Epidemiological Overview of Colorectal Cancer in Kidney Transplant Recipients: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview
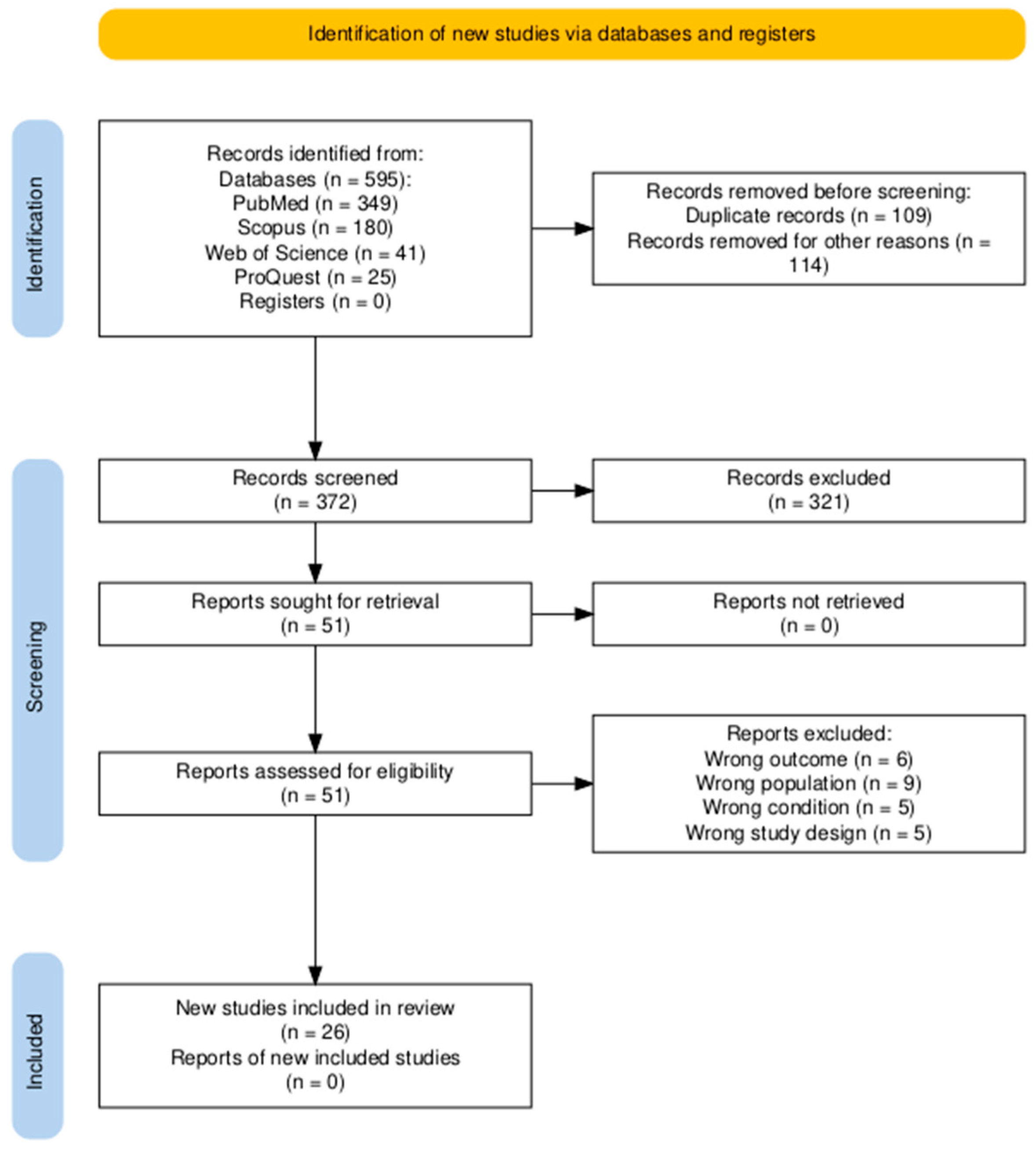
2.2. Search Strategy and Study Selection
2.3. Eligibility Criteria
- Population/Problem (P)
- Exposure/Condition (E)
- Outcomes (O)
- Study designs (S)
- Animal or in vitro studies.
- Reviews, editorials, commentaries, letters to the editor, study protocols without original data, abstracts, case reports, or case series.
- Studies not published in English.
