Prognostic Value of Dynamic Changes in Immune-Inflammatory and Tumor Biomarkers Following Chemoradiotherapy in Locally Advanced Rectal Cancer
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Treatment Protocol
2.3. Data Collection
2.4. Outcomes and Follow-Up
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Patient Demographics and Clinical Characteristics
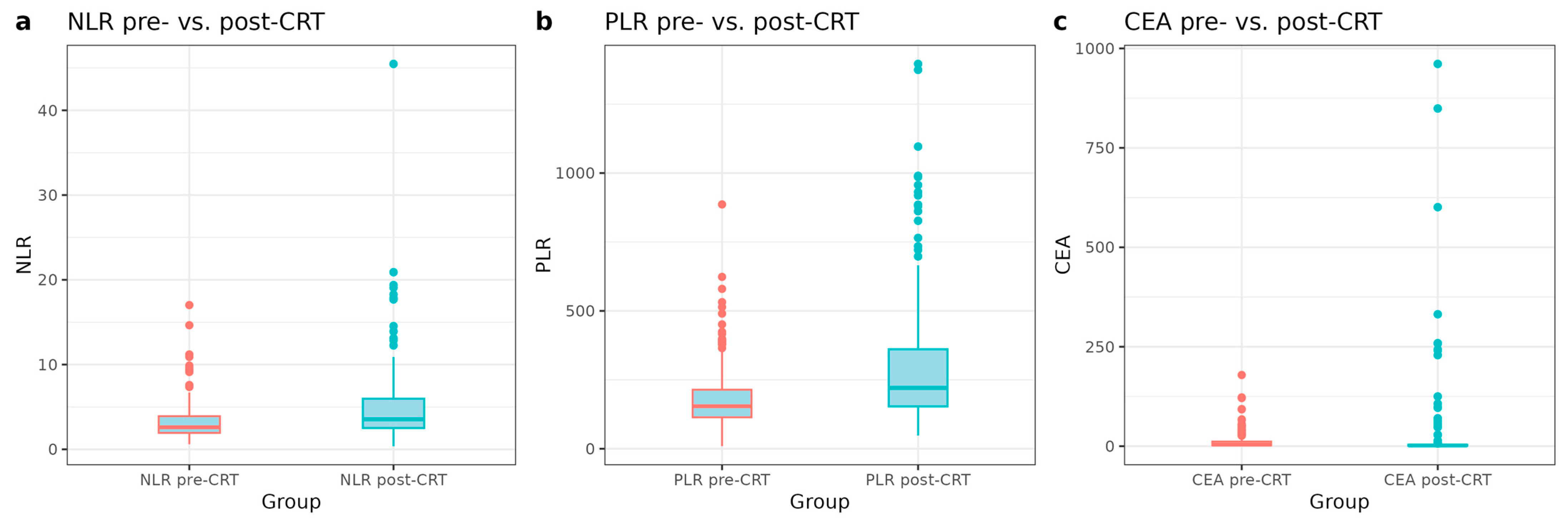
3.2. Changes in Inflammatory and Tumor Markers Pre- and Post-CRT
3.3. Univariable and Multivariable Survival Analysis of Clinical and Biomarker Variables
3.3.1. Overall Survival (OS)
3.3.2. Disease-Free Survival (DFS)
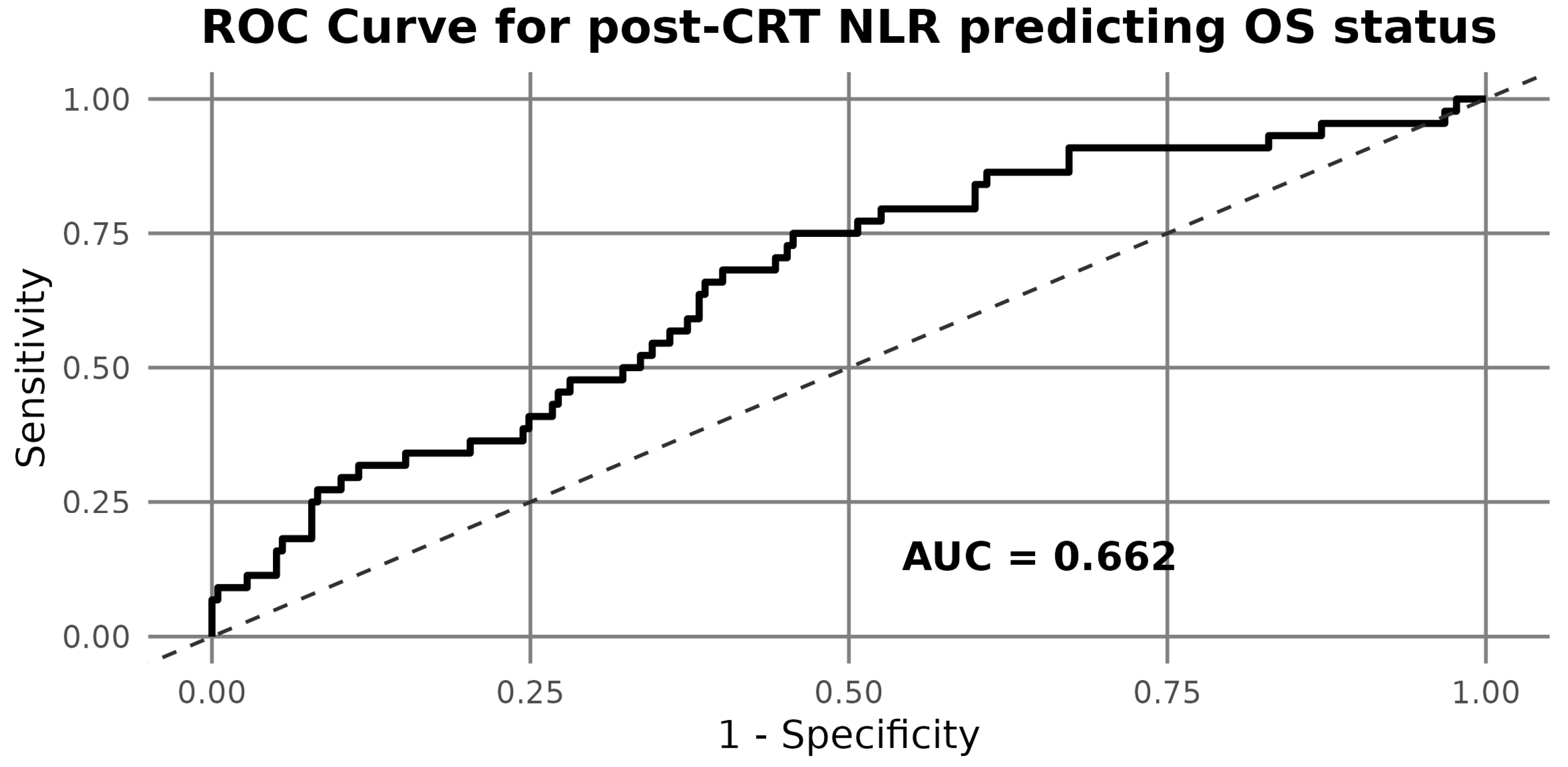
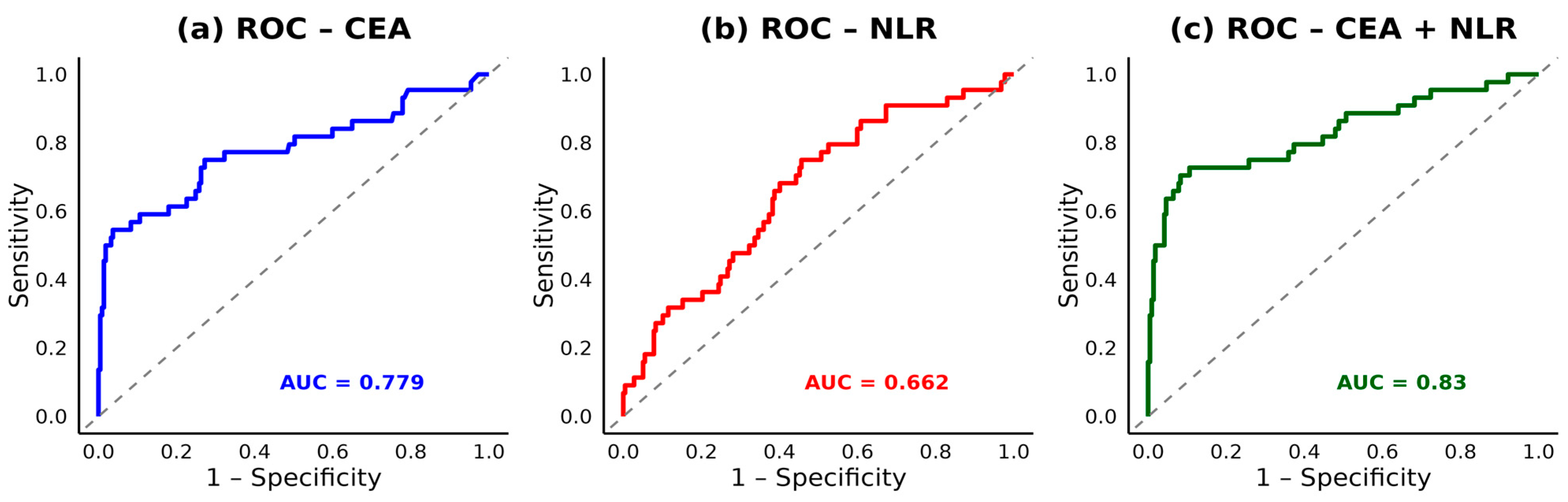
3.4. ROC Analysis and Biomarker Cut-Offs
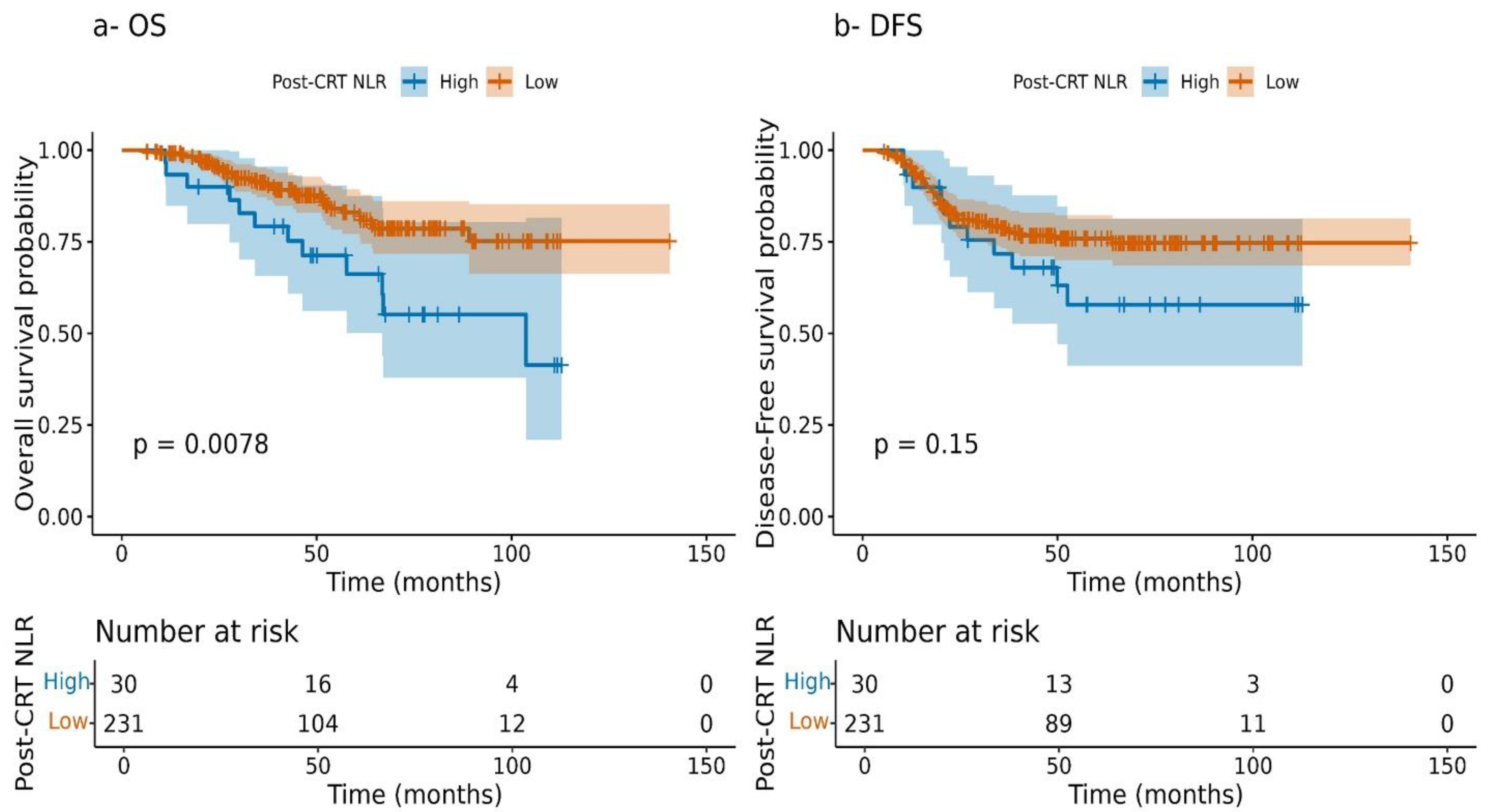
3.5. Kaplan–Meier Survival Stratified by NLR
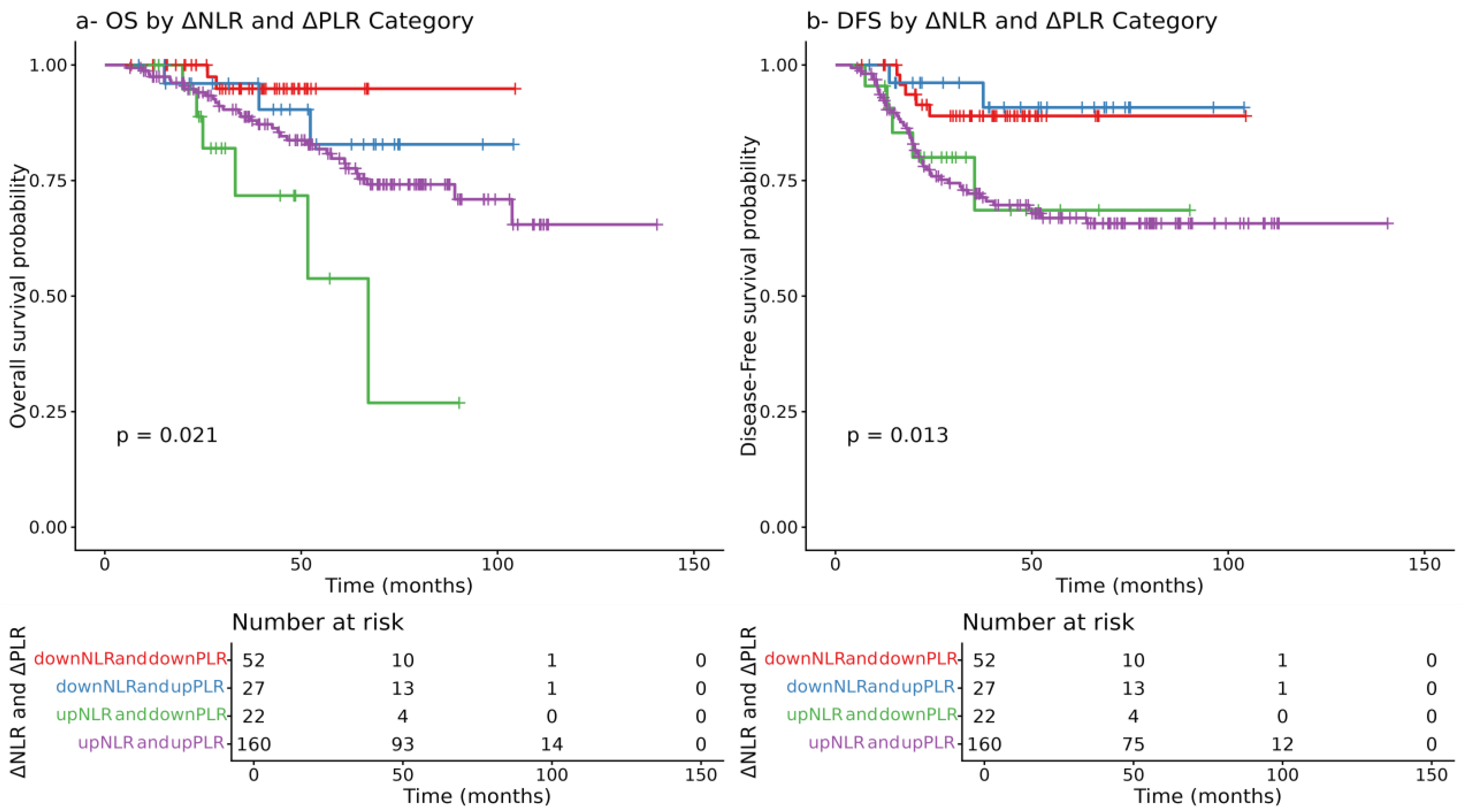
3.6. Prognostic Role of Delta NLR and PLR Combinations
3.7. Combined Predictive Performance of Post-CRT CEA and NLR
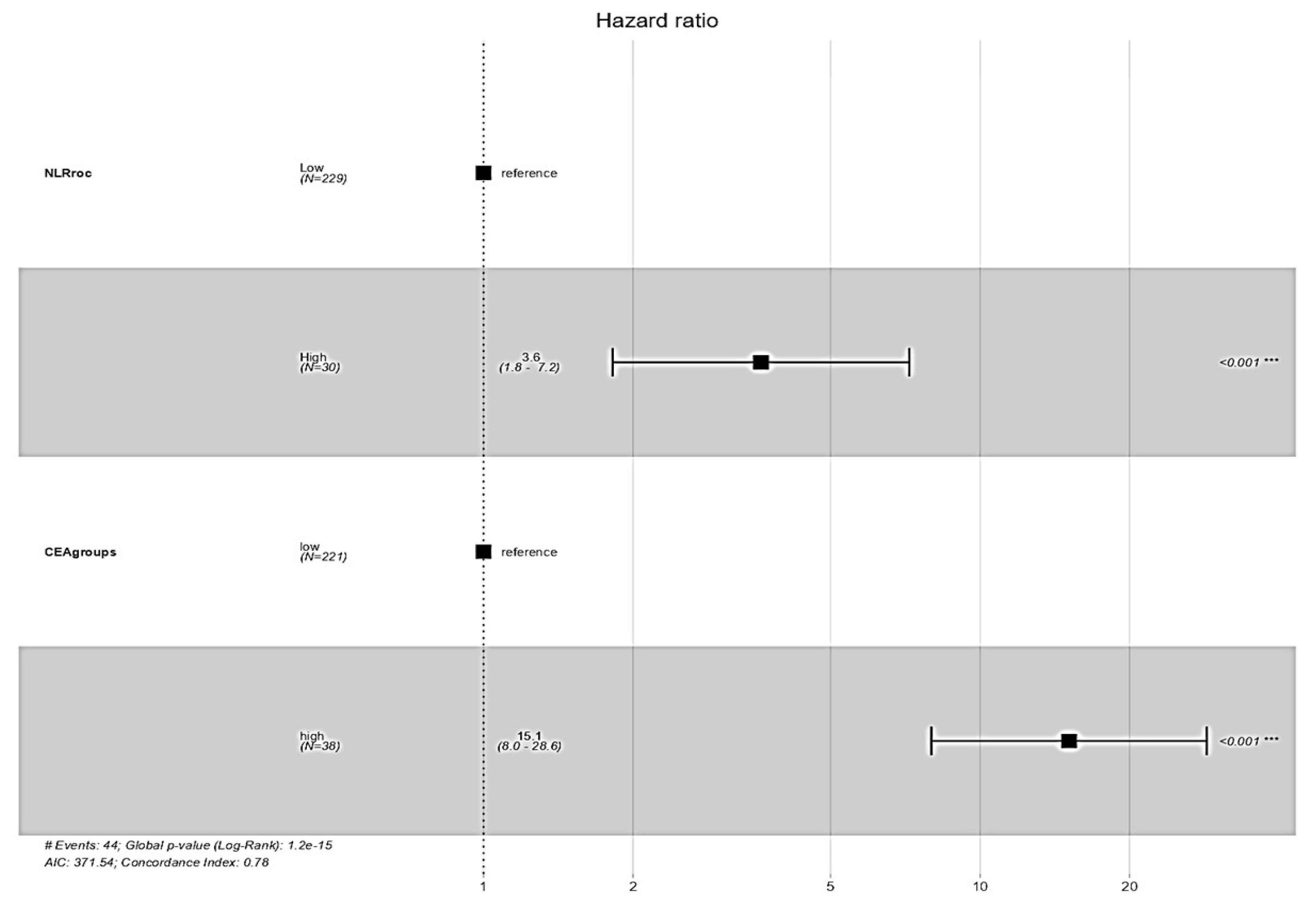
3.8. Survival Impact of NLR and CEA Groups
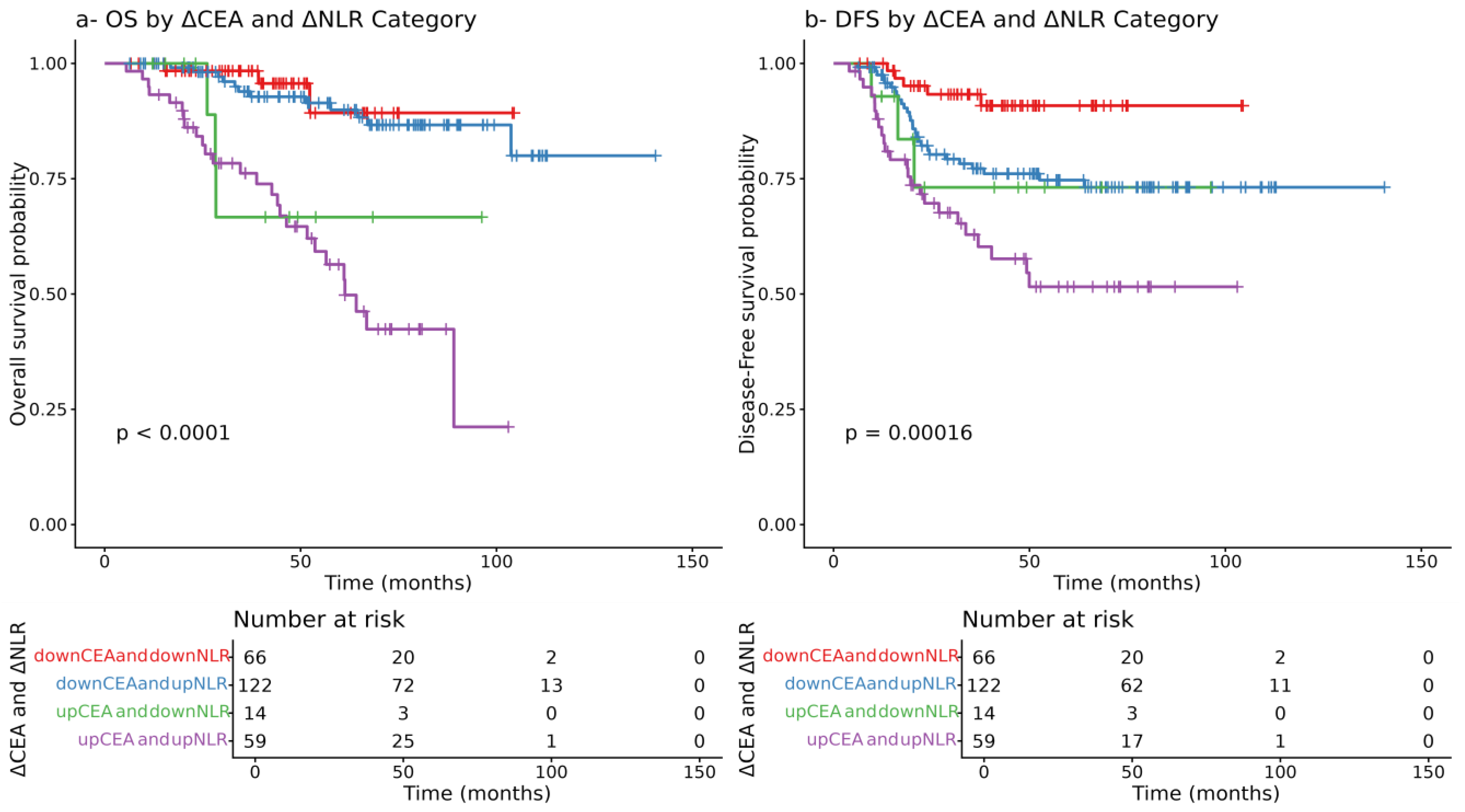
3.9. Prognostic Value of the Dynamic Changes in CEA and NLR
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Mohammed, H.; Mohamed, H.; Mohamed, N.; Sharma, R.; Sagar, J. Early Rectal Cancer: Advances in Diagnosis and Management Strategies. Cancers 2025, 17, 588. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Sharkas, G.F.; Arqoub, K.H.; Khader, Y.S.; Tarawneh, M.R.; Nimri, O.F.; Al-Zaghal, M.J.; Subih, H.S. Colorectal Cancer in Jordan: Survival Rate and Its Related Factors. J. Oncol. 2017, 2017, 3180762. [Google Scholar] [CrossRef]
- Karam, E.; Fredon, F.; Eid, Y.; Muller, O.; Besson, M.; Michot, N.; Giger-Pabst, U.; Alves, A.; Ouaissi, M. Review of definition and treatment of upper rectal cancer. Surg. Oncol. 2024, 57, 102145. [Google Scholar] [CrossRef]
- Chehade, L.; Dagher, K.; Shamseddine, A. Tailoring treatment for locally advanced rectal cancer. Cancer Treat. Res. Commun. 2024, 41, 100847. [Google Scholar] [CrossRef]
- Ali, F.; Keshinro, A.; Wesiser, M.R. Advances in the treatment of locally advanced rectal cancer. Ann. Gastroenterol. Surg. 2020, 5, 32–38. [Google Scholar] [CrossRef]
- Zwart, W.H.; Hotca, A.; Hospers, G.A.; Goodman, K.A.; Garcia-Aguilar, J. The Multimodal Management of Locally Advanced Rectal Cancer: Making Sense of the New Data. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 264–277. [Google Scholar] [CrossRef]
- Conces, M.L.; Mahipal, A. Adoption of Total Neoadjuvant Therapy in the Treatment of Locally Advanced Rectal Cancer. Curr. Oncol. 2024, 31, 366–382. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Azad, N.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 1139–1167. [Google Scholar] [CrossRef] [PubMed]
