Visceral Pleural Invasion as a Determinant of Surgical Strategy in Non–Small Cell Lung Cancer: A Multicenter Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Patient Follow-Up
2.3. Pathological Classification
2.4. Statistical Analysis
3. Result
3.1. Patient Characteristics
3.2. Difference in Histological Subtype and VPI
3.3. Prognostic Factors Related to VPI and Survival Analysis
3.4. VPI and Areas of Lymph Node Metastasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VPI | visceral pleural invasion |
| NSCLC | non-small-cell lung cancer |
| RFS | recurrence-free survival |
| CT | computed tomography |
| FDG | 18F-fluorodeoxyglucose |
| PET/CT | positron emission tomography/computed tomography |
| MRI | magnetic resonance imaging |
| OS | overall survival |
| LUAD | adenocarcinoma |
| AIS | adenocarcinoma in situ |
| MIA | minimally invasive adenocarcinoma |
| Lep | lepidic adenocarcinoma |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Lu, S.; Zhou, Q.; Zhang, L.; Cheng, Y.; Wang, J.; Wang, B.; Hu, C.; Lin, L.; Zhong, W.; et al. Expert consensus on treatment for stage Ⅲ non-small cell lung cancer. Med. Adv. 2023, 1, 3–13. [Google Scholar] [CrossRef]
- Dai, Y.; Tian, X.; Ye, X.; Gong, Y.; Xu, L.; Jiao, L. Role of the TME in immune checkpoint blockade resistance of non-small cell lung cancer. Cancer Drug Resist. 2024, 7, 52. [Google Scholar] [CrossRef]
- Altorki, N.; Wang, X.; Kozono, D.; Watt, C.; Landrenau, R.; Wigle, D.; Port, J.; Jones, D.R.; Conti, M.; Ashrafi, A.S.; et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 388, 489–498. [Google Scholar] [CrossRef]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Aokage, K.; Suzuki, K.; Saji, H.; Wakabayashi, M.; Kataoka, T.; Sekino, Y.; Fukuda, H.; Endo, M.; Hattori, A.; Mimae, T.; et al. Segmentectomy for ground-glass-dominant lung cancer with a tumour diameter of 3 cm or less including ground-glass opacity (JCOG1211): A multicentre, single-arm, confirmatory, phase 3 trial. Lancet Respir. Med. 2023, 11, 540–549. [Google Scholar] [CrossRef]
- Gu, A.; Li, J.; Li, M.Y.; Liu, Y. Patient-derived xenograft model in cancer: Establishment and applications. MedComm 2025, 6, e70059. [Google Scholar] [CrossRef]
