Influence of Residual Disease on the Efficacy of PARP Inhibitors in Advanced Epithelial Ovarian Cancer: A Systematic Review and Meta Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
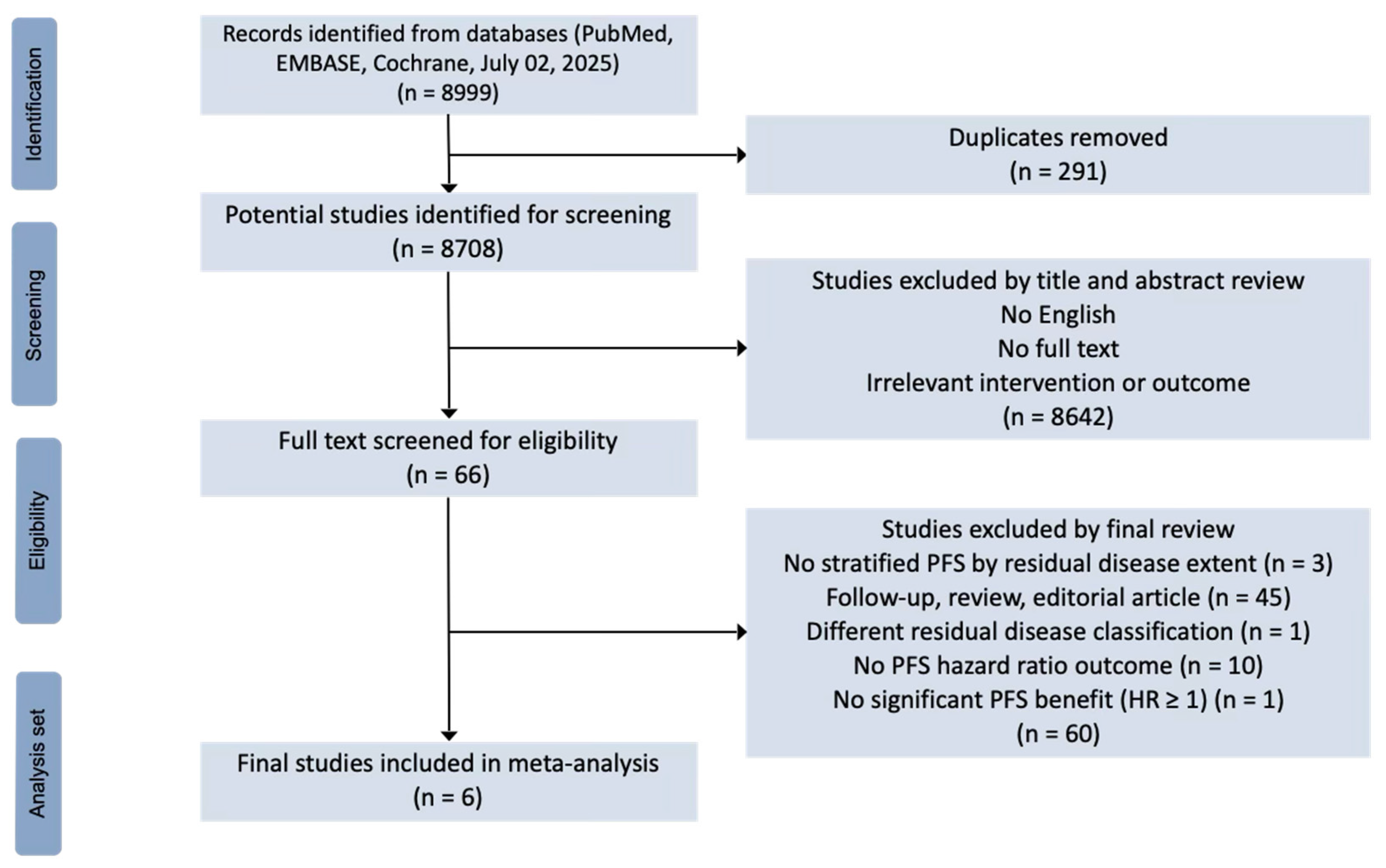
2.1. Search Strategy and Selection Criteria
2.2. Data Analysis
3. Results
4. Discussion
4.1. Summary of Main Results
4.2. Results in the Context of Published Literature
4.3. Strengths and Weaknesses
4.4. Implications for Practice and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Harter, P.; Leary, A.; Lorusso, D.; Miller, R.E.; Pothuri, B.; ESMO Guidelines Committee. Newly diagnosed and relapsed epithelial ovarian cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 833–848. [Google Scholar] [CrossRef] [PubMed]
- du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 2009, 115, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Weroha, S.J.; Cliby, W. Ovarian Cancer: A Review. JAMA 2025, 334, 1278–1291. [Google Scholar] [CrossRef]
- Baert, T.; Ferrero, A.; Sehouli, J.; O’Donnell, D.; González-Martín, A.; Joly, F.; van der Velden, J.; Blecharz, P.; Tan, D.; Querleu, D.; et al. The systemic treatment of recurrent ovarian cancer revisited. Ann. Oncol. 2021, 32, 710–725. [Google Scholar] [CrossRef]
- Dziadkowiec, K.N.; Gąsiorowska, E.; Nowak-Markwitz, E.; Jankowska, A. PARP inhibitors: Review of mechanisms of action and BRCA1/2 mutation targeting. Menopausal Rev. 2016, 4, 215–219. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Fleming, G.F.; Brady, M.F.; Swisher, E.M.; Steffensen, K.D.; Friedlander, M.; Okamoto, A.; Moore, K.N.; Ben-Baruch, N.E.; Werner, T.L.; et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2403–2415. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Parkinson, C.; Lim, M.C.; O’Malley, D.M.; Oaknin, A.; Wilson, M.K.; Coleman, R.L.; Lorusso, D.; Bessette, P.; Ghamande, S.; et al. A Randomized, Phase III Trial to Evaluate Rucaparib Monotherapy as Maintenance Treatment in Patients With Newly Diagnosed Ovarian Cancer (ATHENA–MONO/GOG-3020/ENGOT-ov45). J. Clin. Oncol. 2022, 40, 3952–3964. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- O’Cearbhaill, R.E.; Pérez-Fidalgo, J.