2.4. Screening
2.5. Data Extraction and Synthesis
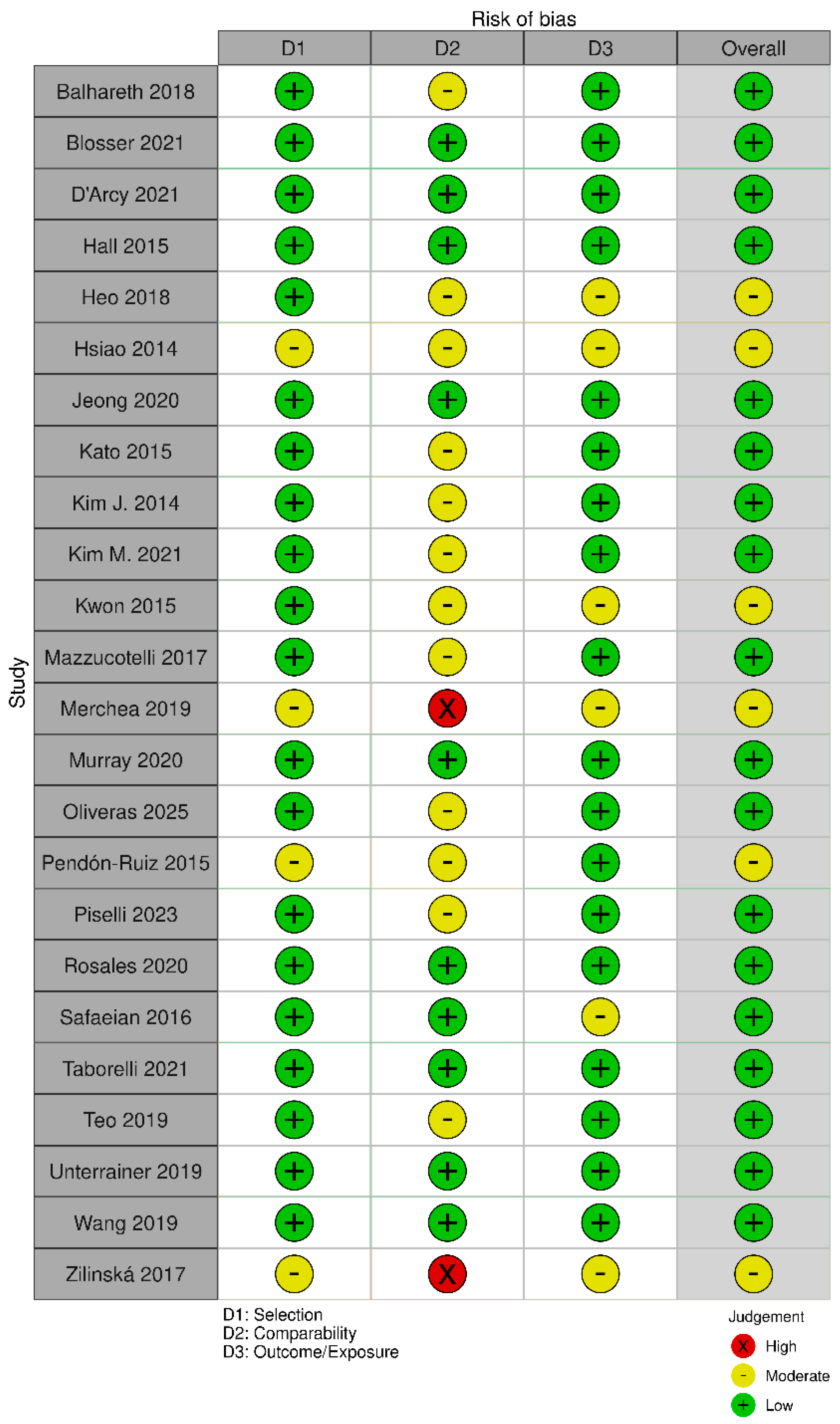
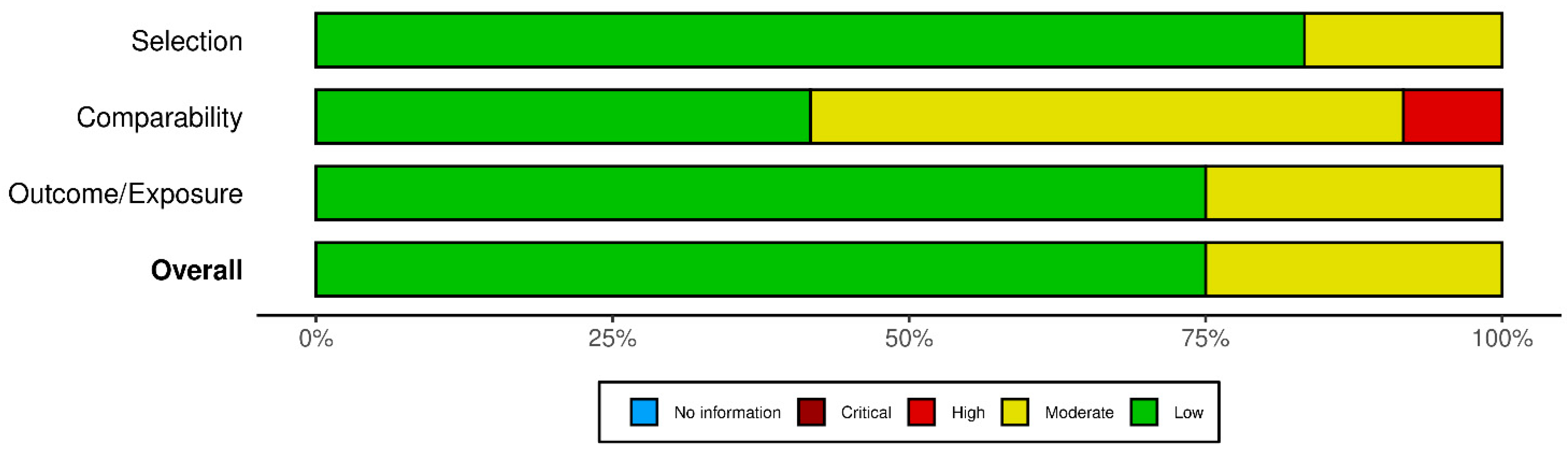
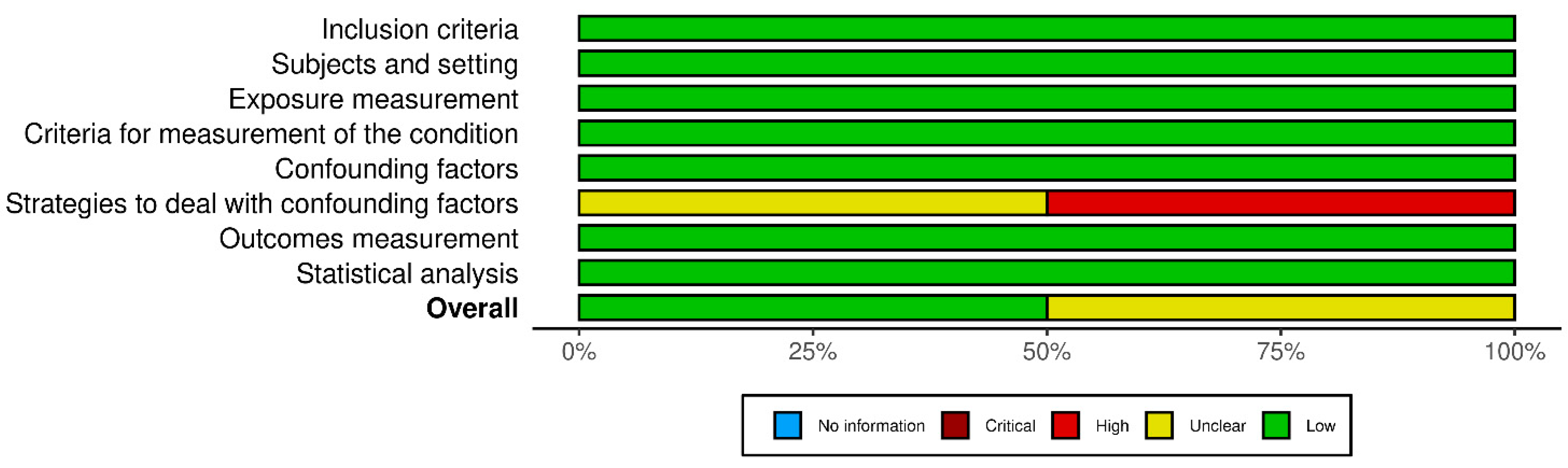
2.6. Risk of Bias Assessment
3. Results
3.1. Overview
3.2. Incidence and Prevalence of Colorectal Cancer
3.3. Risk for Developing Colorectal Cancer
3.4. Prognosis, Mortality, and Five-Year Survival
3.5. Risk Factors for Malignancies
3.6. Immunosuppressive Regimen Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAD | Coronary artery disease |
| CMV | Cytomegalovirus |
| CNI | Calcineurin inhibitor |
| CRC | Colorectal cancer |
| DWFG | Death with functioning graft |
| EAR | Excess absolute risk |
| EBV | Epstein–Barr virus |
| ESRD | End-stage renal disease |
| HPV | Human papillomavirus |
| HR | Hazard ratio |
| IBD | Inflammatory bowel disease |
| IL2-RA | Interleukin-2 receptor antagonist |
| IRR | Incidence rate ratio |
| JCV | JC Polyomavirus |
| KTR | Kidney transplant recipient |
| mTORi | Mammalian target of rapamycine inhibitors |
| NMSC | Non-melanoma skin cancer |
| OR | Odds ratio |
| OS | Overall survival |
| PSC | Primary sclerosing cholangitis |
| PTLD | Post-transplant lymphoproliferative disorder |
| PTM | Post-transplant malignancy |
| rATG | Rabbit Anti-Thymocyte globulin |
| RCC | Renal cell carcinoma |
| RFS | Recurrence-free survival |
| SIR | Standardized incidence ratio |
| SMR | Standardized mortality ratio |
| SOTR | Solid organ transplant recipient |
Appendix A
- PubMed
- Web of Science
- Scopus
- ProQuest
References
- Thongprayoon, C.; Hansrivijit, P.; Leeaphorn, N.; Acharya, P.; Torres-Ortiz, A.; Kaewput, W.; Kovvuru, K.; Kanduri, S.R.; Bathini, T.; Cheungpasitporn, W. Recent Advances and Clinical Outcomes of Kidney Transplantation. J. Clin. Med. 2020, 9, 1193. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, D.A.; Schnitzler, M.A.; Xiao, H.; Irish, W.; Tuttle-Newhall, E.; Chang, S.H.; Kasiske, B.L.; Alhamad, T.; Lentine, K.L. An Economic Assessment of Contemporary Kidney Transplant Practice. Am. J. Transplant. 2018, 18, 1168–1176. [Google Scholar] [CrossRef]