- Shimizu, K.; Yoshida, J.; Nagai, K.; Nishimura, M.; Yokose, T.; Ishii, G.; Nishiwaki, Y. Visceral pleural invasion classification in non-small cell lung cancer: A proposal on the basis of outcome assessment. J. Thorac. Cardiovasc. Surg. 2004, 127, 1574–1578. [Google Scholar] [CrossRef]
- Kudo, Y.; Saji, H.; Shimada, Y.; Nomura, M.; Matsubayashi, J.; Nagao, T.; Kakihana, M.; Usuda, J.; Kajiwara, N.; Ohira, T.; et al. Impact of visceral pleural invasion on the survival of patients with non-small cell lung cancer. Lung Cancer 2012, 78, 153–160. [Google Scholar] [CrossRef]
- Tan, K.S.; Reiner, A.; Emoto, K.; Eguchi, T.; Takahashi, Y.; Aly, R.G.; Rekhtman, N.; Adusumilli, P.S.; Travis, W.D. Novel Insights Into the International Association for the Study of Lung Cancer Grading System for Lung Adenocarcinoma. Mod. Pathol. 2024, 37, 100520. [Google Scholar] [CrossRef]
- Fan, J.; Yao, J.; Si, H.; Xie, H.; Ge, T.; Ye, W.; Chen, J.; Yin, Z.; Zhuang, F.; Xu, L.; et al. Frozen sections accurately predict the IASLC proposed grading system and prognosis in patients with invasive lung adenocarcinomas. Lung Cancer 2023, 178, 123–130. [Google Scholar] [CrossRef]
- Kudo, Y.; Nakamura, T.; Matsubayashi, J.; Ichinose, A.; Goto, Y.; Amemiya, R.; Park, J.; Shimada, Y.; Kakihana, M.; Nagao, T.; et al. AI-driven Characterization of Solid Pulmonary Nodules on CT Imaging for Enhanced Malignancy Prediction in Small-sized Lung Adenocarcinoma. Clin. Lung Cancer 2024, 25, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Kudo, Y.; Maehara, S.; Fukuta, K.; Masuno, R.; Park, J.; Ikeda, N. Artificial intelligence-based radiomics for the prediction of nodal metastasis in early-stage lung cancer. Sci. Rep. 2023, 13, 1028. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Nishimura, K.K.; Giroux, D.J.; Detterbeck, F.; Cardillo, G.; Edwards, J.G.; Fong, K.M.; Giuliani, M.; Huang, J.; Kernstine, K.H., Sr.; et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groups in the Forthcoming (Ninth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2024, 19, 1007–1027. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B. The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Rusch, V.W.; Asamura, H.; Watanabe, H.; Giroux, D.J.; Rami-Porta, R.; Goldstraw, P. The IASLC lung cancer staging project: A proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2009, 4, 568–577. