Simple Summary
Neuroendocrine tumours (NETs) belong to a heterogeneous group of malignant neoplasms that differ in their ability to produce hormones and biogenic amines, which is often associated with a more favourable prognosis for well-differentiated tumours. Despite numerous reports in the literature documenting venous thromboembolism (VTE) events in these patients, data on thromboembolic complications in NETs remain limited, and no specific recommendations exist regarding the use of antithrombotic prophylaxis in this group. Thrombotic risk assessment models have not yet been validated in NETs. This article presents the first prospective analysis of selected coagulation parameters, the incidence and risk factors for VTE, and evaluates thromboembolic risk assessment models as well as survival data in pancreatic and small intestinal NETs. Our aim was to improve patient stratification for VTE risk and to help identify patients with NETs who might benefit from antithrombotic prophylaxis.
Abstract
Background: Data on venous thromboembolism (VTE) in neuroendocrine tumours (NETs) are scarce. To the best of our knowledge, this is the first analysis of coagulation parameters and a comparison of VTE risk assessment scales in NETs. Methods: Patients with well-differentiated NETs (n = 99), including pancreatic (n = 63) and small intestinal (n = 36) primary, as well as 47 healthy controls, were enrolled. Blood levels of coagulation parameters, including D-dimer (DD), fibrinogen, platelets, antithrombin III (AT-III), and tissue factor (TF), were assessed. Venous Doppler ultrasound of the lower extremities was performed in all study participants. Results: DD plasma concentration was significantly higher in NET patients than in the control group (957.59 ± 2021.86 µg/L vs. 400.26 ± 230.55 µg/L, p = 0.007) and positively correlated with chromogranin A (rS = 0.32, p = 0.001). DD and fibrinogen plasma levels were statistically higher in patients with disease progression compared to those with stable disease (2513.7 ± 3624.3 vs. 431.9 ± 244.7 µg/L, p < 0.001; 359.3 mg/mL vs. 305.4 mg/mL, p < 0.001, respectively). None of the VTE risk assessment scales provided a good measure (AUC > 0.7) in ROC analysis. However, higher scores on the Khorana (p = 0.023) and Vienna CATS scale (p < 0.001) were associated with unfavourable survival in NETs. Conclusions: Pancreatic NETs demonstrate increased risk of VTE. The Khorana and Vienna CATS scales best correlated with the NET patients’ outcomes. Routine assessment of DD and fibrinogen may improve risk stratification for VTE in NET patients; however, extensive multicenter validation is necessary for clinical implementation.
1. Introduction
The incidence of venous thromboembolism (VTE) markedly increases mortality in cancer patients, potentially by as much as fourfold [1]. There are various potential mechanisms related to cancer-associated thrombosis. Virchow’s triad highlights three key risk factors that may contribute to the development of thrombosis, including damage to the vessel wall, stasis of the blood flow, and hypercoagulability [2]. VTE may present as deep vein thrombosis (DVT), pulmonary embolism (PE), or atypical thrombosis, which may include abdominal tumour thrombosis (TT) such as portal, splanchnic, and mesenteric vein thrombosis. Superficial venous thrombosis (SVT), regarded as a benign condition, may also lead to the development of DVT and/or PE [3]. Effective blood biomarkers indicating the development of VTE in cancer patients are needed. Candidate biomarkers include blood levels of D-dimer (DD), fibrinogen, platelets (PLT), antithrombin III (AT-III), and tissue factor (TF).
Well-differentiated neuroendocrine tumours (NETs) are biologically and clinically distinct from other malignancies because of their ability to produce hormones in the case of functioning NETs (F-NETs), as well as a typically msore favourable prognosis and a different treatment approach due to the presence of somatostatin receptors on their surfaces [4]. NETs can also exhibit an increased risk of VTE [5], but the mechanisms behind this are still not well understood. It is assumed that some angiogenic factors, such as vascular endothelial growth factor (VEGF), may be good candidates to be prognostic factors of VTE [6,7]. NET cases also presented a high expression of proangiogenic factors [8,9], but the link between VTE and these parameters warrants further research. Additionally, the excessive serotonin production in F-NETs can also contribute to the damage of the vascular endothelium and lead to a loss of anticoagulant properties, facilitating clot formation [10]. However, this requires broadening our knowledge in this area. Despite a large number of reported cases of VTE in NETs [11,12], there are currently no guidelines on the assessment of coagulation parameters or the application of thromboembolic risk assessment models in this group. It is assumed that conventional VTE risk assessment models, such as the Khorana score (KS) and Vienna-CATS score (VC-S), may not apply to NETs, as they were developed and validated on a different group of oncological patients, often with completely different biology and aggressiveness [13]. However, the appropriate assessment of the thromboembolic risk remains vital to effectively identify patients at risk of VTE to implement preventive management such as thromboprophylaxis. The primary objectives of this work were to assess the predictive validity of various VTE risk assessment scales in predicting VTE risk in patients with pancreatic (Pan-NETs) and small intestine (SI-NETs) NETs, and the secondary objective was to determine the clinical utility of blood coagulation biomarkers as indicators of VTE in these patients.
2. Materials and Methods
All patients were enrolled at the Department of Endocrinology and Neuroendocrine Tumours, ENETS Center of Excellence, Medical University of Silesia, in Katowice, Poland. Informed written consent was obtained from all study participants. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The Ethics Committee of the Medical University of Silesia approved the study protocol (approval number: BNW/NWN/0052/KB1/147/I/22/23). The inclusion and exclusion criteria for the study are shown in Table 1.

Table 1.
The main inclusion and exclusion criteria for this study group.
Blood levels of selected parameters, including DD, fibrinogen, PLT, AT-III, and TF, were measured. These parameters were then correlated with four VTE risk assessment scales (the Khorana score [KS], the Vienna CATS score [VC-S], the ONCOTEV score [ONCO-S], and the Padua Prediction Score [PPS], Table 2). The KS and the VC-S are used to predict the risk of VTE in cancer patients, especially those receiving anticancer therapy [14,15]. The ONKO-S can also serve as a VTE risk prediction model for cancer patients to help stratify VTE risk and identify a higher occurrence of VTE [16]. The PPS is employed to evaluate the risk of VTE in hospitalized, non-surgical patients [17]. The coagulation parameters were also correlated with other variables such as survival data, ECOG/WHO scale, primary tumour site, clinical staging, secretory status, concentration of selected NET markers (chromogranin A, serotonin, and urinary 5-hydroxyindoleacetic acid), disease progression (based on radiological evaluation), and treatment history. All study participants underwent screening for DVT or SVT using a venous Doppler ultrasound of the lower extremities and iliac vessels, including a B-mode compression test and a Doppler examination. A qualified specialist in angiology performed all examinations. Patients were followed up for VTE events for 12 months. The overall survival (OS) was calculated as the time from blood sample collection to either the patient’s death or the end of follow-up.