- Paramanathan, A.; Saxena, A.; Morris, D.L. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg. Oncol. 2014, 23, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Mahar, A.L.; Compton, C.; Halabi, S.; Hess, K.R.; Weiser, M.R.; Groome, P.A. Personalizing prognosis in colorectal cancer: A systematic review of the quality and nature of clinical prognostic tools for survival outcomes. J. Surg. Oncol. 2017, 116, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.F.; Karakiewicz, P.I.; Roehrborn, C.G.; Kattan, M.W. An updated catalog of prostate cancer predictive tools. Cancer 2008, 113, 3075–3099. [Google Scholar] [CrossRef]
- Koh, C.-H.; Bhoo-Pathy, N.; Ng, K.-L.; Jabir, R.S.; Tan, G.-H.; See, M.-H.; Jamaris, S.; Taib, N.A. Utility of pre-treatment neutrophil–lymphocyte ratio and platelet–lymphocyte ratio as prognostic factors in breast cancer. Br. J. Cancer 2015, 113, 150–158. [Google Scholar] [CrossRef]
- Hong, X.; Cui, B.; Wang, M.; Yang, Z.; Wang, L.; Xu, Q. Systemic Immune-inflammation Index, Based on Platelet Counts and Neutrophil-Lymphocyte Ratio, Is Useful for Predicting Prognosis in Small Cell Lung Cancer. Tohoku J. Exp. Med. 2015, 236, 297–304. [Google Scholar] [CrossRef]
- Jia, J.; Zheng, X.; Chen, Y.; Wang, L.; Lin, L.; Ye, X.; Chen, Y.; Chen, D.; Dettke, M. Stage-dependent changes of preoperative neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in colorectal cancer. Tumor Biol. 2015, 36, 9319–9325. [Google Scholar] [CrossRef]
- Madore, J.; Strbenac, D.; Vilain, R.; Menzies, A.M.; Yang, J.Y.; Thompson, J.F.; Long, G.V.; Mann, G.J.; Scolyer, R.A.; Wilmott, J.S. PD-L1 Negative Status is Associated with Lower Mutation Burden, Differential Expression of Immune-Related Genes, and Worse Survival in Stage III Melanoma. Clin. Cancer Res. 2016, 22, 3915–3923. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Wu, W.; Zhang, L.; Ju, H.; Fan, Y.; Zhu, Y.; Luo, J.; Liu, P.; Zhou, N.; et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Locally Advanced Rectal Cancer Treated with Neoadjuvant Chemoradiotherapy. Med. Sci. Monit. 2017, 23, 315–324. [Google Scholar] [CrossRef]
- Kankanala, V.L.; Zubair, M.; Mukkamalla, S.K.R. Carcinoembryonic Antigen; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Niedzielska, J.; Jastrzębski, T. Carcinoembryonic Antigen (CEA): Origin, Role in Oncology, and Concentrations in Serum and Peritoneal Fluid. J. Clin. Med. 2025, 14, 3189. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.-J.; Chung, S.-M.; Kim, J.-Y.; Ryu, M.