Radioligand Therapy in Cancer Management: A Global Perspective
Simple Summary
Abstract
1. Introduction
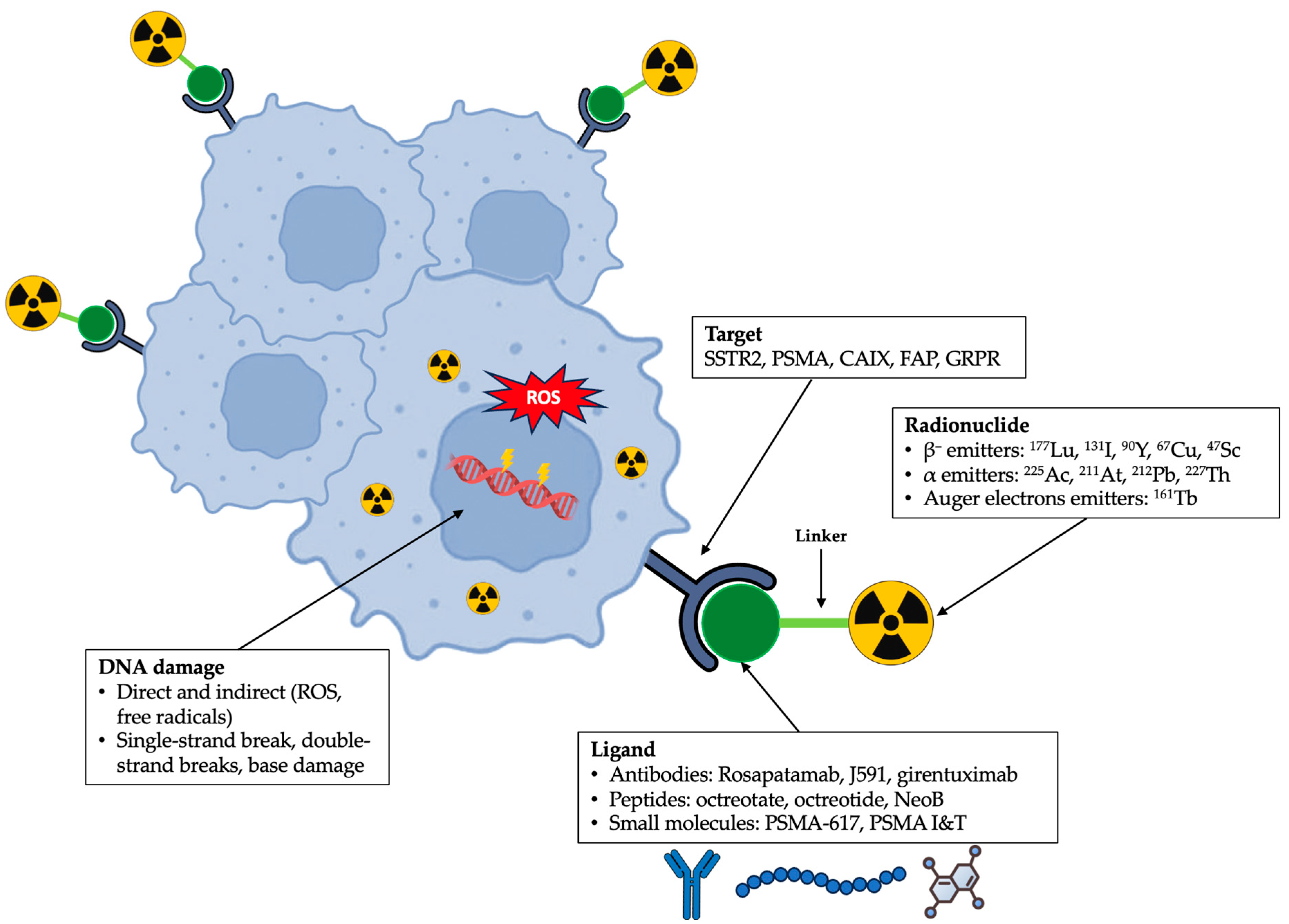
2. Radioligand Therapy: Design and Mechanism of Action
2.1. Design
2.1.1. Ligand
2.1.2. Linker
2.1.3. Radionuclide
2.2. Mechanism of Action
3. Clinical Applications of Radioligand Therapy
3.1. SSTR-Targeted Radioligand Therapy
3.2. PSMA-Targeted Radioligand Therapy
3.3. FAP-Targeted Radioligand Therapy
3.4. CAIX-Targeted Radioligand Therapy
3.5. GRPR-Targeted Radioligand Therapy
3.6. Emerging Targets
4. Global Access and Availability of Radioligand Therapy
5. Optimising Radioligand Therapy: Current Clinical Challenges
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARPI | Androgen receptor pathway inhibitor |
| CAIX | Carbonic anhydrase IX |
| ccRCC | Clear cell renal cell carcinoma |
| CD | Cluster of differentiation |
| CXCR4 | C-X-C chemokine receptor type 4 |
| DCR | Disease control rate |
| DLL3 | Delta-like ligand 3 |
| EANM | European Association of Nuclear Medicine |
| EMA | European Medicines Agency |
| FAP | Fibroblast activation protein |
| FDA | U.S. Food and Drug Administration |
| FRα | Folate receptor alpha |
| GEP | Gastroenteropancreatic |
| GRPR | Gastrin-releasing peptide receptor |
| HER2 | Human epidermal growth factor receptor 2 |
| IAEA | International Atomic Energy Agency |
| IGF-1R | Insulin-like growth factor 1 receptor |
| LET | Linear energy transfer |
| MC1R | Melanocortin 1 receptor |
| mCRPC | Metastatic castration-resistant prostate cancer |
| mHSPC | Metastatic hormone-sensitive prostate cancer |
| NET | Neuroendocrine tumour |
| NSCLC | Non-small-cell lung cancer |
| ORR | Objective response rate |
| OS | Overall survival |
| PET | Positron emission tomography |
| PFS | Progression-free survival |
| PSMA | Prostate-specific membrane antigen |
| ROS | Reactive oxygen species |
| RLT | Radioligand therapy |
| SNMMI | Society of Nuclear Medicine and Molecular Imaging |
| SSTR | Somatostatin receptor |
| STEAP2 | Six-transmembrane epithelial antigen of the prostate 2 |
| TKI | Tyrosine kinase inhibitor |
References
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H. A Review of Cancer Immunotherapy: From the Past, to the Present, to the Future. Curr. Oncol. 2020, 27, S87–S97. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, S.; Shi, Y. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001–2020). J. Hematol. Oncol. 2020, 13, 143. [Google Scholar] [CrossRef]
- Joshi, D.C.; Sharma, A.; Prasad, S.; Singh, K.; Kumar, M.; Sherawat, K.; Singh Tuli, H.; Gupta, M. Novel therapeutic agents in clinical trials: Emerging approaches in cancer therapy. Discov. Onc. 2024, 15, 342. [Google Scholar] [CrossRef]
- Bodei, L.; Herrmann, K.; Schöder, H.; Scott, A.M.; Lewis, J.S. Radiotheranostics in oncology: Current challenges and emerging opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 534–550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lei, H.; Chen, X.; Dou, Z.; Yu, B.; Su, W.; Wang, W.; Jin, X.; Katsube, T.; Wang, B.; et al. Carrier systems of radiopharmaceuticals and the application in cancer therapy. Cell Death Discov. 2024, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Parakh, S.; Lee, S.T.; Gan, H.K.; Scott, A.M. Radiolabeled Antibodies for Cancer Imaging and Therapy. Cancers 2022, 14, 1454. [Google Scholar] [CrossRef]
- Rowe, S.P.; Werner, R.A.; Garg, T.; Gafita, A.; Voter, A.F.; Sadaghiani, M.S.; Markowski, M.C.; Paller, C.J.; Zalutsky, M.R.; Solnes, L.B.; et al. Small Molecules as Vectors for Radiopharmaceutical Therapy. In Radiopharmaceutical Therapy; Bodei, L., Lewis, J.S., Eds.; Springer: Cham, Switzerland, 2023; pp. 349–367. [Google Scholar]
- Nock, B.A.; Kanellopoulos, P.; Joosten, L.; Mansi, R.; Maina, T. Peptide Radioligands in Cancer Theranostics: Agonists and Antagonists. Pharmaceuticals 2023, 16, 674. [Google Scholar] [CrossRef]
- Lamb, H.O.; Benfield, A.H.; Henriques, S.T. Peptides as innovative strategies to combat drug resistance in cancer therapy. Drug Discov. Today. 2024, 29, 104206. [Google Scholar] [CrossRef]
- Kaur, J.; Mukherjee, M. An Overview of Structural Framework and Classification of Theranostic Radiopharmaceuticals. ChemistrySelect 2024, 9, e202403431. [Google Scholar] [CrossRef]
- Lau, J.; Lee, H.; Rousseau, J.; Bénard, F.; Lin, K.-S. Application of Cleavable Linkers to Improve Therapeutic Index of Radioligand Therapies. Molecules 2022, 27, 4959. [Google Scholar] [CrossRef]
