The Clinical Utility of Selected Coagulation Parameters in Predicting the Risk of Venous Thromboembolism in Neuroendocrine Tumours: A Prospective, Single-Centre Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Sampling and Laboratory Analysis
2.2. Statistical Analysis
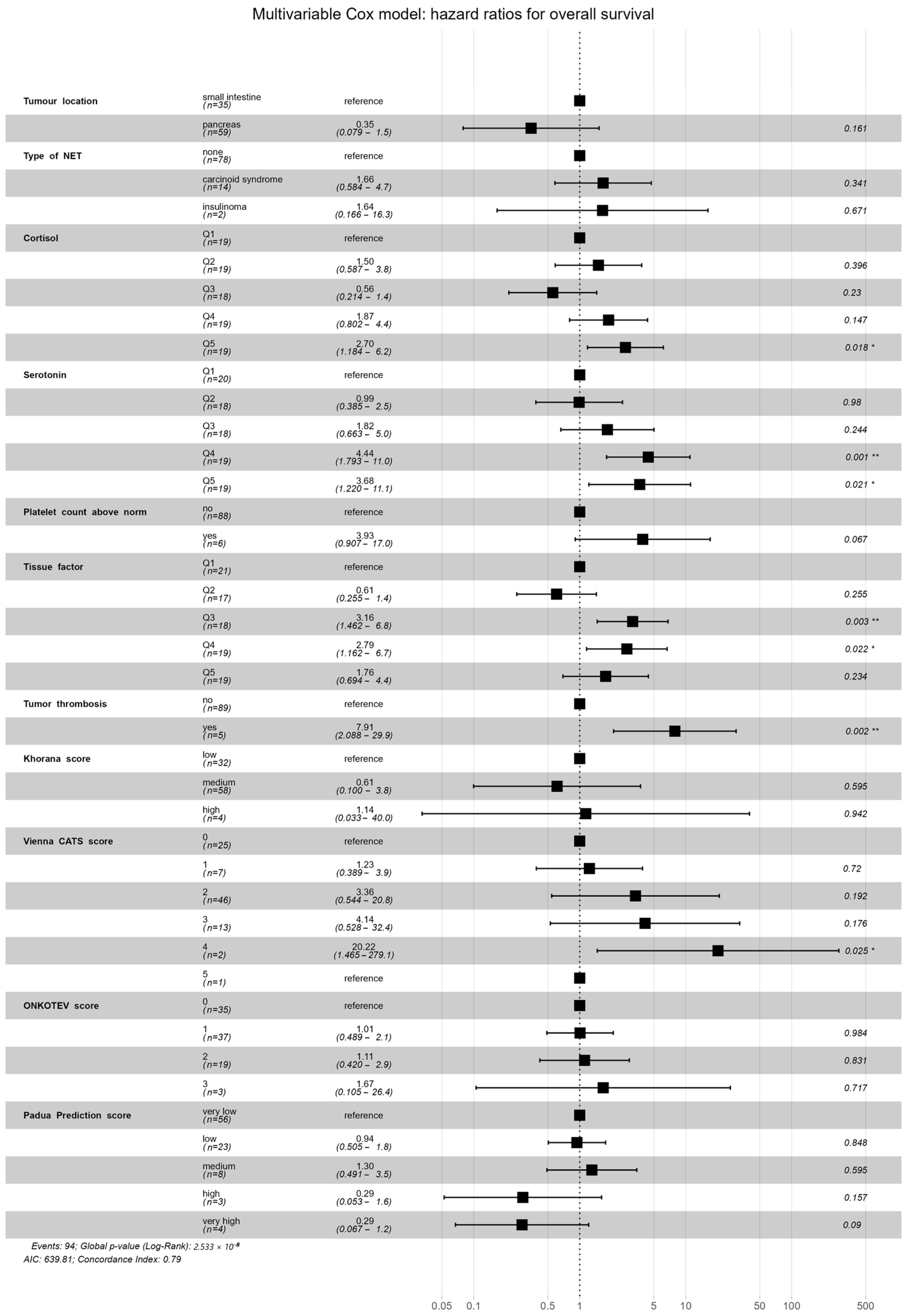
2.3. Multivariate Survival Analysis
2.4. Missing Data Handling
3. Results
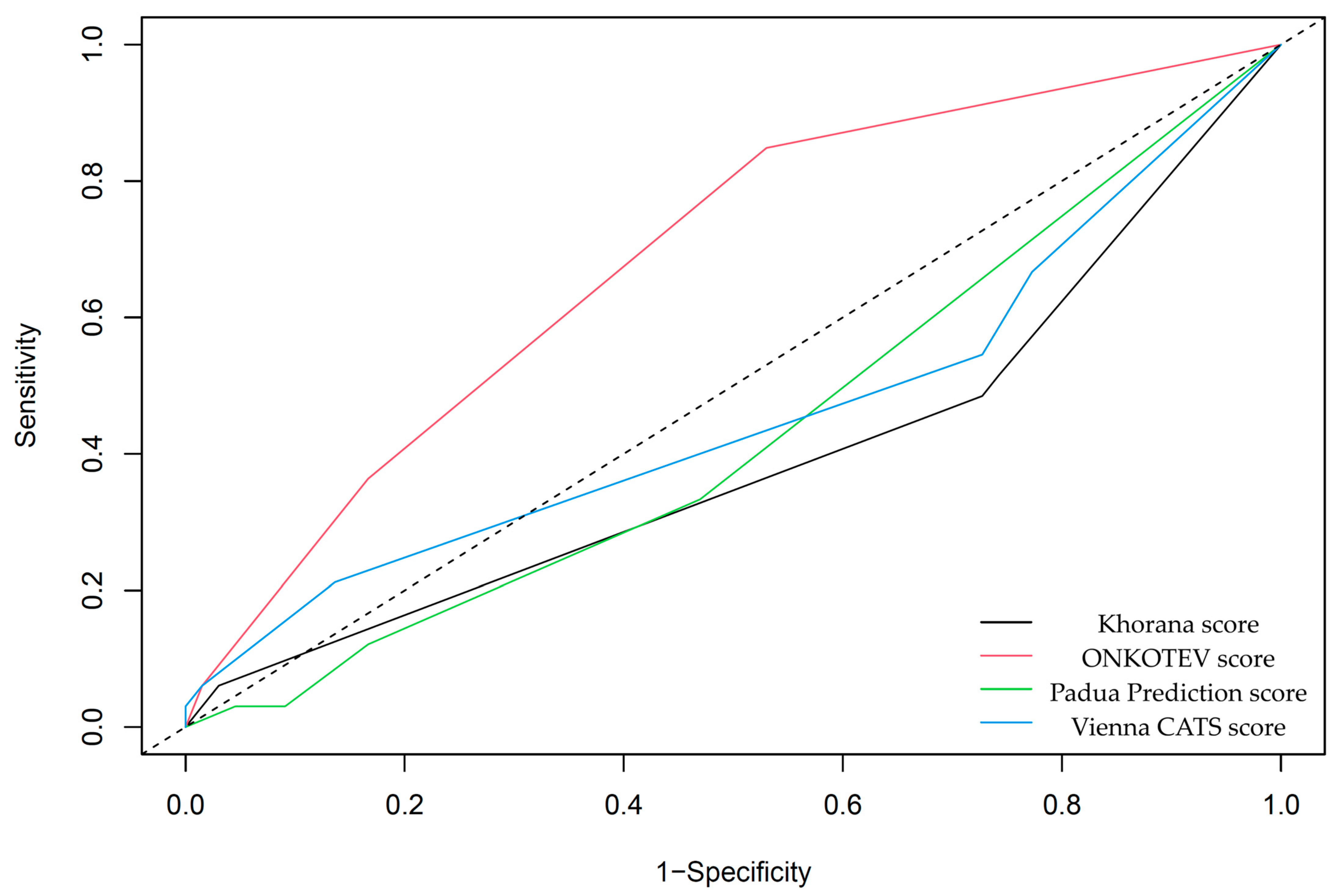
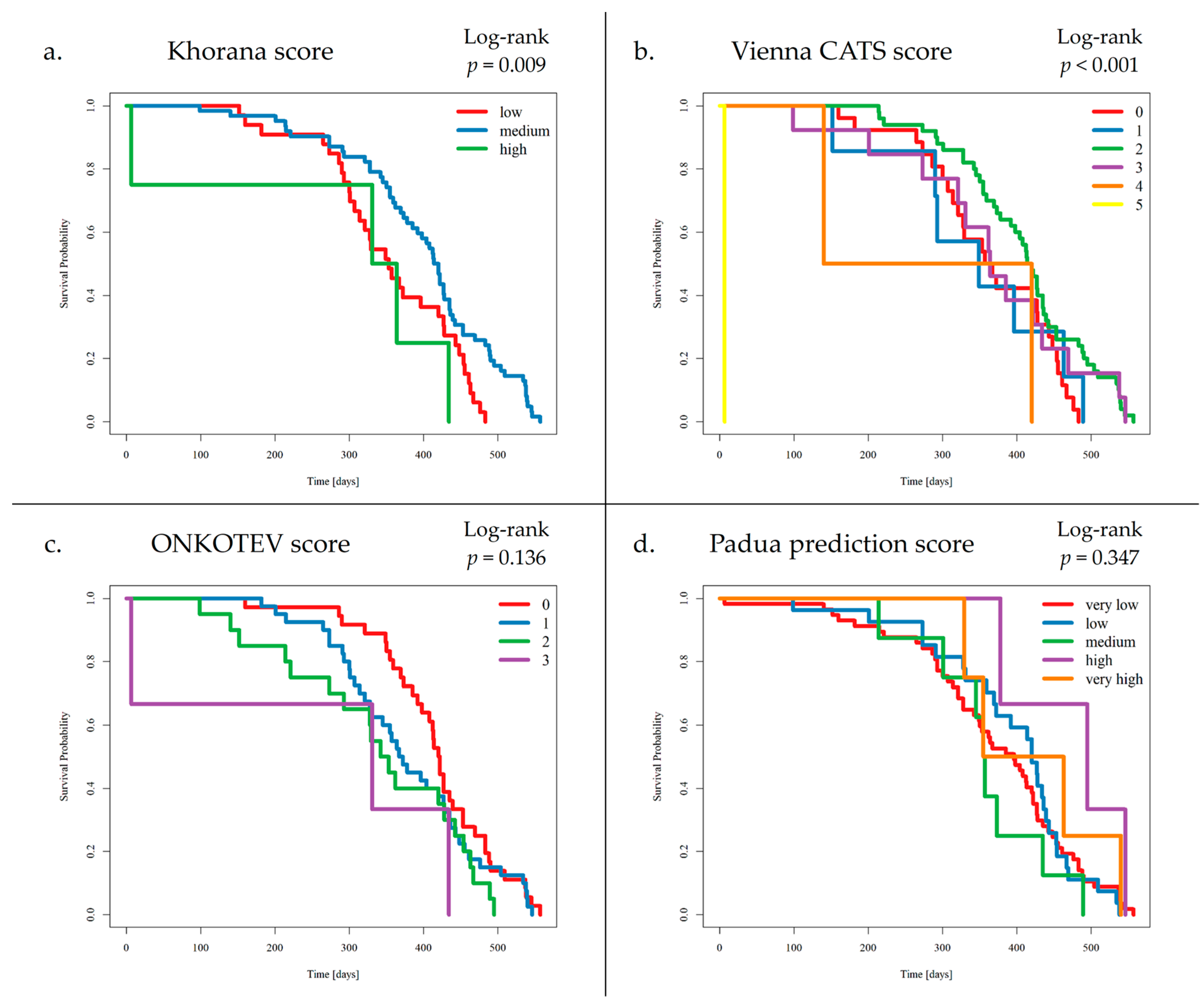