-R. Prognostic Significance of Serum Carcinoembryonic Antigen Normalization on Survival in Rectal Cancer Treated with Preoperative Chemoradiation. Cancer Res. Treat. 2013, 45, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Stremitzer, S.; Stift, J.; Graf, A.; Singh, J.; Starlinger, P.; Gruenberger, B.; Tamandl, D.; Gruenberger, T. CEA Change After Neoadjuvant Chemotherapy Including Bevacizumab and Clinical Outcome in Patients Undergoing Liver Resection for Colorectal Liver Metastases. Ann. Surg. Oncol. 2014, 22, 1315–1323. [Google Scholar] [CrossRef]
- Muñoz-Montaño, W.R.; López-Basave, H.N.; Castillo-Morales, A.; Castillo-Morales, C.; Sánchez-Trejo, K.; Catalán, R.; Díaz-Romero, C.; Lino-Silva, L.S.; Maliachi-Díaz, A.; Ruiz-García, E.; et al. Persistent high levels of carcinoembryonic antigen after tumor resection are associated with poorer survival outcomes in patients with resected colon cancer. BMC Cancer 2023, 23, 678. [Google Scholar] [CrossRef]
- Fakih, M.G.; Padmanabhan, A. CEA monitoring in colorectal cancer. What you should know. Oncology 2006, 20, 579–587. [Google Scholar] [PubMed]
- Zeng, Z.; Ma, D.; Zhu, P.; Niu, K.; Fu, S.; Di, X.; Zhu, J.; Xie, P. Prognostic value of the ratio of pretreatment carcinoembryonic antigen to tumor volume in rectal cancer. J. Gastrointest. Oncol. 2023, 14, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, D.; Chu, L.; Huang, Y.; Zhang, F.; Li, W.; Chen, J. Potential Diagnostic Value Of Combining Inflammatory Cell Ratios With Carcinoembryonic Antigen For Colorectal Cancer. Cancer Manag. Res. 2019, 11, 9631–9640. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-L.; Su, W.-J.; Leung, W.-H.; Lai, C.-T.; Liu, C.-K. Neutrophil–lymphocyte ratio and CEA level as prognostic and predictive factors in colorectal cancer: A systematic review and meta-analysis. J. Cancer Res. Ther. 2016, 12, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.-K. Individualized Cutoff Value of the Serum Carcinoembryonic Antigen Level According to TNM Stage in Colorectal Cancer. Ann. Coloproctol. 2013, 29, 91–92. [Google Scholar] [CrossRef]
- Malietzis, G.; Giacometti, M.; Kennedy, R.H.; Athanasiou, T.; Aziz, O.; Jenkins, J.T. The Emerging Role of Neutrophil to Lymphocyte Ratio in Determining Colorectal Cancer Treatment Outcomes: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2014, 21, 3938–3946. [Google Scholar] [CrossRef]
- Tóth, Z.; Lőczi, L.; Sebők, B.; Merkely, P.; Keszthelyi, E.; Lintner, B.; Ács, N.; Keszthelyi, A.; Várbíró, S.; Tóth, R.; et al. Neutrophil/Lymphocyte Ratio (NLR) as a Predictive Marker for p16 Positivity and Cervical Cancer Progression: Insights from the SCOPE Study. Cancers 2025, 17, 921. [Google Scholar] [CrossRef]
