet-derived Extracellular Vesicle |
| PFS | Progression-Free Survival |
| PGI2 | Prostacyclin (Prostaglandin I2) |
| PLT | Platelet Count |
| p-y | Person Years |
| PVT | Portal Vein Thrombosis |
| ROTEM | Rotational Thromboelastometry |
| ROS | Reactive Oxygen Species |
| SVT | Splanchnic Vein Thrombosis |
| TAFI | Thrombin-Activatable Fibrinolysis Inhibitor |
| TEG-PM | Thromboelastography with Platelet Mapping |
| TF | Tissue Factor |
| TGA | Thrombin Generation Assay |
| TM | Thrombomodulin |
| TMPs | Tumor-derived Microparticles |
| TNF-α | Tumor Necrosis Factor alpha |
| TPO | Thrombopoietin |
| uPA/uPAR | Urokinase-type Plasminogen Activator/uPA Receptor |
| vWF | von Willebrand Factor |
| VKA | Vitamin K Antagonist |
| VEGF/VEGFR | Vascular Endothelial Growth Factor/VEGF Receptor |
| VTE | Venous Thromboembolism |
| YAP | Yes-associated Protein |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Sangro, B.; Argemi, J.; Ronot, M.; Paradis, V.; Meyer, T.; Mazzaferro, V.; Jepsen, P.; Golfieri, R.; Galle, P.; Dawson, L.; et al. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J. Hepatol. 2025, 82, 315–374. [Google Scholar] [CrossRef]
- Flores, B.; Trivedi, H.D.; Robson, S.C.; Bonder, A. Hemostasis, bleeding and thrombosis in liver disease. J. Transl. Sci. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Abdulla, A.; Davis, W.M.; Ratnaweera, N.; Szefer, E.; Ballantyne Scott, B.; Lee, A.Y.Y. A Meta-Analysis of Case Fatality Rates of Recurrent Venous Thromboembolism and Major Bleeding in Patients with Cancer. Thromb. Haemost. 2020, 120, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Mackman, N.; Falanga, A.; Pabinger, I.; Noble, S.; Ageno, W.; Moik, F.; Lee, A.Y.Y. Cancer-associated venous thromboembolism. Nat. Rev. Dis. Primers 2022, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Connolly, G.C. Assessing risk of venous thromboembolism in the patient with cancer. J. Clin. Oncol. 2009, 27, 4839–4847. [Google Scholar] [CrossRef]
- Stein, P.D.; Beemath, A.; Meyers, F.A.; Skaf, E.; Sanchez, J.; Olson, R.E. Incidence of venous thromboembolism in patients hospitalized with cancer. Am. J. Med. 2006, 119, 60–68. [Google Scholar] [CrossRef]
- Francoz, C.; Valla, D.; Durand, F. Portal vein thrombosis, cirrhosis, and liver transplantation. J. Hepatol. 2012, 57, 203–212. [Google Scholar] [CrossRef]
- Campello, E.; Zanetto, A.; Bulato, C.; Maggiolo, S.; Spiezia, L.; Russo, F.P.; Gavasso, S.; Mazzeo, P.; Tormene, D.; Burra, P.; et al. Coagulopathy is not predictive of bleeding in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. Liver Int. 2021, 41, 2455–2466. [Google Scholar] [CrossRef]
- Zanetto, A.; Rinder, H.M.; Senzolo, M.; Simioni, P.; Garcia-Tsao, G. Reduced Clot Stability by Thromboelastography as a Potential Indicator of Procedure-Related Bleeding in Decompensated Cirrhosis. Hepatol. Commun. 2020, 5, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Nonami, T.; Yokoyama, I.; Iwatsuki, S.; Starzl, T.E. The incidence of portal vein thrombosis at liver transplantation. Hepatology 1992, 16, 1195–1198. [Google Scholar] [CrossRef]
- Davidson, B.R.; Gibson, M.; Dick, R.; Burroughs, A.; Rolles, K. Incidence, risk factors, management, and outcome of portal vein abnormalities at orthotopic liver transplantation. Transplantation 1994, 57, 1174–1177. [Google Scholar] [CrossRef]
- Ravaioli, M.; Zanello, M.; Grazi, G.L.; Ercolani, G.; Cescon, M.; Del Gaudio, M.; Cucchetti, A.; Pinna, A.D. Portal vein thrombosis and liver transplantation: Evolution during 10 years of experience at the University of Bologna. Ann. Surg. 2011, 253, 378–384. [Google Scholar] [CrossRef]
- Ogren, M.; Bergqvist, D.; Björck, M.; Acosta, S.; Eriksson, H.; Sternby, N.H. Portal vein thrombosis: Prevalence, patient characteristics and lifetime risk: A population study based on 23,796 consecutive autopsies. World J. Gastroenterol. 2006, 12, 2115–2119. [Google Scholar] [CrossRef]
- Connolly, G.C.; Chen, R.; Hyrien, O.; Mantry, P.; Bozorgzadeh, A.; Abt, P.; Khorana, A.A. Incidence, risk factors and consequences of portal vein and systemic thromboses in hepatocellular carcinoma. Thromb. Res. 2008, 122, 299–306. [Google Scholar] [CrossRef]
- Tzeng, C.-W.D.; Katz, M.H.G.; Fleming, J.B.; Pisters, P.W.T.; Lee, J.E.; Abdalla, E.K.; Curley, S.A.; Vauthey, J.N.; Aloia, T.A. Risk of venous thromboembolism outweighs post-hepatectomy bleeding complications: Analysis of 5651 National Surgical Quality Improvement Program patients. HPB 2012, 14, 506–513. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Zocco, M.A.; Senzolo, M.; Pompili, M.; Gasbarrini, A.; Avolio, A.W. Portal vein thrombosis and liver transplantation: Implications for waiting list period, surgical approach, early and late follow-up. Transplant. Rev. 2014, 28, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Zanetto, A.; Senzolo, M.; Vitale, A.; Cillo, U.; Radu, C.; Sartorello, F.; Spiezia, L. Thromboelastometry hypercoagulable profiles and portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma. Dig. Liver Dis. 2017, 49, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Cagin, Y.F.; Atayan, Y.; Erdogan, M.A.; Dagtekin, F.; Colak, C. Incidence and clinical presentation of portal vein thrombosis in cirrhotic patients. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Attar, B.M.; Fuentes, H.E.; Yu, J.; Zhang, H.; Tafur, A.J. Performance of Khorana Risk Score for Prediction of Venous Thromboembolism in Patients with Hepatocellular Carcinoma. Clin. Appl. Thromb. Hemost. 2018, 24, 471–476. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Latteri, S.; Bertino, G.; Madeddu, R.; Catania, V.E.; Currò, G.; Borzì, A.M.; Drago, F.; Malaguarnera, G. D-dimer plasmatic levels as a marker for diagnosis and prognosis of hepatocellular carcinoma patients with portal vein thrombosis. CEG 2018, 11, 373–380. [Google Scholar] [CrossRef]