- Chigoho, D.M.; Bridoux, J.; Hernot, S. Reducing the renal retention of low- to moderate-molecular-weight radiopharmaceuticals. Curr. Opin. Chem. Biol. 2021, 63, 219–228. [Google Scholar] [CrossRef]
- Stokke, C.; Kvassheim, M.; Blakkisrud, J. Radionuclides for Targeted Therapy: Physical Properties. Molecules 2022, 27, 5429. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.; Choi, Y.; Kim, E.S.; Ryu, J.H.; Kwon, I.C. Receptor-ligand interactions for optimized endocytosis in targeted therapies. J. Control. Release 2025, 380, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Ayzman, A.; Pachynski, R.K.; Reimers, M.A. PSMA-based Therapies and Novel Therapies in Advanced Prostate Cancer: The Now and the Future. Curr. Treat. Options Oncol. 2025, 26, 375–384. [Google Scholar] [CrossRef]
- Rousseau, J.; Lau, J.; Bénard, F. Radiolabeled Antibodies for Cancer Radioimmunotherapy. In Nuclear Medicine and Immunology; Harsini, S., Alavi, A., Eds.; Springer: Cham, Switzerland, 2022; pp. 297–345. [Google Scholar]
- Abusalem, M.; Martiniova, L.; Soebianto, S.; DePalatis, L.; Ravizzini, G. Current Status of Radiolabeled Monoclonal Antibodies Targeting PSMA for Imaging and Therapy. Cancers 2023, 15, 4537. [Google Scholar] [CrossRef]
- Hennrich, U.; Kopka, K. Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Gaze, M.N.; Handkiewicz-Junak, D.; Hladun, R.; Laetsch, T.W.; Sorge, C.; Sparks, R.; Wan, S.; Ceraulo, A.; Kluczewska-Galka, A.; Gámez-Cenzano, C.; et al. Safety and dosimetry of [177Lu]Lu-DOTA-TATE in adolescent patients with somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumours, or pheochromocytomas and paragangliomas: Primary analysis of the Phase II NETTER-P study. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 4001–4015. [Google Scholar] [CrossRef]
- Clement, D.; Navalkissoor, S.; Srirajaskanthan, R.; Courbon, F.; Dierickx, L.; Eccles, A.; Lewington, V.; Mitjavila, M.; Percovich, J.C.; Lequoy, B.; et al. Efficacy and safety of 177Lu-DOTATATE in patients with advanced pancreatic neuroendocrine tumours: Data from the NETTER-R international, retrospective study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3529–3537. [Google Scholar] [CrossRef]
- Baudin, E.; Walter, T.A.; Beron, A.; Smith, D.; Hadoux, J.; Lachachi, C.; Taieb, D.; Ansquer, C.; Dierickx, L.O.; de Mestier du Bourg, L.; et al. 887O First multicentric randomized phase II trial investigating the antitumor efficacy of peptide receptor radionucleide therapy with 177Lutetium-Octreotate (OCLU) in unresectable progressive neuroendocrine pancreatic tumor: Results of the OCLURANDOM trial. Ann. Oncol. 2022, 33, S954. [Google Scholar] [CrossRef]
- Singh, S.; Halperin, D.; Myrehaug, S.; Herrmann, K.; Pavel, M.; Kunz, P.L.; Chasen, B.; Tafuto, S.; Lastoria, S.; Capdevila, J.; et al. [177Lu]Lu-DOTA-TATE plus long-acting octreotide versus high-dose long-acting octreotide for the treatment of newly diagnosed, advanced grade 2–3, well-differentiated, gastroenteropancreatic neuroendocrine tumours (NETTER-2): An open-label, randomised, phase 3 study. Lancet 2024, 403, 2807–2817. [Google Scholar]
- Perez, K.; Del Rivero, J.; Kennedy, E.B.; Basu, S.; Chauhan, A.; Connolly, H.M.; Dasari, A.N.; Gangi, A.; Clarke, C.N.; Hallet, J.; et al. Symptom Management for Well-Differentiated Gastroenteropancreatic Neuroendocrine Tumors: ASCO Guideline. JCO Oncol. Pract. 2025, in press. [Google Scholar] [CrossRef]
- Theiler, D.; Cattaneo, M.; Dierickx, L.O.; Igaz, P.; Grozinsky-Glasberg, S.; Bournaud, C.; O’Dorisio, T.; O’Dorisio, M.S.; Wild, D.; Christ, E.; et al. Safety and Efficacy of Peptide-Receptor Radionuclide Therapy in Elderly Neuroendocrine Tumor Patients. Cancers 2021, 13, 6290. [Google Scholar] [CrossRef]
- Baum, R.P.; Fan, X.; Jakobsson, V.; Yu, F.; Schuchardt, C.; Chen, X.; Zhang, J. Long-term Nephrotoxicity after PRRT: Myth or Reality. Theranostics 2024, 14, 451–459. [Google Scholar] [CrossRef]
- Bodei, L.; Kidd, M.; Paganelli, G.; Grana, C.M.; Drozdov, I.; Cremonesi, M.; Lepensky, C.; Kwekkeboom, D.J.; Baum, R.P.; Krenning, E.P.; et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: The value and limitations of clinical factors. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 5–19. [Google Scholar] [CrossRef]