- Matsas, S.; Aguiar, P.N., Jr.; Del Giglio, A. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as biomarkers to prognosticate survival in advanced gastric cancer patients in the era of immunotherapy: A systematic review and meta-analysis. J. Gastrointest. Oncol. 2024, 15, 33–51. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.Y.; Kim, H.K.; Lee, J.W.; Jung, S.G.; Jung, K.; Kim, S.E.; Moon, W.; Park, M.I.; Park, S.J. Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with stage III and IV colorectal cancer. World J. Gastroenterol. 2017, 23, 505–515. [Google Scholar] [CrossRef]
- Misiewicz, A.; Dymicka-Piekarska, V. Fashionable, but What is Their Real Clinical Usefulness? NLR, LMR, and PLR as a Promising Indicator in Colorectal Cancer Prognosis: A Systematic Review. J. Inflamm. Res. 2023, 16, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Yuan, L.; Ni, H.; Xu, B.; Zhao, P. Prognostic Value of Platelet-to-Lymphocyte Ratio, Neutrophil-to-Lymphocyte Ratio, and Lymphocyte-to-White Blood Cell Ratio in Colorectal Cancer Patients Who Received Neoadjuvant Chemotherapy. Technol. Cancer Res. Treat. 2021, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pine, J.K.; Morris, E.; Hutchins, G.G.; West, N.P.; Jayne, D.G.; Quirke, P.; Prasad, K.R. Systemic neutrophil-to-lymphocyte ratio in colorectal cancer: The relationship to patient survival, tumour biology and local lymphocytic response to tumour. Br. J. Cancer 2015, 113, 204–211. [Google Scholar] [CrossRef]
- Absenger, G.; Szkandera, J.; Stotz, M.; Postlmayr, U.; Pichler, M.; Ress, A.L.; Schaberl-Moser, R.; Loibner, H.; Samonigg, H.; Gerger, A. Preoperative neutrophil-to-lymphocyte ratio predicts clinical outcome in patients with stage II and III colon cancer. Anticancer Res. 2013, 33, 4591–4594. [Google Scholar]
- El Mohtaseb, M.S.; Ismail, L.N.; Haddad, T.A.; Ghanayem, H.; Qudah, S.; Al Aruri, D.O.; Abufaraj, M. Neutrophil-to-Lymphocyte Ratio (Nlr) and Platelet-to-Lymphocyte Ratio (Plr) as Prognostic Factors in Patients with Colorectal Cancer: A Retrospective Study. 2025; published online first. [Google Scholar] [CrossRef]
- Cha, Y.J.; Park, E.J.; Baik, S.H.; Lee, K.Y.; Kang, J.; Ahmad, A. Prognostic impact of persistent lower neutrophil-to-lymphocyte ratio during preoperative chemoradiotherapy in locally advanced rectal cancer patients: A propensity score matching analysis. PLoS ONE 2019, 14, e0214415. [Google Scholar] [CrossRef]
- Jeon, B.H.; Shin, U.S.; Moon, S.M.; Choi, J.I.; Kim, M.-S.; Kim, K.H.; Sung, S.-J. Neutrophil to Lymphocyte Ratio: A Predictive Marker for Treatment Outcomes in Patients With Rectal Cancer Who Underwent Neoadjuvant Chemoradiation Followed by Surgery. Ann. Coloproctol. 2019, 35, 100–106. [Google Scholar] [CrossRef]