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, C.; Lu, Y.; Yu, B.; Lv, F.; Zhu, Z. The predictive and prognostic values of factors associated with visceral pleural involvement in resected lung adenocarcinomas. Onco Targets Ther. 2016, 9, 2337–2348. [Google Scholar] [CrossRef]
- Nitadori, J.I.; Colovos, C.; Kadota, K.; Sima, C.S.; Sarkaria, I.S.; Rizk, N.P.; Rusch, V.W.; Travis, W.D.; Adusumilli, P.S. Visceral pleural invasion does not affect recurrence or overall survival among patients with lung adenocarcinoma ≤ 2 cm: A proposal to reclassify T1 lung adenocarcinoma. Chest 2013, 144, 1622–1631. [Google Scholar] [CrossRef]
- Jeon, H.W.; Kim, Y.D.; Sim, S.B.; Moon, M.H. Comparison of clinical results between high grade patterns in stage I lung adenocarcinoma. Thorac. Cancer 2022, 13, 2473–2479. [Google Scholar] [CrossRef]
- Liang, M.; Tang, W.; Tan, F.; Zeng, H.; Guo, C.; Feng, F.; Wu, N. Preoperative prognostic prediction for stage I lung adenocarcinomas: Impact of the computed tomography features associated with the new histological grading system. Front. Oncol. 2023, 13, 1103269. [Google Scholar] [CrossRef]
- Ohtani-Kim, S.J.; Taki, T.; Tane, K.; Miyoshi, T.; Samejima, J.; Aokage, K.; Nagasaki, Y.; Kojima, M.; Sakashita, S.; Watanabe, R.; et al. Efficacy of Preoperative Biopsy in Predicting the Newly Proposed Histologic Grade of Resected Lung Adenocarcinoma. Mod. Pathol. 2023, 36, 100209. [Google Scholar] [CrossRef]
- Zhu, E.; Xie, H.; Dai, C.; Zhang, L.; Huang, Y.; Dong, Z.; Guo, J.; Su, H.; Ren, Y.; Shi, P.; et al. Intraoperatively measured tumor size and frozen section results should be considered jointly to predict the final pathology for lung adenocarcinoma. Mod. Pathol. 2018, 31, 1391–1399. [Google Scholar] [CrossRef]
- Takizawa, H.; Kondo, K.; Kawakita, N.; Tsuboi, M.; Toba, H.; Kajiura, K.; Kawakami, Y.; Sakiyama, S.; Tangoku, A.; Morishita, A.; et al. Autofluorescence for the diagnosis of visceral pleural invasion in non-small-cell lung cancer. Eur. J. Cardio-Thoracic Surg. 2018, 53, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Ojima, T.; Takaoka, Y.; Sugano, A.; Someya, Y.; Hirabayashi, K.; Homma, T.; Kitamura, N.; Akemoto, Y.; Tanabe, K.; et