- Violi, F.; Corazza, G.R.; Caldwell, S.H.; Talerico, G.; Romiti, G.F.; Napoleone, L.; Perticone, F.; Bolondi, L.; Pietrangelo, A.; Vestri, A.R.; et al. Incidence and Recurrence of Portal Vein Thrombosis in Cirrhotic Patients. Thromb. Haemost. 2019, 119, 496–499. [Google Scholar] [CrossRef]
- Al-Taee, A.M.; Mohammed, K.A.; Khneizer, G.W.; Neuschwander-Tetri, B.A. Correlates, Trends, and Short-Term Outcomes of Venous Thromboembolism in Hospitalized Patients with Hepatocellular Carcinoma. J. Gastrointest. Cancer 2019, 50, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Serag, W.M.; Mohammed, B.S.E.; Mohamed, M.M.; Elsayed, B.E. Predicting the risk of portal vein thrombosis in patients with liver cirrhosis and hepatocellular carcinoma. Heliyon 2020, 6, e04677. [Google Scholar] [CrossRef]
- Senzolo, M.; Riva, N.; Dentali, F.; Rodriguez-Castro, K.; Sartori, M.T.; Bang, S.M.; Martinelli, I.; Schulman, S.; Alatri, A.; Beyer-Westendorf, J.; et al. Long-Term Outcome of Splanchnic Vein Thrombosis in Cirrhosis. Clin. Transl. Gastroenterol. 2018, 9, 176. [Google Scholar] [CrossRef]
- Ow, T.W.; Fatourou, E.; Rabinowich, L.; van den Boom, B.; Nair, S.; Patel, V.C.; Hogan, B.; McPhail, M.; Roberts, L.N.; Bernal, W. Prevalence of Bleeding and Thrombosis in Critically Ill Patients with Chronic Liver Disease. Thromb. Haemost. 2021, 122, 1006–1016. [Google Scholar] [CrossRef]
- Grasso, M.; Vitale, A.; Pizzirani, E.; Zanetto, A.; Gringeri, E.; D’Amico, F.; Gambato, M.; Cillo, U.; Burra, P.; Senzolo, M. Analysis of clinical course of portal vein thrombosis in cirrhosis with hepatocellular carcinoma. Dig. Liver Dis. 2021, 53, S43. [Google Scholar] [CrossRef]
- Faccia, M.; Santopaolo, F.; Gasbarrini, A.; Pompili, M.; Zocco, M.A.; Ponziani, F.R. Risk factors for portal vein thrombosis or venous thromboembolism in a large cohort of hospitalized cirrhotic patients. Intern. Emerg. Med. 2022, 17, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.T.U.-H.; Fareed, G.; Khan, M.R.; Riaz, A.; Hamid, S.S. Portal vein thrombosis in patients with hepatocellular carcinoma and early cirrhosis—Prevalence and risk factors. eCancer 2023, 17, 1581. [Google Scholar] [CrossRef]
- Lesmana, C.R.A.; Inggriani, S.; Cahyadinata, L.; Lesmana, L.A. Deep vein thrombosis in patients with advanced liver cirrhosis: A rare condition? Hepatol. Int. 2010, 4, 433. [Google Scholar] [CrossRef]
- Campello, E.; Zanetto, A.; Spiezia, L.; Radu, C.M.; Gavasso, S.; Ferrarese, A.; Farinati, F.; Senzolo, M.; Simioni, P. Hypercoagulability detected by circulating microparticles in patients with hepatocellular carcinoma and cirrhosis. Thromb. Res. 2016, 143, 118–121. [Google Scholar] [CrossRef]
- Stefanini, B.; Iavarone, M.; Marra, F.; Cabibbo, G.; Vivaldi, C.; Palloni, A.; on behalf of the ARTE study group. Efficacy and safety of atezolizumab/bevacizumab for hepatocellular carcinoma in a real-life prospective cohort: A 2024 update. Dig. Liver Dis. 2025, 57, S28–S29. [Google Scholar] [CrossRef]
- Schlick, C.J.R.; Ellis, R.J.; Merkow, R.P.; Yang, A.D.; Bentrem, D.J. Development and validation of a risk calculator for post-discharge venous thromboembolism following hepatectomy for malignancy. HPB 2021, 23, 723–732. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, J.; Zhang, Z.; Ding, Z.; Chen, Y.; Chen, X.; Zhang, W. Construction and Validation of a Nomogram for Predicting the Risk of Deep Vein Thrombosis in Hepatocellular Carcinoma Patients After Laparoscopic Hepatectomy: A Retrospective Study. J. Hepatocell. Carcinoma 2021, 8, 783–794. [Google Scholar] [CrossRef]
- Liu, L.; Liu, D.; He, W.; Zhang, W. Incidence of thrombosis in patients with hepatocellular carcinoma: Systematic review and meta-analysis. J. Thromb. Thrombolysis 2025. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.; Mannucci, P.M. The Coagulopathy of Chronic Liver Disease. N. Engl. J. Med. 2011, 365, 147–156. [Google Scholar] [CrossRef]
- Islam, R.; Kundu, S.; Jha, S.B.; Rivera, A.P.; Flores Monar, G.V.; Islam, H.; Puttagunta, S.M.; Sange, I. Cirrhosis and Coagulopathy: Mechanisms of Hemostasis Changes in Liver Failure and Their Management. Cureus 2022, 14, e23785. [Google Scholar] [CrossRef] [PubMed]
- Zanetto, A.; Campello, E.; Bulato, C.; Gavasso, S.; Saggiorato, G.; Shalaby, S.; Spiezia, L.; Cillo, U.; Farinati, F.; Russo, F.P.; et al. More Pronounced Hypercoagulable State and Hypofibrinolysis in Patients with Cirrhosis with Versus without HCC. Hepatol. Commun. 2021, 5, 1987–2000. [Google Scholar] [CrossRef]
- Willems, R.A.L.; Zanetto, A.; Campello, E.; Simone Ide Bulato, C.; Konings, J.; Kramer, M.; Tufaha, S.; Russo, F.P.; Senzolo, M.; Burra, P.; et al. Patients with cirrhosis have a disbalance between coagulation and fibrinolysis resulting in a prothrombotic phenotype. J. Thromb. Haemost. 2025, 23, 1974–1987. [Google Scholar] [CrossRef]