- Sung, S.; Son, S.H.; Park, E.Y.; Kay, C.S. Prognosis of locally advanced rectal cancer can be predicted more accurately using pre- and post-chemoradiotherapy neutrophil-lymphocyte ratios in patients who received preoperative chemoradiotherapy. PLoS ONE 2017, 12, e0173955. [Google Scholar] [CrossRef]
- Morais, M.; Fonseca, T.; Machado-Neves, R.; Honavar, M.; Coelho, A.R.; Lopes, J.; Guerreiro, E.; Carneiro, S. Prognostic value of neutrophil-to-lymphocyte ratio (NLR) and platelet-neutrophil (PN) index in locally advanced rectal cancer patients: A retrospective cohort study. Ann. Med. Surg. 2024, 86, 2474–2480. [Google Scholar] [CrossRef]
- Partl, R.; Paal, K.; Stranz, B.; Hassler, E.; Magyar, M.; Brunner, T.B.; Langsenlehner, T. The Pre-Treatment Platelet-to-Lymphocyte Ratio as a Prognostic Factor for Loco-Regional Control in Locally Advanced Rectal Cancer. Diagnostics 2023, 13, 679. [Google Scholar] [CrossRef]
- Tan, D.; Fu, Y.; Su, Q.; Wang, H. Prognostic role of platelet–lymphocyte ratio in colorectal cancer. Medicine 2016, 95, e3837. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, K.; Park, H.J. Meta-Analysis on the Neutrophil-Lymphocyte Ratio in Rectal Cancer Treated With Preoperative Chemoradiotherapy: Prognostic Value of Pre- and Post-Chemoradiotherapy Neutrophil-Lymphocyte Ratio. Front. Oncol. 2022, 12, 778607. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xi, Y.; He, G.; Li, X.; Zhan, F. Dynamics of neutrophil-to-lymphocyte ratio predict outcomes of metastatic colorectal carcinoma patients treated by FOLFOX. J. Gastrointest. Oncol. 2021, 12, 2846–2853. [Google Scholar] [CrossRef]
- Acun, M.; Alikanoglu, A.; Onder, A.; Ozturk, B.; Alparslan, A.; Karaca, M. P-291 The prognostic and predictive factors for patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Ann. Oncol. 2021, 32, S196. [Google Scholar] [CrossRef]
- Soykut, E.D. Pre-Treatment and Post-Treatment Neutrophil-to-Lymphocyte Ratio Predict Pathological Tumor Response and Survival in Rectal Cancer Patients Treated with Neoadjuvant Chemoradiotherapy. Turk. J. Oncol. 2022, 37, 139–149. [Google Scholar] [CrossRef]
- Joo, J.I.; Lim, S.W.; Oh, B.Y. Prognostic Impact of Carcinoembryonic Antigen Levels in Rectal Cancer Patients Who Had Received Neoadjuvant Chemoradiotherapy. Ann. Coloproctol. 2021, 37, 179–185. [Google Scholar] [CrossRef]