3.1. The Occurrence of VTE Events and VTE Risk Assessment Scales
3.2. Selected Blood Parameters of Coagulation
3.2.1. D-Dimer
3.2.2. Fibrinogen
3.2.3. Platelets
3.2.4. Antithrombin III Activity
3.2.5. Tissue Factor
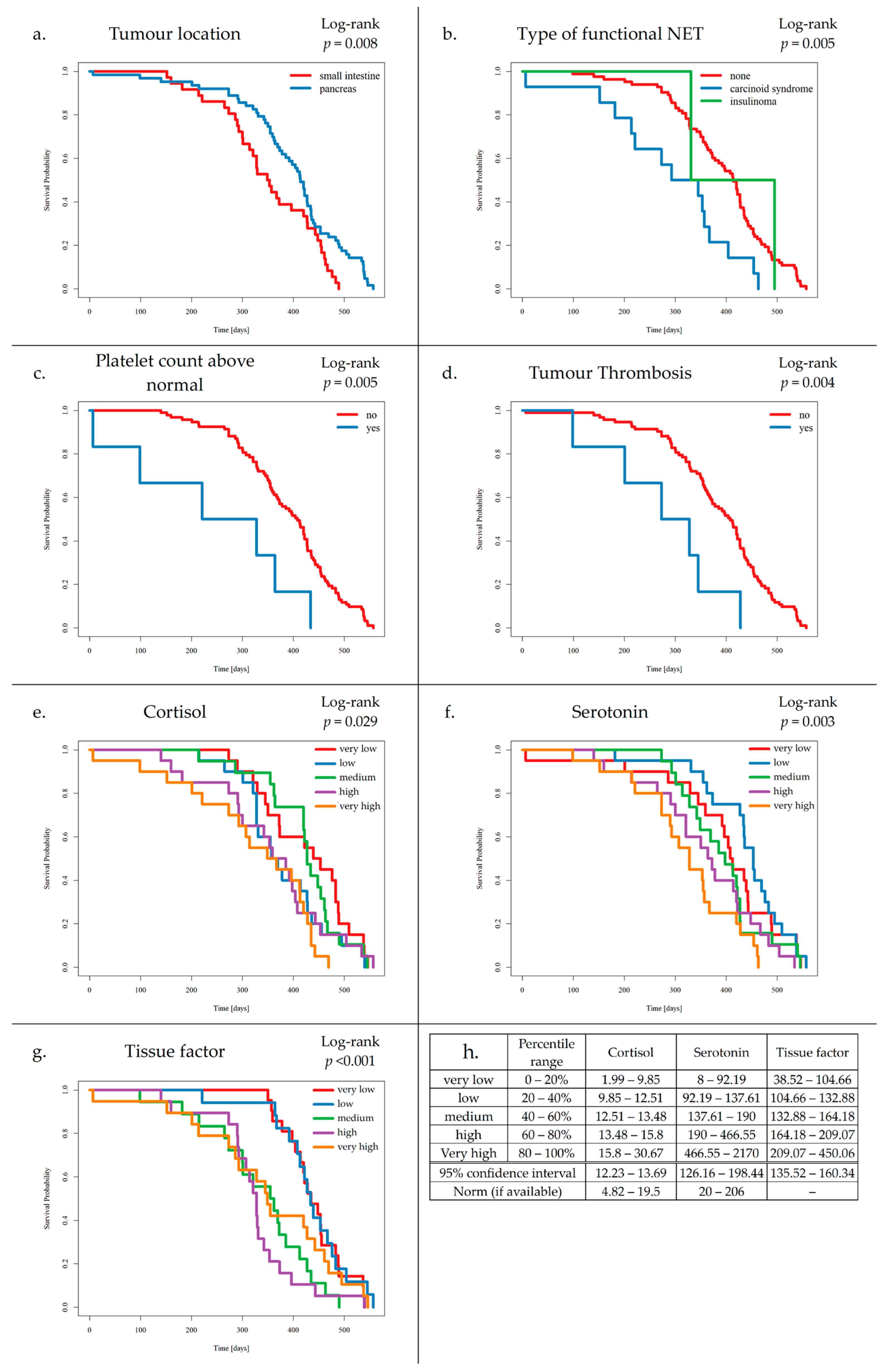
3.3. Survival Analysis and VTE Risk Assessment Scales
- (a)
- Patients with SI-NETs compared to Pan-NETs exhibited poorer survival (p = 0.008).
- (b)
- Patients with carcinoid syndrome, compared to NF-NETs, exhibited poorer survival (p = 0.005).
- (c)
- Increased platelet levels were found to negatively affect survival (p = 0.005).
- (d)
- Increased TF levels were found to negatively affect survival (p ≤ 0.004).
- (e)
- Elevated cortisol levels were associated with worse outcomes compared to those with normal levels of cortisol (p = 0.029).
- (f)
- Additionally, elevated serotonin levels were associated with worse outcomes compared to those with normal levels of serotonin (p = 0.003).
- (g)
- Increased TF levels were found to negatively affect survival (p ≤ 0.001).