Table 2.
Characteristics of VTE Risk Assessment Scales. The table presents the parameters considered in assessing the risk scale.
2.1. Blood Sampling and Laboratory Analysis
Venous blood samples were collected from all study participants. The testing tubes contained trisodium citrate. The ACL TOP 350 analyzer was used to assess plasma levels of DD, fibrinogen, and AT-III, and the Sysmex XN-1000 analyzer was used to measure the blood count. Plasma levels of TF were determined using enzyme-linked immunosorbent assay (ELISA).
2.2. Statistical Analysis
Data analysis and visualization were conducted using R version 4.5.0 (The R Foundation for Statistical Computing, Vienna, Austria) in R Studio version 2024.12.1 build 563 (PBC, Boston, MA, USA). Kruskal–Wallis and Fisher-exact tests were used for group comparisons. The Spearman rank correlation coefficient (rS) was used to correlate the biochemical, clinical, and demographic parameters using the stats package. Cox proportional hazards models and Kaplan–Meier survival curves were generated using R with the survival package, survminer package, and dplyr package. Continuous parameters were converted to categorical variables by dividing the dataset into 5 groups, where each group contained 20% of the cases, ordered from the lowest to the highest values (labelled very low, low, medium, high, and very high) due to Kaplan–Meier analysis requirements. All the data were presented as numbers of cases (n) and mean values ± standard deviation (SD). The value of p < 0.05 was considered statistically significant.
2.3. Multivariate Survival Analysis
Given the limited sample size, multivariate survival analyses were performed on selected parameters, chosen based on the study’s scope, preliminary survival results, and statistical analyses (including correlations with survival and other sociodemographic and biochemical parameters). Age and metastasis did not demonstrate a statistically significant association with patient survival, whereas the impact of clinical staging was consistent with findings reported in previous studies. Notably, clinical staging differed between tumour locations (Pan-NETs vs. SI-NETs) in our study; therefore, in the Cox proportional hazards analysis, clinical staging was excluded in favour of tumour location. For consistency, the results of the Cox proportional hazards model in this article are presented with continuous variables categorized in the same manner as in the Kaplan–Meier analysis. Results of the Cox model for continuous variables are available in the Supplementary Materials (Supplementary Figure S1).
2.4. Missing Data Handling
A review of the dataset in the study group showed no missing values for sociodemographic parameters (age and sex), body mass index (BMI), selected coagulation parameters (DD, fibrinogen, PLT count, and AT-III), survival data, ECOG/WHO scale, primary tumour site, clinical staging, grading, secretory status, concentration of selected NET markers (chromogranin A, serotonin, urinary 5-hydroxyindoleacetic acid), cortisol, disease progression, and treatment and VTE risk assessment scales. The only missing data pertained to TF, where levels for 5 patients (5.05%) were undetermined. Given that this represented a small portion of the total sample, a complete case analysis was deemed appropriate for survival modelling. For all other statistical analyses that did not involve the TF variable, data from the full cohort of patients were included.
No missing data for measured parameters were reported in the control group.
3. Results
The study group included 99 NET patients, of which Pan-NETs comprised 63.6% (n = 63) and 36.4% of SI-NETs (n = 36). The control group included 47 healthy volunteers. No significant differences in age, sex, or BMI between the study and control groups. The follow-up for VTE events ranged due to differences in the timing of patient inclusion during the over 1.5-year recruitment phase but was approximately 12 months. The clinicopathological characteristics of both the study and control groups are shown in Table 3.

Table 3.
Characteristic of the study and control group.
3.1. The Occurrence of VTE Events and VTE Risk Assessment Scales
VTE events were observed at the start of the study, before the follow-up period, in 10% of patients (n = 10). These included DVT (n = 1), both DVT and SVT (n = 1), SVT alone (n = 2), and TT (n = 6), which comprised splanchnic vein thrombosis (n = 5) and portal vein thrombosis (n = 1) (Table 4). None of the patients exhibited PE symptoms, nor was this condition detected during follow-up imaging (CT scans) (Figure 1). No VTE events were observed in the control group. The assessment of study and control groups according to the VTE risk assessment scales is presented in Table 3.

Table 4.
Characteristic of the patients with VTE events.
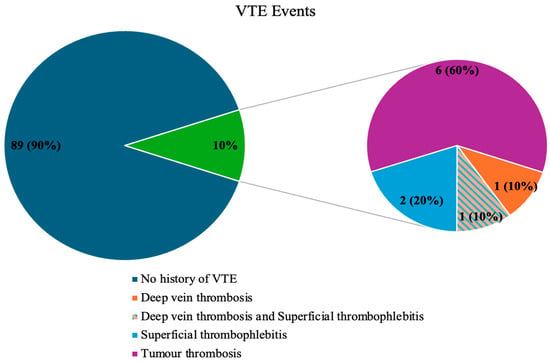
Figure 1.
The occurrence of VTE events in the study group.
3.2. Selected Blood Parameters of Coagulation
3.2.1. D-Dimer
The mean plasma concentration of DD was significantly higher in the study group compared to the control group (957.59 ± 2021.86 vs. 400.26 ± 230.55 µg/L, p = 0.007) (Table 5), but no differences between Pan-NETs and SI-NETs were observed (Table 6). The highest DD levels were noted in patients with liver and bone metastases (1717 ± 3151.1 vs. 2073.4 ± 2288.9 µg/L, respectively), and in patients with progressive disease compared to stable disease (2513.7 ± 3624.3 vs. 431.9 ± 244.7 µg/L, p ≤ 0.001). DD levels were statistically higher in patients who passed away compared to those who remained alive at the last follow-up (1749.2 ± 445.2 vs. 915.5 ± 2064.9 µg/L, p = 0.002). No statistical differences were observed in DD concentrations depending on the treatment used.

Table 5.
The results of the coagulation parameters in the study and control groups.

Table 6.
The results of the coagulation parameters in the Pan-NETs and SI-NETs groups.
3.2.2. Fibrinogen
The mean plasma concentration of fibrinogen showed no difference between the study and control groups (318.98 ± 78.74 vs. 303.40 ± 55.45 mg/dL, p = 0.301, respectively) (Table 5), and no differences between Pan-NETs and SI-NETs (Table 6). Patients with progressive disease had higher fibrinogen levels compared to those with stable disease (359.3 ± 106.3 vs. 305.4 ± 62.2 mg/dL, p = 0.041). Similarly, patients who died, compared to those who remained alive at the last follow-up, also exhibited higher fibrinogen levels (421.4 ± 123 vs. 313.5 ± 72.7 mg/dL, p = 0.025). No statistical differences were observed in fibrinogen levels depending on the treatment used.