- Legendre, C.; Canaud, G.; Martinez, F. Factors Influencing Long-term Outcome after Kidney Transplantation. Transpl. Int. 2014, 27, 19–27. [Google Scholar] [CrossRef]
- Au, E.; Wong, G.; Chapman, J.R. Cancer in Kidney Transplant Recipients. Nat. Rev. Nephrol. 2018, 14, 508–520. [Google Scholar] [CrossRef]
- Lees, J.S.; Elyan, B.M.P.; Herrmann, S.M.; Lang, N.N.; Jones, R.J.; Mark, P.B. The “other” Big Complication: How Chronic Kidney Disease Impacts on Cancer Risks and Outcomes. Nephrol. Dial. Transplant. 2023, 38, 1071–1079. [Google Scholar] [CrossRef]
- Gallagher, M.P.; Kelly, P.J.; Jardine, M.; Perkovic, V.; Cass, A.; Craig, J.C.; Eris, J.; Webster, A.C. Long-Term Cancer Risk of Immunosuppressive Regimens after Kidney Transplantation. J. Am. Soc. Nephrol. 2010, 21, 852–858. [Google Scholar] [CrossRef]
- Rama, I.; Grinyó, J.M. Malignancy after Renal Transplantation: The Role of Immunosuppression. Nat. Rev. Nephrol. 2010, 6, 511–519. [Google Scholar] [CrossRef]
- Sprangers, B.; Nair, V.; Launay-Vacher, V.; Riella, L.V.; Jhaveri, K.D. Risk Factors Associated with Post-Kidney Transplant Malignancies: An Article from the Cancer-Kidney International Network. Clin. Kidney J. 2018, 11, 315–329. [Google Scholar] [CrossRef]
- Al-Adra, D.; Al-Qaoud, T.; Fowler, K.; Wong, G. De Novo Malignancies after Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2022, 17, 434–443. [Google Scholar] [CrossRef]
- Massicotte-Azarniouch, D.; Noel, J.A.; Knoll, G.A. Epidemiology of Cancer in Kidney Transplant Recipients. Semin. Nephrol. 2024, 44, 151494. [Google Scholar] [CrossRef]
- Saidi, R.F.; Dudrick, P.S.; Goldman, M.H. Colorectal Cancer after Renal Transplantation. Transpl. Proc. 2003, 35, 1410–1412. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.D.; López-Andréu, M.; Rodríguez-Benot, A.; Agüera, M.L.; Del Castillo, D.; Aljama, P. Cancer Incidence and Survival in Kidney Transplant Patients. Transpl. Proc. 2008, 40, 2936–2940. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.A.; Pfeiffer, R.M.; Fraumeni, J.F.; Kasiske, B.L.; Israni, A.K.; Snyder, J.J.; Wolfe, R.A.; Goodrich, N.P.; Bayakly, A.R.; Clarke, C.A.; et al. Spectrum of Cancer Risk among US Solid Organ Transplant Recipients. JAMA 2011, 306, 1891–1901. [Google Scholar] [CrossRef]