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NETs | Neuroendocrine Tumours |
| Pan-NETs | Pancreatic Neuroendocrine Tumours |
| SI-NETs | Small Intestinal Neuroendocrine Tumours |
| NF-NETs | Non-functioning Neuroendocrine Tumours |
| F-NETs | Functioning Neuroendocrine Tumours |
| VTE | Venous Thromboembolism |
| DD | D-dimer |
| PLT | Platelets |
| AT-III | Antithrombin-III |
| TF | Tissue Factor |
| DVT | Deep Vein Thrombosis |
| PE | Pulmonary Embolism |
| TT | Tumour Thrombosis |
| SVT | Superficial Venous Thrombosis |
| KS | Khorana Score |
| VC-S | Vienna CATS Score |
| ONCO-S | ONCOTEV Score |
| PPS | Padua Prediction Score |
| ECOG | Eastern Cooperative Oncology Group Performance Status |
| WHO | World Health Organization |
References
- Leiva, O.; Newcomb, R.; Connors, J.M.; Al-Samkari, H. Cancer and Thrombosis: New Insights to an Old Problem. J. Med. Vasc. 2020, 45, 6S8–6S16. [Google Scholar] [CrossRef]
- Bagot, C.N.; Arya, R. Virchow and His Triad: A Question of Attribution. Br. J. Haematol. 2008, 143, 180–190. [Google Scholar] [CrossRef]
- Mangiafico, M.; Costanzo, L. Superficial Venous Thrombosis: A Comprehensive Review. Healthcare 2024, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Kos-Kudła, B.; Foltyn, W.; Malczewska, A.; Bednarczuk, T.; Bolanowski, M.; Borowska, M.; Chmielik, E.; Ćwikła, J.B.; Gisterek, I.; Handkiewicz-Junak, D. Update of the Diagnostic and Therapeutic Guidelines for Gastro-Entero-Pancreatic Neuroendocrine Neoplasms (Recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol. Pol. 2022, 73, 387–423. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Cavalcoli, F.; Artoni, A.; Sciola, V.; Zilli, A.; Ciafardini, C.; Elisa Rossi, R. Thrombotic Risk in Gastroenteropancreatic Neuroendocrine Tumor Patients: A Single-Center Experience. Ann. Gastroenterol. 2021, 34, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, F.L.; Ciusani, E.; Silvani, A.; Corsini, E.; Frigerio, S.; Pogliani, S.; Parati, E.; Croci, D.; Boiardi, A.; Salmaggi, A. Genetic and Plasma Markers of Venous Thromboembolism in Patients with High Grade Glioma. Clin. Cancer Res. 2004, 10, 1312–1317. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X.; Zhang, J.; Wang, B.; Tian, Q.; Meng, X.; Zhang, J.; Jiang, M.; Zhang, Y.; Zheng, D.; et al. Vascular Endothelial Growth Factor and the Risk of Venous Thromboembolism: A Genetic Correlation and Two-Sample Mendelian Randomization Study. Thromb. J. 2022, 20, 67. [Google Scholar] [CrossRef]
- Cigrovski Berković, M.; Čačev, T.; Catela Ivković, T.; Marout, J.; Ulamec, M.; Zjačić-Rotkvić, V.; Kapitanović, S. High VEGF Serum Values Are Associated with Locoregional Spread of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs). Mol. Cell. Endocrinol. 2016, 425, 61–68. [Google Scholar] [CrossRef]
- Berardi, R.; Torniai, M.; Partelli, S.; Rubini, C.; Pagliaretta, S.; Savini, A.; Polenta, V.; Santoni, M.; Giampieri, R.; Onorati, S.; et al. Impact of Vascular Endothelial Growth Factor (VEGF) and Vascular Endothelial Growth Factor Receptor (VEGFR) Single Nucleotide Polymorphisms on Outcome in Gastroenteropancreatic Neuroendocrine Neoplasms. PLoS ONE 2018, 13, e0197035. [Google Scholar] [CrossRef]
- Hurtado-Cordovi, J.; Lipka, S.; Avezbakiyev, B.; Multz, A.S. Budd-Chiari Syndrome Induced by Stage IV Rectal Carcinoid. Am. J. Med. Sci. 2013, 345, 246–247. [Google Scholar] [CrossRef]
- Wójcik-Giertuga, M.; Malczewska-Herman, A.; Kos-Kudła, B. The Risk of Venous Thromboembolism in Neuroendocrine Neoplasms. Cancers 2023, 15, 5477. [Google Scholar] [CrossRef]
- Massironi, S.; Gervaso, L.; Fanizzi, F.; Preatoni, P.; Dell’Anna, G.; Fazio, N.; Danese, S. Venous Thromboembolism in Patients with Neuroendocrine Neoplasms: A Systematic Review of Incidence, Types, and Clinical Outcomes. Cancers 2025, 17, 212. [Google Scholar] [CrossRef] [PubMed]
- Verzeroli, C.; Giaccherini, C.; Russo, L.; Bolognini, S.; Gamba, S.; Tartari, C.J.; Schieppati, F.; Ticozzi, C.; Vignoli, A.; Masci, G.; et al. Utility of the Khorana and the New-Vienna CATS Prediction Scores in Cancer Patients of the HYPERCAN Cohort. J. Thromb. Haemost. 2023, 21, 1869–1881. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and Validation of a Predictive Model for Chemotherapy- Associated Thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef] [PubMed]
- Englisch, C.; Nopp, S.; Moik, F.; Starzer, A.M.; Quehenberger, P.; Preusser, M.; Berghoff, A.S.; Ay, C.; Pabinger, I. The Vienna CATScore for Predicting Cancer-Associated Venous Thromboembolism: An External Validation across Multiple Time Points. ESMO Open 2025, 10, 104130. [Google Scholar] [CrossRef]
- Cella, C.A.; Knoedler, M.; Hall, M.; Arcopinto, M.; Bagnardi, V.; Gervaso, L.; Pellicori, S.; Spada, F.; Zampino, M.G.; Ravenda, P.S.; et al. Validation of the ONKOTEV Risk Prediction Model for Venous Thromboembolism in Outpatients with Cancer. JAMA Netw. Open 2023, 6, E230010. [Google Scholar] [CrossRef] [PubMed]
- Barbar, S.; Noventa, F.; Rossetto, V.; Ferrari, A.; Brandolin, B.; Perlati, M.; De Bon, E.; Tormene, D.; Pagnan, A.; Prandoni, P. A Risk Assessment Model for the Identification of Hospitalized Medical Patients at Risk for Venous Thromboembolism: The Padua Prediction Score. J. Thromb. Haemost. 2010, 8, 2450–2457. [Google Scholar] [CrossRef]
- Wojtukiewicz, M.Z.; Sierko, E.; Tomkowski, W.; Zawilska, K.; Undas, A.; Podolak-Dawidziak, M.; Wysocki, P.; Krzakowski, M.; Warzocha, K.; Windyga, J. Guidelines for the Prevention and Treatment of Venous Thromboembolism in Patients with Cancers Treated Conservatively. Hematologia 2016, 7, 128–160. Available online: https://journals.viamedica.pl/hematology_in_clinical_practice/article/view/49469 (accessed on 20 October 2025).