3.2.3. Platelets
The mean PLT count was similar between the study and control groups (255.28 ± 97.22 vs. 256.11 ± 54.93, p = 0.435) (Table 5), and no differences between Pan-NETs and SI-NETs were observed (Table 6). Patients with higher serotonin levels had higher PLT levels (282.9 ± 99.4 vs. 238.1 ± 100.4, p = 0.004). No statistical differences were observed in PLT count depending on the treatment used.
3.2.4. Antithrombin III Activity
The average activity of AT-III was similar in both the study and control groups (101.71 ± 14.95 vs. 102.47 ± 11.34%, p = 0.795) (Table 5) and was higher in Pan-NETs compared to Si-NETs (104.63 ± 14.56 vs. 96.58 ± 14.42%, p = 0.010) (Table 6). Moreover, patients with TT exhibited higher AT-III levels than those without TT (122 ± 17.1 vs. 100.4 ± 13.9%, p = 0.011). Patients with metastatic disease had lower AT-III levels compared to those without metastases (98.3 ± 16.7 vs. 106.4 ± 10.7%, p = 0.007). Patients with higher serotonin levels had lower AT-III levels (96.8 ± 14.1 vs. 104.7 ± 14.8%, p = 0.013). The lower AT-III levels were noted in patients during somatostatin analogue treatment compared to those without this type of treatment (95.1 ± 15.6 vs. 105.2 ± 13.5%, p = 0.003). No statistical differences were observed in AT-III depending on the other treatment used.
3.2.5. Tissue Factor
The mean concentration of TF was similar in both the study and control groups (157.71 ± 63.96 vs. 161.77 ± 25.56 pg/mL, p = 0.160) (Table 5) or between Pan-NETs and SI-NETs (Table 6). Patients with localized disease had lower levels of TF than those with metastases (143.8 ± 71.2 vs. 167.2 ± 57.3 pg/mL, p = 0.023). No statistical differences were observed in TF levels depending on the treatment used.
The correlations between the measured coagulation parameters and selected clinical parameters are presented in Table 7.

Table 7.
The correlations (rS) between coagulation parameters and selected clinical parameters.
3.3. Survival Analysis and VTE Risk Assessment Scales
Preliminary survival analysis with a median follow-up of 12 months uncovered that 5.1% of patients passed away (n = 5). All deceased patients were at the IV clinical stage. The cause of death was unknown. The OS was calculated at 94.9%. The selected parameters, which negatively affected survival in NET patients, are presented in Figure 2. Multivariate Cox regression analysis of selected parameters and VTE risk assessment scales was presented in Figure 3. None of the VTE risk assessment scales provided a good measure (AUC > 0.7) in ROC analysis (Table 8, Figure 4). A higher score on the Khorana or Vienna CATS scale compared to other scales was linked to worse survival (p = 0.009 and p ≤ 0.001, respectively) (Figure 5).
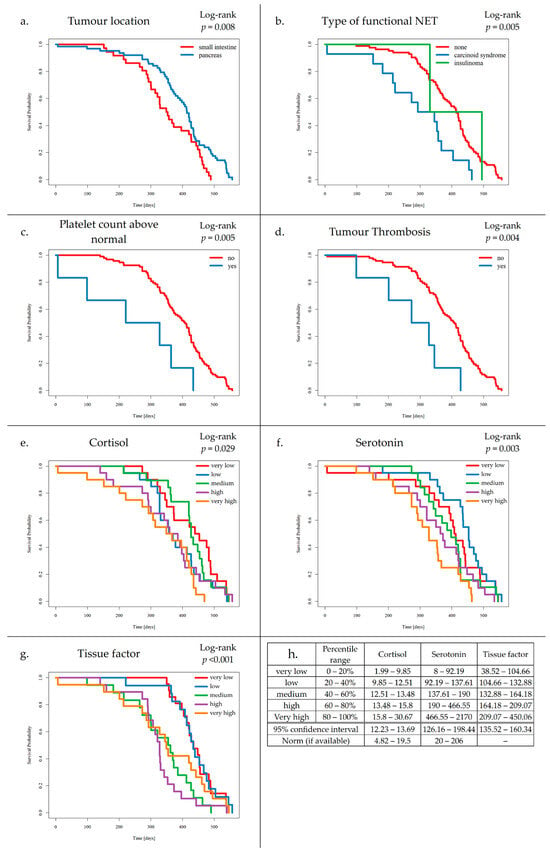
Figure 2.
Kaplan–Meier survival curves for tumour location (a), type of functional NET (b), platelet count above normal (c), tumour thrombosis (d), cortisol (e), serotonin (f), tissue factor (g), and range legend for categorized levels of cortisol, serotonin, and tissue factor, with 95% confidence interval range and polish norm range (h).
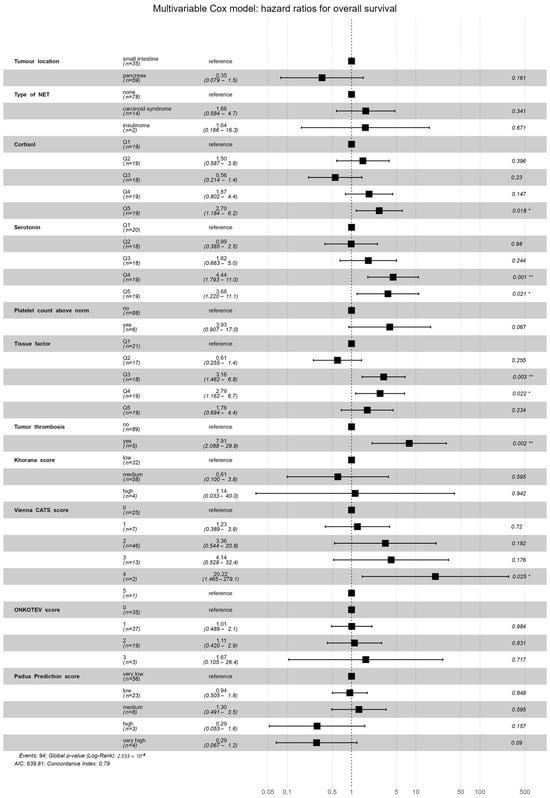
Figure 3.