- Zanetto, A.; Campello, E.; Pelizzaro, F.; Farinati, F.; Burra, P.; Simioni, P.; Zanetto, A.; Campello, E.; Pelizzaro, F.; Farinati, F.; et al. Haemostatic alterations in patients with cirrhosis and hepatocellular carcinoma: Laboratory evidence and clinical implications. Liver Int. 2022, 42, 1229–1240. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Han, B.; Zhu, Z.; Sun, L.; Cui, X. The Efficacy and Safety of Anticoagulants in the Treatment of Cirrhotic Portal Vein Thrombosis: A Systematic Review and Meta-Analysis. Clin. Appl. Thromb. Hemost. 2022, 28, 1–10. [Google Scholar] [CrossRef]
- Groeneveld, D.J.; Poole, L.G.; Luyendyk, J.P. Targeting von Willebrand factor in liver diseases: A novel therapeutic strategy? J. Thromb. Haemost. 2021, 19, 1390–1408. [Google Scholar] [CrossRef] [PubMed]
- Stine, J.G.; Wang, J.; Shah, P.M.; Argo, C.K.; Intagliata, N.; Uflacker, A.; Caldwell, S.H.; Northup, P.G. Decreased Portal Vein Velocity is Predictive of the Development of Portal Vein Thrombosis: A Matched Case-Control Study. Liver Int. 2017, 38, 94. [Google Scholar] [CrossRef]
- Airola, C.; Severino, A.; Porcari, S.; Fusco, W.; Mullish, B.H.; Gasbarrini, A.; Cammarota, G.; Ponziani, F.R.; Ianiro, G. Future Modulation of Gut Microbiota: From Eubiotics to FMT, Engineered Bacteria, and Phage Therapy. Antibiotics 2023, 12, 868. [Google Scholar] [CrossRef]
- Galasso, L.; Cerrito, L.; Termite, F.; Mignini, I.; Esposto, G.; Borriello, R.; Galasso, L.; Cerrito, L.; Termite, F.; Mignini, I.; et al. The Molecular Mechanisms of Portal Vein Thrombosis in Hepatocellular Carcinoma. Cancers 2024, 16, 3247. [Google Scholar] [CrossRef]
- El-Mokhtar, S.A.; Afifi, N.A.; Abdel-Malek, M.O.; Hassan, W.A.; Hetta, H.; El-Badawy, O. Aberrant cytokine and VCAM-1 expression in patients with viral and non-viral related liver cirrhosis. Cytokine 2023, 171, 156385. [Google Scholar] [CrossRef]
- Jiang, S.; Ai, Y.; Ni, L.; Wu, L.; Huang, X.; Chen, S. Platelet-derived TGF-β1 is related to portal vein thrombosis in cirrhosis by promoting hypercoagulability and endothelial dysfunction. Front. Cardiovasc. Med. 2022, 9, 938397. [Google Scholar] [CrossRef] [PubMed]
- Terbah, R.; Testro, A.; Gow, P.; Majumdar, A.; Sinclair, M. Portal Hypertension in Malnutrition and Sarcopenia in Decompensated Cirrhosis—Pathogenesis, Implications and Therapeutic Opportunities. Nutrients 2023, 16, 35. [Google Scholar] [CrossRef]
- Molinari, M.; Fernandez-Carrillo, C.; Dai, D.; Dana, J.; Clemente-Sanchez, A.; Dharmayan, S.; Kaltenmeier, C.; Liu, H.; Behari, J.; Rachakonda, V.; et al. Portal vein thrombosis and renal dysfunction: A national comparative study of liver transplant recipients for NAFLD versus alcoholic cirrhosis. Transpl. Int. 2021, 34, 1105. [Google Scholar] [CrossRef]
- Dalbeni, A.; Cattazzo, F.; Marco, L.D.; Bevilacqua, M.; Zoncapè, M.; Lombardi, R.; Stupia, R.; Mantovani, A.; Sacerdoti, D. Bacterial infections as a risk factor for non-neoplastic portal vein thrombosis development in cirrhotic patients. Dig. Liver Dis. 2024, 56, 477–483. [Google Scholar] [CrossRef]
- Amitrano, L.; Guardascione, M.A.; Brancaccio, V.; Margaglione, M.; Manguso, F.; Iannaccone, L.; Grandone, E.; Balzano, A. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J. Hepatol. 2004, 40, 736–741. [Google Scholar] [CrossRef]
- He, Q.; Yang, J.; Jin, Y. Immune infiltration and clinical significance analyses of the coagulation-related genes in hepatocellular carcinoma. Brief. Bioinform. 2022, 23, bbac291. [Google Scholar] [CrossRef]
- Ahmadi, S.E.; Shabannezhad, A.; Kahrizi, A.; Akbar, A.; Safdari, S.M.; Hoseinnezhad, T.; Zahedi, M.; Sadeghi, S.; Golizadeh Mojarrad, M.; Safa, M. Tissue factor (coagulation factor III): A potential double-edge molecule to be targeted and re-targeted toward cancer. Biomark. Res. 2023, 11, 60. [Google Scholar] [CrossRef]
- Poon, R.T.-P.; Lau, C.P.-Y.; Ho, J.W.-Y.; Yu, W.-C.; Fan, S.-T.; Wong, J. Tissue factor expression correlates with tumor angiogenesis and invasiveness in human hepatocellular carcinoma. Clin. Cancer Res. 2003, 9, 5339–5345. [Google Scholar]
- Hwang, S.-J.; Luo, J.-C.; Li, C.-P.; Chu, C.-W.; Wu, J.-C.; Lai, C.-R.; Chiang, J.-H.; Chau, G.-Y.; Lui, W.-Y.; Lee, C.-C.; et al. Thrombocytosis: A paraneoplastic syndrome in patients with hepatocellular carcinoma. World J. Gastroenterol. 2004, 10, 2472–2477. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Wu, J.-D.; Fang, M.-M.; Pu, L.-Y. Clinicopathological significance and prognostic value of the expression of the cancer stem cell marker CD133 in hepatocellular carcinoma: A meta-analysis. Tumor Biol. 2015, 36, 7623–7630. [Google Scholar] [CrossRef]
- Abdelgawad, I.A. Epithelial Cell Adhesion Molecule mRNA Can be a Potential Marker to Predict Metastasis in Hepatocellular Carcinoma Patients. Asian Pac. J. Cancer Prev. 2020, 21, 861–866. [Google Scholar] [CrossRef]
- Wang, T.; Hu, H.S.; Feng, Y.X.; Shi, J.; Li, N.; Guo, W.X.; Xue, J.; Xie, D.; Liu, S.R.; Wu, M.C.; et al. Characterisation of a novel cell line (CSQT-2) with high metastatic activity derived from portal vein tumour thrombus of hepatocellular carcinoma. Br. J. Cancer 2010, 102, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.-Y.; Jin, C.; Ma, L.; Shi, Y.-X.; Li, X.-S.; Jiang, P.; Gao, S.; Lin, J.R.; Song, Y. Urokinase plasminogen activator predicts poor prognosis in hepatocellular carcinoma. J. Gastrointest. Oncol. 2021, 12, 1851. [Google Scholar] [CrossRef]
- Azam, A.; Klisic, A.; Mercantepe, F.; Faseeh, H.; Mercantepe, T.; Rafaqat, S. Role of Coagulation Factors in Hepatocellular Carcinoma: A Literature Review. Life 2025, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Marquard, S.; Thomann, S.; Weiler, S.M.E.; Bissinger, M.; Lutz, T.; Sticht, C.; Tóth, M.; de la Torre, C.; Gretz, N.; Straub, B.K.; et al. Yes-associated protein (YAP) induces a secretome phenotype and transcriptionally regulates plasminogen activator Inhibitor-1 (PAI-1) expression in hepatocarcinogenesis. Cell Commun. Signal. 2020, 18, 166. [Google Scholar] [CrossRef]