-A.; Monk, B.J.; Tusquets, I.; McCormick, C.; Fuentes, J.; Moore, R.G.; Vulsteke, C.; Shahin, M.S.; Forget, F.; et al. Efficacy of niraparib by time of surgery and postoperative residual disease status: A post hoc analysis of patients in the PRIMA/ENGOT-OV26/GOG-3012 study. Gynecol. Oncol. 2022, 166, 36–43. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.; Wang, J.; Wang, L.; Lin, Z.; Wang, X.; Zhu, J.; Kong, B.; Fei, J.; Tang, Y.; et al. Senaparib as first-line maintenance therapy in advanced ovarian cancer: A randomized phase 3 trial. Nat. Med. 2024, 30, 1612–1621. [Google Scholar] [CrossRef]
- Bianchi, T.; Grassi, T.; Bazzurini, L.; Testa, F.; Corti, J.; Cavagna, G.P.; Bombelli, M.; Lissoni, A.A.; Di Martino, G.; Trezzi, G.; et al. The paradigm shift in advanced ovarian cancer: Outcomes of extensive primary cytoreductive surgery. A single-center retrospective analysis. Eur. J. Surg. Oncol. 2024, 50, 108523. [Google Scholar] [CrossRef]
- Korn, R.L.; Crowley, J.J. Overview: Progression-Free Survival as an Endpoint in Clinical Trials with Solid Tumors. Clin. Cancer Res. 2013, 19, 2607–2612. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Banerjee, S.; Moore, K.N.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1721–1731. [Google Scholar] [CrossRef]
- Harter, P.; Mouret-Reynier, M.A.; Pignata, S.; Cropet, C.; González-Martín, A.; Bogner, G.; Fujiwara, K.; Vergote, I.; Colombo, N.; Nøttrup, T.J.; et al. Efficacy of maintenance olaparib plus bevacizumab according to clinical risk in patients with newly diagnosed, advanced ovarian cancer in the phase III PAOLA-1/ENGOT-ov25 trial. Gynecol. Oncol. 2022, 164, 254–2564. [Google Scholar] [CrossRef]
- Sedgwick, P. Meta-analyses: What is heterogeneity? BMJ 2015, 350, h1435. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Lee, C.H.; Cook, S.; Lee, J.S.; Han, B. Comparison of Two Meta-Analysis Methods: Inverse-Variance-Weighted Average and Weighted Sum of Z-Scores. Genom. Inform. 2016, 14, 173. [Google Scholar] [CrossRef]
- Chapter 10: Analysing Data and Undertaking Meta-Analyses|Cochrane [Internet]. 2024. Available online: https://www.cochrane.org/authors/handbooks-and-manuals/handbook/current/chapter-10 (accessed on 24 July 2025).