- Kim, H.; Jung, H.I.; Kwon, S.H.; Bae, S.H.; Kim, H.C.; Baek, M.-J.; Lee, M.S. Preoperative neutrophil-lymphocyte ratio and CEA is associated with poor prognosis in patients with synchronous colorectal cancer liver metastasis. Ann. Surg. Treat. Res. 2019, 96, 191–200. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, M.; Qin, Y.; Lin, F.; Lai, M. Single and combined use of neutrophil–lymphocyte ratio, platelet–lymphocyte ratio and carcinoembryonic antigen in diagnosing gastric cancer. Clin. Chim. Acta 2018, 481, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-X.; Yang, L.; He, B.-S.; Pan, Y.-Q.; Ying, H.-Q.; Sun, H.-L.; Lin, K.; Hu, X.-X.; Xu, T.; Wang, S.-K. Combination of preoperative NLR, PLR and CEA could increase the diagnostic efficacy for I-III stage CRC. J. Clin. Lab. Anal. 2016, 31, e22075. [Google Scholar] [CrossRef]
- Liu, X.-F.; Zhou, L.-Y.; Wei, Z.-H.; Liu, J.-X.; Li, A.; Wang, X.-Z.; Ying, H.-Q. The Diagnostic Role of Circulating Inflammation-Based Biomarker in Gallbladder Carcinoma. Biomark. Med. 2018, 12, 1095–1103. [Google Scholar] [CrossRef]
- An, S.H.; Kim, I.Y. Can pretreatment platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios predict long-term oncologic outcomes after preoperative chemoradiation followed by surgery for locally advanced rectal cancer? Ann. Coloproctol. 2022, 38, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Xu, M.; Chen, K.; Li, S.; Guan, G. Prognostic value of pretreatment systemic inflammatory markers in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy. Sci. Rep. 2020, 10, 8017. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, Y.; Ran, Y.; Lu, F.; Qin, Y. Biomarkers of inflammation and colorectal cancer risk. Front. Oncol. 2025, 15, 1514009. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.M.; Han, N. The expression of tumor necrosis factor receptor 2 is correlated with the prognosis of cancer: A systematic review and meta-analysis. Transl. Cancer Res. 2024, 13, 4231–4241. [Google Scholar] [CrossRef]
- Hua, X.; Kratz, M.; Malen, R.C.; Dai, J.Y.; Lindström, S.; Zheng, Y.; Newcomb, P.A. Association between post-treatment circulating biomarkers of inflammation and survival among stage II–III colorectal cancer patients. Br. J. Cancer 2021, 125, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Babic, A.; Shah, S.M.; Song, M.; Wu, K.; Meyerhardt, J.A.; Ogino, S.; Yuan, C.; Giovannucci, E.L.; Chan, A.T.; Stampfer, M.J.; et al. Soluble tumour necrosis factor receptor type II and survival in colorectal cancer. Br. J. Cancer 2016, 114, 995–1002. [Google Scholar] [CrossRef]
- Vahed, I.E.; Moshgelgosha, M.; Kor, A.; Minadi, M.; Ebrahimi, F.; Azhdarian, A.; Arjmandi, M.; Alamdar, A.; Zare, M.; Shabani, N.; et al. The role of Adiponectin and Leptin in Colorectal Cancer and Adenoma: A systematic review and meta-analysis. BMC Cancer 2025, 25, 968. [Google Scholar] [CrossRef]