al. Prediction of visceral pleural invasion of clinical stage I lung adenocarcinoma using thoracoscopic images and deep learning. Surg. Today 2024, 54, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Saito, A.; Horiuchi, T.; Murakami, K.; Kobayashi, M.; Matsubayashi, J.; Nagao, T.; Ohira, T.; Kuroda, M.; Ikeda, N. Preoperative evaluation of visceral pleural invasion in peripheral lung cancer utilizing deep learning technology. Surg. Today 2025, 55, 18–28. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Xiao, X.; Huo, W.; Li, X.; Zhong, B.; Gan, X.; Cao, Q. A deep learning approach for predicting visceral pleural invasion in cT1 lung adenocarcinoma. J. Thorac. Dis. 2024, 16, 5675–5687. [Google Scholar] [CrossRef]
- Wang, Y.; Lyu, D.; Hu, S.; Ma, Y.; Duan, S.; Geng, Y.; Zhou, T.; Tu, W.; Xiao, Y.; Fan, L.; et al. Nomogram using intratumoral and peritumoral radiomics for the preoperative prediction of visceral pleural invasion in clinical stage IA lung adenocarcinoma. J. Cardiothorac. Surg. 2024, 19, 307. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.H.; Zhang, J.M.; Shi, B.; Gao, F.; Zhang, Z.X.; Qian, L.T. The value of CT radiomics features to predict visceral pleural invasion in ≤3 cm peripheral type early non-small cell lung cancer. J. Xray Sci. Technol. 2022, 30, 1115–1126. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, T.; Wang, B.; Satishkumar, B.M.; Ding, L.; Sun, X.; Chen, C. Deep learning radiomics fusion model to predict visceral pleural invasion of clinical stage IA lung adenocarcinoma: A multicenter study. J. Cardiothorac. Surg. 2025, 20, 246. [Google Scholar] [CrossRef] [PubMed]
- Altorki, N.; Wang, X.; Damman, B.; Jones, D.R.; Wigle, D.; Port, J.; Conti, M.; Ashrafi, A.S.; Lieberman, M.; Landreneau, R.; et al. Recurrence of Non-Small Cell Lung Cancer With Visceral Pleural Invasion: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2024, 10, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Adachi, H.; Tsuboi, M.; Nishii, T.; Yamamoto, T.; Nagashima, T.; Ando, K.; Ishikawa, Y.; Woo, T.; Watanabe, K.; Kumakiri, Y.; et al. Influence of visceral pleural invasion on survival in completely resected non-small-cell lung cancer. Eur. J. Cardio-Thoracic Surg. 2015, 48, 691–697; discussion 697. [Google Scholar] [CrossRef] [PubMed]