- Lauri, C.; Chiurchioni, L.; Russo, V.M.; Zannini, L.; Signore, A. PSMA Expression in Solid Tumors beyond the Prostate Gland: Ready for Theranostic Applications? J. Clin. Med. 2022, 11, 6590. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; De Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Hsiang Tan, T.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Castellano, D.; Herrmann, K.; De Bono, J.S.; Shore, N.D.; Chi, K.N.; Crosby, M.; Piulats, J.M.; Fléchon, A.; Wei, X.X.; et al. 177Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naive patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): A phase 3, randomised, controlled trial. Lancet 2024, 404, 1227–1239. [Google Scholar] [CrossRef]
- Fizazi, K.; Chi, K.N.; Shore, N.D.; Herrmann, K.; de Bono, J.S.; Castellano, D.; Piulats, J.M.; Fléchon, A.; Wei, X.X.; Mahammedi, H.; et al. Final overall survival and safety analyses of the phase III PSMAfore trial of [177Lu]Lu-PSMA-617 versus change of androgen receptor pathway inhibitor in taxane-naive patients with metastatic castration-resistant prostate cancer. Ann. Oncol. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Subramaniam, S.; Crumbaker, M.; Nguyen, A.; Joshua, A.M.; Weickhardt, A.; Lee, S.T.; Ng, S.; Francis, R.J.; Goh, J.C.; et al. [177Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2024, 25, 563–571. [Google Scholar] [CrossRef]
- Emmett, L.; Subramaniam, S.; Crumbaker, M.; Joshua, A.M.; Sandhu, S.; Nguyen, A.; Weickhardt, A.; Lee, S.T.; Ng, S.; Francis, R.J.; et al. Overall survival and quality of life with [177Lu]Lu-PSMA-617 plus enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer (ENZA-p): Secondary outcomes from a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2025, 26, 291–299. [Google Scholar] [CrossRef]
- Azad, A.A.; Bressel, M.; Tan, H.; Voskoboynik, M.; Suder, A.; Weickhardt, A.J.; Guminski, A.; Francis, R.J.; Saghebi, J.; Dhiantravan, N.; et al. Sequential [177Lu]Lu-PSMA-617 and docetaxel versus docetaxel in patients with metastatic hormone-sensitive prostate cancer (UpFrontPSMA): A multicentre, open-label, randomised, phase 2 study. Lancet Oncol. 2024, 25, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Ninatti, G.; Al-Ibraheem, A.; Lee, S.T.; Scott, A.M. Tracing the global evolution of PSMA-targeted radioligand therapy in prostate cancer: Clinical advancements, future directions, and challenges. Q. J. Nucl. Med. Mol. Imaging 2025, 69, 86–98. [Google Scholar] [CrossRef]
- Wenzel, M.; Siech, C.; Garcia, C.C.; Humke, C.; Groener, D.; Kriegmair, M.; Graefen, M.; Maurer, T.; Salomon, G.; Banek, S.; et al. Cancer-control outcomes with [177Lu]Lu-PSMA Radioligand Therapy in elderly, frail or comorbid mCRPC patients. Theranostics 2025, 15, 2672–2679. [Google Scholar] [CrossRef]
- Groener, D.; Nguyen, C.T.; Baumgarten, J.; Bockisch, B.; Davis, K.; Happel, C.; Mader, N.; Nguyen Ngoc, C.; Wichert, J.; Banek, S.; et al. Hematologic safety of 177Lu-PSMA-617 radioligand therapy in patients with metastatic castration-resistant prostate cancer. EJNMMI Res. 2021, 11, 61. [Google Scholar] [CrossRef]
- Steinhelfer, L.; Lunger, L.; Cala, L.; Pfob, C.H.; Lapa, C.; Hartrampf, P.E.; Buck, A.K.; Schäfer, H.; Schmaderer, C.; Tauber, R.; et al. Long-Term Nephrotoxicity of 177Lu-PSMA Radioligand Therapy. J. Nucl. Med. 2024, 65, 79–84. [Google Scholar] [CrossRef]
- Murthy, V.; Voter, A.F.; Nguyen, K.; Allen-Auerbach, M.; Chen, L.; Caputo, S.; Ledet, E.; Akerele, A.; Moradi Tuchayi, A.; Lawhn-Heath, C.; et al. Efficacy and Toxicity of [177Lu]Lu-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer: Results from the U.S. Expanded-Access Program and Comparisons with Phase 3 VISION Data. J. Nucl. Med. 2024, 65, 1740–1744. [Google Scholar] [CrossRef] [PubMed]
- Sathekge, M.M.; Lawal, I.O.; Bal, C.; Bruchertseifer, F.; Ballal, S.; Cardaci, G.; Davis, C.; Eiber, M.; Hekimsoy, T.; Knoesen, O.; et al. Actinium-225-PSMA radioligand therapy of metastatic castration-resistant prostate cancer (WARMTH Act): A multicentre, retrospective study. Lancet Oncol. 2024, 25, 175–183. [Google Scholar] [CrossRef]
- Ninatti, G.; Scilipoti, P.; Pini, C.; Barletta, F.; Longoni, M.; Gelardi, F.; Sollini, M.; Gandaglia, G.; Sathekge, M.; Montorsi, F.; et al. Time for action: Actinium-225 PSMA-targeted alpha therapy for metastatic prostate cancer—A systematic review and meta-analysis. Theranostics 2025, 15, 3386–3399. [Google Scholar] [CrossRef]
- Dondi, F.; Miceli, A.; Rovera, G.; Feudo, V.; Battisti, C.; Rondini, M.; Marongiu, A.; Mura, A.; Camedda, R.; De Feo, M.S.; et al. Prostate-Specific Membrane Antigen Radioligand Therapy in Non-Prostate Cancers: Where Do We Stand? Bioengineering 2024, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, A.A.; Weiner, L.M. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020, 39, 783–803. [Google Scholar] [CrossRef] [PubMed]