- Vajdic, C.M.; McDonald, S.P.; McCredie, M.R.E.; Van Leeuwen, M.T.; Stewart, J.H.; Law, M.; Chapman, J.R.; Webster, A.C.; Kaldor, J.M.; Grulich, A.E. Cancer Incidence before and after Kidney Transplantation. JAMA 2006, 296, 2823–2831. [Google Scholar] [CrossRef]
- Park, J.M.; Choi, M.G.; Kim, S.W.; Chung, I.S.; Yang, C.W.; Kim, Y.S.; Jung, C.K.; Lee, K.Y.; Kang, J.H. Increased Incidence of Colorectal Malignancies in Renal Transplant Recipients: A Case Control Study. Am. J. Transplant. 2010, 10, 2043–2050. [Google Scholar] [CrossRef]
- Veroux, M.; Puliatti, C.; Fiamingo, P.; Cappello, D.; Macarone, M.; Puliatti, D.; Vizcarra, D.; Gagliano, M.; Veroux, P. Early de Novo Malignancies after Kidney Transplantation. Transpl. Proc. 2004, 36, 718–720. [Google Scholar] [CrossRef]
- Collins, M.G.; Teo, E.; Cole, S.R.; Chan, C.Y.; McDonald, S.P.; Russ, G.R.; Young, G.P.; Bampton, P.A.; Coates, P.T. Screening for Colorectal Cancer and Advanced Colorectal Neoplasia in Kidney Transplant Recipients: Cross Sectional Prevalence and Diagnostic Accuracy Study of Faecal Immunochemical Testing for Haemoglobin and Colonoscopy. BMJ 2012, 345, e4657. [Google Scholar] [CrossRef]
- Watt, K.D.; Rolak, S.; Foley, D.P.; Plichta, J.K.; Pruthi, S.; Farr, D.; Zwald, F.O.; Carvajal, R.D.; Dudek, A.Z.; Sanger, C.B.; et al. Cancer Surveillance in Solid Organ Transplant Recipients with a Pretransplant History of Malignancy: Multidisciplinary Collaborative Expert Opinion. Transplantation 2024, 108, 2336–2350. [Google Scholar] [CrossRef] [PubMed]
- Manickavasagar, R.; Thuraisingham, R. Post Renal-Transplant Malignancy Surveillance. Clin. Med. 2020, 20, 142–145. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Tang, S.C.W. An Update on Cancer after Kidney Transplantation. Nephrol. Dial. Transplant. 2019, 34, 914–920. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 9 May 2025).
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic Reviews of Etiology and Risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020; Available online: https://synthesismanual.jbi.global (accessed on 1 May 2025).