- Gorai, A.; Sakao, Y.; Kuroda, H.; Uehara, H.; Mun, M.; Ishikawa, Y.; Nakagawa, K.; Masuda, M.; Okumura, S. The clinicopathological features associated with skip N2 metastases in patients with clinical stage IA non-small-cell lung cancer. Eur. J. Cardio-Thoracic Surg. 2015, 47, 653–658. [Google Scholar] [CrossRef] [PubMed]
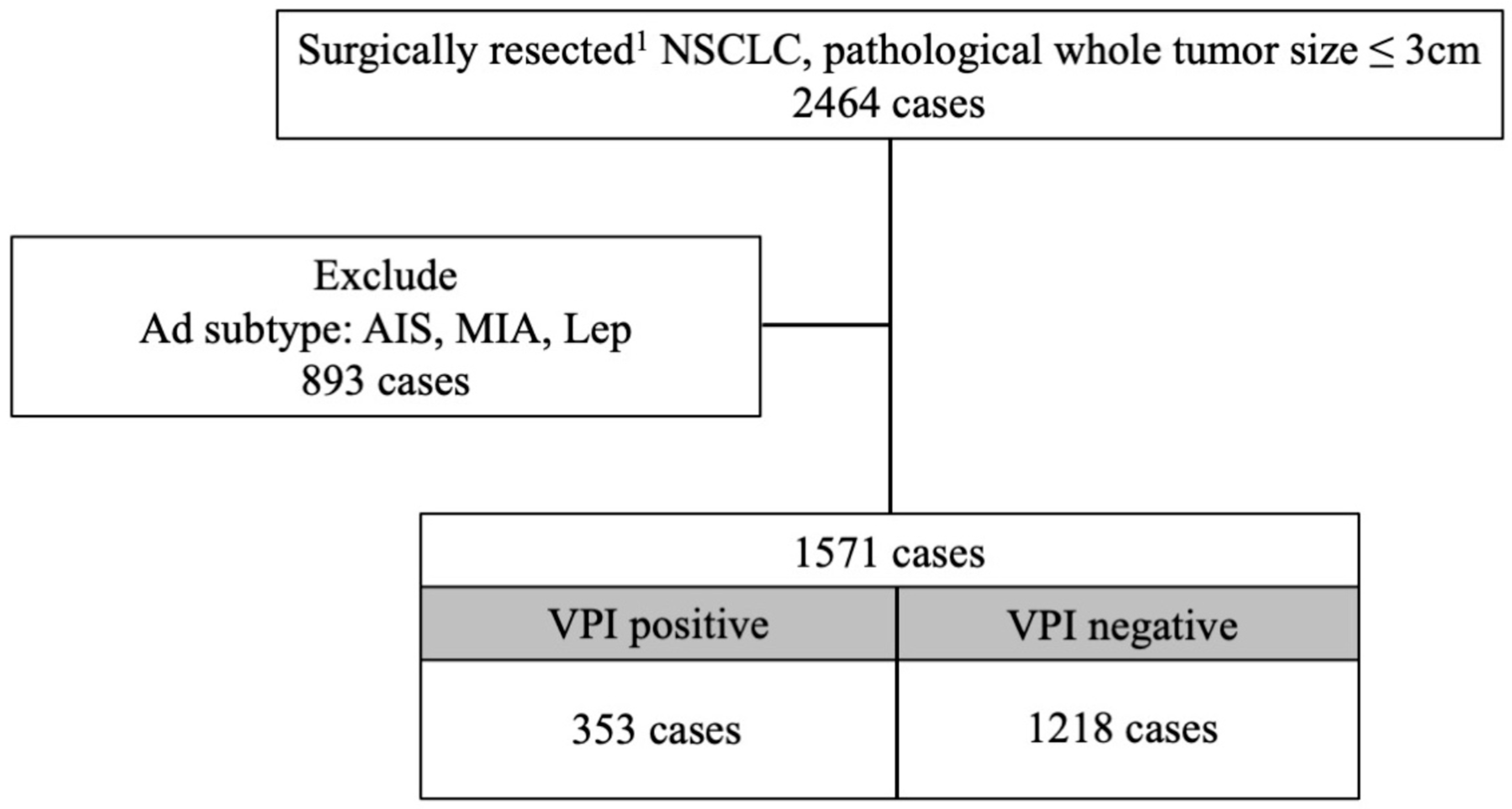
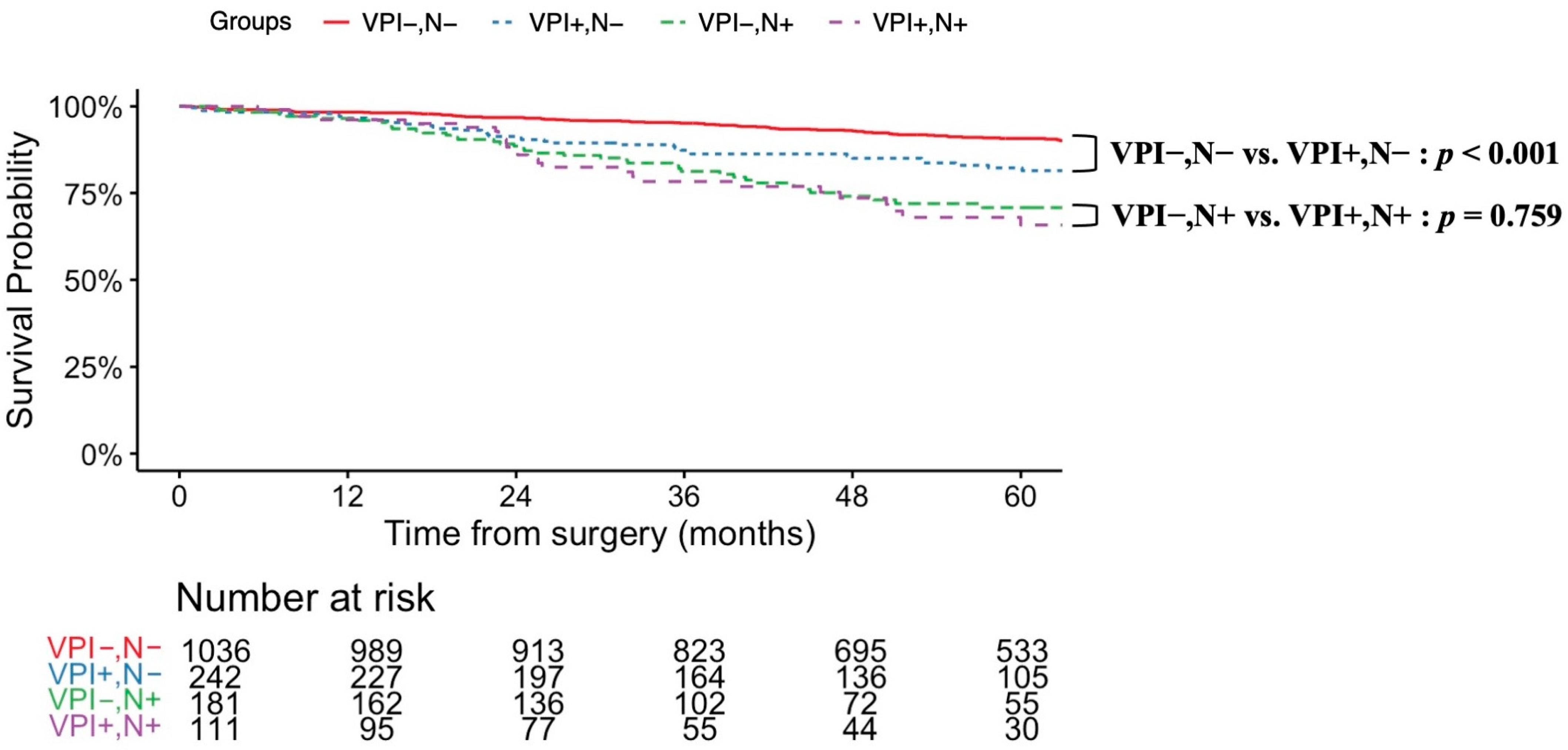
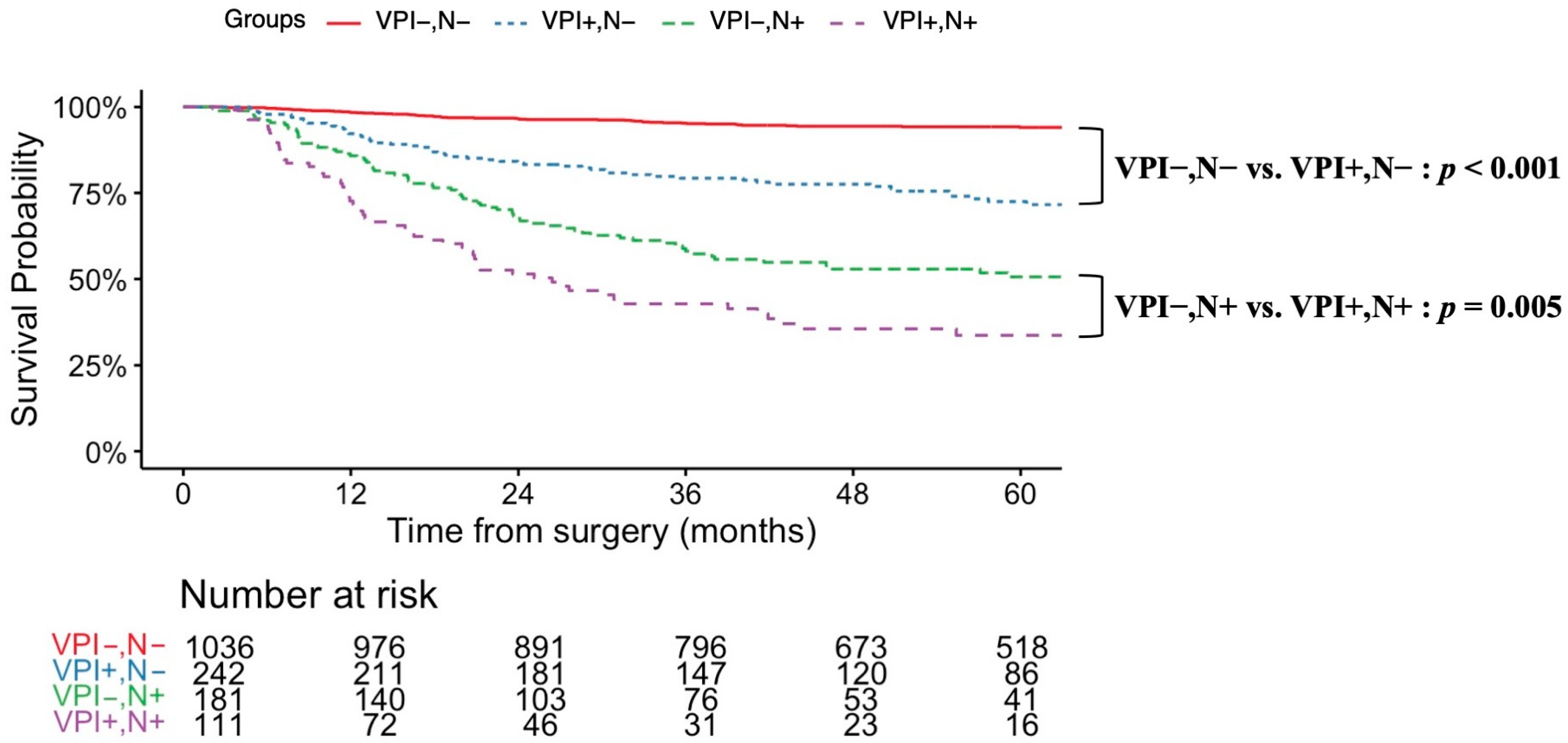
| Variable | Overall, n = 1571 | Non-VPI, n = 1218 | VPI, n = 353 | p-Value (Non-VPI vs. VPI) |
|---|---|---|---|---|
| Age, Median (Minimum, Maximum) | 69 (62, 75) | 69 (63, 75) | 68 (61, 75) | 0.067 |
| Sex, n (%) | <0.001 1 | |||
| Men | 945 (60.2) | 702 (57.6) | 243 (68.8) | |
| Women | 626 (39.8) | 516 (42.4) | 110 (31.2) | |
| Smoker, n (%) | 1025 (65.3) | 766 (63.0) | 259 (73.4) | <0.001 1 |
| CT component of tumor, n (%) | <0.001 1 | |||
| Solid | 1018 (64.8) | 739 (60.7) | 279 (79.0) | |
| Part solid | 496 (31.6) | 429 (35.2) | 67 (19.0) | |
| Pure GGO | 27 (1.7) | 27 (2.2) | 0 (0.0) | |
| Histological type | 0.233 1 | |||
| Adenocarcinoma | 1221 (77.7) | 952 (78.2) | 269 (76.2) | |
| Squamous cell carcinoma | 238 (15.1) | 187 (15.4) | 51 (14.4) | |
| Others | 112 (7.1) | 79 (6.5) | 33 (9.3) | |
| Surgical procedure, n (%) | <0.001 1 | |||
| Segmentectomy | 320 (20.4) | 277 (22.7) | 43 (12.2) | |
| Lobectomy | 1251 (79.6) | 941 (77.3) | 310 (87.8) | |
| Tumor location, n (%) | 0.546 1 | |||
| Left | 627 (39.9) | 491 (40.3) | 136 (38.5) | |
| Right | 944 (60.1) | 727 (59.7) | 217 (61.5) | |
| Lymph node dissection, n (%) | <0.001 1 | |||
| Not conducted | 35 (2.2) | 31 (2.5) | 4 (1.1) | |
| ND1 | 240 (15.3) | 196 (16.1) | 44 (12.5) | |
| ND2a-1 | 1077 (68.6) | 844 (69.3) | 233 (66.0) | |
| ND2a-2 | 219 (13.9) | 147 (12.1) | 72 (20.4) | |
| Median pathological tumor size, (Minimum, Maximum) | 2.0 (0.2, 3.0) | 2.00 (0.2, 3.0) | 2.2 (0.8, 3.0) | <0.001 1 |
| Pathological T factor, 9th edition, n (%) | ||||
| T1 | 1196 (76.1) | 1196 (98.2) | 0 (0.0) | |
| T2 | 312 (19.9) | 12 (1.0) | 300 (85.0) | |