Multivariate analysis of selected parameters and VTE risk assessment scales in the Cox regression model. High levels of serotonin (Q4 p = 0.001, Q5 p = 0.021) and cortisol (Q5 p = 0.018), medium and high levels of TF (Q3 p = 0.003, Q4 p = 0.022), presence of TT (p = 0.002), and Vienna CATS score (score = 4, p = 0.025) were statistically significant in the specific Cox model results. Model concordance index = 0.79, model p < 0.001. Statistical significance is indicated as follows: ‘*’ for p-values lower than 0.05; ‘**’ for p-values lower than 0.005. The black square represents the hazard ratio, the black lines represent the hazard ratio 95% confidence interval, and the dotted line represents the hazard ratio of the reference (HR = 1.0).

Table 8.
ROC analysis of VTE risk assessment scales and survival.
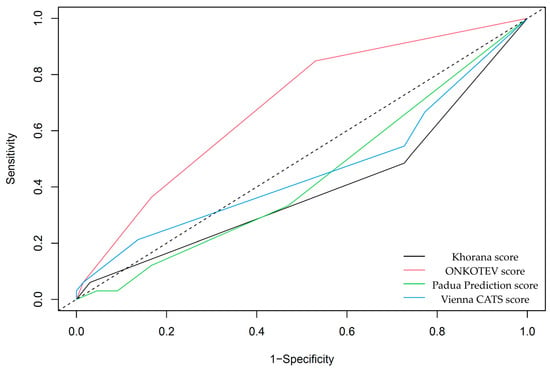
Figure 4.
ROC analysis of VTE risk assessment scales and survival. None of the VTE risk assessment scales are considered good measures (AUC > 0.7) in the context of survival analysis. Among others, only the ONCOTEV score showed an AUC of 0.68 ± 0.05, p ≤ 0.001.
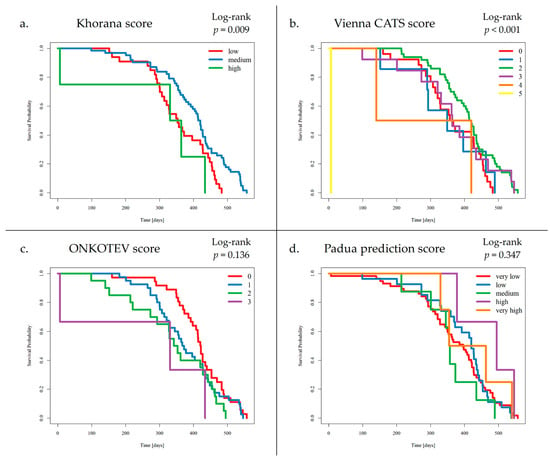
Figure 5.
Survival analysis by VTE Risk Assessment Scales: (a) Khorana score, (b) Vienna CATS score, (c) ONCOTEV score, (d) Padua Prediction score. Patients who developed VTE events had a medium score on the KS (1–2 points) in 9 cases and a low score on the KS (0 points) in 1 case. Additionally, VTE events were detected in patients with 3 points, 2 points, and 1 point on the VC-S, in 3 cases, 5 cases, and 2 cases, respectively. A higher score on the KS or VC-S was associated with reduced survival (p = 0.009 and p < 0.001, respectively).
- (a)
- Patients with SI-NETs compared to Pan-NETs exhibited poorer survival (p = 0.008).
- (b)
- Patients with carcinoid syndrome, compared to NF-NETs, exhibited poorer survival (p = 0.005).
- (c)
- Increased platelet levels were found to negatively affect survival (p = 0.005).
- (d)
- Increased TF levels were found to negatively affect survival (p ≤ 0.004).
- (e)
- Elevated cortisol levels were associated with worse outcomes compared to those with normal levels of cortisol (p = 0.029).
- (f)
- Additionally, elevated serotonin levels were associated with worse outcomes compared to those with normal levels of serotonin (p = 0.003).
- (g)
- Increased TF levels were found to negatively affect survival (p ≤ 0.001).
4. Discussion
The most commonly predisposed cancer sites associated with VTE include the digestive system (in particular, the pancreas and stomach) [18,19,20]. NETs were also reported to present an increase in thrombotic risk [11,12]. To the best of our knowledge, the current study is the first to analyze thromboembolic risk using VTE risk assessment scales and selected coagulation parameters in patients with NETs. According to Massironi et al., in a retrospective cohort of 160 GEP-NET patients, 12 patients developed VTE, and an elevated risk of VTE was found to be associated with the primary tumour site location (particularly in the pancreas) or the higher tumour grade [5]. We also confirmed the most common VTE events in Pan-NETs, and presented that routinely available tests, such as DD and fibrinogen assessment, could help improve risk stratification of VTE in NET cases. Typically, VTE in NETs may occur as DVT and PE. However, NET patients may also develop thrombosis in atypical locations, such as abdominal veins [11]. Nearly 33% of Pan-NETs were found to develop venous tumour thrombi [21]. Almost 33% of non-functioning NETs (NF-NETs), that are typically slow-growing and late presenting tumours, were diagnosed with venous thrombosis within the tumour [22]. TT is common in advanced Pan-NETs [23], particularly at T3 and T4 stages [24,25]. TT was associated with an unfavourable prognosis [26], similar to our findings. In metastatic pancreatic cancer, the occurrence of TT was associated with almost a three-fold higher risk of mortality [26]. Of note, diagnosis of the TT, acute symptomatic or incidentally detected splanchnic vein thrombosis, requires anticoagulation treatment for usually 3–6 months (or sometimes longer) [26].
Plasma levels of DD and fibrinogen are frequently used as indicators of hypercoagulability [27,28]. DD concentration exceeding the upper limit of normal by two-fold correlated with a higher risk of VTE in cancer patients [18,29], and similarly, we observed elevated levels of DD in most cases of VTE events in our study. However, DD remains a non-specific biomarker that can rise due to various causes other than VTE [18]. We also showed, similarly to many different malignancies [28,30,31,32,33,34], that DD and fibrinogen correlated with disease progression in NET patients. Cancer cells are reported to possess potent procoagulant properties that can activate the coagulation system by local activation of thrombin or by secretion of inflammatory factors [35] and increase plasma DD and fibrinogen levels [36]. In the literature, a correlation between DD and VEGF concentration has been noted [37,38]. It is known that VEGF is recognized as a crucial factor that promotes angiogenesis, and this process may assist the migration of cancer cells, increasing blood and oxygen supply to the tumour, and facilitating disease progression [39,40]. The role of fibrinogen in promoting metastasis remains unclear, potentially being the crucial mediator in establishing the tumour microenvironment [41]. Palumbo et al. noticed that fibrinogen is considered an essential determinant of metastasis of cancer cells [42]. In a study on cell lines, fibrinogen administration was shown to induce the expression of adhesion molecules such as ICAM-1 and enhance tumour cell migration, as well as increase angiogenesis and vascular endothelial permeability [43], but further research in NETs is needed.