- Ruzzeh, S.; Abdlkadir, A.S.; Paez, D.; Kang, K.W.; Watabe, T.; Moghrabi, S.; Scott, A.M.; Al-Ibraheem, A. Therapeutic potential of FAPI RLT in oncology: A systematic review. Theranostics 2025, 15, 4084–4100. [Google Scholar] [CrossRef] [PubMed]
- Perrone, E.; Ghai, K.; Eismant, A.; Hackermüller, N.; Moretti, R.; Greifenstein, L.; Baum, R.P. A retrospective analysis of safety, efficacy and survival of metastatic colorectal cancer patients treated with Actinium-225 FAP-directed radiopharmaceutical therapy. J. Nucl. Med. 2025, 66, 251921. [Google Scholar]
- Fu, H.; Huang, J.; Zhao, T.; Wang, H.; Chen, Y.; Xu, W.; Pang, Y.; Guo, W.; Sun, L.; Wu, H.; et al. Fibroblast Activation Protein-Targeted Radioligand Therapy with 177Lu-EB-FAPI for Metastatic Radioiodine-Refractory Thyroid Cancer: First-in-Human, Dose-Escalation Study. Clin. Cancer Res. 2023, 29, 4740–4750. [Google Scholar] [CrossRef]
- Fu, H.; Huang, J.; Zhao, L.; Chen, Y.; Xu, W.; Cai, J.; Yu, L.; Pang, Y.; Guo, W.; Su, B.; et al. 177Lu-LNC1004 Radioligand Therapy in Patients with End-stage Metastatic Cancers: A Single-Center, Single-Arm, Phase II Study. Clin. Cancer Res. 2025, 31, 1415–1426. [Google Scholar] [CrossRef]
- Juneau, D.; Kavan, P.; Metser, U.; Abikhzer, G.; Bodei, L.; Liberman, M.; Taunk, N.; O’Reilly, E.M.; Ma, J.; Cioci, R.; et al. FRONTIER: FAPi Radioligand OpeN-label, phase 1 study to evaluate safety, Tolerability and dosImetry of [Lu-177]-PNT6555; a dose Escalation study for treatment of patients with select solid tumors. J. Nucl. Med. 2024, 65, 241162. [Google Scholar]
- Courcier, J.; De La Taille, A.; Nourieh, M.; Leguerney, I.; Lassau, N.; Ingels, A. Carbonic Anhydrase IX in Renal Cell Carcinoma, Implications for Disease Management. Int. J. Mol. Sci. 2020, 21, 7146. [Google Scholar] [CrossRef]
- Shuch, B.; Pantuck, A.J.; Bernhard, J.-C.; Morris, M.A.; Master, V.; Scott, A.M.; van Praet, C.; Bailly, C.; Önal, B.; Aksoy, T.; et al. [89Zr]Zr-girentuximab for PET–CT imaging of clear-cell renal cell carcinoma: A prospective, open-label, multicentre, phase 3 trial. Lancet Oncol. 2024, 25, 1277–1287. [Google Scholar] [CrossRef]
- Stillebroer, A.B.; Boerman, O.C.; Desar, I.M.E.; Boers-Sonderen, M.J.; Van Herpen, C.M.L.; Langenhuijsen, J.F.; Smith-Jones, P.M.; Oosterwijk, E.; Oyen, W.J.G.; Mulders, P.F.A. Phase 1 Radioimmunotherapy Study with Lutetium 177–labeled Anti-Carbonic Anhydrase IX Monoclonal Antibody Girentuximab in Patients with Advanced Renal Cell Carcinoma. Eur. Urol. 2013, 64, 478–485. [Google Scholar] [CrossRef]
- Muselaers, C.H.J.; Boers-Sonderen, M.J.; Van Oostenbrugge, T.J.; Boerman, O.C.; Desar, I.M.E.; Stillebroer, A.B.; Mulder, S.F.; van Herpen, C.M.L.; Langenhuijsen, J.F.; Oosterwijk, E.; et al. Phase 2 Study of Lutetium 177–Labeled Anti–Carbonic Anhydrase IX Monoclonal Antibody Girentuximab in Patients with Advanced Renal Cell Carcinoma. Eur. Urol. 2016, 69, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, C.N.; Osellame, L.D.; Cao, Z.; McDonald, A.F.; Rigopoulos, A.; Burvenich, I.J.G.; Wichmann, C.W.; Guo, N.; Ivashkevich, A.N.; Wheatcroft, M.P.; et al. DNA-Dependent Protein Kinase Inhibitor Peposertib Enhances Efficacy of 177Lu-Based Radioimmunotherapy in Preclinical Models of Prostate and Renal Cell Carcinoma. J. Nucl. Med. 2025, 66, 385–390. [Google Scholar] [CrossRef]
- Massière, F.; Wiedemann, N.; Borrego, I.; Hoehne, A.; Osterkamp, F.; Paschke, M.; Zboralski, D.; Schumann, A.; Bredenbeck, A.; Brichory, F.; et al. Preclinical Characterization of DPI-4452: A 68Ga/177Lu Theranostic Ligand for Carbonic Anhydrase IX. J. Nucl. Med. 2024, 65, 761–767. [Google Scholar] [CrossRef]
- Chatzopoulos, K.; Aubry, M.-C.; Gupta, S. Immunohistochemical expression of carbonic anhydrase 9, glucose transporter 1, and paired box 8 in von Hippel-Lindau disease–related lesions. Hum. Pathol. 2022, 123, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Cornelio, D.B.; Roesler, R.; Schwartsmann, G. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Ann. Oncol. 2007, 18, 1457–1466. [Google Scholar] [CrossRef]
- Domanska, U.M.; Kruizinga, R.C.; Nagengast, W.B.; Timmer-Bosscha, H.; Huls, G.; De Vries, E.G.E.; Walenkamp, A.M.E. A review on CXCR4/CXCL12 axis in oncology: No place to hide. Eur. J. Cancer. 2013, 49, 219–230. [Google Scholar] [CrossRef]