| T3 | 58 (3.7) | 10 (0.8) | 48 (13.6) | |
| T4 | 5 (0.3) | 0 (0.0) | 5 (1.4) | |
| Pathological N factor, 9th edition, n (%) | <0.001 1 | |||
| 0 | 1278 (81.4) | 1036 (85.1) | 242 (68.6) | |
| Lymph node metastasis | 292 (18.6) | 181 (14.9) | 111 (31.4) | |
| N1 | 156 (9.9) | 102 (8.4) | 54 (15.3) | |
| N2a | 102 (6.5) | 64 (5.3) | 38 (10.8) | |
| N2b | 32 (2.0) | 14 (1.1) | 18 (5.1) | |
| NA 2 | 3 (0.2) | 2 (0.2) | 1 (0.3) | |
| Pathological stage, 9th edition, n (%) | <0.001 1 | |||
| 0 | 3 (0.2) | 3 (0.2) | 0 (0.0) | |
| ⅠA1 | 173 (11.0) | 173 (14.2) | 0 (0.0) | |
| ⅠA2 | 595 (37.9) | 595 (48.9) | 0 (0.0) | |
| ⅠA3 | 252 (16.0) | 252 (20.7) | 0 (0.0) | |
| ⅠB | 214 (13.6) | 6 (0.5) | 208 (58.9) | |
| IIA | 97 (6.2) | 97 (8.0) | 0 (0.0) | |
| ⅡB | 147 (9.4) | 70 (5.7) | 77 (21.8) | |
| ⅢA | 67 (4.3) | 20 (1.6) | 47 (13.3) | |
| ⅢB | 19 (1.2) | 0 (0.0) | 19 (5.4) | |
| ⅣA | 1 (0.1) | 0 (0.0) | 1 (0.3) | |
| Unknown | 3 (0.2) | 2 (0.2) | 1 (0.3) | |
| Recurrence, n (%) | 264 (16.8) | 142 (11.7) | 122 (34.6) | <0.001 |
| Variable | Overall, n = 842 | Non-VPI, n = 687 | VPI, n = 155 | p-Value (Non-VPI vs. VPI) |
|---|---|---|---|---|
| Surgical procedure, n (%) | <0.001 1 | |||
| Segmentectomy | 239 (28.4) | 212 (30.9) | 27 (17.4) | |
| Lobectomy | 603 (71.6) | 475 (69.1) | 128 (82.6) | |
| Tumor location, n (%) | 0.617 1 | |||
| Left | 330 (39.2) | 272 (39.6) | 58 (37.4) | |
| Right | 512 (60.8) | 415 (60.4) | 97 (62.6) | |
| Lymph node dissection, n (%) | 0.057 1 | |||
| Not conducted | 24 (2.9) | 22 (3.2) | 2 (1.3) | |
| ND1 | 159 (18.9) | 135 (19.7) | 24 (15.5) | |
| ND2a-1 | 569 (67.6) | 465 (67.7) | 104 (67.1) | |
| ND2a-2 | 90 (10.7) | 65 (9.5) | 25 (16.1) | |
| Median pathological tumor size, (Minimum, Maximum) | 1.5 (0.2, 2.0) | 1.5 (0.2, 2.0) | 1.6 (0.8, 2.0) | 0.084 1 |
| Pathological T factor, 9th edition, n (%) | <0.001 1 | |||
| T1 | 684 (81.2) | 684 (99.6) | 0 (0.0) | |
| T2 | 130 (15.4) | 1 (0.1) | 129 (83.2) | |
| T3 | 26 (3.1) | 2 (0.3) | 24 (15.5) | |
| T4 | 2 (0.2) | 0 (0.0) | 2 (1.3) | |
| Pathological N factor, 9th edition, n (%) | <0.001 1 | |||
| 0 | 727 (86.3) | 609 (88.6) | 118 (76.1) | |
| Lymph node metastasis | 112 (13.3) | 76 (11.1) | 36 (23.2) | |
| N1 | 65 (7.7) | 45 (6.6) | 20 (12.9) | |