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-Bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-Bias Assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Balhareth, A.; Reynolds, I.S.; Solon, J.G.; Harte, E.G.; Boland, F.; O’Sullivan, J.M.; Burke, J.P.; Little, D.; McNamara, D.A. Thirty-Seven-Year Population-Based Study of Colorectal Cancer Rates in Renal Transplant Recipients in Ireland. Transpl. Proc. 2018, 50, 3434–3439. [Google Scholar] [CrossRef]
- Blosser, C.D.; Haber, G.; Engels, E.A. Changes in Cancer Incidence and Outcomes among Kidney Transplant Recipients in the United States over a Thirty-Year Period. Kidney Int. 2021, 99, 1430–1438. [Google Scholar] [CrossRef]
- D’Arcy, M.E.; Castenson, D.; Lynch, C.F.; Kahn, A.R.; Morton, L.M.; Shiels, M.S.; Pfeiffer, R.M.; Engels, E.A. Risk of Rare Cancers among Solid Organ Transplant Recipients. J. Natl. Cancer Inst. 2021, 113, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.C.; Engels, E.A.; Pfeiffer, R.M.; Segev, D.L. Association of Antibody Induction Immunosuppression with Cancer after Kidney Transplantation. Transplantation 2015, 99, 1051–1057. [Google Scholar] [CrossRef]
- Heo, J.; Noh, O.K.; Oh, Y.T.; Chun, M.; Kim, L. Cancer Risk after Renal Transplantation in South Korea: A Nationwide Population-Based Study. BMC Nephrol. 2018, 19, 311. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, F.Y.; Hsu, W.W.Y. Epidemiology of Post-Transplant Malignancy in Asian Renal Transplant Recipients: A Population-Based Study. Int. Urol. Nephrol. 2014, 46, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Lee, H.S.; Kong, S.G.; Kim, D.J.; Lee, S.; Park, M.J.; Song, W.; Rim, J.H.; Kim, H.J. Incidence of Malignancy and Related Mortality after Kidney Transplantation: A Nationwide, Population-Based Cohort Study in Korea. Sci. Rep. 2020, 10, 21398. [Google Scholar] [CrossRef]
- Kato, T.; Kakuta, Y.; Abe, T.; Yamanaka, K.; Imamura, R.; Okumi, M.; Ichimaru, N.; Takahara, S.; Nonomura, N. The Benefits of Cancer Screening in Kidney Transplant Recipients: A Single-Center Experience. Cancer Med. 2016, 5, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.O.; Han, D.J.; Park, S.K. Post-Transplant Malignancy: A Burdensome Complication in Renal Allograft Recipients in Korea. Clin. Transpl. 2014, 28, 434–442. [Google Scholar] [CrossRef]
- Kim, M.; Kim, C.W.; Hwang, S.; Kim, Y.H.; Lee, J.L.; Yoon, Y.S.; Park, I.J.; Lim, S.B.; Yu, C.S.; Kim, J.C.; et al. Characteristics and Prognosis of Colorectal Cancer after Liver or Kidney Transplantation. World J. Surg. 2021, 45, 3206–3213. [Google Scholar] [CrossRef]
- Kwon, J.H.; Koh, S.J.; Kim, J.Y.; Kim, J.W.; Lee, K.L.; Kim, B.G.; Im, J.P.; Kim, J.S. Prevalence of Advanced Colorectal Neoplasm After Kidney Transplantation: Surveillance Based on the Results of Screening Colonoscopy. Dig. Dis. Sci. 2015, 60, 1761–1769. [Google Scholar] [CrossRef]
- Mazzucotelli, V.; Piselli, P.; Verdirosi, D.; Cimaglia, C.; Cancarini, G.; Serraino, D.; Sandrini, S. De Novo Cancer in Patients on Dialysis and after Renal Transplantation: North-Western Italy, 1997–2012. J. Nephrol. 2017, 30, 851–857. [Google Scholar] [CrossRef]
- Merchea, A.; Shahjehan, F.; Croome, K.P.; Cochuyt, J.J.; Li, Z.; Colibaseanu, D.T.; Kasi, P.M. Colorectal Cancer Characteristics and Outcomes after Solid Organ Transplantation. J. Oncol. 2019, 2019, 5796108. [Google Scholar] [CrossRef]
- Murray, S.L.; O’Leary, E.; de Bhailís, Á.M.; Deady, S.; Daly, F.E.; O’Kelly, P.; Williams, Y.; O’Neill, J.P.; Sexton, D.J.; Conlon, P.J. Cancer Survival in Kidney Transplant Recipients in Ireland. Nephrol. Dial. Transplant. 2020, 35, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Oliveras, L.; Pareja, L.; Ribes, J.; Comas, J.; Couceiro, C.; Favà, À.; Codina, S.; Coloma, A.; Manonelles, A.; Lloberas, N.; et al. Cancer Risks in People on Dialysis and Kidney Transplant Recipients: A Catalan Cohort Study, 2003–2021. Clin. Kidney. J. 2025, 18, sfaf077. [Google Scholar] [CrossRef]
- Pendón-Ruiz De Mier, V.; Navarro Cabello, M.D.; Martínez Vaquera, S.; Lopez-Andreu, M.; Aguera Morales, M.L.; Rodriguez-Benot, A.; Aljama Garcia, P. Incidence and Long-Term Prognosis of Cancer after Kidney Transplantation. Transplant. Proc. 2015, 47, 2618–2621. [Google Scholar] [CrossRef] [PubMed]
- Piselli, P.; Serraino, D.; Cimaglia, C.; Furian, L.; Biancone, L.; Busnach, G.; Bossini, N.; Todeschini, P.; Iaria, M.; Citterio, F.; et al. Variation in Post-Transplant Cancer Incidence among Italian Kidney Transplant Recipients over a 25-Year Period. Cancers 2023, 15, 1347. [Google Scholar] [CrossRef]
- Privitera, F.; Gioco, R.; Civit, A.I.; Corona, D.; Cremona, S.; Puzzo, L.; Costa, S.; Trama, G.; Mauceri, F.; Cardella, A.; et al. Colorectal Cancer after Kidney Transplantation: A Screening Colonoscopy Case-Control Study. Biomedicines 2021, 9, 937. [Google Scholar] [CrossRef]