- Thomsen, M.; Kersten, C.; Sorbye, H.; Skovlund, E.; Glimelius, B.; Pfeiffer, P.; Johansen, J.S.; Kure, E.H.; Ikdahl, T.; Tveit, K.M.; et al. Interleukin-6 and C-reactive protein as prognostic biomarkers in metastatic colorectal cancer. Oncotarget 2016, 7, 75013–75022. [Google Scholar] [CrossRef]
| Overall (N = 261) | |
|---|---|
| Age | |
| Mean (SD) | 55.5 (11.4) |
| Range | 25.0–80.0 |
| Gender | |
| Female | 115 (44.1%) |
| Male | 146 (55.9%) |
| BMI | |
| Mean (SD) | 29.5 (5.9) |
| Range | 11.6–52.9 |
| Comorbidity | |
| No | 98 (37.5%) |
| Yes | 163 (62.5%) |
| Smoking | |
| Yes | 98 (37.5%) |
| no | 163 (62.5%) |
| Clinical Stage—Radiological | |
| 2 | 17 (6.5%) |
| 3 | 244 (93.5%) |
| Therapy group | |
| CCRT | 169 (64.8%) |
| TNT | 92 (35.2%) |
| Surgery | |
| APR | 71 (27.2%) |
| LAR | 187 (71.6%) |
| Pelvic Exenteration | 3 (1.1%) |
| Surgical approach | |
| Minimally invasive | 101 (38.3%) |
| Open | 161 (61.7%) |
| Postoperative pathological AJCC | |
| 0 | 41 (15.7%) |
| 1 | 50 (19.2%) |
| 2 | 95 (36.4%) |
| 3 | 75 (28.7%) |
| Distance from anal verge | |
| N-Miss | 4 |
| Mean (SD) | 6.6 (3.1) |
| Range | 1.0–17.0 |
| Complication | |
| No | 159 (60.9%) |
| Yes | 102 (39.1%) |
| Pre-CRT | Post-CRT | Paired t-Test p | Wilcoxon p | |
|---|---|---|---|---|
| CEA | 0.126 | <0.01 | ||
| Mean (SD) | 11.4 (20.2) | 20.6 (95.8) | ||
| Median (Range) | 4.2 (0.4–179.0) | 1.9 (0.2–961.0) | ||
| NLR | <0.001 | <0.001 | ||
| Mean (SD) | 3.2 (2.1) | 4.9 (4.3) | ||
| Median (Range) | 2.6 (0.6–17.0) | 3.53 (0.4–45.5) | ||
| PLR | <0.001 | <0.001 | ||
| Mean (SD) | 185.4 (110.2) | 293.5 (217.6) | ||
| Median (Range) | 154 (9.5–886.3) | 221 (48.4–1395.9) |
| Overall Survival | Disease-Free Survival | ||||
|---|---|---|---|---|---|
| HR (Univariable) | HR (Multivariable) | HR (Univariable) | HR (Multivariable) | ||
| Gender | Female | - | - | ||
| Male | 1.24 (0.67–2.27, p = 0.495) | 1.34 (0.79–2.27, p = 0.271) | |||
| Comorbidity | No | - | - | ||
| Yes | 3.49 (1.48–8.27, p = 0.004) | 2.17 (0.86–5.46, p = 0.099) | 1.70 (0.96–3.02, p = 0.068) | ||
| Smoking | Yes | - | - | ||
| no | 0.99 (0.54–1.84, p = 0.978) | 1.13 (0.66–1.92, p = 0.657) | |||
| Clinical Stage | 2 | - | - | ||
| 3 | 0.55 (0.22–1.39, p = 0.207) | 0.54 (0.23–1.25, p = 0.150) | |||
| Treatment Group | CCRT | - | |||
| TNT | 0.7 (0.26–1.88, p = 0.484) | 0.9 (0.46–1.94, p = 0.612) | |||
| Surgery | APR | - | - | ||
| LAR | 0.80 (0.42–1.52, p = 0.490) | 0.90 (0.51–1.56, p = 0.699) | |||
| Pelvic Exenteration | 4.23 (0.96–18.66, p = 0.057) | 3.29 (0.76–14.20, p = 0.110) | |||
| Surgical approach | Minimally invasive | - | - | ||
| Open | 1.32 (0.91–2.64, p = 0.721) | 1.21 (0.69–2.10, p = 0.509) | |||
| Complication | No | - | - | ||