- Chen, H.; Chen, J.; Yuan, H.; Li, X.; Li, W. Hypoxia-inducible factor-1α: A critical target for inhibiting the metastasis of hepatocellular carcinoma. Oncol. Lett. 2022, 24, 284. [Google Scholar] [CrossRef]
- Chun, K. Molecular Targets and Signaling Pathways of microRNA-122 in Hepatocellular Carcinoma. Pharmaceutics 2022, 14, 1380. [Google Scholar] [CrossRef] [PubMed]
- Amr, K.S.; Elmawgoud Atia, H.A.; Elazeem Elbnhawy, R.A.; Ezzat, W.M. Early diagnostic evaluation of miR-122 and miR-224 as biomarkers for hepatocellular carcinoma. Genes Dis. 2017, 4, 215–221. [Google Scholar] [CrossRef]
- Zanetto, A.; Senzolo, M.; Campello, E.; Bulato, C.; Gavasso, S.; Shalaby, S.; Gambato, M.; Vitale, A.; Cillo, U.; Farinati, F.; et al. Influence of Hepatocellular Carcinoma on Platelet Aggregation in Cirrhosis. Cancers 2021, 13, 1150. [Google Scholar] [CrossRef]
- Gade, I.L.; Braekkan, S.K.; Naess, I.A.; Hansen, J.-B.; Cannegieter, S.C.; Overvad, K.; Jensvoll, H.; Hammerstrøm, J.; Blix, K.; Tjønneland, A.; et al. The impact of initial cancer stage on the incidence of venous thromboembolism: The Scandinavian Thrombosis and Cancer (STAC) Cohort. J. Thromb. Haemost. 2017, 15, 1567–1575. [Google Scholar] [CrossRef]
- Liu, P.-H.; Hsu, C.-Y.; Su, C.-W.; Huang, Y.-H.; Hou, M.-C.; Rich, N.E.; Fujiwara, N.; Hoshida, Y.; Singal, A.G.; Huo, T.I. Thrombocytosis is associated with worse survival in patients with hepatocellular carcinoma. Liver Int. 2020, 40, 2522–2534. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, B.; Kirstein, M.; Popp, S.; Hucke, F.; Bota, S.; Rohr-Udilova, N.; Reiberger, T.; Müller, C.; Trauner, M.; Peck-Radosavljevic, M.; et al. Association of Platelet Count and Mean Platelet Volume with Overall Survival in Patients with Cirrhosis and Unresectable Hepatocellular Carcinoma. Liver Cancer 2019, 8, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Yeini, E.; Satchi-Fainaro, R. The role of P-selectin in cancer-associated thrombosis and beyond. Thromb. Res. 2022, 213, S22–S28. [Google Scholar] [CrossRef]
- Ferroni, P.; Spila, A.; D’Alessandro, R.; Martini, F.; Iacovone, F.; Ettorre, G.M.; Vennarecci, G.; Santoro, R.; Puoti, C.; Guadagni, F. Platelet activation and vascular endothelial growth factor 165 release in hepatocellular cancer. Clin. Chim. Acta 2011, 412, 450–454. [Google Scholar] [CrossRef]
- Giuli, L.; Pallozzi, M.; Venturini, G.; Gasbarrini, A.; Ponziani, F.R.; Santopaolo, F. Molecular Mechanisms Underlying Vascular Liver Diseases: Focus on Thrombosis. Int. J. Mol. Sci. 2023, 24, 12754. [Google Scholar] [CrossRef]
- Lou, C.; Cai, X. The emerging roles of platelet-derived extracellular vesicles in disease. Ann. Med. 2024, 57, 2499029. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Hu, Y.-X.; Huang, R.-Z.; Xu, Y.-Y.; Dong, S.-H.; Guo, F.-H.; Guo, J.J.; Qiu, J.J.; Cao, Z.Y.; Wei, L.J.; et al. Intraplatelet miRNA-126 regulates thrombosis and its reduction contributes to platelet inhibition. Cardiovasc. Res. 2024, 120, 1622–1635. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, D.; Sheng, Y.; Huang, J.; Ha, X. MicroRNA-126 Regulates Thrombosis Through Endothelial Progenitor Cells. DNA Cell Biol. 2023, 42, 6. [Google Scholar] [CrossRef]
- Kinoshita, A.; Onoda, H.; Imai, N.; Iwaku, A.; Oishi, M.; Tanaka, K.; Fushiya, N.; Koike, K.; Nishino, H.; Matsushima, M.; et al. Elevated Plasma Fibrinogen Levels Are Associated with a Poor Prognosis in Patients with Hepatocellular Carcinoma. Oncology 2013, 85, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Hrnčár, M.; Chudej, J.; Pritzová, E.; Jablonicka, M.; Sokol, J. P-64 P-selectin and factor VIII as risk factors of thromboembolic disease in patients with hepatocellular carcinoma. Ann. Oncol. 2020, 31, S110. [Google Scholar] [CrossRef]
- Bitto, N.; Tosetti, G.; La Mura, V.; Primignani, M. AB0, von Willebrand factor/factor VIII and portal vein thrombosis in decompensated cirrhosis: Too late to unmask the culprit? Liver Int. 2020, 40, 1788–1789. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R.; Sacco, M.; Tardugno, M.; Santopaolo, F.; Marsico, A.; Manna, S.; Lancellotti, S.; Gasbarrini, A.; De Cristofaro, R.; Pompili, M.; et al. Low ADAMTS-13/VWF ratio and altered gut-liver axis predict complications of advanced chronic liver disease: A pilot study. Gastroenterol. Rep. 2022, 10, goac065. [Google Scholar] [CrossRef] [PubMed]
- Sacco, M.; Tardugno, M.; Lancellotti, S.; Ferretti, A.; Ponziani, F.R.; Riccardi, L.; Zocco, M.A.; De Magistris, A.; Santopaolo, F.; Pompili, M.; et al. ADAMTS-13/von Willebrand factor ratio: A prognostic biomarker for portal vein thrombosis in compensated cirrhosis. A prospective observational study. Dig. Liver Dis. 2022, 54, 1672–1680. [Google Scholar] [CrossRef]
- Suda, T.; Takatori, H.; Hayashi, T.; Kaji, K.; Nio, K.; Terashima, T.; Shimakami, T.; Arai, K.; Yamashita, T.; Mizukoshi, E.; et al. Plasma Antithrombin III Levels Can Be a Prognostic Factor in Liver Cirrhosis Patients with Portal Vein Thrombosis. Int. J. Mol. Sci. 2023, 24, 7732. [Google Scholar] [CrossRef]