| N2a | 39 (4.6) | 28 (4.1) | 11 (7.1) | |
| N2b | 8 (1.0) | 3 (0.4) | 5 (3.2) | |
| NA | 3 (0.4) | 2 (0.3) | 1 (0.6) | |
| Pathological stage, 9th edition, n (%) | <0.001 1 | |||
| 0 | 3 (0.4) | 3 (0.4) | 0 (0.0) | |
| IA1 | 160 (19.0) | 160 (23.3) | 0 (0.0) | |
| IA2 | 441 (52.4) | 441 (64.2) | 0 (0.0) | |
| IA3 | 3 (0.4) | 3 (0.4) | 0 (0.0) | |
| IB | 98 (11.6) | 0 (0.0) | 98 (63.2) | |
| IIA | 45 (5.3) | 45 (6.6) | 0 (0.0) | |
| IIB | 64 (7.6) | 29 (4.2) | 35 (22.6) | |
| IIIA | 20 (2.4) | 4 (0.6) | 16 (10.3) | |
| IIIB | 5 (0.6) | 0 (0.0) | 5 (3.2) | |
| Unknown | 3 (0.4) | 2 (0.3) | 1 (0.6) |
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| OR 1 | 95% CI 1 | p-Value | OR 1 | 95% CI 1 | p-Value | |
| Age: (>70 vs. ≤70) | 0.83 | 0.64–1.08 | 0.20 | 0.85 | 0.65–1.12 | 0.26 |
| Sex: (women vs. men) | 0.67 | 0.51–0.87 | 0.003 | 0.58 | 0.41–0.82 | 0.002 |
| Smoking habit: (smoker vs. non-smoker) | 1.13 | 0.86–1.48 | 0.40 | 0.78 | 0.54–1.12 | 0.17 |
| Tumor location: (Left vs. Right) | 1.24 | 0.96–1.60 | 0.10 | 1.48 | 1.13–1.94 | 0.005 |
| Pathological tumor size: (>2 cm vs. ≤2 cm) | 2.06 | 1.59–2.68 | <0.001 | 1.66 | 1.27–2.19 | <0.001 |
| Histological type: (Ad vs. non-Ad) | 1.08 | 0.80–1.48 | 0.60 | 1.15 | 0.82–1.62 | 0.42 |
| Visceral pleural invasion: (present vs. absent) | 2.63 | 1.99–3.46 | <0.001 | 2.24 | 1.68–2.98 | <0.001 |
| Characteristic | Overall, n = 1562 | Non-VPI, n = 1212 | VPI, n = 350 | p-Value 2 (Non-VPI vs. VPI) |
|---|---|---|---|---|
| Surgical procedure, n (%) | <0.001 2 | |||
| Segmentectomy | 320 (20.5) | 277 (22.9) | 43 (12.3) | |
| Lobectomy | 1242 (79.5) | 935 (77.1) | 307 (87.7) | |
| Lymph node dissection, n (%) | ||||
| Not conducted | 35 (2.2) | 31 (2.6) | 4 (1.1) | |
| ND1 | 240 (15.4) | 196 (16.2) | 44 (12.6) | |
| ND2a-1 | 1069 (68.4) | 839 (69.2) | 230 (65.7) | |
| ND2a-2 | 218 (14.0) | 146 (12.0) | 72 (20.6) | |
| Pathological N factor, 9th edition, n (%) | <0.001 2 | |||
| N1 | 149 (9.5) | 97 (8.0) | 52 (14.9) | |
| N2a | 102 (6.5) | 64 (5.3) | 38 (10.9) | <0.001 2 |
| N2b | 32 (2.0) | 14 (1.2) | 18 (5.1) | <0.001 2 |
| Lymph node metastasis to N2 area, n (%) | 134 (8.6) | 78 (6.4) | 56 (16.0) | |
| Skip N2 metastasis | 38 (2.4) | 24 (2.0) | 14 (4.0) | 0.0031 2 |