- Davis, A. How to Interpret the p-Value for the Chi-Square Test for Goodness of Fit [Internet]. 2023. Available online: https://study.com/skill/learn/how-to-interpret-the-p-value-for-the-chi-square-test-for-goodness-of-fit-explanation.html (accessed on 24 July 2025).
- Fang, J.; Zhang, M. What is the minimum number of effect sizes required in meta-regression? An estimation based on statistical power and estimation precision. Adv. Psychol. Sci. 2020, 28, 673. [Google Scholar] [CrossRef]
- Prasad, M. Introduction to the GRADE tool for rating certainty in evidence and recommendations. Clin. Epidemiol. Glob. Health 2024, 25, 101484. [Google Scholar] [CrossRef]
- DiSilvestro, P.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Efficacy of maintenance olaparib for newly diagnosed, advanced ovarian cancer patients with a BRCA mutation: Subgroup analysis findings from the SOLO1 trial. J. Clin. Oncol. 2020, 38, 3528–3537. [Google Scholar] [CrossRef] [PubMed]
| Outcomes | No. of Studies (Participants) | Pooled Effect (95% CI) | Starting Certainty | Downgrading Factors | Certainty of the Evidence (GRADE) |
|---|---|---|---|---|---|
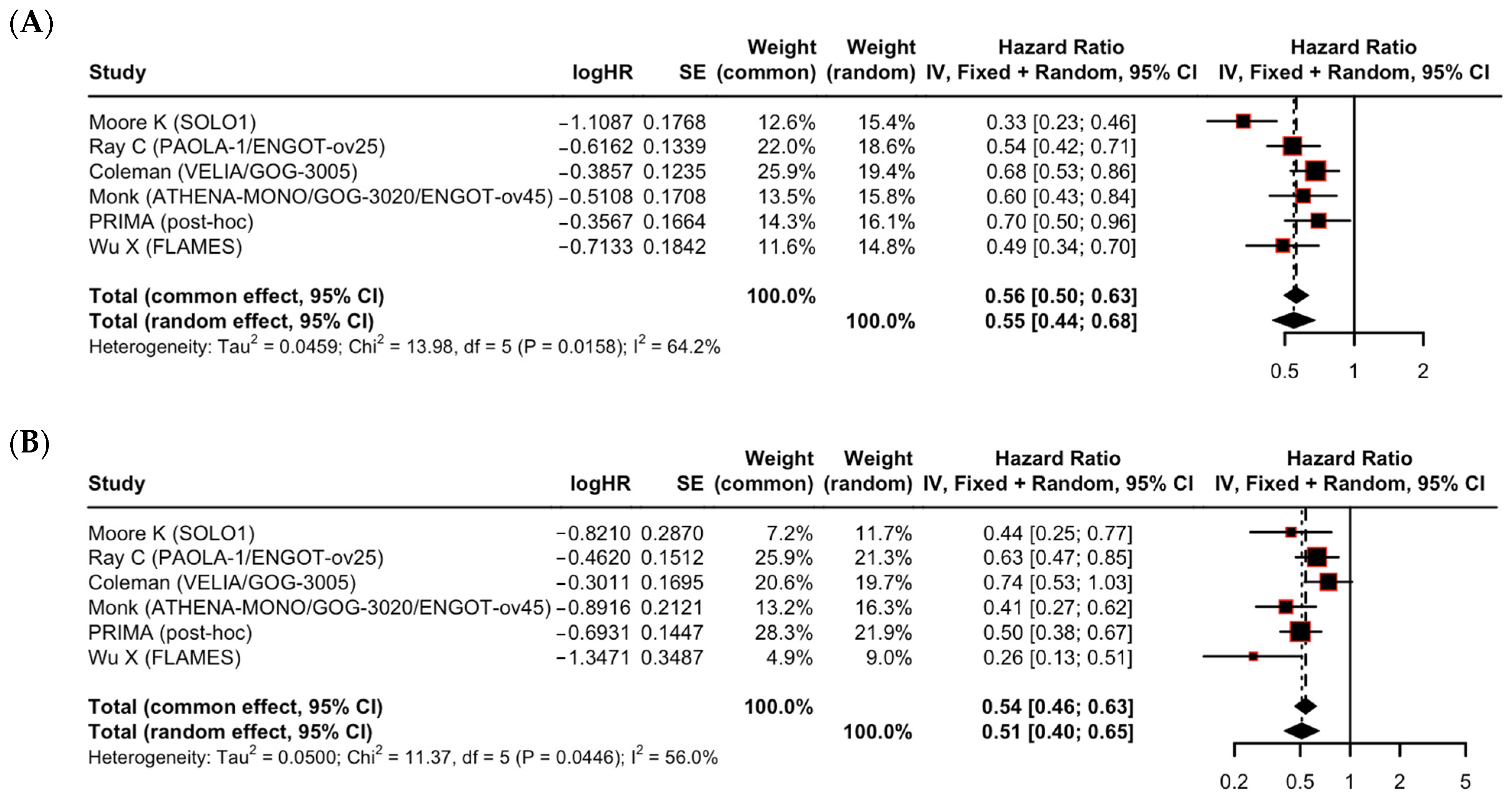
| PFS benefit in patients with no residual disease (R0) | 6 RCTs (n = 2015) | HR 0.55 (95% CI 0.44–0.68) | High | Downgraded one level due to concerns regarding inconsistency (I2 = 64.2%). | Moderate ⊕⊕⊕⊖ |
| PFS benefit in patients with macroscopic residual disease (R1/R2) | 6 RCTs (n = 1093) | HR 0.51 (95% CI 0.40–0.65) | High | Downgraded one level due to concerns regarding inconsistency (I2 = 56.0%). | Moderate ⊕⊕⊕⊖ |