- Pyrża, M.; Małyszko, J.; Głogowski, T.; Wieliczko, M.; Żebrowski, P.; Małyszko, J. Kidney Transplant Recipients Have Higher Malignancy Prevalence Than Hemodialyzed Patients. Transpl. Proc. 2022, 54, 972–975. [Google Scholar] [CrossRef]
- Rosales, B.M.; De La Mata, N.; Vajdic, C.M.; Kelly, P.J.; Wyburn, K.; Webster, A.C. Cancer Mortality in Kidney Transplant Recipients: An Australian and New Zealand Population-Based Cohort Study, 1980–2013. Int. J. Cancer 2020, 146, 2703–2711. [Google Scholar] [CrossRef]
- Safaeian, M.; Robbins, H.A.; Berndt, S.I.; Lynch, C.F.; Fraumeni, J.F.; Engels, E.A. Risk of Colorectal Cancer after Solid Organ Transplantation in the United States. Am. J. Transplant. 2016, 16, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Taborelli, M.; Serraino, D.; Cimaglia, C.; Furian, L.; Biancone, L.; Busnach, G.; Todeschini, P.; Bossini, N.; Iaria, M.; Campise, M.R.; et al. The Impact of Cancer on the Risk of Death with a Functioning Graft of Italian Kidney Transplant Recipients. Am. J. Transplant. 2022, 22, 588–598. [Google Scholar] [CrossRef]
- Teo, S.H.; Lee, K.G.; Lim, G.H.; Koo, S.X.; Ramirez, M.E.; Chow, K.Y.; Kee, T. Incidence, Risk Factors and Outcomes of Malignancies after Kidney Transplantation in Singapore: A 12-Year Experience. Singap. Med. J. 2019, 60, 253–259. [Google Scholar] [CrossRef]
- Unterrainer, C.; Opelz, G.; Döhler, B.; Süsal, C. Pretransplant Cancer in Kidney Recipients in Relation to Recurrent and de Novo Cancer Incidence Posttransplantation and Implications for Graft and Patient Survival. Transplantation 2019, 103, 581–587. [Google Scholar] [CrossRef]
- Wang, H.E.; Liao, Y.C.; Hu, J.M.; Wu, W.C.; Chou, W.Y.; Chen, Y.C.; Chou, Y.C.; Hung, C.F.; Tian, Y.F.; You, S.L.; et al. Correlation between Kidney Transplantation and Colorectal Cancer in Hemodialysis Patients: A Nationwide, Retrospective, Population-Based Cohort Study. BMC Cancer 2019, 19, 1120. [Google Scholar] [CrossRef] [PubMed]
- Zilinska, Z.; Sersenova, M.; Chrastina, M.; Breza, J.; Bena, L.; Baltesova, T.; Jurcina, A.; Roland, R.; Lackova, E.; Cellar, M.; et al. Occurrence of Malignancies after Kidney Transplantation in Adults: Slovak Multicenter Experience. Neoplasma 2017, 64, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Komaki, Y.; Komaki, F.; Micic, D.; Ido, A.; Sakuraba, A. Risk of Colorectal Cancer in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2018, 52, 796–804. [Google Scholar] [CrossRef]
- Au, E.H.; Chapman, J.R.; Craig, J.C.; Lim, W.H.; Teixeira-Pinto, A.; Ullah, S.; McDonald, S.; Wong, G. Overall and Site-Specific Cancer Mortality in Patients on Dialysis and after Kidney Transplant. J. Am. Soc. Nephrol. 2019, 30, 471–480. [Google Scholar] [CrossRef]
- Meguid, R.A.; Slidell, M.B.; Wolfgang, C.L.; Chang, D.C.; Ahuja, N. Is There a Difference in Survival between Right- versus Left-Sided Colon Cancers? Ann. Surg. Oncol. 2008, 15, 2388–2394. [Google Scholar] [CrossRef]
- Kiberd, B.A.; Rose, C.; Gill, J.S. Cancer Mortality in Kidney Transplantation. Am. J. Transplant. 2009, 9, 1868–1875. [Google Scholar] [CrossRef]
- Piselli, P.; Serraino, D.; Segoloni, G.P.; Sandrini, S.; Piredda, G.B.; Scolari, M.P.; Rigotti, P.; Busnach, G.; Messa, P.; Donati, D.; et al. Risk of de Novo Cancers after Transplantation: Results from a Cohort of 7217 Kidney Transplant Recipients, Italy 1997–2009. Eur. J. Cancer 2013, 49, 336–344. [Google Scholar] [CrossRef]
- Merchea, A.; Abdelsattar, Z.M.; Taner, T.; Dean, P.G.; Colibaseanu, D.T.; Larson, D.W.; Dozois, E.J. Outcomes of Colorectal Cancer Arising in Solid Organ Transplant Recipients. J. Gastrointest. Surg. 2014, 18, 599–604. [Google Scholar] [CrossRef]
- Collins, M.G.; Bampton, P.A.; Teo, E.; Chan, C.; Cole, S.R.; Young, G.P.; Russ, G.R.; McDonald, S.P.; Coates, P.T. Colorectal Cancer and Advanced Colorectal Neoplasia in Kidney Transplant Recipients: A Prospective Screening Study Comparing Faecal Immunochemical Testing for Occult Blood with Colonoscopy. Transplantation 2010, 90, 259. [Google Scholar] [CrossRef]
- Rostami, Z.; Einollahi, B.; Lessan-Pezeshki, M.; Nourbala, M.H.; Nemati, E.; Pourfarziani, V.; Shahbazian, H.; Khosravi, M.; Jalalzadeh, M. Old Male Living Renal Transplant Recipients More Likely to Be at Risk for Colorectal Cancer. Transpl. Proc. 2011, 43, 588–589. [Google Scholar] [CrossRef]