Thrombocytosis is often observed in cancers of the gastrointestinal tract, breast, lung, and ovaries [44]. F-NETs associated with carcinoid syndrome and carcinoid heart disease had a heightened risk of thrombosis. The underlying mechanism may include the role of the increased serotonin levels in platelet activation [45]. In a study by Lopez-Vilchez et al., serotonin was shown to enhance the procoagulant activity of PLT, which engulfs TF-microparticles (TF-MVs) [46]. In this way, serotonin may enhance the PLT response by increasing thrombin generation, which plays a role in thrombus formation and can be significantly associated with increased cardiovascular risk [46]. According to Llobet D et al., human vesicle-associated membrane protein 8 (VAMP8) and serotonin transporter (SERT) may also be linked with platelet hyper-reactivity and VTE in a female Spanish population [47]. We found in our NET cohort that higher serotonin levels positively correlated with platelet levels, and an increased platelet count was associated with poorer survival.
AT-III acts as an endogenous serine protease inhibitor, and it plays a role in the inhibition of several enzymes related to the coagulation cascade, including thrombin [48]. Antithrombin deficiency is regarded as a risk factor for thrombosis in the general population, but its association with the risk of cancer-related thrombosis remains unclear. In an observational cohort study of 1127 cancer patients, AT-III was not associated with the risk of VTE; however, it showed a u-shaped association with the risk of all-cause mortality; patients with either very high or very low levels had worse OS [49]. We confirmed that AT-III levels were significantly lower in patients with metastatic disease compared to those without metastases, and lower levels of AT-III were observed in SI-NETs and were associated with increased levels of serotonin. We also noticed that patients during somatostatin analogues treatment had lower AT-III levels compared to those not receiving this treatment. However, further research is required in a larger population group to establish the impact of the applied treatment on coagulation parameters.
TF also leads to excessive thrombin generation and a subsequent hypercoagulable state [50]. It plays multiple roles, including its influence on cell migration and cell survival, interacting with integrins and receptor tyrosine kinases [51]. TF plays a critical role in the metastasis process [52,53]. Many types of cancer cells, including pancreatic, lung, gastric, breast, and brain cancer, were shown to express TF and release microparticles [TF-MVs] [44]. TF and TF-MVs correlated with poorer outcomes in cancer patients, especially those with pancreatic cancer [54]; however, no statistically significant association between TF and the risk of VTE was observed in all cases [55]. The downregulation of TF expression in Pan-NETs was shown to inhibit tumour cell proliferation in vitro. Additionally, the mTOR kinase inhibitor sapanisertib suppressed TF expression and activity, reducing Pan-NET growth in vivo [56]. TF was proposed as a potential molecule in cancer therapy for other types of cancer, e.g., tisotumab for the treatment of cervical cancer [53]. Given the promising reports on the role of TF in the survival assessment of patients with various cancers, including NETs, further studies are needed.
There are various available models for predicting the risk of VTE in cancer patients. Notably, these tools were neither developed nor validated for NETs, which exhibit many distinct features from common cancers. Since PP-S is intended to assess the risk of VTE for hospitalized or acutely ill patients, this may potentially result in their limited utility in NETs. Although Godinho et al. suggested that the ONCO-S may help stratify VTE risk in pancreatic cancers and identify patients who may benefit from thromboprophylaxis [57], in our study, this scale approached an AUC nearing the acceptable threshold, but it also did not meet the criterion and may require further research. The KS and new-VC-S can be useful for identifying patients at higher risk of mortality in other types of cancers [13]. Similarly, we found that higher scores on the KS or VC-S were linked to poorer survival in NET patients, which may relate to the location of the primary tumour, advanced clinical stage, and previously mentioned coagulation disorders that increase the risk of VTE in NET cases.
5. Limitations of the Study
This study has several potential limitations. The population size and the number of recorded VTE events (n = 10) were limited, which could impact the statistical multivariate power of the analyses. Additionally, follow-up was restricted to 12 months. Unknown cause of death, lack of long-term survival data (>12 months), and limited population size in relation to analyzed variables strongly limit the viability of survival analysis and require further studies and more extended observation periods. Furthermore, there is a possible underestimation of asymptomatic PE, as patients were not screened for asymptomatic PE cases due to the invasive nature of the computed tomography pulmonary angiography. Another aspect is the impact of the applied treatment, which also requires a more in-depth analysis of a larger population group. Finally, acute-phase proteins and family history, which may affect coagulation parameters, were not assessed. All the above warrant further research in this area.
6. Conclusions
Patients with NETs, particularly Pan-NETs, exhibited a higher risk of VTE. Plasma DD and fibrinogen levels may assist with predicting disease progression in NET patients. The use of the Khorana and Vienna CATS scales may be useful in NET patients Routine assessment of DD and fibrinogen may improve risk stratification for VTE in NET patients; however, extensive multicenter validation is necessary for clinical implementation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17213405/s1, Figure S1: Multivariate analysis of selected parameters without categorization of cortisol, serotonin and tissue factor, and VTE risk assessment scales in the Cox regression model.
Author Contributions
Conceptualization, M.W.-G.; methodology, M.W.-G. and A.M.-H.; software, M.W.-G.; validation, M.W.-G.; formal analysis, M.W.-G.; investigation, M.W.-G., A.M.-H. and A.O.; resources, M.W.-G.; data curation, M.W.-G.; writing—original draft preparation, M.W.-G., A.M.-H.; writing—review and editing, M.W.-G., A.M.-H. and B.K.-K.; visualization, M.W.-G.; supervision, A.M.-H. and B.K.-K.; project administration, M.W.-G.; funding acquisition, M.W.-G. and B.K.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Grant Number BNW-NWN-/641/352/2/2023 from the Ministry of Science and Higher Education, Medical University of Silesia in Katowice.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Committee of the Medical University of Silesia (approval number: BNW/NWN/0052/KB1/147/I/22/23, date of approval: 28 February 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used to support the findings of this research are available upon request from the corresponding author.