- Alkim, H.; Ayaz, S.; Sasmaz, N.; Oguz, P.; Sahin, B. Hemostatic abnormalities in cirrhosis and tumor-related portal vein thrombosis. Clin. Appl. Thromb. Hemost. 2012, 18, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.H.; Garand, M.; Zagorac, B.; Schadinger, S.L.; Scipione, C.; Koschinsky, M.L.; Boffa, M.B. Identification of human thrombin-activatable fibrinolysis inhibitor in vascular and inflammatory cells. Thromb. Haemost. 2011, 105, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Lee, J.M.; Yoon, J.H.; Jang, S.; Chung, J.W.; Lee, K.B.; Yi, N.J.; Lee, J.H. How to Best Detect Portal Vein Tumor Thrombosis in Patients with Hepatocellular Carcinoma Meeting the Milan Criteria: Gadoxetic Acid-Enhanced MRI versus Contrast-Enhanced CT. Liver Cancer 2020, 9, 293–307. [Google Scholar] [CrossRef]
- Abdelhamed, W.; Shousha, H.; El-Kassas, M. Portal vein tumor thrombosis in hepatocellular carcinoma patients: Is it the end? Liver Res. 2024, 8, 141–151. [Google Scholar] [CrossRef]
- Serag, W.M.; Eysa, B.E. Diagnosis of portal vein thrombosis in cirrhotic patients with and without hepatocellular carcinoma. Egypt. Liver J. 2022, 12, 39. [Google Scholar] [CrossRef]
- Fan, X.; Li, Y.; Yi, X.; Chen, G.; Jin, S.; Dai, Y.; Cui, B.; Dai, B.; Lin, H.; Zhou, D. Epigenome-wide DNA methylation profiling of portal vein tumor thrombosis (PVTT) tissues in hepatocellular carcinoma patients. Neoplasia 2020, 22, 630–643. [Google Scholar] [CrossRef]
- Nie, J.; Lu, L.; Du, C.; Gao, X. FAM83D promotes the proliferation and migration of hepatocellular carcinoma cells by inhibiting the FBXW7/MCL1 pathway. Transl. Cancer Res. 2022, 11, 10. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, L.; Xu, Y.; Song, D.; He, W.; Ji, X.; Shao, J. Hypermethylation of SCAND3 and Myo1g Gene Are Potential Diagnostic Biomarkers for Hepatocellular Carcinoma. Cancers 2020, 12, 2332. [Google Scholar] [CrossRef]
- Wang, J.; Wu, S.; Huang, T. Expression and role of VEGFA and miR-381 in portal vein tumor thrombi in patients with hepatocellular carcinoma. Exp. Ther. Med. 2018, 15, 5450–5456. [Google Scholar] [CrossRef]
- Li, M.; Wei, L.; Liu, P.-Y.; Zhang, X.-M.; Liu, F.; Yang, F.; Hu, X.-S.; Mo, Z.-C. Lnc-ATG9B-4 aggravates progress of hepatocellular carcinoma through cell proliferation and migration by upregulating CDK5. Exp. Biol. Med. 2021, 246, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Song, L.-N.; Qiao, G.-L.; Yu, J.; Yang, C.-M.; Chen, Y.; Deng, Z.-F.; Song, L.H.; Ma, L.J.; Yan, H.L. Hsa_circ_0003998 promotes epithelial to mesenchymal transition of hepatocellular carcinoma by sponging miR-143-3p and PCBP1. J. Exp. Clin. Cancer Res. 2020, 39, 114. [Google Scholar] [CrossRef] [PubMed]
- Nenu, I.; Toadere, T.M.; Topor, I.; Țichindeleanu, A.; Bondor, D.A.; Trella Șerban, E.; Sparchez, Z.; Filip, G.A. Interleukin-6 in Hepatocellular Carcinoma: A Dualistic Point of View. Biomedicines 2023, 11, 2623. [Google Scholar] [CrossRef]
- Xiao, Z.; Yeung, C.L.S.; Yam, J.W.P.; Mao, X. An update on the role of complement in hepatocellular carcinoma. Front. Immunol. 2022, 13, 1007382. [Google Scholar] [CrossRef]
- Xing, Y.; Jiang, Y.; Xing, S.; Mao, T.; Guan, G.; Niu, Q.; Zhao, X.; Zhou, J.; Jing, X. Neutrophil extracellular traps are associated with enhanced procoagulant activity in liver cirrhosis patients with portal vein thrombosis. J. Clin. Lab. Anal. 2022, 36, e24433. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Mackowiak, B.; Feng, D.; Lu, H.; Guan, Y.; Lehner, T.; Pan, H.; Wang, X.W.; He, Y.; Gao, B. MicroRNA-223 attenuates hepatocarcinogenesis by blocking hypoxia-driven angiogenesis and immunosuppression. Gut 2023, 72, 1942–1958. [Google Scholar] [CrossRef]
- Favero, A.; Segatto, I.; Perin, T.; Belletti, B. The many facets of miR-223 in cancer: Oncosuppressor, oncogenic driver, therapeutic target, and biomarker of response. WIREs RNA 2021, 12, e1659. [Google Scholar] [CrossRef]
- Tsutsui, H.; Nishiguchi, S. Importance of Kupffer Cells in the Development of Acute Liver Injuries in Mice. Int. J. Mol. Sci. 2014, 15, 7711–7730. [Google Scholar] [CrossRef]
- Casari, M.; Siegl, D.; Deppermann, C.; Schuppan, D. Macrophages and platelets in liver fibrosis and hepatocellular carcinoma. Front. Immunol. 2023, 14, 1277808. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; Yi, S.; Lei, L.; Ma, T.; Huang, R.; Yang, L.; Li, Z.M.; Zhang, D. Potential contribution of the gut microbiota to the development of portal vein thrombosis in liver cirrhosis. Front. Microbiol. 2023, 14, 1217338. [Google Scholar] [CrossRef]
- Qi, P.; Yang, X.; Wang, C.; Sang, W.; Zhang, W.; Bai, Y. Analysis of gut and circulating microbiota characteristics in patients with liver cirrhosis and portal vein thrombosis. Front. Microbiol. 2025, 16, 1597145. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, R.R.; Nieuwenhuis, L.M.; Karmi, N.; Zhang, S.; Swarte, J.C.; Björk, J.R.; Gacesa, R.; Blokzijl, H.; Connelly, M.A.; Weersma, R.K.; et al. Gut microbiome dysbiosis is not associated with portal vein thrombosis in patients with end-stage liver disease: A cross-sectional study. J. Thromb. Haemost. 2025, 23, 1407–1415. [Google Scholar] [CrossRef]
- Rossetto, V.; Spiezia, L.; Senzolo, M.; Rodriguez-Castro, K.I.; Maggiolo, S.; Simioni, P. Whole blood rotation thromboelastometry (ROTEM®) profiles in subjects with non-neoplastic portal vein thrombosis. Thromb. Res. 2013, 132, e131–e134. [Google Scholar] [CrossRef]