- Baron, J.A.; Gridley, G.; Weiderpass, E.; Nyrén, O.; Linet, M. Venous thromboembolism and cancer. Lancet 1998, 351, 1077–1080, Erratum in: Lancet 2000, 355, 758. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G. Venous thromboembolism and cancer: A two-way clinical association. Thromb. Haemost. 1997, 78, 117–120. [Google Scholar] [PubMed]
- De Robertis, R.; Paiella, S.; Cardobi, N.; Landoni, L.; Tinazzi Martini, P.; Ortolani, S.; De Marchi, G.; Gobbo, S.; Giardino, A.; Butturini, G.; et al. Tumor Thrombosis: A Peculiar Finding Associated with Pancreatic Neuroendocrine Neoplasms. A Pictorial Essay. Abdom. Radiol. 2018, 43, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, A.; Tamm, E.P.; Bhosale, P.R.; Katz, M.H.; Fleming, J.B.; Yao, J.C.; Charnsangavej, C. Venous Tumor Thrombus in Nonfunctional Pancreatic Neuroendocrine Tumors. Am. J. Roentgenol. 2012, 199, 602–608. [Google Scholar] [CrossRef]
- Addeo, P.; d’Alessandro, A.; Averous, G.; Imperiale, A.; Faitot, F.; Goichot, B.; Bachellier, P. Macrovascular Venous Invasion of Pancreatic Neuroendocrine Tumours: Impact on Surgical Outcomes and Survival. HPB 2019, 21, 653–661. [Google Scholar] [CrossRef]
- Watase, M.; Sakon, M.; Monden, M.; Miyoshi, Y.; Tono, T.; Ichikawa, T.; Kubota, N.; Shiozaki, H.; Okuda, H.; Okamura, J.; et al. A Case of Splenic Vein Occlusion Caused by the Intravenous Tumor Thrombus of Nonfunctioning Islet Cell Carcinoma. Surg. Today 1992, 22, 62–65. [Google Scholar] [CrossRef]
- Rodriguez, R.A.; Overton, H.; Morris, K.T. Pancreatic Neuroendocrine Tumor with Splenic Vein Tumor Thrombus: A Case Report. Int. J. Surg. Case Rep. 2014, 5, 1271–1274. [Google Scholar] [CrossRef]
- Borbély, R.Z.; Teutsch, B.; Hegyi, P. Incidence and Management of Splanchnic Vein Thrombosis in Pancreatic Diseases. United Eur. Gastroenterol. J. 2025, 13, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.C.; Wang, T.F.; Lun, R.; Zahrai, A.; Mallick, R.; Burger, D.; Zitikyte, G.; Hawken, S.; Wells, P. Circulating Blood Biomarkers and Risk of Venous Thromboembolism in Cancer Patients: A Systematic Review and Meta-Analysis. Thromb. Haemost. 2024, 124, 1117–1133. [Google Scholar] [CrossRef] [PubMed]
- Ohara, S.; Suda, K.; Tomizawa, K.; Takemoto, T.; Fujino, T.; Hamada, A.; Koga, T.; Nishino, M.; Chiba, M.; Sato, K.; et al. Prognostic Value of Plasma Fibrinogen and D-Dimer Levels in Patients with Surgically Resected Non-Small Cell Lung Cancer. Surg. Today 2020, 50, 1427–1433. [Google Scholar] [CrossRef]
- Cohen, A.T.; Harrington, R.; Goldhaber, S.Z.; Hull, R.; Gibson, C.M.; Hernandez, A.F.; Kitt, M.M.; Lorenz, T.J. The Design and Rationale for the Acute Medically Ill Venous Thromboembolism Prevention with Extended Duration Betrixaban (APEX) Study. Am. Heart J. 2014, 167, 335–341. [Google Scholar] [CrossRef]
- Watanabe, A.; Araki, K.; Hirai, K.; Kubo, N.; Igarashi, T.; Tsukagoshi, M.; Ishii, N.; Hoshino, K.; Kuwano, H.; Shirabe, K. A Novel Clinical Factor, D-Dimer Platelet Multiplication, May Predict Postoperative Recurrence and Prognosis for Patients with Cholangiocarcinoma. Ann. Surg. Oncol. 2016, 23, 886–891. [Google Scholar] [CrossRef]
- Giaccherini, C.; Verzeroli, C.; Russo, L.; Gamba, S.; Tartari, C.J.; Bolognini, S.; Schieppati, F.; Ticozzi, C.; Sarmiento, R.; Celio, L.; et al. Thrombin Generation and D-Dimer for Prediction of Disease Progression and Mortality in Patients with Metastatic Gastrointestinal Cancer. Cancers 2022, 14, 4347. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zhou, H.; Sun, Y.; Xu, Z.H.E.; Wang, S.; Feng, T.; Zhang, P. D-Dimer as a Potential Clinical Marker for Predicting Metastasis and Progression in Cancer. Biomed. Rep. 2018, 9, 453–457. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, J.; Deng, Y.; Huang, Y.; Li, R.; Lin, G.; Dong, M.; Huang, Z. Pretreatment Plasma Fibrinogen Level as a Prognostic Biomarker for Patients with Lung Cancer. Clinics 2020, 75, e993. [Google Scholar] [CrossRef]
- Tian, Y.; Hong, M.; Jing, S.; Liu, X.; Wang, H.; Wang, X.; Kaushik, D.; Rodriguez, R.; Wang, Z. Clinical and Prognostic Effect of Plasma Fibrinogen in Renal Cell Carcinoma: A Meta-Analysis. BioMed Res. Int. 2017, 2017, 95915060. [Google Scholar] [CrossRef]
- Lugassy, G.; Falanga, A.; Kakkar, A.K.; Rickles Frederick, R. Thrombosis and Cancer; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9781841842875. [Google Scholar]
- Izuegbuna, O.O.; Agodirin, O.S.; Olawumi, H.O.; Olatoke, S.A. Plasma D-Dimer and Fibrinogen Levels Correlates with Tumor Size and Disease Progression in Nigerian Breast Cancer Patients. Cancer Investig. 2021, 39, 597–606. [Google Scholar] [CrossRef]