Acknowledgments
The authors thank Krzysztof Biernacki for advice on statistical analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NETs | Neuroendocrine Tumours |
| Pan-NETs | Pancreatic Neuroendocrine Tumours |
| SI-NETs | Small Intestinal Neuroendocrine Tumours |
| NF-NETs | Non-functioning Neuroendocrine Tumours |
| F-NETs | Functioning Neuroendocrine Tumours |
| VTE | Venous Thromboembolism |
| DD | D-dimer |
| PLT | Platelets |
| AT-III | Antithrombin-III |
| TF | Tissue Factor |
| DVT | Deep Vein Thrombosis |
| PE | Pulmonary Embolism |
| TT | Tumour Thrombosis |
| SVT | Superficial Venous Thrombosis |
| KS | Khorana Score |
| VC-S | Vienna CATS Score |
| ONCO-S | ONCOTEV Score |
| PPS | Padua Prediction Score |
| ECOG | Eastern Cooperative Oncology Group Performance Status |
| WHO | World Health Organization |
References
- Leiva, O.; Newcomb, R.; Connors, J.M.; Al-Samkari, H. Cancer and Thrombosis: New Insights to an Old Problem. J. Med. Vasc. 2020, 45, 6S8–6S16. [Google Scholar] [CrossRef]
- Bagot, C.N.; Arya, R. Virchow and His Triad: A Question of Attribution. Br. J. Haematol. 2008, 143, 180–190. [Google Scholar] [CrossRef]
- Mangiafico, M.; Costanzo, L. Superficial Venous Thrombosis: A Comprehensive Review. Healthcare 2024, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Kos-Kudła, B.; Foltyn, W.; Malczewska, A.; Bednarczuk, T.; Bolanowski, M.; Borowska, M.; Chmielik, E.; Ćwikła, J.B.; Gisterek, I.; Handkiewicz-Junak, D. Update of the Diagnostic and Therapeutic Guidelines for Gastro-Entero-Pancreatic Neuroendocrine Neoplasms (Recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol. Pol. 2022, 73, 387–423. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Cavalcoli, F.; Artoni, A.; Sciola, V.; Zilli, A.; Ciafardini, C.; Elisa Rossi, R. Thrombotic Risk in Gastroenteropancreatic Neuroendocrine Tumor Patients: A Single-Center Experience. Ann. Gastroenterol. 2021, 34, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, F.L.; Ciusani, E.; Silvani, A.; Corsini, E.; Frigerio, S.; Pogliani, S.; Parati, E.; Croci, D.; Boiardi, A.; Salmaggi, A. Genetic and Plasma Markers of Venous Thromboembolism in Patients with High Grade Glioma. Clin. Cancer Res. 2004, 10, 1312–1317. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X.; Zhang, J.; Wang, B.; Tian, Q.; Meng, X.; Zhang, J.; Jiang, M.; Zhang, Y.; Zheng, D.; et al. Vascular Endothelial Growth Factor and the Risk of Venous Thromboembolism: A Genetic Correlation and Two-Sample Mendelian Randomization Study. Thromb. J. 2022, 20, 67. [Google Scholar] [CrossRef]
- Cigrovski Berković, M.; Čačev, T.; Catela Ivković, T.; Marout, J.; Ulamec, M.; Zjačić-Rotkvić, V.; Kapitanović, S. High VEGF Serum Values Are Associated with Locoregional Spread of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs). Mol. Cell. Endocrinol. 2016, 425, 61–68. [Google Scholar] [CrossRef]
- Berardi, R.; Torniai, M.; Partelli, S.; Rubini, C.; Pagliaretta, S.; Savini, A.; Polenta, V.; Santoni, M.; Giampieri, R.; Onorati, S.; et al. Impact of Vascular Endothelial Growth Factor (VEGF) and Vascular Endothelial Growth Factor Receptor (VEGFR) Single Nucleotide Polymorphisms on Outcome in Gastroenteropancreatic Neuroendocrine Neoplasms. PLoS ONE 2018, 13, e0197035. [Google Scholar] [CrossRef]
- Hurtado-Cordovi, J.; Lipka, S.; Avezbakiyev, B.; Multz, A.S. Budd-Chiari Syndrome Induced by Stage IV Rectal Carcinoid. Am. J. Med. Sci. 2013, 345, 246–247. [Google Scholar] [CrossRef]
- Wójcik-Giertuga, M.; Malczewska-Herman, A.; Kos-Kudła, B. The Risk of Venous Thromboembolism in Neuroendocrine Neoplasms. Cancers 2023, 15, 5477. [Google Scholar] [CrossRef]
- Massironi, S.; Gervaso, L.; Fanizzi, F.; Preatoni, P.; Dell’Anna, G.; Fazio, N.; Danese, S. Venous Thromboembolism in Patients with Neuroendocrine Neoplasms: A Systematic Review of Incidence, Types, and Clinical Outcomes. Cancers 2025, 17, 212. [Google Scholar] [CrossRef] [PubMed]
- Verzeroli, C.; Giaccherini, C.; Russo, L.; Bolognini, S.; Gamba, S.; Tartari, C.J.; Schieppati, F.; Ticozzi, C.; Vignoli, A.; Masci, G.; et al. Utility of the Khorana and the New-Vienna CATS Prediction Scores in Cancer Patients of the HYPERCAN Cohort. J. Thromb. Haemost. 2023, 21, 1869–1881. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and Validation of a Predictive Model for Chemotherapy- Associated Thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef] [PubMed]
- Englisch, C.; Nopp, S.; Moik, F.; Starzer, A.M.; Quehenberger, P.; Preusser, M.; Berghoff, A.S.; Ay, C.; Pabinger, I. The Vienna CATScore for Predicting Cancer-Associated Venous Thromboembolism: An External Validation across Multiple Time Points. ESMO Open 2025, 10, 104130. [Google Scholar] [CrossRef]
- Cella, C.A.; Knoedler, M.; Hall, M.; Arcopinto, M.; Bagnardi, V.; Gervaso, L.; Pellicori, S.; Spada, F.; Zampino, M.G.; Ravenda, P.S.; et al. Validation of the ONKOTEV Risk Prediction Model for Venous Thromboembolism in Outpatients with Cancer. JAMA Netw. Open 2023, 6, E230010. [Google Scholar] [CrossRef] [PubMed]
- Barbar, S.; Noventa, F.; Rossetto, V.; Ferrari, A.; Brandolin, B.; Perlati, M.; De Bon, E.; Tormene, D.; Pagnan, A.; Prandoni, P. A Risk Assessment Model for the Identification of Hospitalized Medical Patients at Risk for Venous Thromboembolism: The Padua Prediction Score. J. Thromb. Haemost. 2010, 8, 2450–2457. [Google Scholar] [CrossRef]
- Wojtukiewicz, M.Z.; Sierko, E.; Tomkowski, W.; Zawilska, K.; Undas, A.; Podolak-Dawidziak, M.; Wysocki, P.; Krzakowski, M.; Warzocha, K.; Windyga, J. Guidelines for the Prevention and Treatment of Venous Thromboembolism in Patients with Cancers Treated Conservatively. Hematologia 2016, 7, 128–160. Available online: https://journals.viamedica.pl/hematology_in_clinical_practice/article/view/49469 (accessed on 20 October 2025).