- Angriman, I.; Furian, L.; Scarpa, M.; Fassan, M.; Morgan, S.; Porzionato, A.; Kotsafti, A.; Saadeh, L.; Silvestre, C.; De Caro, R.; et al. Effects of Immune Suppression for Transplantation on Inflammatory Colorectal Cancer Progression. Oncogenesis 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Sapir-Pichhadze, R.; Laprise, C.; Beauchamp, M.E.; Kaouache, M.; Zhang, X.; Della Vecchia, A.; Azoulay, L.; Franco, E.L.; Abrahamowicz, M.; Nicolau, B. Immunosuppression and Cancer Risk in Kidney Transplant Recipients: A Retrospective Cohort Study. Int. J. Cancer 2024, 154, 2043–2053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mizuuchi, Y.; Ohuchida, K.; Hisano, K.; Shimada, Y.; Katayama, N.; Tsutsumi, C.; Tan, B.C.; Nagayoshi, K.; Tamura, K.; et al. Exploring the Tumor Microenvironment of Colorectal Cancer Patients Post Renal Transplantation by Single-Cell Analysis. Cancer Sci. 2025, 116, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.; Weinstein, T.; Korzets, A.; Chagnac, A.; Ori, Y.; Zevin, D.; Malachi, T.; Gafter, U. Effect of Cyclosporin A on DNA Repair and Cancer Incidence in Kidney Transplant Recipients. J. Lab. Clin. Med. 2001, 137, 14–20. [Google Scholar] [CrossRef]
- Fu, Y.; Liao, C.; Cui, K.; Liu, X.; Fang, W. Antitumor Pharmacotherapy of Colorectal Cancer in Kidney Transplant Recipients. Ther. Adv. Med. Oncol. 2019, 11, 1758835919876196. [Google Scholar] [CrossRef]
- Juul, F.E.; Cross, A.J.; Schoen, R.E.; Senore, C.; Pinsky, P.F.; Miller, E.A.; Segnan, N.; Wooldrage, K.; Wieszczy-Szczepanik, P.; Armaroli, P.; et al. Effectiveness of Colonoscopy Screening vs Sigmoidoscopy Screening in Colorectal Cancer. JAMA Netw. Open 2024, 7, E240007. [Google Scholar] [CrossRef]
- Buskermolen, M.; Cenin, D.R.; Helsingen, L.M.; Guyatt, G.; Vandvik, P.O.; Haug, U.; Bretthauer, M.; Lansdorp-Vogelaar, I. Colorectal Cancer Screening with Faecal Immunochemical Testing, Sigmoidoscopy or Colonoscopy: A Microsimulation Modelling Study. BMJ 2019, 367, l5383. [Google Scholar] [CrossRef]
- Webster, A.C.; Craig, J.C.; Simpson, J.M.; Jones, M.P.; Chapman, J.R. Identifying High Risk Groups and Quantifying Absolute Risk of Cancer after Kidney Transplantation: A Cohort Study of 15 183 Recipients. Am. J. Transplant. 2007, 7, 2140–2151. [Google Scholar] [CrossRef]
- Wong, G.; Chapman, J.R.; Craig, J.C. Cancer Screening in Renal Transplant Recipients: What Is the Evidence? Clin. J. Am. Soc. Nephrol. 2008, 3, S87–S100. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ju, M.K.; Kim, M.S.; Kim, N.K.; Sohn, S.K.; Kim, S.I.; Kim, Y.S. Clinical Characteristics and Treatment Outcomes of Colorectal Cancer in Renal Transplant Recipients in Korea. Yonsei Med. J. 2011, 52, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.E.; Leverson, G.E.; Pirsch, J.D.; Heise, C.P. A 30-Year Analysis of Colorectal Adenocarcinoma in Transplant Recipients and Proposal for Altered Screening. J. Gastrointest. Surg. 2007, 11, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Levin, T.R.; Lieberman, D.; Robertson, D.J. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017, 153, 307–323. [Google Scholar] [CrossRef]
- Kobiela, J.; Dobrzycka, M.; Danielewicz, R.; Jończyk, J.; Łachiński, A.J.; Śledziński, Z.; Dębska-Ślizień, A. Colonoscopy as Part of Pre-Transplant Work-Up in Successful Kidney Transplant Candidates: Single-Center Experience and Review of Literature. Ann. Transpl. 2018, 23, 782–788. [Google Scholar] [CrossRef]
- Granata, R.; Zanghì, A.; Scribano, M.; Riccioli, G.; Privitera, F.; La Vignera, S.; Condorelli, R.A.; Leonforte, F.; Mistretta, A.; Calogero, A.E.; et al. Incidental Parathyroidectomy After Thyroid Surgery: A Single-Center Study. Biomedicines 2024, 12, 2372. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Country | Study Design | Sample Size and Characteristics | Immunosuppressive Regimens and Dosages | Key Findings |
|---|---|---|---|---|---|
| Balhareth et al. (2018) [26] | Ireland | Retrospective cohort (1980–2017) | 4731 KTRs; 33 developed CRC (20 male, 13 female); mean age at transplantation 51.5 years; mean time to CRC diagnosis 10.9 years | Immunosuppressive details not specified | Incidence: 697.5/100,000; CRC diagnosed 33/4731 (0.6975%); 60.6% were in right colon |
| Blosser et al. (2021) [27] | United States | Retrospective cohort (1987–2016) | 101,014 KTRs (60.2% male, 39.8% female); cancer outcomes evaluated over 3 decades with 351,127 person-years of follow-up | Increasing use of polyclonal antibodies, tacrolimus, mycophenolate, IL-2R inhibitors; reduced maintenance corticosteroids over time | No significant trend changes in CRC incidence; IRR and SIR for CRC declined over time but not significant in adjusted analyses |
| D’Arcy et al. (2021) [28] | United States | Retrospective cohort (1987–2014) | 262,455 SOTRs (61.9% KTRs); median age 48 years; median follow-up 139,3047 person-years | 48% received induction therapy, details unspecified | SIR for signet ring cell adenocarcinoma of colon: 4.45 (95% CI: 3.02–6.31) |
| Hall et al. (2015) [29] | United States | Retrospective cohort (1987–2009) | 111,857 KTRs; median follow-up 3.5 years | Muromonab-CD3, alemtuzumab, polyclonal antibodies, IL2R antagonists | Alemtuzumab associated with increased CRC risk (aIRR = 2.46); other regimens not associated with CRC significantly |