- Gieseler, F.; Lühr, I.; Kunze, T.; Mundhenke, C.; Maass, N.; Erhart, T.; Denker, M.; Beckmann, D.; Tiemann, M.; Schulte, C.; et al. Activated coagulation factors in human malignant effusions and their contribution to cancer cell metastasis and therapy. Thromb. Haemost. 2007, 97, 1023–1030. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Li, J.; Che, G. Prognostic Value of Pretreatment D-Dimer Level in Small-Cell Lung Cancer: A Meta-Analysis. Technol. Cancer Res. Treat. 2021, 20, 1533033821989822. [Google Scholar] [CrossRef] [PubMed]
- Chekhonin, V.P.; Shein, S.A.; Korchagina, A.A.; Gurina, O.I. VEGF in Tumor Progression and Targeted Therapy. Curr. Cancer Drug Targets 2013, 13, 423–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jia, Y.; Jia, Y.; Zhang, X.; Li, K. Prognostic and Predictive Value of Plasma D-Dimer Levels in Patients with Small-Cell Lung Cancer. Int. J. Clin. Oncol. 2018, 23, 1070–1075. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Zhang, J.; Mafa, T.; Zhang, J.; Zhu, H.; Chen, L.; Zong, Z.; Yang, L. Fibrinogen: A New Player and Target on the Formation of Pre-Metastatic Niche in Tumor Metastasis. Crit. Rev. Oncol. Hematol. 2025, 207, 104625. [Google Scholar] [CrossRef]
- Palumbo, J.S.; Kombrinck, K.W.; Drew, A.F.; Grimes, T.S.; Kiser, J.H.; Degen, J.L.; Bugge, T.H. Fibrinogen Is an Important Determinant of the Metastatic Potential of Circulating Tumor Cells. Blood 2000, 96, 3302–3309. [Google Scholar] [CrossRef]
- Jiang, C.; Li, Y.; Li, Y.; Liu, L.; Wang, X.A.; Wu, W.; Bao, R.; Weng, H.; Li, M.; Geng, Y.; et al. Fibrinogen Promotes Gallbladder Cancer Cell Metastasis and Extravasation by Inducing ICAM1 Expression. Med. Oncol. 2023, 40, 10. [Google Scholar] [CrossRef]
- Hisada, Y.; Mackman, N. Cancer-Associated Pathways and Biomarkers of Venous Thrombosis. Blood 2017, 130, 1499–1506. [Google Scholar] [CrossRef]
- Hollander, K.N.; Joshi, B.L.; Joshi, B.L. Bioprosthetic Valve Thrombosis in Carcinoid Heart Disease. Ann. Card. Anaesth. 2019, 22, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vilchez, I.; Diaz-Ricart, M.; White, J.G.; Escolar, G.; Galan, A.M. Serotonin Enhances Platelet Procoagulant Properties and Their Activation Induced during Platelet Tissue Factor Uptake. Cardiovasc. Res. 2009, 84, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Llobet, D.; Vallvé, C.; Tirado, I.; Vilalta, N.; Murillo, J.; Cuevas, B.; Román, L.; Carrasco, M.; Oliver, A.; Mateo, J.; et al. VAMP8 and Serotonin Transporter Levels Are Associated with Venous Thrombosis Risk in a Spanish Female Population. Results from the RETROVE Project. Thromb. Res. 2019, 181, 99–105. [Google Scholar] [CrossRef]
- Wulftange, W.J.; Kucukal, E.; Man, Y.; An, R.; Monchamp, K.; Sevrain, C.D.; Dashora, H.R.; Owusu-Ansah, A.T.; Bode, A.; Ilich, A.; et al. Antithrombin-III Mitigates Thrombin-Mediated Endothelial Cell Contraction and Sickle Red Blood Cell Adhesion in Microscale Flow. Br. J. Haematol. 2022, 198, 893–902. [Google Scholar] [CrossRef]
- Englisch, C.; Königsbrügge, O.; Nopp, S.; Moik, F.; Quehenberger, P.; Preusser, M.; Pabinger, I.; Ay, C. Antithrombin Activity and Association with Risk of Thrombosis and Mortality in Patients with Cancer. Int. J. Mol. Sci. 2022, 23, 15770. [Google Scholar] [CrossRef]
- Lundbech, M.; Krag, A.E.; Christensen, T.D.; Hvas, A.M. Thrombin Generation, Thrombin-Antithrombin Complex, and Prothrombin Fragment F1+2 as Biomarkers for Hypercoagulability in Cancer Patients. Thromb. Res. 2020, 186, 80–85. [Google Scholar] [CrossRef]
- Åberg, M.; Eriksson, O.; Siegbahn, A. Tissue Factor Noncoagulant Signaling: Mechanisms and Implications for Cell Migration and Apoptosis. Semin. Thromb. Hemost. 2015, 41, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Tieken, C.; Versteeg, H.H. Anticoagulants versus Cancer. Thromb. Res. 2016, 140, S148–S153. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.E.; Shabannezhad, A.; Kahrizi, A.; Akbar, A.; Safdari, S.M.; Hoseinnezhad, T.; Zahedi, M.; Sadeghi, S.; Mojarrad, M.G.; Safa, M. Tissue Factor (Coagulation Factor III): A Potential Double-Edge Molecule to Be Targeted and Re-Targeted toward Cancer. Biomark. Res. 2023, 11, 60. [Google Scholar] [CrossRef]
- Hisada, Y.; Mackman, N. Tissue Factor and Extracellular Vesicles: Activation of Coagulation and Impact on Survival in Cancer. Cancers 2021, 13, 3839. [Google Scholar] [CrossRef]