| Difference in effect between R0 and R1/R2 subgroups | N/A | Test for difference: p = 0.66 | High | Downgraded two levels due to concerns regarding inconsistency and concerns regarding imprecision (insufficient power to rule out a clinically important difference) | Low ⊕⊕⊖⊖ |
| (A) | |||||||
| Trial | Study Design | N (Experimental Arm) | N (Control Arm) | Primary Endpoint | Secondary Endpoint | HR for PFS (95% CI) Whole Population | OS HR (95% CI) |
| Moore K (SOLO1), 2018 [10] | Phase III RCT, double-blind, placebo-controlled | 260, Olaparib | 131, Placebo | PFS | OS, PFS2, time until first and second subsequent therapies, QoL | 0.30 (0.23–0.41) | 0.55 (0.40–0.76) |
| Ray C (PAOLA-1/ENGOT-ov25), 2019 [11] | Phase III randomized, double-blind, placebo-controlled | 537, Olaparib + Bev * | 269, Placebo + Bev * | PFS | OS, PFS2, Time until subsequent therapy, QoL | 0.59 (0.49–0.72) | - |
| Coleman (VELIA/GOG-3005), 2019 [12] | Phase III, randomized, double-blind, placebo-controlled trial | 382, Veliparib throughout | 375, Placebo | PFS | OS, NFOSI-18 symptom score | 0.68 (0.56–0.83) | - |
| Monk (ATHENA-MONO/GOG-3020)/ENGOT-ov45), 2022 [13] | Phase III, randomized, double-blind | 427, Rucaparib | 111, Placebo | PFS | OS, ORR, Duration of response, Safety | 0.52 (0.40–0.68) | - |
| O’Cearbhaill (PRIMA post-hoc), 2019 [14,15] | Phase III, double-blind, placebo-controlled | 487, Niraparib | 246, Placebo | PFS | - | 0.62 (0.50–0.76) | 1.01 (0.84–1.23) |