- Peddio, A.; Pietroluongo, E.; Lamia, M.R.; Luciano, A.; Caltavituro, A.; Buonaiuto, R.; Pecoraro, G.; De Placido, P.; Palmieri, G.; Bianco, R.; et al. DLL3 as a potential diagnostic and therapeutic target in neuroendocrine neoplasms: A narrative review. Crit. Rev. Oncol. Hematol. 2024, 204, 104524. [Google Scholar] [CrossRef] [PubMed]
- Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the folate receptor α in oncology. Nat. Rev. Clin. Oncol. 2020, 17, 349–359. [Google Scholar] [CrossRef]
- Torrez, C.Z.; Easley, A.; Bouamar, H.; Zheng, G.; Gu, X.; Yang, J.; Chiu, Y.C.; Chen, Y.; Halff, G.A.; Cigarroa, F.G.; et al. STEAP2 promotes hepatocellular carcinoma progression via increased copper levels and stress-activated MAP kinase activity. Sci. Rep. 2024, 14, 12753. [Google Scholar] [CrossRef]
- Burnell, S.E.A.; Spencer-Harty, S.; Howarth, S.; Bodger, O.; Kynaston, H.; Morgan, C.; Doak, S.H. STEAP2 Knockdown Reduces the Invasive Potential of Prostate Cancer Cells. Sci. Rep. 2018, 8, 6252. [Google Scholar] [CrossRef]
- Soni, U.K.; Jenny, L.; Hegde, R.S. IGF-1R targeting in cancer—Does sub-cellular localization matter? J. Exp. Clin. Cancer Res. 2023, 42, 273. [Google Scholar] [CrossRef]
- Bergonzini, C.; Kroese, K.; Zweemer, A.J.M.; Danen, E.H.J. Targeting Integrins for Cancer Therapy—Disappointments and Opportunities. Front. Cell Dev. Biol. 2022, 10, 863850. [Google Scholar] [CrossRef]
- Yan, M.; Schwaederle, M.; Arguello, D.; Millis, S.Z.; Gatalica, Z.; Kurzrock, R. HER2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev. 2015, 34, 157–164. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Giammarile, F.; Carrara, M.; Paez, D.; Hricak, H.; Ayati, N.; Li, J.J.; Mueller, M.; Aggarwal, A.; Al-Ibraheem, A.; et al. Radiotherapy and theranostics: A Lancet Oncology Commission. Lancet Oncol. 2024, 25, e545–e580. [Google Scholar] [CrossRef]
- Al-Ibraheem, A.; Abdlkadir, A.S.; Albalooshi, B.; Alkuwari, M.; Al Mahariq, D.; Al-Nabhani, K.; Alqarni, A.; Al-Rowaily, M.; AlSalloum, R.; Bozkurt, M.F.; et al. Current Status of Theranostics in West Asia: A Country-based Surveillance Study. Nucl. Med. Mol. Imaging 2025, 59, 351–359. [Google Scholar] [CrossRef]
- Scott, A.M.; Zeglis, B.M.; Lapi, S.E.; Scott, P.J.H.; Windhorst, A.D.; Abdel-Wahab, M.; Giammarile., F.; Piaez, D.; Jalilian, A.; Knoll, P.; et al. Trends in nuclear medicine and the radiopharmaceutical sciences in oncology: Workforce challenges and training in the age of theranostics. Lancet Oncol. 2024, 25, e250–e259. [Google Scholar] [CrossRef]
- Giammarile, F.; Paez, D.; Zimmermann, R.; Cutler, C.S.; Jalilian, A.; Korde, A.; Knoll, P.; Ayati, N.; Lewis, J.S.; Lapi, S.E.; et al. Production and regulatory issues for theranostics. Lancet Oncol. 2024, 25, e260–e269. [Google Scholar] [CrossRef] [PubMed]
- Al-Ibraheem, A.; Brink, A.; Lee, S.T.; De Los Reyes, A.; Paez, D.; Selemo Craviolatti, P.; Llamas Olier, A.; Giammarile, F.; Abdlkadir, A.S.; Estrada-Lobato, E.; et al. Implementation of Radiotheranostics: Challenges, Barriers, and IAEA-Driven Strategies for Sustainable Access. Semin. Nucl. Med. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Yusufaly, T.; Roncali, E.; Brosch-Lenz, J.; Uribe, C.; Jha, A.K.; Currie, G.; Dutta, J.; El-Fakhri, G.; McMeekin, H.; Pandit-Taskar, N.; et al. Computational Nuclear Oncology Toward Precision Radiopharmaceutical Therapies: Current Tools, Techniques, and Uncharted Territories. J. Nucl. Med. 2025, 66, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Seifert, R.; Karkampouna, S.; Gomes, C.V.; Xue, S.; Afshar-Ormieh, A.; Rominger, A.; Shi, K. Influence of dosimetry accuracy on the correlation with treatment outcome in a preliminary PSMA radiopharmaceutical therapy study. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 1649–1657. [Google Scholar] [CrossRef]
- Aerts, A.; Eberlein, U.; Holm, S.; Hustinx, R.; Konijnenberg, M.; Strigari, L.; van Leeuwen, F.W.B.; Glatting, G.; Lassmann, M. EANM position paper on the role of radiobiology in nuclear medicine. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3365–3377. [Google Scholar] [CrossRef]
- Pouget, J.-P.; Gabina, P.M.; Herrmann, K.; Deandreis, D.; Konijnenberg, M.; Taieb, D.; van Leeuwen, F.W.B.; Kurth, J.; Eberlein, U.; Lassmann, M.; et al. EANM expert opinion: How can lessons from radiobiology be applied to the design of clinical trials? Part I: Back to the basics of absorbed dose–response and threshold absorbed doses. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 1210–1222. [Google Scholar] [CrossRef]
- Lawhn-Heath, C.; Hope, T.A.; Martinez, J.; Fung, E.K.; Shin, J.; Seo, Y.; Flavell, R.R. Dosimetry in radionuclide therapy: The clinical role of measuring radiation dose. Lancet Oncol. 2022, 23, e75–e87. [Google Scholar] [CrossRef]