- Baron, J.A.; Gridley, G.; Weiderpass, E.; Nyrén, O.; Linet, M. Venous thromboembolism and cancer. Lancet 1998, 351, 1077–1080, Erratum in: Lancet 2000, 355, 758. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G. Venous thromboembolism and cancer: A two-way clinical association. Thromb. Haemost. 1997, 78, 117–120. [Google Scholar] [PubMed]
- De Robertis, R.; Paiella, S.; Cardobi, N.; Landoni, L.; Tinazzi Martini, P.; Ortolani, S.; De Marchi, G.; Gobbo, S.; Giardino, A.; Butturini, G.; et al. Tumor Thrombosis: A Peculiar Finding Associated with Pancreatic Neuroendocrine Neoplasms. A Pictorial Essay. Abdom. Radiol. 2018, 43, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, A.; Tamm, E.P.; Bhosale, P.R.; Katz, M.H.; Fleming, J.B.; Yao, J.C.; Charnsangavej, C. Venous Tumor Thrombus in Nonfunctional Pancreatic Neuroendocrine Tumors. Am. J. Roentgenol. 2012, 199, 602–608. [Google Scholar] [CrossRef]
- Addeo, P.; d’Alessandro, A.; Averous, G.; Imperiale, A.; Faitot, F.; Goichot, B.; Bachellier, P. Macrovascular Venous Invasion of Pancreatic Neuroendocrine Tumours: Impact on Surgical Outcomes and Survival. HPB 2019, 21, 653–661. [Google Scholar] [CrossRef]
- Watase, M.; Sakon, M.; Monden, M.; Miyoshi, Y.; Tono, T.; Ichikawa, T.; Kubota, N.; Shiozaki, H.; Okuda, H.; Okamura, J.; et al. A Case of Splenic Vein Occlusion Caused by the Intravenous Tumor Thrombus of Nonfunctioning Islet Cell Carcinoma. Surg. Today 1992, 22, 62–65. [Google Scholar] [CrossRef]
- Rodriguez, R.A.; Overton, H.; Morris, K.T. Pancreatic Neuroendocrine Tumor with Splenic Vein Tumor Thrombus: A Case Report. Int. J. Surg. Case Rep. 2014, 5, 1271–1274. [Google Scholar] [CrossRef][Green Version]
- Borbély, R.Z.; Teutsch, B.; Hegyi, P. Incidence and Management of Splanchnic Vein Thrombosis in Pancreatic Diseases. United Eur. Gastroenterol. J. 2025, 13, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.C.; Wang, T.F.; Lun, R.; Zahrai, A.; Mallick, R.; Burger, D.; Zitikyte, G.; Hawken, S.; Wells, P. Circulating Blood Biomarkers and Risk of Venous Thromboembolism in Cancer Patients: A Systematic Review and Meta-Analysis. Thromb. Haemost. 2024, 124, 1117–1133. [Google Scholar] [CrossRef] [PubMed]
- Ohara, S.; Suda, K.; Tomizawa, K.; Takemoto, T.; Fujino, T.; Hamada, A.; Koga, T.; Nishino, M.; Chiba, M.; Sato, K.; et al. Prognostic Value of Plasma Fibrinogen and D-Dimer Levels in Patients with Surgically Resected Non-Small Cell Lung Cancer. Surg. Today 2020, 50, 1427–1433. [Google Scholar] [CrossRef]
- Cohen, A.T.; Harrington, R.; Goldhaber, S.Z.; Hull, R.; Gibson, C.M.; Hernandez, A.F.; Kitt, M.M.; Lorenz, T.J. The Design and Rationale for the Acute Medically Ill Venous Thromboembolism Prevention with Extended Duration Betrixaban (APEX) Study. Am. Heart J. 2014, 167, 335–341. [Google Scholar] [CrossRef]
- Watanabe, A.; Araki, K.; Hirai, K.; Kubo, N.; Igarashi, T.; Tsukagoshi, M.; Ishii, N.; Hoshino, K.; Kuwano, H.; Shirabe, K. A Novel Clinical Factor, D-Dimer Platelet Multiplication, May Predict Postoperative Recurrence and Prognosis for Patients with Cholangiocarcinoma. Ann. Surg. Oncol. 2016, 23, 886–891. [Google Scholar] [CrossRef]
- Giaccherini, C.; Verzeroli, C.; Russo, L.; Gamba, S.; Tartari, C.J.; Bolognini, S.; Schieppati, F.; Ticozzi, C.; Sarmiento, R.; Celio, L.; et al. Thrombin Generation and D-Dimer for Prediction of Disease Progression and Mortality in Patients with Metastatic Gastrointestinal Cancer. Cancers 2022, 14, 4347. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zhou, H.; Sun, Y.; Xu, Z.H.E.; Wang, S.; Feng, T.; Zhang, P. D-Dimer as a Potential Clinical Marker for Predicting Metastasis and Progression in Cancer. Biomed. Rep. 2018, 9, 453–457. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, J.; Deng, Y.; Huang, Y.; Li, R.; Lin, G.; Dong, M.; Huang, Z. Pretreatment Plasma Fibrinogen Level as a Prognostic Biomarker for Patients with Lung Cancer. Clinics 2020, 75, e993. [Google Scholar] [CrossRef]
- Tian, Y.; Hong, M.; Jing, S.; Liu, X.; Wang, H.; Wang, X.; Kaushik, D.; Rodriguez, R.; Wang, Z. Clinical and Prognostic Effect of Plasma Fibrinogen in Renal Cell Carcinoma: A Meta-Analysis. BioMed Res. Int. 2017, 2017, 95915060. [Google Scholar] [CrossRef]
- Lugassy, G.; Falanga, A.; Kakkar, A.K.; Rickles Frederick, R. Thrombosis and Cancer; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9781841842875. [Google Scholar]
- Izuegbuna, O.O.; Agodirin, O.S.; Olawumi, H.O.; Olatoke, S.A. Plasma D-Dimer and Fibrinogen Levels Correlates with Tumor Size and Disease Progression in Nigerian Breast Cancer Patients. Cancer Investig. 2021, 39, 597–606. [Google Scholar] [CrossRef]
- Gieseler, F.; Lühr, I.; Kunze, T.; Mundhenke, C.; Maass, N.; Erhart, T.; Denker, M.; Beckmann, D.; Tiemann, M.; Schulte, C.; et al. Activated coagulation factors in human malignant effusions and their contribution to cancer cell metastasis and therapy. Thromb. Haemost. 2007, 97, 1023–1030. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Li, J.; Che, G. Prognostic Value of Pretreatment D-Dimer Level in Small-Cell Lung Cancer: A Meta-Analysis. Technol. Cancer Res. Treat. 2021, 20, 1533033821989822. [Google Scholar] [CrossRef] [PubMed]