| Wu X (FLAMES), 2024 [16] | Phase III, randomized, double-blind | 271, Senaparib | 133, Placebo | PFS | OS, PFS2, Time until subsequent therapy/death, QoL | 0.43 (0.31–0.58) | - |
| (B) | |||||||
| Trial | HR for PFS (95% CI) Higher Risk | HR for PFS (95% CI) Lower Risk | HR for PFS (95% CI) R0 | HR for PFS (95% CI) R1/R2 | HR for PFS (95% CI) PCS | HR for PFS (95% CI) ICS | |
| Moore K (SOLO1), 2018 [10] | 0.34 (0.24–0.48) | 0.33 (0.20–0.52) | 0.33 (0.23–0.46) | 0.44 (0.25–0.77) | 0.31 (0.21–0.46) | 0.37 (0.24–0.58) | |
| Ray C (PAOLA-1/ENGOT-ov25), 2019 [11] | 0.60 (0.49–0.74) | 0.46 (0.30–0.72) | 0.54 (0.42–0.71) | 0.63 (0.47–0.85) | 0.52 (0.40–0.69) | 0.66 (0.50–0.87) | |
| Coleman (VELIA/GOG-3005), 2019 [12] | - | - | After PDS: 0.77 (0.58–1.04)/ After IDS: 0.52 (0.34–0.81) | After PDS: 0.60 (0.40–0.91)/ After IDS: 1.12 (0.62–2.00) | 0.72 (0.57–0.92) | 0.64 (0.45–0.90) | |
| Monk (ATHENA-MONO/GOG-3020)/ENGOT-ov45), 2022 [13] | - | - | 0.60 (0.43–0.84) | 0.41 (0.27–0.62) | 0.64 (0.43–0.95) | 0.44 (0.31–0.62) | |
| O’Cearbhaill (PRIMA post-hoc), 2019 [14,15] | 0.62 (0.50–0.76) | - | 0.70 (0.50–0.96) | 0.50 (0.38–0.67) | 0.67 (0.47–0.96) | 0.57 (0.44–0.73) | |
| Wu X (FLAMES), 2024 [16] | - | - | 0.49 (0.34–0.70) | 0.26 (0.13–0.51) | - | - | |
| Subgroup Analysis | Subgroups Compared | Number of Studies (k) | Pooled HR (95% CI) per Subgroup | Heterogeneity (I2) | p-Value for Subgroup Difference |
|---|---|---|---|---|---|
| Residual disease | R0 | 6 | 0.55 (0.44–0.68) | 64.2% | 0.66 |