- Warfvinge, C.F.; Gustafsson, J.; Roth, D.; Tennvall, J.; Svensson, J.; Bernhardt, P.; Åkesson, A.; Wieslander, E.; Sundlöv, A.; Sjögreen Gleisner, K. Relationship Between Absorbed Dose and Response in Neuroendocrine Tumors Treated with [177Lu]Lu-DOTATATE. J. Nucl. Med. 2024, 65, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Grkovski, M.; Krebs, S.S.; O’Donoghue, J.A.; Kuten, J.; Mauguen, A.; Shobeiri, P.; Lafontaine, D.; Thor, M.; Augensen, F.; Fox, J.J.; et al. Lesion Absorbed Dose–Response Relationship in Patients with Metastatic Castration-Resistant Prostate Cancer Undergoing [177Lu]Lu-PSMA-617 Radiopharmaceutical Therapy. J. Nucl. Med. 2025, 66, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Tamborino, G.; Engbers, P.; Wolf THde Reuvers, T.G.A.; Verhagen, R.; Konijnenberg, M.; Nonnekens, J. Establishing In Vitro Dosimetric Models and Dose–Effect Relationships for 177Lu-DOTATATE in Neuroendocrine Tumors. J. Nucl. Med. 2025, 66, 1291–1298. [Google Scholar] [CrossRef]
- Kiess, A.P.; O’Donoghue, J.; Uribe, C.; Bodei, L.; Hobbs, R.F.; Hesterman, J.; Kesner, A.L.; Sgouros, G. How Can Radiopharmaceutical Therapies Reach Their Full Potential? Improving Dose Reporting and Phase I Clinical Trial Design. J. Clin. Oncol. 2024, 42, 1734–1737. [Google Scholar] [CrossRef]
- Hebert, K.; Santoro, L.; Monnier, M.; Castan, F.; Berkane, I.; Assénat, E.; Fersing, C.; Gélibert, P.; Pouget, J.P.; Bardiès, M.; et al. Absorbed Dose–Response Relationship in Patients with Gastroenteropancreatic Neuroendocrine Tumors Treated with [177Lu]Lu-DOTATATE: One Step Closer to Personalized Medicine. J. Nucl. Med. 2024, 65, 923–930. [Google Scholar] [CrossRef]
- Maccauro, M.; Cuomo, M.; Bauckneht, M.; Bagnalasta, M.; Mazzaglia, S.; Scalorbi, F.; Argiroffi, G.; Kirienko, M.; Lorenzoni, A.; Aliberti, G.; et al. The LUTADOSE trial: Tumour dosimetry after the first administration predicts progression free survival in gastro-entero-pancreatic neuroendocrine tumours (GEP NETs) patients treated with [177Lu]Lu-DOTATATE. Eur. J. Nucl. Med. Mol. Imaging 2024, 52, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Di Dia, A.; Ferrari, M.E.; Travaini, L.L.; Grappeja, L.; Guglielmo, P.; Barone, A.; Mei, R.; Papi, S.; Ceci, F.; Grana, M.C. The value of dosimetry in Peptide Receptor Radionuclide Therapy (PRRT) for neuroendocrine tumors: Literature and practical aspects. Clin. Transl. Imaging 2025, 13, 121–129. [Google Scholar] [CrossRef]
- Jackson, P.; Hofman, M.; McIntosh, L.; Buteau, J.P.; Ravi Kumar, A. Radiation Dosimetry in 177Lu-PSMA-617 Therapy. Semin. Nucl. Med. 2022, 52, 243–254. [Google Scholar] [CrossRef]
- Bodei, L.; Cremonesi, M.; Ferrari, M.; Mittra, E.S.; Kulkarni, H.R.; Deroose, C.M.; Srirajaskanthan, R.; Ramage, J.; Grana, M.C.; Botta, F.; et al. Dosimetry of [177Lu]Lu-DOTATATE in Patients with Advanced Midgut Neuroendocrine Tumors: Results from a Substudy of the Phase III NETTER-1 Trial. J. Nucl. Med. 2025, 66, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Tran-Gia, J.; Cicone, F.; Koole, M.; Giammarile, F.; Gear, J.; Deshayes, E.; Minguez Gabiña, P.; Cremonsesi, M.; Wadsley, J.; Bernhardt, P.; et al. Rethinking Dosimetry: A European Perspective. J. Nucl. Med. 2025, 66, 1323–1330. [Google Scholar] [CrossRef]
- Shi, C.; Morse, M.A. Mechanisms of Resistance in Gastroenteropancreatic Neuroendocrine Tumors. Cancers 2022, 14, 6114. [Google Scholar] [CrossRef]
- Sartor, O.; Ledet, E.; Huang, M.; Schwartz, J.; Lieberman, A.; Lewis, B.; Layton, J.; Barata, P.; Jang, A.; Hawkins, M.; et al. Prediction of Resistance to 177Lu-PSMA Therapy by Assessment of Baseline Circulating Tumor DNA Biomarkers. J. Nucl. Med. 2023, 64, 1721–1725. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Heussel, C.-P.; Kazdal, D.; Endris, V.; Nientiedt, C.; Bruchertseifer, F.; Kippenberger, M.; Rathke, H.; Leichsenring, J.; et al. Patients Resistant Against PSMA-Targeting α-Radiation Therapy Often Harbor Mutations in DNA Damage-Repair–Associated Genes. J. Nucl. Med. 2020, 61, 683–688. [Google Scholar] [CrossRef]
- Baum, R.P.; Fan, X.; Jakobsson, V.; Schuchardt, C.; Chen, X.; Yu, F.; Zhang, J. Extended peptide receptor radionuclide therapy: Evaluating nephrotoxicity and therapeutic effectiveness in neuroendocrine tumor patients receiving more than four treatment cycles. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Telli, T.; Lapa, C.; Desaulniers, M.; Hekimsoy, T.; Weber, W.A.; Pfob, C.; Hadaschik, B.; Bögemann, M.; Schäfers, M.; et al. Safety and Efficacy of Extended Therapy with [177Lu]Lu-PSMA: A German Multicenter Study. J. Nucl. Med. 2024, 65, 909–916. [Google Scholar] [CrossRef]