- Chekhonin, V.P.; Shein, S.A.; Korchagina, A.A.; Gurina, O.I. VEGF in Tumor Progression and Targeted Therapy. Curr. Cancer Drug Targets 2013, 13, 423–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jia, Y.; Jia, Y.; Zhang, X.; Li, K. Prognostic and Predictive Value of Plasma D-Dimer Levels in Patients with Small-Cell Lung Cancer. Int. J. Clin. Oncol. 2018, 23, 1070–1075. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Zhang, J.; Mafa, T.; Zhang, J.; Zhu, H.; Chen, L.; Zong, Z.; Yang, L. Fibrinogen: A New Player and Target on the Formation of Pre-Metastatic Niche in Tumor Metastasis. Crit. Rev. Oncol. Hematol. 2025, 207, 104625. [Google Scholar] [CrossRef]
- Palumbo, J.S.; Kombrinck, K.W.; Drew, A.F.; Grimes, T.S.; Kiser, J.H.; Degen, J.L.; Bugge, T.H. Fibrinogen Is an Important Determinant of the Metastatic Potential of Circulating Tumor Cells. Blood 2000, 96, 3302–3309. [Google Scholar] [CrossRef]
- Jiang, C.; Li, Y.; Li, Y.; Liu, L.; Wang, X.A.; Wu, W.; Bao, R.; Weng, H.; Li, M.; Geng, Y.; et al. Fibrinogen Promotes Gallbladder Cancer Cell Metastasis and Extravasation by Inducing ICAM1 Expression. Med. Oncol. 2023, 40, 10. [Google Scholar] [CrossRef]
- Hisada, Y.; Mackman, N. Cancer-Associated Pathways and Biomarkers of Venous Thrombosis. Blood 2017, 130, 1499–1506. [Google Scholar] [CrossRef]
- Hollander, K.N.; Joshi, B.L.; Joshi, B.L. Bioprosthetic Valve Thrombosis in Carcinoid Heart Disease. Ann. Card. Anaesth. 2019, 22, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vilchez, I.; Diaz-Ricart, M.; White, J.G.; Escolar, G.; Galan, A.M. Serotonin Enhances Platelet Procoagulant Properties and Their Activation Induced during Platelet Tissue Factor Uptake. Cardiovasc. Res. 2009, 84, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Llobet, D.; Vallvé, C.; Tirado, I.; Vilalta, N.; Murillo, J.; Cuevas, B.; Román, L.; Carrasco, M.; Oliver, A.; Mateo, J.; et al. VAMP8 and Serotonin Transporter Levels Are Associated with Venous Thrombosis Risk in a Spanish Female Population. Results from the RETROVE Project. Thromb. Res. 2019, 181, 99–105. [Google Scholar] [CrossRef]
- Wulftange, W.J.; Kucukal, E.; Man, Y.; An, R.; Monchamp, K.; Sevrain, C.D.; Dashora, H.R.; Owusu-Ansah, A.T.; Bode, A.; Ilich, A.; et al. Antithrombin-III Mitigates Thrombin-Mediated Endothelial Cell Contraction and Sickle Red Blood Cell Adhesion in Microscale Flow. Br. J. Haematol. 2022, 198, 893–902. [Google Scholar] [CrossRef]
- Englisch, C.; Königsbrügge, O.; Nopp, S.; Moik, F.; Quehenberger, P.; Preusser, M.; Pabinger, I.; Ay, C. Antithrombin Activity and Association with Risk of Thrombosis and Mortality in Patients with Cancer. Int. J. Mol. Sci. 2022, 23, 15770. [Google Scholar] [CrossRef]
- Lundbech, M.; Krag, A.E.; Christensen, T.D.; Hvas, A.M. Thrombin Generation, Thrombin-Antithrombin Complex, and Prothrombin Fragment F1+2 as Biomarkers for Hypercoagulability in Cancer Patients. Thromb. Res. 2020, 186, 80–85. [Google Scholar] [CrossRef]
- Åberg, M.; Eriksson, O.; Siegbahn, A. Tissue Factor Noncoagulant Signaling: Mechanisms and Implications for Cell Migration and Apoptosis. Semin. Thromb. Hemost. 2015, 41, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Tieken, C.; Versteeg, H.H. Anticoagulants versus Cancer. Thromb. Res. 2016, 140, S148–S153. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.E.; Shabannezhad, A.; Kahrizi, A.; Akbar, A.; Safdari, S.M.; Hoseinnezhad, T.; Zahedi, M.; Sadeghi, S.; Mojarrad, M.G.; Safa, M. Tissue Factor (Coagulation Factor III): A Potential Double-Edge Molecule to Be Targeted and Re-Targeted toward Cancer. Biomark. Res. 2023, 11, 60. [Google Scholar] [CrossRef]
- Hisada, Y.; Mackman, N. Tissue Factor and Extracellular Vesicles: Activation of Coagulation and Impact on Survival in Cancer. Cancers 2021, 13, 3839. [Google Scholar] [CrossRef]
- Hernández, C.; Orbe, J.; Roncal, C.; Alvarez-Hernandez, M.; Martinez de Lizarrondo, S.; Alves, M.T.; García Mata, J.; Páramo, J.A. Tissue factor expressed by microparticles is associated with mortality but not with thrombosis in cancer patients. Thromb. Haemost. 2013, 110, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.S.; Thomas, H.E.; Orr-asman, M.; Green, L.C.; Matiash, K.; Karve, A.; Hisada, Y.M.; Davis, H.W.; Mercer, C.; Lucas, F.V.; et al. mTOR Kinase Inhibition Reduces Tissue Factor Expression and Growth of Pancreatic Neuroendocrine Tumors. J. Thromb. Haemost. 2019, 17, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Godinho, J.; Casa-Nova, M.; Moreira-Pinto, J.; Simões, P.; Paralta Branco, F.; Leal-Costa, L.; Faria, A.; Lopes, F.; Teixeira, J.A.; Passos-Coelho, J.L. ONKOTEV Score as a Predictive Tool for Thromboembolic Events in Pancreatic Cancer—A Retrospective Analysis. Oncologist 2020, 25, e284–e290. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).