- Hernández, C.; Orbe, J.; Roncal, C.; Alvarez-Hernandez, M.; Martinez de Lizarrondo, S.; Alves, M.T.; García Mata, J.; Páramo, J.A. Tissue factor expressed by microparticles is associated with mortality but not with thrombosis in cancer patients. Thromb. Haemost. 2013, 110, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.S.; Thomas, H.E.; Orr-asman, M.; Green, L.C.; Matiash, K.; Karve, A.; Hisada, Y.M.; Davis, H.W.; Mercer, C.; Lucas, F.V.; et al. mTOR Kinase Inhibition Reduces Tissue Factor Expression and Growth of Pancreatic Neuroendocrine Tumors. J. Thromb. Haemost. 2019, 17, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Godinho, J.; Casa-Nova, M.; Moreira-Pinto, J.; Simões, P.; Paralta Branco, F.; Leal-Costa, L.; Faria, A.; Lopes, F.; Teixeira, J.A.; Passos-Coelho, J.L. ONKOTEV Score as a Predictive Tool for Thromboembolic Events in Pancreatic Cancer—A Retrospective Analysis. Oncologist 2020, 25, e284–e290. [Google Scholar] [CrossRef] [PubMed]

| The Inclusion Criterion | The Exclusion Criteria |
|---|---|
| Histologically confirmed diagnosis of a well-differentiated Pan-NET or SI-NET (NET G1 or G2) | Individuals under 18 years of age |
| A history of any other cancers or blood disorders | |
| Heart or respiratory failure | |
| Acute cardiac or neurological events, recent surgeries or fractures (within the last month) | |
| Pregnancy | |
| Use of oral contraceptives | |
| Use of anticoagulants |
| Khorana Score 1 | Vienna CATS Score 2 | ONCOTEV Score 3 | Padua Prediction Score 4 |
|---|---|---|---|
Cancer type:
Platelet count ≥ 350 × 109/L (1 score) Hemoglobin < 10 g/dL or using red blood cells growth factors (1 score) Leukocyte count > 11 × 109/L) (1 score) | Khorana score (maximum 6 score) Soluble P-selectin ≥ 53.1 mg/mL (1 score) D-dimer ≥ 1.44 ug/mL (1 score) | Khorana score > 2 (1 score) Presence of metastatic disease (1 score) Compression of vascular/lymphatic structures (1 score) History of previous VTE (1 score) | Active cancer (patients with metastases to regional lymph nodes or with distant metastases who received chemotherapy or radiotherapy within the last 6 months) (3 score) Previous VTE (excluding superficial vein thrombosis) (3 score) Reduced mobility (3 score) Already known thrombophilic condition (3 score) Recent (≤1 month) trauma and/or surgery (2 score) Elderly age (≥70 years) (1 score) Heart and/or respiratory failure (1 score) Acute myocardial infarct and/or ischemic stroke (1 score) Acute infection and/or rheumatologic disorder (1 score) Body-mass index (BMI ≥ 30 kg/m2) (1 score) Ongoing hormonal treatment (1 score) |
| Parameters | Study Group n = 99 | Control Group n= 47 | p Value | |||
|---|---|---|---|---|---|---|
| Age (years) | 57.63 ± 12.96 | 54.6 ± 12.57 | 0.119 | |||
| Sex (female/male) | 55/44 | 29/18 | 0.591 | |||
| Body Mass Index (BMI) | 25.98 ± 4.57 | 26.76 ± 4.29 | 0.389 | |||
| Primary tumour location | Pancreas n (% of patients) | Small intestine n (% of patients) | - | - | ||
| 63 (63.6) | 36 (36.4) | - | - | |||
| Histological grade | G1 (Ki-67 < 3%) | 40 (40.4) | 27 (27.2) | - | - | |
| G2 (Ki-67 3–20%) | 23 (23.2) | 9 (9.1) | - | - | ||
| Clinical staging | I | 24 (24.2) | 5 (5.1) | - | - | |
| II | 11 (11.1) | 0 (0.0) | - | - | ||
| III | 10 (10.1) | 6 (6.1) | - | - | ||
| IV | 18 (18.1) | 25 (25.3) | - | - | ||
| Lymph node metastases | 24 (24.2) | 27 (27.2) | - | - | ||
| Liver metastases | 16 (16.1) | 21 (21.2) | - | - | ||
| Bone metastases | 2 (2.0) | 3 (3.0) | - | - | ||
| Other metastases | 4 (4.0) | 12 (12.1) | - | - | ||
| Secretory status | Non-functioning | 57 (57.6) | 26 (26.3) | - | - | |
| Functioning | 4 (carcinoid syndrome), 2 (insulinoma) (6.1) | 10 (carcinoid syndrome) (10.1) | - | - | ||
| Treatment | Treatment-naïve | 36 (36.4) | 17 (17.2) | - | - | |
| Somatostatin analogues | 17 (17.2) | 17 (17.2) | - | - | ||
| Molecular targeted treatment | 1 (everolimus) and 1 (sunitinib) (2.0) | 0 (0.0) | - | - | ||
| Chemotherapy with capecitabine and temozolomide | 0 (0.0) | 1 (1.0) | - | - | ||
| Disease progression | Progressive | 15 (15.2) | 10 (10.1) | - | - | |
| Stable | 48 (48.5) | 26 (26.3) | - | - | ||
| VTE Risk Assessment Scales | Khorana score | Low (0 score) | 0 (0.0) | 32 (32.3) | 0 (0.0) | - |
| Medium (1–2 score) | 58 (58.6) | 4 (4.0) | 0 (0.0) | |||
| High (>2 score) | 4 (4.0) | 0 (0.0) | 0 (0.0) | |||
| Vienna CATS score | 0 score | 0 (0.0) | 25 (25.3) | 31(66.0) | - | |
| 1 score | 0 (0.0) | 7 (7.1) | 16 (34.0) | |||
| 2 score | 47 (47.4) | 4 (4.0) | 0 (0.0) | |||
| 3 score | 13 (13.1) | 0 (0.0) | 0 (0.0) | |||
| 4 score | 2 (2.0) | 0 (0.0) | 0 (0.0) | |||
| 5 score | 1 (1.0) | 0 (0.0) | 0 (0.0) | |||
| ONCOTEV score | Low (0–1 score) | 49 (49.5) | 27 (27.3) | 0 (0.0) | - | |
| Medium (2 score) | 11 (11.1) | 9 (9.1) | 0 (0.0) | |||
| High (≥3 score) | 3 (3.0) | 0 (0.0) | 0 (0.0) | |||
| Padua Prediction Score | 0 score | 35 (35.4) | 22 (22.2) | 32 (68.1) | - | |
| 1 score | 19 (19.2) | 8 (8.1) | 12 (25.5) | |||
| 2 score | 4 (4.0) | 4 (4.0) | 3 (6.4) | |||
| 3 score | 3 (3.0) | 0 (0.0) | 0 (0.0) | |||