- Hindorf, C.; Glatting, G.; Chiesa, C.; Lindén, O.; Flux, G. EANM Dosimetry Committee guidelines for bone marrow and whole-body dosimetry. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Farolfi, A.; Armstrong, W.R.; Djaileb, L.; Gafita, A.; Hotta, M.; Allen-Auerbach, M.; Unterrainer, L.M.; Fendler, W.P.; Rettig, M.; Eiber, M.; et al. Differences and Common Ground in 177Lu-PSMA Radioligand Therapy Practice Patterns: International Survey of 95 Theranostic Centers. J. Nucl. Med. 2024, 65, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Aalbersberg, E.A.; Huizing, D.M.V.; Walraven, I.; De Wit-van Der Veen, B.J.; Kulkarni, H.R.; Singh, A.; Stokkel, M.P.; Baum, R.P. Parameters to Predict Progression-Free and Overall Survival After Peptide Receptor Radionuclide Therapy: A Multivariate Analysis in 782 Patients. J. Nucl. Med. 2019, 60, 1259–1265. [Google Scholar] [CrossRef]
- Gafita, A.; Calais, J.; Grogan, T.R.; Hadaschik, B.; Wang, H.; Weber, M.; Sandhu, S.; Kratochwil, C.; Esfandiari, R.; Tauber, R.; et al. Nomograms to predict outcomes after 177Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: An international, multicentre, retrospective study. Lancet Oncol. 2021, 22, 1115–1125. [Google Scholar] [CrossRef]
- Akhavanallaf, A.; Joshi, S.; Mohan, A.; Worden, F.P.; Krauss, J.C.; Zaidi, H.; Frey, K.; Suresh, K.; Dewaraja, Y.K.; Wong, K.K. Enhancing precision: A predictive model for 177Lu-DOTATATE treatment response in neuroendocrine tumors using quantitative 68Ga-DOTATATE PET and clinicopathological biomarkers. Theranostics 2024, 14, 3708–3718. [Google Scholar] [CrossRef]
- Buteau, J.P.; Martin, A.J.; Emmett, L.; Iravani, A.; Sandhu, S.; Joshua, A.M.; Francis, R.J.; Zhang, A.Y.; Scott, A.M.; Lee, S.T.; et al. PSMA and FDG-PET as predictive and prognostic biomarkers in patients given [177Lu]Lu-PSMA-617 versus cabazitaxel for metastatic castration-resistant prostate cancer (TheraP): A biomarker analysis from a randomised, open-label, phase 2 trial. Lancet Oncol. 2022, 23, 1389–1397. [Google Scholar] [CrossRef]
- Strigari, L.; Schwarz, J.; Bradshaw, T.; Brosch-Lenz, J.; Currie, G.; El-Fakhri, G.; Jha, A.K.; Mežinska, S.; Pandit-Taskar, N.; Roncali, E.; et al. Computational Nuclear Oncology Toward Precision Radiopharmaceutical Therapies: Ethical, Regulatory, and Socioeconomic Dimensions of Theranostic Digital Twins. J. Nucl. Med. 2025, 66, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.H. Avatar: Personalized Precision Radio-Genomic Theranostic Oncology. Cancer Biother. Radiopharm. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Gafita, A.; Djaileb, L.; Rauscher, I.; Fendler, W.P.; Hadaschik, B.; Rowe, S.P.; Herrmann, K.; Calais, J.; Rettig, M.; Eiber, M.; et al. Response Evaluation Criteria in PSMA PET/CT (RECIP 1.0) in Metastatic Castration-resistant Prostate Cancer. Radiology 2023, 308, e222148. [Google Scholar] [CrossRef]
- Gafita, A.; Djaileb, L.; Rauscher, I.; Fendler, W.P.; Hadaschik, B.; Rowe, S.P.; Herrmann, K.; Solnes, L.B.; Calais, J.; Rettig, M.B.; et al. RECIP 1.0 Predicts Progression-Free Survival After [177Lu]Lu-PSMA Radiopharmaceutical Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2024, 65, 917–922. [Google Scholar] [CrossRef]
- Kong, G.; Noe, G.; Chiang, C.; Herrmann, K.; Hope, T.A.; Michael, M. Assessment of response to PRRT including anatomical and molecular imaging as well as novel biomarkers. J. Neuroendocrinol. 2025, 37, e13461. [Google Scholar] [CrossRef]
- Al-Ibraheem, A.; Scott, A.M.; Abdlkadir, A.S.; Vrachimis, A.; Lamoureux, F.; Bernal Trujillo, P.; Bailey, D.L.; More, S.; Giammarile, F.; Kumar, R.; et al. Consensus Nomenclature for Radionuclide Therapy: Initial Recommendations from Nuclear Medicine Global Initiative. J. Nucl. Med. 2025, 66, 757–763. [Google Scholar] [CrossRef] [PubMed]

| [177Lu]Lu-DOTA-TATE (Lutathera®) | [177Lu]Lu-PSMA-617 (Pluvicto ®) | |
|---|---|---|
| Target | SSTR2 | PSMA |
| Ligand | TATE (peptide) | PSMA-617 (small molecule) |
| Linker | DOTA (non-cleavable) | DOTA (non-cleavable) |
| Radionuclide | Lutetium-177 (β-emitter) | Lutetium-177 (β-emitter) |
| Year of FDA approval | 2018 | 2022 |
| Year of EMA approval | 2017 | 2022 |