| Yes | 1.68 (0.91–3.10, p = 0.097) | 1.41 (0.85–2.35, p = 0.182) | |||
| AJCC—postoperative pathologic stage | 0 | - | - | - | - |
| 1 | 1.40 (0.34–5.87, p = 0.643) | 1.38 (0.33–5.79, p = 0.658) | 1.42 (0.42–4.87, p = 0.572) | 1.42 (0.41–4.84, p = 0.578) | |
| 2 | 2.33 (0.67–8.05, p = 0.181) | 1.89 (0.54–6.60, p = 0.317) | 2.09 (0.71–6.19, p = 0.181) | 2.05 (0.69–6.06, p = 0.194) | |
| 3 | 3.67 (1.09–12.33, p = 0.036) | 2.01 (0.57–7.08, p = 0.277) | 5.10 (1.80–14.46, p = 0.002) | 4.12 (1.43–11.82, p = 0.009) | |
| Age | Mean (SD) | 1.03 (1.01–1.06, p = 0.011) | 1.03 (1.00–1.06, p = 0.070) | 1.02 (1.00–1.04, p = 0.125) | |
| BMI | Mean (SD) | 1.00 (0.95–1.05, p = 0.981) | 1.00 (0.96–1.05, p = 0.845) | ||
| CEA pre-CRT | Mean (SD) | 1.00 (0.99–1.01, p = 0.640) | 1.00 (1.00–1.01, p = 0.309) | ||
| CEA post-CRT | Mean (SD) | 1.00 (1.00–1.01, p < 0.001) | 1.00 (1.00–1.01, p < 0.001) | 1.01 (1.00–1.01, p < 0.001) | 1.00 (1.00–1.01, p < 0.001) |
| NLR pre-CRT | Mean (SD) | 1.04 (0.90–1.21, p = 0.592) | 0.89 (0.76–1.05, p = 0.169) | ||
| PLR pre-CRT | Mean (SD) | 1.00 (1.00–1.00, p = 0.659) | 1.00 (1.00–1.00, p = 0.365) | ||
| NLR post-CRT | Mean (SD) | 1.06 (1.02–1.10, p = 0.003) | 1.05 (1.01–1.09, p = 0.007) | 1.03 (0.98–1.07, p = 0.246) | |
| PLN post-CRT | Mean (SD) | 1.00 (0.98–1.00, p = 0.674) | 1.00 (1.00–1.00, p = 0.072) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Masri, M.; Safi, Y.; Almasri, M.; Kardan, R.; Mustafa, D.; Alayyan, O.; Kahalah, B.; AlMasri, R. Prognostic Value of Dynamic Changes in Immune-Inflammatory and Tumor Biomarkers Following Chemoradiotherapy in Locally Advanced Rectal Cancer. Cancers 2025, 17, 3383. https://doi.org/10.3390/cancers17203383
Al-Masri M, Safi Y, Almasri M, Kardan R, Mustafa D, Alayyan O, Kahalah B, AlMasri R. Prognostic Value of Dynamic Changes in Immune-Inflammatory and Tumor Biomarkers Following Chemoradiotherapy in Locally Advanced Rectal Cancer. Cancers. 2025; 17(20):3383. https://doi.org/10.3390/cancers17203383
Chicago/Turabian StyleAl-Masri, Mahmoud, Yasmin Safi, Mohammad Almasri, Ramiz Kardan, Daliana Mustafa, Osama Alayyan, Bilal Kahalah, and Rama AlMasri. 2025. "Prognostic Value of Dynamic Changes in Immune-Inflammatory and Tumor Biomarkers Following Chemoradiotherapy in Locally Advanced Rectal Cancer" Cancers 17, no. 20: 3383. https://doi.org/10.3390/cancers17203383
APA StyleAl-Masri, M., Safi, Y., Almasri, M., Kardan, R., Mustafa, D., Alayyan, O., Kahalah, B., & AlMasri, R. (2025). Prognostic Value of Dynamic Changes in Immune-Inflammatory and Tumor Biomarkers Following Chemoradiotherapy in Locally Advanced Rectal Cancer. Cancers, 17(20), 3383. https://doi.org/10.3390/cancers17203383