| ≥4 score | 2 (2.0) | 2 (2.0) | 0 (0.0) | |||
| VTE events | Deep vein thrombosis | 1 (1.0) | 0 (0.0) | 0 (0.0) | - | |
| Deep vein thrombosis and Superficial vein thrombosis | 0 (0.0) | 1 (1.0) | 0 (0.0) | |||
| Superficial vein thrombosis | 1 (1.0) | 1 (1.0) | 0 (0.0) | - | ||
| Tumour thrombosis | 6 (6.1) | 0 (0.0) | 0 (0.0) | - | ||
| VTE Events | Primary Tumour Location | Histological Grade | Clinical Staging | Secretory Status | Disease Progression | Treatment | Time from Diagnosis <6 Months | DD Levels [µg/L, n: <500 µg/L] |
|---|---|---|---|---|---|---|---|---|
| Deep vein thrombosis | Pan-NET | G2 | III | NF | no | Treatment-naïve | yes | 680 |
| Deep vein thrombosis and Superficial vein thrombosis | SI-NET | G1 | IV | NF | yes | Treatment-naïve | yes | 3676 |
| Superficial vein thrombosis | Pan-NET | G2 | IV | NF | yes | Treatment-naïve | yes | 3382 |
| SI-NET | G2 | III | NF | no | Treatment-naïve | no | 313 | |
| Tumour thrombosis (splanchnic vein thrombosis) | Pan-NET | G1 | IV | NF | yes | Somatostatin analogue, Everolimus | yes | 590 |
| Pan-NET | G2 | III | F (carcinoid syndrome) | yes | Treatment-naïve | yes | 477 | |
| Pan-NET | G2 | III | NF | yes | Treatment-naïve | yes | 198 | |
| Pan-NET | G1 | III | NF | yes | Treatment-naïve | yes | 1651 | |
| Pan-NET | G1 | IV | NF | yes | Treatment-naïve | yes | 393 | |
| Tumour thrombosis (portal vein thrombosis) | Pan-NET | G1 | IV | NF | yes | Treatment-naïve | yes | 2331 |
| Parameters [Unit] | Study Group | Control Group | p Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| DD [µg/L] | 957.59 ± 2021.86 | 400.26 ± 230.55 | 0.007 |
| Fibrinogen [mg/dL] | 318.98 ± 78.74 | 303.40 ± 55.45 | 0.301 |
| PLT [109/L] | 255.28 ± 97.22 | 256.11 ± 54.93 | 0.435 |
| AT-III [%] | 101.71 ± 14.95 | 102.47 ± 11.34 | 0.795 |
| TF [pg/mL] | 157.71 ± 63.96 | 161.77 ± 25.56 | 0.160 |
| Parameters [Unit] | Pan-NETs | SI-NETs | p Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| DD [µg/L] | 1023.92 ± 2371.65 | 841.50 ± 1208.51 | 0.558 |
| Fibrinogen [mg/dL] | 311.73 ± 65.72 | 331.67 ± 97.21 | 0.302 |
| PLT [109/L] | 260.56 ± 97.92 | 246.03 ± 96.65 | 0.430 |
| AT-III [%] | 104.63 ± 14.56 | 96.58 ± 14.42 | 0.010 |
| TF [pg/mL] | 156.36 ± 69.53 | 159.99 ± 54.16 | 0.474 |
| Parameters | Age | WHO/ECOG Scale | Clinical Staging | Tumour Size | Lymph Node Metastases | CgA | Serotonin | 5-HIO | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rS | p | rS | p | rS | p | rS | p | rS | p | rS | p | rS | p | rS | p | |
| DD | 0.38 | <0.001 | 0.51 | <0.001 | 0.32 | 0.001 | 0.23 | 0.022 | 0.25 | 0.014 | 0.32 | 0.001 | 0.05 | 0.657 | 0.06 | 0.578 |
| Fibrinogen | 0.23 | 0.021 | 0.21 | 0.040 | 0.15 | 0.128 | 0.16 | 0.127 | −0.04 | 0.675 | 0.15 | 0.127 | 0.15 | 0.136 | 0.15 | 0.129 |
| PLT | 0.02 | 0.831 | 0.07 | 0.487 | 0.08 | 0.432 | −0.03 | 0.744 | −0.01 | 0.951 | −0.16 | 0.107 | 0.22 | 0.029 | −0.13 | 0.203 |
| AT-III | −0.01 | 0.897 | 0.00 | 0.998 | −0.22 | 0.031 | −0.15 | 0.130 | −0.20 | 0.044 | 0.05 | 0.615 | −0.21 | 0.041 | −0.04 | 0.659 |
| TF | 0.12 | 0.238 | 0.23 | 0.024 | 0.21 | 0.043 | 0.10 | 0.323 | 0.19 | 0.074 | 0.05 | 0.632 | 0.00 | 0.983 | 0.14 | 0.165 |
| VTE Risk Assessment Scales | AUC ± SD | p-Value |
|---|---|---|
| Khorana score | 0.39 ± 0.05 | 0.059 |
| Vienna CATS score | 0.46 ± 0.06 | 0.501 |
| ONCOTEV score | 0.68 ± 0.05 | <0.001 |
| Padua Prediction Score | 0.43 ± 0.05 | 0.191 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik-Giertuga, M.; Malczewska-Herman, A.; Orzeł, A.; Kos-Kudła, B. The Clinical Utility of Selected Coagulation Parameters in Predicting the Risk of Venous Thromboembolism in Neuroendocrine Tumours: A Prospective, Single-Centre Study. Cancers 2025, 17, 3405. https://doi.org/10.3390/cancers17213405
Wójcik-Giertuga M, Malczewska-Herman A, Orzeł A, Kos-Kudła B. The Clinical Utility of Selected Coagulation Parameters in Predicting the Risk of Venous Thromboembolism in Neuroendocrine Tumours: A Prospective, Single-Centre Study. Cancers. 2025; 17(21):3405. https://doi.org/10.3390/cancers17213405
Chicago/Turabian StyleWójcik-Giertuga, Monika, Anna Malczewska-Herman, Arkadiusz Orzeł, and Beata Kos-Kudła. 2025. "The Clinical Utility of Selected Coagulation Parameters in Predicting the Risk of Venous Thromboembolism in Neuroendocrine Tumours: A Prospective, Single-Centre Study" Cancers 17, no. 21: 3405. https://doi.org/10.3390/cancers17213405
APA StyleWójcik-Giertuga, M., Malczewska-Herman, A., Orzeł, A., & Kos-Kudła, B. (2025). The Clinical Utility of Selected Coagulation Parameters in Predicting the Risk of Venous Thromboembolism in Neuroendocrine Tumours: A Prospective, Single-Centre Study. Cancers, 17(21), 3405. https://doi.org/10.3390/cancers17213405