- Ben Khaled, N.; Möller, M.; Jochheim, L.S.; Leyh, C.; Ehmer, U.; Böttcher, K.; Pinter, M.; Balcar, L.; Scheiner, B.; Weich, A.; et al. Atezolizumab/bevacizumab or lenvatinib in hepatocellular carcinoma: Multicenter real-world study with focus on bleeding and thromboembolic events. JHEP Rep. 2024, 6, 101065. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.; Primignani, M.; Chantarangkul, V.; Clerici, M.; Dell’Era, A.; Fabris, F.; Salerno, F.; Mannucci, P.M. Thrombin generation in patients with cirrhosis: The role of platelets. Hepatology 2006, 44, 440–445. [Google Scholar] [CrossRef]
- Cen, G.; Song, Y.; Chen, S.; Liu, L.; Wang, J.; Zhang, J.; Li, J.; Li, G.; Li, H.; Liang, H.; et al. The investigation on the hypercoagulability of hepatocellular carcinoma-related cerebral infarction with thromboelastography. Brain Behav. 2023, 13, e2961. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wan, J.; Roberts, L.N.; Hendrix, W.; Konings, J.; Ow, T.-W.; Rabinowich, L.; Barbouti, O.; de Laat, B.; Arya, R.; Patel, V.C.; et al. Whole blood thrombin generation profiles of patients with cirrhosis explored with a near patient assay. J. Thromb. Haemost. 2020, 18, 834–843. [Google Scholar] [CrossRef]
- Drotarova, M.; Zolkova, J.; Belakova, K.M.; Brunclikova, M.; Skornova, I.; Stasko, J.; Simurda, T. Basic Principles of Rotational Thromboelastometry (ROTEM®) and the Role of ROTEM-Guided Fibrinogen Replacement Therapy in the Management of Coagulopathies. Diagnostics 2023, 13, 3219. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Ng, L.X.L.; Wong, Y.J.; Tan, C.K.; Wang, L.Z.; Qiu, T.Y.; Wong, B.; Lin, K.W.; Li, J.W.; Kwek, A.B.E.; et al. Rotational Thromboelastometry Reduces the Need for Preemptive Transfusion in Cirrhosis: A Randomized Controlled Trial (NCT:05698134). J. Clin. Exp. Hepatol. 2025, 15, 102409. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, S.Y.; Gu, J.-Y.; Kim, H.K. Assessment of Rotational Thromboelastometry and Thrombin Generation Assay to Identify Risk of High Blood Loss and Re-Operation After Cardiac Surgery. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221123310. [Google Scholar] [CrossRef] [PubMed]
- Benevento, F.; Pecorelli, A.; Stefanescu, H.; Sparchez, Z.; Vukotic, R.; Pettinari, I.; Grigoras, C.A.; Tovoli, F.; Ravaioli, F.; Stefanini, B.; et al. Presence of Hepatocellular Carcinoma Does Not Affect Course and Response to Anticoagulation of Bland Portal Vein Thrombosis in Cirrhotic Patients. J. Hepatocell. Carcinoma 2023, 10, 473–482. [Google Scholar] [CrossRef]
- Senzolo, M.; Shalaby, S.; Grasso, M.; Vitale, A.; Pizzirani, E.; Barbiero, G.; Zanetto, A.; Feltracco, P.; Simioni, P.; Burra, P.; et al. Role of nonneoplastic PVT in the natural history of patients with cirrhosis and first diagnosis of HCC. Hepatology 2024, 79, 355–367. [Google Scholar] [CrossRef]
- Mahmoudi, T.M.; Marquez, V.; Kayal, A.; Carvalho, R.; Weiss, A.A. HCC complicated by PVT: Outcome and the role of anticoagulation therapy. Can. Liver J. 2019, 2, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Balcar, L.; Mrekva, A.; Scheiner, B.; Pomej, K.; Meischl, T.; Mandorfer, M.; Reiberger, T.; Trauner, M.; Tamandl, D.; Pinter, M. Management of varices but not anticoagulation is associated with improved outcome in patients with HCC and macrovascular tumour invasion. Cancer Imaging 2024, 24, 9. [Google Scholar] [CrossRef] [PubMed]
- Antonios, K.; Antonios, B.; Al-Banaa, K.; Chisti, M.M. Anticoagulation for portal vein thrombosis in patients with hepatocellular carcinoma: A retrospective observational study. JCO 2023, 41, e16212. [Google Scholar] [CrossRef]
- Talerico, R.; Pellegrino, S.; Plessier, A.; Ponziani, F.R.; Porfidia, A.; Landi, F.; Gasbarrini, A.; Pola, R.; Santopaolo, F. Safety of Anticoagulant Treatment in Patients with Splanchnic Vein Thrombosis and History of Portal Hypertension-Related Bleeding. Liver Int. 2025, 45, e70114. [Google Scholar] [CrossRef]
- Semmler, G.; Pomej, K.; Bauer, D.J.M.; Balcar, L.; Simbrunner, B.; Binter, T.; Hartl, L.; Becker, J.; Pinter, M.; Quehenberger, P.; et al. Safety of direct oral anticoagulants in patients with advanced liver disease. Liver Int. 2021, 41, 2159–2170. [Google Scholar] [CrossRef]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients with Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef]
- Villa, E.; Cammà, C.; Marietta, M.; Luongo, M.; Critelli, R.; Colopi, S.; Tata, C.; Zecchini, R.; Gitto, S.; Petta, S.; et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 2012, 143, 1253–1260.e4. [Google Scholar] [CrossRef]
- Puente, Á.; Turón, F.; Martínez, J.; Fortea, J.I.; Guerra, M.H.; Alvarado, E.; Pons, M.; Magaz, M.; Llop, E.; Alvarez-Navascués, C.; et al. Rivaroxaban to prevent complications of portal hypertension in cirrhosis: The CIRROXABAN study. J. Hepatol. 2025, 83, 1069–1076. [Google Scholar] [CrossRef]
- Serrano, P.E.; Parpia, S.; Linkins, L.-A.; Elit, L.; Simunovic, M.; Ruo, L.; Bhandari, M.; Levine, M.N. Venous Thromboembolic Events Following Major Pelvic and Abdominal Surgeries for Cancer: A Prospective Cohort Study. Ann. Surg. Oncol. 2018, 25, 3214–3221. [Google Scholar] [CrossRef]
- Vivarelli, M.; Zanello, M.; Zanfi, C.; Cucchetti, A.; Ravaioli, M.; Gaudio, M.D.; Cescon, M.; Lauro, A.; Montanari, E.; Grazi, G.L.; et al. Prophylaxis for venous thromboembolism after resection of hepatocellular carcinoma on cirrhosis: Is it necessary? World J. Gastroenterol. 2010, 16, 2146–2150. [Google Scholar] [CrossRef]
- Zhang, X.; Ai, Z. Efficacy and safety of reduced thromboprophylaxis with low molecular weight heparin for hepatocellular carcinoma after conversion therapy. BMC Gastroenterol. 2025, 25, 606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, B.J.; Day, R.W.; Davis, C.H.; Narula, N.; Kroll, M.H.; Tzeng, C.W.D.; Aloia, T.A. Extended pharmacologic thromboprophylaxis in oncologic liver surgery is safe and effective. J. Thromb. Haemost. 2017, 15, 2158–2164. [Google Scholar] [CrossRef] [PubMed]