| N2 lymph node metastasis area, n (%) | <0.001 2 | |||
| Superior mediastinal nodes | 79 (5.1) | 48 (4.0) | 31 (8.9) | |
| Inferior mediastinal nodes | 38 (2.4) | 24 (2.0) | 14 (4.0) | |
| Both | 16 (1.0) | 6 (0.5) | 10 (2.9) | |
| Any N1 lymph node metastasis, n (%) | 245 (15.7) | 151 (12.5) | 94 (26.9) | <0.001 2 |
| Single N1 station metastasis, n (%) | 153 (9.8) | 97 (8.0) | 56 (16.0) | <0.001 2 |
| Multiple N1 stationmetastasis, n (%) | 92 (5.9) | 54 (4.5) | 38 (10.9) | <0.001 2 |
| N1 lymph node metastasis area, n (%) | ||||
| Hilar zone (#10) | 44 (2.8) | 21 (1.7) | 23 (6.6) | <0.001 2 |
| Interlobar zone (#11) | 68 (4.4) | 39 (3.2) | 29 (8.3) | <0.001 2 |
| Lobar zone (#12u, #12m, #12l) | 157 (10.1) | 98 (8.1) | 59 (16.9) | <0.001 2 |
| Segmental subsegmental zone (#13, 14) | 87 (5.6) | 57 (4.7) | 30 (8.6) | 0.005 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagase, W.; Kudo, Y.; Nagashima, T.; Mimae, T.; Shimada, Y.; Hagiwara, M.; Kakihana, M.; Ohira, T.; Miyata, Y.; Ito, H.; et al. Visceral Pleural Invasion as a Determinant of Surgical Strategy in Non–Small Cell Lung Cancer: A Multicenter Study. Cancers 2025, 17, 3382. https://doi.org/10.3390/cancers17203382
Nagase W, Kudo Y, Nagashima T, Mimae T, Shimada Y, Hagiwara M, Kakihana M, Ohira T, Miyata Y, Ito H, et al. Visceral Pleural Invasion as a Determinant of Surgical Strategy in Non–Small Cell Lung Cancer: A Multicenter Study. Cancers. 2025; 17(20):3382. https://doi.org/10.3390/cancers17203382
Chicago/Turabian StyleNagase, Wakako, Yujin Kudo, Takuya Nagashima, Takahiro Mimae, Yoshihisa Shimada, Masaru Hagiwara, Masatoshi Kakihana, Tatsuo Ohira, Yoshihiro Miyata, Hiroyuki Ito, and et al. 2025. "Visceral Pleural Invasion as a Determinant of Surgical Strategy in Non–Small Cell Lung Cancer: A Multicenter Study" Cancers 17, no. 20: 3382. https://doi.org/10.3390/cancers17203382
APA StyleNagase, W., Kudo, Y., Nagashima, T., Mimae, T., Shimada, Y., Hagiwara, M., Kakihana, M., Ohira, T., Miyata, Y., Ito, H., Okada, M., & Ikeda, N. (2025). Visceral Pleural Invasion as a Determinant of Surgical Strategy in Non–Small Cell Lung Cancer: A Multicenter Study. Cancers, 17(20), 3382. https://doi.org/10.3390/cancers17203382