| Approved indication(s) | Progressive, unresectable or metastatic, SSTR-positive G1-G2 GEP NETs in adults (FDA, EMA) Progressive, unresectable or metastatic, SSTR-positive G1-G2 GEP NETs in paediatric patients ≥ 12 years (FDA) | PSMA-positive mCRPC previously treated with ARPI and taxane-based CT (FDA, EMA) PSMA-positive mCRPC previously treated with ARPI and considered appropriate to delay taxane-based chemotherapy (FDA) |
| Standard activity administration | 7.4 GBq (200 mCi) IV every 8 weeks for 4 cycles; co-infusion of amino acids for renal protection | 7.4 GBq (200 mCi) IV every 6 weeks for up to 6 cycles |
| Common adverse effects | Fatigue, nausea, vomiting, diarrhea, abdominal pain, myelosuppression, (anaemia, thrombocytopaenia, lymphocytopaenia), nephrotoxicity | Fatigue, nausea, vomiting, diarrhea, myelosuppression (anaemia, thrombocytopaenia, lymphocytopaenia), nephrotoxicity, xerostomia, dry eyes |
| NCT Number | Completion, y | Radiopharmaceutical | Patients, n | Patient Population | Combination | Comparator Arm | Sponsor | Outcome Data |
|---|---|---|---|---|---|---|---|---|
| NCT01578239 (NETTER-1) | 2021 | [177Lu]Lu-DOTA-TATE | 231 | Progressive, inoperable or metastatic SSTR+ G1-G2 midgut NETs | Octreotide LAR | Octreotide LAR | Advanced Accelerator Applications * | mPFS: 28.4 vs. 8.4 mo (p < 0.001) mOS: 48 mo vs. 36.3 mo (p = 0.30) |
| NCT03972488 (NETTER-2) | 2027 | [177Lu]Lu-DOTA-TATE | 226 | Treatment-naïve inoperable or metastatic G2-G3 GEP NETs | Octreotide LAR | Octreotide LAR | Advanced Accelerator Applications * | mPFS: 22.8 vs. 8.5 (p < 0.001) |
| NCT04919226 (COMPOSE) | 2027 | [177Lu]Lu-DOTA-TOC | 259 | Advanced G2-G3 GEP NETs (first or second line of treatment) | None | Best SoC | ITM Solucin GmbH | NA |
| NCT03049189 (COMPETE) | 2029 | [177Lu]Lu-DOTA-TOC | 309 | Progressive, inoperable or metastatic SSTR+ G1-G2 GEP NETs | None | Everolimus | ITM Solucin GmbH | mPFS: 29.3 vs. 14.1 mo (p = 0.022) † mOS: 63.4 vs. 58.7 mo (p = 0.206) † |
| NCT06784752 (NETTER-3) | 2034 | [177Lu]Lu-DOTA-TATE | 240 | Newly diagnosed metastatic or locally advanced G1-G2 GEP NET | Octreotide LAR | Octreotide LAR | Novartis Pharmaceuticals | NA |
| NCT Number | Completion, y | Radiopharmaceutical | Patients, n | Patient Population | Combination | Comparator Arm | Sponsor | Outcome Data |
|---|---|---|---|---|---|---|---|---|
| NCT03511664 (VISION) | 2023 | [177Lu]Lu-PSMA-617 | 831 | mCRPC post ARPI, post taxane CT | SoC | SoC | Endocyte | mPFS: 8.7 vs. 3.4 mo (p < 0.001) mOS: 15.3 vs. 11.3 mo (p < 0.001) |
| NCT04689828 (PSMAfore) | 2025 | [177Lu]Lu-PSMA-617 | 470 | mCRPC post ARPI | None | ARPI change | Novartis Pharmaceuticals | mPFS: 11.6 vs. 5.6 mo (p < 0.001) mOS: 24.5 vs. 23.1 mo (p = 0.20) |
| NCT04720157 (PSMAddition) | 2026 | [177Lu]Lu-PSMA-617 | 1145 | mHSPC | SoC (ARPI + ADT) | SoC (ARPI + ADT) | Novartis Pharmaceuticals | NA |
| NCT04647526 (SPLASH) | 2028 | [177Lu]Lu-PSMA-I&T | 415 | mCRPC post ARPI | None | ARPI change | POINT Biopharma | mPFS: 9.5 vs. 6.0 mo (p = 0.0088) * mOS: 20.8 vs. NE mo (p = 0.615) * |
| NCT04876651 (ProstACT GLOBAL) | 2028 | [177Lu]Lu-DOTA- rosopatamab | 392 | mCRPC post ARPI | SoC | SoC | Telix Pharmaceuticals | NA |
| NCT05204927 (ECLIPSE) | 2029 | [177Lu]Lu-PSMA-I&T | 439 | mCRPC post ARPI | None | ARPI change | Curium US LLC | NA |
| NCT05939414 (PSMA-DC) | 2030 | [177Lu]Lu-PSMA-617 | 450 | OMPC progressing after primary treatment | SBRT | SBRT | Novartis Pharmaceuticals | NA |
| NCT06520345 (ProstACT GLOBAL) | 2030 | [177Lu]Lu-DOTA- rosopatamab | 430 | mCRPC post ARPI | SoC | SoC | Telix Pharmaceuticals | NA |
| NCT06320067 (STAMPEDE2) | 2034 | [177Lu]Lu-PSMA-617 | 1756 | mHSPC | SoC | SoC | University College, London | NA |
| NCT06496581 (PEACE6-Poor Responders) | 2039 | [177Lu]Lu-PSMA-617 | 500 | mHSPC | SoC | SoC | UNICANCER | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ninatti, G.; Lee, S.T.; Scott, A.M. Radioligand Therapy in Cancer Management: A Global Perspective. Cancers 2025, 17, 3412. https://doi.org/10.3390/cancers17213412
Ninatti G, Lee ST, Scott AM. Radioligand Therapy in Cancer Management: A Global Perspective. Cancers. 2025; 17(21):3412. https://doi.org/10.3390/cancers17213412
Chicago/Turabian StyleNinatti, Gaia, Sze Ting Lee, and Andrew M. Scott. 2025. "Radioligand Therapy in Cancer Management: A Global Perspective" Cancers 17, no. 21: 3412. https://doi.org/10.3390/cancers17213412
APA StyleNinatti, G., Lee, S. T., & Scott, A. M. (2025). Radioligand Therapy in Cancer Management: A Global Perspective. Cancers, 17(21), 3412. https://doi.org/10.3390/cancers17213412