- Carlin, S.; Cuker, A.; Gatt, A.; Gendron, N.; Hernández-Gea, V.; Meijer, K.; Siegal, D.M.; Stanworth, S.; Lisman, T.; Roberts, L.N. Anticoagulation for stroke prevention in atrial fibrillation and treatment of venous thromboembolism and portal vein thrombosis in cirrhosis: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2024, 22, 2653–2669. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.A.; Puleo, C.R.; Kovalic, A.J.; Nisly, S.A. Efficacy and safety of direct oral anticoagulant therapy for the treatment of venous thromboembolism in patients with chronic liver disease. Thromb. Res. 2019, 176, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Jiménez, D.; Garcia-Tsao, G.; Barba, R.; Font, C.; Díaz-Pedroche, M.D.C.; Mazzolai, L.; Little, D.H.W.; Tufano, A.; Tafur, A.J.; et al. Venous Thromboembolism in Patients with Liver Cirrhosis: Findings from the RIETE (Registro Informatizado de la Enfermedad TromboEmbolica) Registry. Semin. Thromb. Hemost. 2019, 45, 793–801. [Google Scholar] [CrossRef]
- Candeloro, M.; Valeriani, E.; Monreal, M.; Ageno, W.; Riva, N.; Lopez-Reyes, R.; Peris, M.L.; Beyer Westendorf, J.; Schulman, S.; Rosa, V.; et al. Anticoagulant therapy for splanchnic vein thrombosis: An individual patient data meta-analysis. Blood Adv. 2022, 6, 4516–4523. [Google Scholar] [CrossRef]
- Mahé, I.; Carrier, M.; Mayeur, D.; Chidiac, J.; Vicaut, E.; Falvo, N.; Sanchez, O.; for the API-CAT Investigators. Extended Reduced-Dose Apixaban for Cancer-Associated Venous Thromboembolism. N. Engl. J. Med. 2025, 392, 1363–1373. [Google Scholar] [CrossRef]
- Rajani, R.; Björnsson, E.; Bergquist, A.; Danielsson, Å.; Gustavsson, A.; Grip, O.; Melin, T.; Sangfelt, P.; Wallerstedt, S.; Almer, S. The epidemiology and clinical features of portal vein thrombosis: A multicentre study. Aliment. Pharmacol. Ther. 2010, 32, 1154–1162. [Google Scholar] [CrossRef]
- Rimini, M.; Rimassa, L.; Ueshima, K.; Burgio, V.; Shigeo, S.; Tada, T.; Suda, G.; Yoo, C.; Cheon, J.; Pinato, D.J.; et al. Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: An international propensity score matching analysis. ESMO Open 2022, 7, 100591. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Cheon, J.; Kim, H.; Kang, B.; Ha, Y.; Kim, D.Y.; Hwang, S.G.; Chon, Y.E.; Chon, H. Atezolizumab/Bevacizumab vs. Lenvatinib as First-Line Therapy for Unresectable Hepatocellular Carcinoma: A Real-World, Multi-Center Study. Cancers 2022, 14, 1747. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Rimassa, L.; Sun, H.-C.; Vogel, A.; Kaseb, A.O. Immunotherapy in hepatocellular carcinoma: Evaluation and management of adverse events associated with atezolizumab plus bevacizumab. Ther. Adv. Med Oncol. 2021, 13, 17588359211031141. [Google Scholar] [CrossRef]
- Allaire, M.; Sultanik, P.; Thabut, D. Anticoagulation is not associated with an increased risk of variceal bleeding under systemic therapy for advanced HCC. JHEP Rep. 2024, 6, 101120. [Google Scholar] [CrossRef]
- Moriguchi, M.; Okuda, K.; Horiguchi, G.; Kataoka, S.; Seko, Y.; Yamaguchi, K.; Nishimura, T.; Fujii, H.; Mitsumoto, Y.; Miyagawa, M.; et al. Safety/efficacy of atezolizumab + bevacizumab during anti-platelet/anticoagulation therapy in unresectable hepatocellular carcinoma. Liver Int. 2024, 44, 1751–1761. [Google Scholar] [CrossRef]
- Nakabori, T.; Kunimasa, K.; Kawabata, M.; Higashi, S.; Mukai, K.; Kawamura, T.; Inoue, T.; Tamiya, M.; Nishino, K.; Ohkawa, K. Feasibility of atezolizumab and bevacizumab combination regimens in patients with hepatocellular carcinoma and lung cancer taking direct oral anticoagulants. Cancer Med. 2024, 13, e7430. [Google Scholar] [CrossRef]
- Larrey, E.; Campion, B.; Evain, M.; Sultanik, P.; Blaise, L.; Giudicelli, H.; Wagner, M.; Cluzel, P.; Rudler, M.; Ganne-Carrié, N.; et al. A history of variceal bleeding is associated with further bleeding under atezolizumab–bevacizumab in patients with HCC. Liver Int. 2022, 42, 2843–2854. [Google Scholar] [CrossRef] [PubMed]

| Study | Design | Year | Thrombotic Site | Population | HCC Treatment | Incidence/Prevalence |
|---|---|---|---|---|---|---|
| Nonami T. et al. [11] | Retrospective | 1991 | PVT | 401 cirrhotic patients, 87 with HCC (21.7%) | OLT | 15.7% PVT prevalence in cirrhosis without HCC. 34.8% prevalence in cirrhosis with HCC. |
| Davidson et al. [12] | Prospective | 1994 | PVT | 132 patients with cirrhosis, 22 with HCC (16.7%) | OLT | 27.3% (6/22) PVT prevalence in HCC vs. 9.1% (10/110) in non-HCC. |
| Ögren M et al. [14] | Retrospective | 2006 | PVT | 392 HCC autoptic report, 182 with cirrhosis | No treatment | PVT prevalence 14.3% in cirrhosis with HCC (OR 17.1), 9.7% in HCC without (OR 5.2), and 4.5% in cirrhosis without HCC. |
| Connolly GC. et al. [15] | Retrospective | 2008 | PVT + DVT/PE | 194 patients with cirrhosis and HCC | OLT or LRT | PVT: 31%; VTE: 6.7%; significantly impacting survival (mOS 2.3 m in PVT vs. 17.6 m without PVT); PVT patients with higher risk of VTE compared to patients. without PVT (11.5% vs. 4.4%; p = 0.04). |
| Lesmana et al. [30] | Case–control, single center | 2010 | DVT/PE | 256 patients with cirrhosis, 87 with HCC | Unknown | 4.6% (4/87) VTE prevalence without HCC vs. 4.3% (8/169) with HCC. |