| R1/R2 | 6 | 0.51 (0.40–0.65) | 56.0% | ||
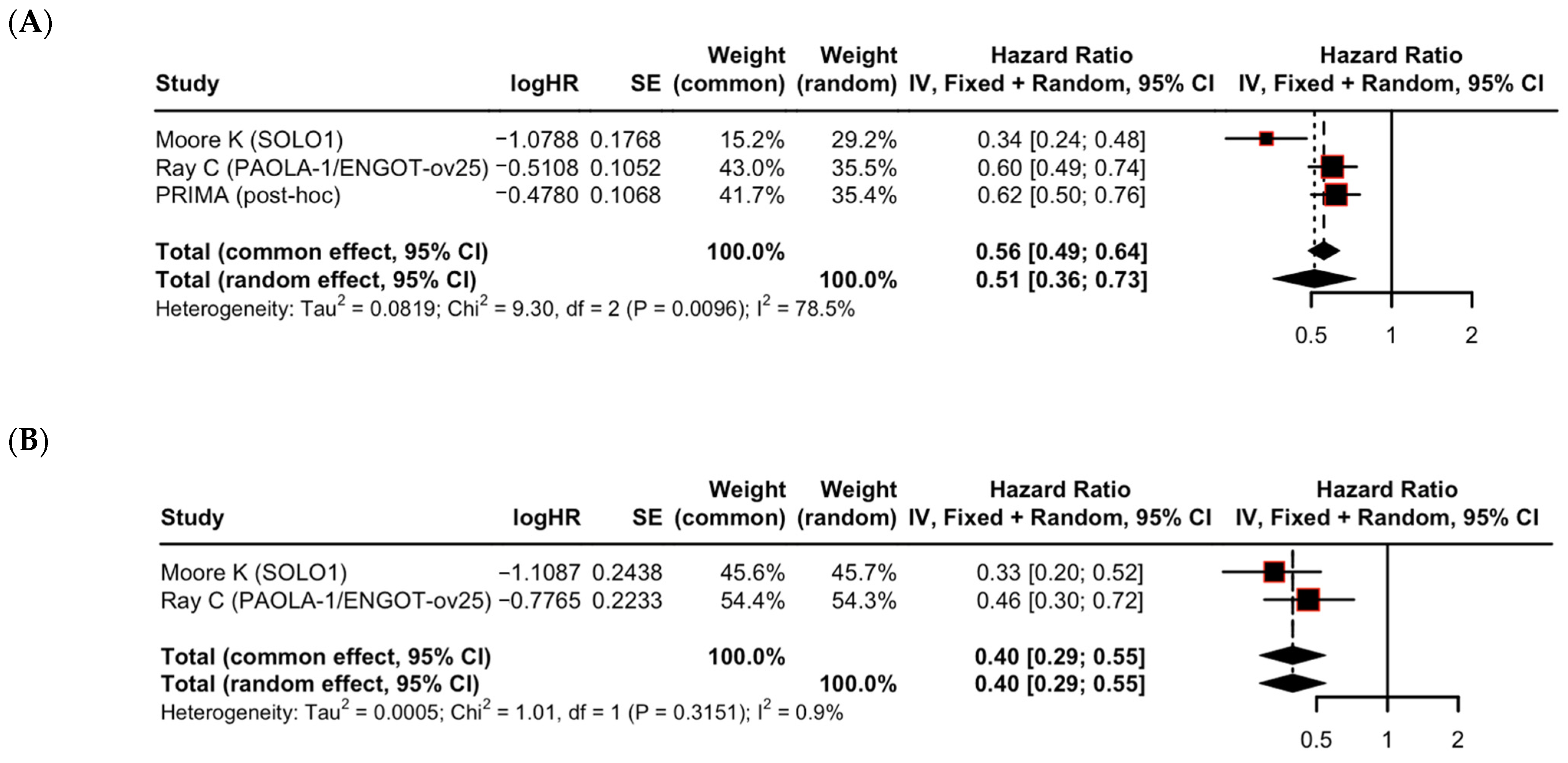
| Clinical risk | Higher risk | 3 | 0.51 (0.36–0.73) | 78.5% | 0.30 |
| Lower risk | 2 | 0.40 (0.29–0.55) | 0.9% | ||
| Timing of surgery | PCS | 5 | 0.56 (0.42–0.74) | 72.3% | 0.88 |
| ICS | 5 | 0.54 (0.45–0.66) | 44.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.; Kim, J.H.; Kim, U.; Ha, H.I.; Park, S.-Y.; Lim, M.C. Influence of Residual Disease on the Efficacy of PARP Inhibitors in Advanced Epithelial Ovarian Cancer: A Systematic Review and Meta Analysis. Cancers 2025, 17, 3365. https://doi.org/10.3390/cancers17203365
Hwang S, Kim JH, Kim U, Ha HI, Park S-Y, Lim MC. Influence of Residual Disease on the Efficacy of PARP Inhibitors in Advanced Epithelial Ovarian Cancer: A Systematic Review and Meta Analysis. Cancers. 2025; 17(20):3365. https://doi.org/10.3390/cancers17203365
Chicago/Turabian StyleHwang, Sekyoung, Ji Hyun Kim, Uisuk Kim, Hyeong In Ha, Sang-Yoon Park, and Myong Cheol Lim. 2025. "Influence of Residual Disease on the Efficacy of PARP Inhibitors in Advanced Epithelial Ovarian Cancer: A Systematic Review and Meta Analysis" Cancers 17, no. 20: 3365. https://doi.org/10.3390/cancers17203365
APA StyleHwang, S., Kim, J. H., Kim, U., Ha, H. I., Park, S.-Y., & Lim, M. C. (2025). Influence of Residual Disease on the Efficacy of PARP Inhibitors in Advanced Epithelial Ovarian Cancer: A Systematic Review and Meta Analysis. Cancers, 17(20), 3365. https://doi.org/10.3390/cancers17203365