| Rajani et al. [32] | Retrospective | 2010 | PVT | 173 patients, 40% (80) with cirrhosis, 27% (47) with malignancy. | Unknown | PVT incidence rate was 0.7 per 100,000 per year and prevalence rate was 3.7 per 100,000 inhabitants. |
| Ravaioli et al. [13] | Retrospective | 2011 | PVT | 889 patients with cirrhosis, 282 with HCC (31.7%) | OLT | PVT prevalence in cirrhosis with HCC is 40.7% vs. 30.7% in patients without HCC (p < 0.05). |
| Tzeng et al. [16] | Retrospective | 2012 | PVT + DVT/PE | 5651 hepatectomies | Surgery | DVT (1.93%), PE (1.31%), venous thromboembolism (VTE) (2.88%). |
| Ponziani et al. [17] | Systematic review | 2014 | PVT | 23,932 cirrhotic patients, (39%) with HCC | OLT | PVT incidence: 10.6% overall, 3.2/100 p-y (new), 7.4/100 p-y (total). |
| Cagin et al. [19] | Retrospective | 2016 | PVT | 461 patients with cirrhosis, 69 (15%) with HCC | Unknown | 9.8% (45/461) PVT overall prevalence vs. 18.8% (13/69) in HCC. |
| Zanetto et al. [31] | Prospective | 2016 | PVT + DVT/PE | 76 patients with cirrhosis, 41 with HCC (53.9%) | Surgery, LRTs, systemic | 24.4% (10/41) PVT incidence at 1 year in HCC vs. 11.4% (4/35) in non-HCC. Among HCC, 50% of PVT occurred in Child A. No DVT/PE |
| Wang Y et al. [20] | Retrospective | 2018 | PVT and DVT/PE | 270 patients with cirrhosis and HCC | 11.5% curative treatment, and 35.9% sorafenib | 5.9% (16 cases) of VTE, including 7 (43.8%) pulmonary embolism, 4 (25%) peripheral deep vein thrombosis, and 6 (37.5%) intra-abdominal thrombosis. |
| Malaguarnera et al. [21] | Retrospective | 2018 | PVT | 118 patients with liver cirrhosis and HCC | Unknown | 49.2% (58 patients) PVT prevalence. |
| Senzolo et al. [25] | Review | 2018 | PVT | 2721 cirrhotic patients, 41 with HCC | Unknown | ~10% PVT prevalence is in CPA cirrhosis, 17% in CPB/C, and up to 26% in OLT candidates. 1-year incidence ranges from 3.7% to 24.4%, lower in cohorts with mostly compensated cirrhosis. |
| Violi et al. [22] | Prospective | 2019 | PVT | 753 cirrhotic patients, 152 with HCC | Unknown | PVT incidence: 6.05/100 p-y (4.1 without prior PVT, 18.9 with prior PVT). |
| Al-Taee et al. [23] | Retrospective | 2019 | DVT/PE | 54,275 hospitalized patients with HCC | Unknown | 2.8% VTE prevalence (2.5% in 2008 to 3.0% in 2013, a significant increase). |
| Serag et al. [24] | Retrospective | 2020 | PVT | 91 patients with cirrhosis, 44 (48.4%) with HCC | Unknown | 12.7% (6/47) PVT prevalence in cirrhosis without HCC vs. 22.7% (10/44) in cirrhosis with HCC. |
| Schlick et al. [33] | Retrospective | 2021 | DVT/PE | Patients undergoing hepatectomy for malignancy (n = 11,172) | Hepatectomy (partial, left, right, trisegmentectomy) | Post-discharge VTE: 0.9% (overall VTE: 3.1%). |
| Ow et al. [26] | Retrospective | 2021 | PVT + DVT/PE | 632 cirrhotic patients in ICU, 77 with HCC (12%) | Unknown | 13% incidence of early VTE; 7.2% of late VTE (80% were PVT). No difference in survival between patients with and without VTE |
| Chen et al. [34] | Retrospective | 2021 | DVT | 355 patients with HCC | Surgery | 18.6% (66/355) DVT incidence after surgery. |
| Grasso et al. [27] | Retrospective | 2021 | PVT | 750 patients with cirrhosis and HCC | LPS MWA | 11.7% (88/750) prevalence of PVT. Cirrhosis severity, lack of response to HCC treatments, and complete/progressive PVT predictive of death. |
| Faccia et al. [28] | Retrospective | 2022 | PVT + DVT/PE | 7445 cirrhotic patients accessing ED, 1524 with HCC | Unknown | In cirrhotic patients with PVT: 5.13% (382/7445), VTE: 1.27% (95/7445). Among HCC patients: PVT 10.63%, VTE 1.70% |
| Siddiqui et al. [29] | Retrospective cross-sectional analysis of prospective registry | 2023 | PVT | 316 patients with HCC; subgroup analysis on Child–Pugh A) | All treatment allowed | PVT prevalence: 31% overall; 24.5% in Child–Pugh A; |
| Liu et al. [35] | Meta-analysis | 2025 | DVT/PE | 935,639 patients with HCC | Unknown | VTE incidence in liver cancer was 35.85‰ overall, but higher with longer follow-up (up to 47.19‰) and markedly elevated in HCC with cirrhosis (229.56‰) vs. HCC alone (2.66‰). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stella, L.; De Siati, M.; Talerico, R.; Pallozzi, M.; Cerrito, L.; Sorrentino, S.; Gasbarrini, A.; De Candia, E.; Pola, R.; Ponziani, F.R. Thrombotic Risk and Coagulation Imbalance in Cirrhosis and Hepatocellular Carcinoma: Clinical Implications and Management. Cancers 2025, 17, 3413. https://doi.org/10.3390/cancers17213413
Stella L, De Siati M, Talerico R, Pallozzi M, Cerrito L, Sorrentino S, Gasbarrini A, De Candia E, Pola R, Ponziani FR. Thrombotic Risk and Coagulation Imbalance in Cirrhosis and Hepatocellular Carcinoma: Clinical Implications and Management. Cancers. 2025; 17(21):3413. https://doi.org/10.3390/cancers17213413
Chicago/Turabian StyleStella, Leonardo, Matteo De Siati, Rosa Talerico, Maria Pallozzi, Lucia Cerrito, Silvia Sorrentino, Antonio Gasbarrini, Erica De Candia, Roberto Pola, and Francesca Romana Ponziani. 2025. "Thrombotic Risk and Coagulation Imbalance in Cirrhosis and Hepatocellular Carcinoma: Clinical Implications and Management" Cancers 17, no. 21: 3413. https://doi.org/10.3390/cancers17213413
APA StyleStella, L., De Siati, M., Talerico, R., Pallozzi, M., Cerrito, L., Sorrentino, S., Gasbarrini, A., De Candia, E., Pola, R., & Ponziani, F. R. (2025). Thrombotic Risk and Coagulation Imbalance in Cirrhosis and Hepatocellular Carcinoma: Clinical Implications and Management. Cancers, 17(21), 3413. https://doi.org/10.3390/cancers17213413