| Heo et al. (2018) [30] | South Korea | Retrospective cohort (2010–2014) | 1343 KTRs (64.9% male, 35.1% female); mean age 48.5; 7.7% developed malignancy | Azathioprine, cyclosporine, prednisolone, mycophenolate mofetil, tacrolimus, and sirolimus | CRC incidence data not directly provided: colon (n = 1), rectum (n = 2). Non-statistically significant SIR for colon and rectum malignancies |
| Hsiao et al. (2014) [31] | Taiwan | Retrospective cohort (2000–2010) | 642 KTRs; mean age 54.5 years (with cancer) or 42.4 years (without cancer); ~50% male and ~50% female; median follow-up 46.2 months; 8.4% developed cancers | Cyclosporine, Tacrolimus, Mycophenolate | CRC accounted for 5.6% of cancers (3/54 cases), incidence not reported in absolute terms |
| Jeong et al. (2020) [32] | South Korea | Retrospective cohort (2003–2016) | 9915 KTRs (60.2% male and 39.8% female); median follow-up 4.87 years; median age at diagnosis 52.0 years; 6.0% PTMs | Use of basiliximab and anti-thymocyte globulin for induction; CNIs, mycophenolate, steroids for maintenance | CRC was 11.2% of post-transplant cancers (67/598); overall SIR for CRC = 3.6; SIR in male = 2.3; SIR in female = 6.0; SIR in <40y = 40.0; cancer-specific mortality for CRC = 7.9% |
| Kato et al. (2015) [33] | Japan | Retrospective cohort (1972–2013) | 750 KTRs (454 male, 256 female); mean age at transplantation 38.9 years; 77 PTMs (10.3%); mean interval to cancer 134.5 months | CNIs, mycophenolate, prednisolone, antilymphocyte globulin or basiliximab for induction | CRC detected in 6.5% (5/77 cases, 4 in males and 1 in females), difficult to detect via routine screening methods |
| Kim J. et al. (2014) [34] | South Korea | Retrospective cohort (1989–2009) | 2365 KTRs (61% male, 39% female); mean age at transplantation 39.4 years; 140 cancers (5.7%); mean follow-up 9.8 years | Predominantly basiliximab, daclizumab, OKT3, or antithymocyte globulin for induction; calcineurin inhibitors, corticosteroids, and either mycophenolic acid or azathioprine for maintenance | CRC accounted for ~6.4% of cancers (9/140, 4 in males, 5 in females); overall SIR of CRC = 1.0 (95% CI: 0.5–1.9), SIR in males = 0.6 (95% CI: 0.2–1.6), SIR in females (95% CI: 0.5–4.2) |
| Kim M. et al. (2021) [35] | South Korea | Retrospective cohort (2005–2016) | 4264 KTRs; 66 de novo CRC; median follow-up 5.8 years; mean age at transplantation: 44.1 years; compared to matched sporadic CRC patients | CNIs, mycophenolate, and prednisolone | SIR for de novo CRC: 1.67 (95% CI: 0.98–2.64) in men, 2.54 (95% CI: 1.21–4.68) in women in KT recipients; survival like sporadic CRC; more colon than rectal cancer (p = 0.041) |
| Kwon et al. (2015) [36] | South Korea | Case–control (1996–2008) | 248 KTRs with colonoscopy (155 male, 93 female); mean age at transplantation 52.6; mean follow-up 67.7 months; 900 age/sex-matched controls | Cyclosporine- or tacrolimus-based triple therapy, mycophenolate, azathioprine, mTORi | Advanced colonic neoplasms 8.1% vs. 3.7% in controls; CRC prevalence 1.6% (4/248); OR for advanced neoplasms 2.305 (95% CI: 1.29–4.09); stronger in >50y (OR 5.37) |
| Mazzucotelli et al. (2017) [37] | Italy | Retrospective cohort (1997–2012) | 735 KTRs; follow-up 4858 patients-years | CNIs; monoclonal or polyclonal antibodies against T-cell antigens combined with conventional immunosuppressive drugs for induction | CRC reported in 5 cases (3 in males, 2 in females) among solid cancers; SIR = 1.4 (95% CI: 0.4–3.2) |
| Merchea et al. (2019) [38] | USA | Retrospective cohort (1987–2016) | 63 patients with CRC after solid organ transplant (55.6% male, 44.4% female); majority kidney (44.4%); mean age at transplantation 57.3 years; median time to CRC 59.3 months | Immunosuppressive regimen not detailed | 24.6% diagnosed at stage IV; 5-year OS 42.5%; right colon predilection (60.9%); poor survival in advanced stages. Precise incidence in KTRs unspecified |
| Murray et al. (2020) [39] | Ireland | Retrospective cohort (1994–2014) | 3267 KTRs; 907 with cancer | Immunosuppression details not reported | CRC in 33 KTRs (3.6%), 18 in males and 15 in females. No increased risk of mortality in KTRs vs. general population for CRC; HR for all-cause mortality of 0.91 (95% CI: 0.58–1.43) and HR for cancer-specific mortality of 0.91 (95% CI: 0.58–1.43) for CRC |
| Oliveras et al. (2025) [40] | Spain | Retrospective cohort (2003–2021) | 8037 KTRs (64.7% male, 35.3% female); median age at transplantation 57 years; median time to cancer diagnosis 5 years. 2013 PTMs | Polyclonal antibodies, CNIs, mTORi during the first 6 weeks post-transplant | 122 CRC cases; SIR = 1.55 (95% CI: 1.28–1.85) |
| Pendon-Ruiz de Mier et al. (2015) [41] | Spain | Retrospective cohort (1979–2015) | 1450 KTRs; 90 developed PTMs; mean age at transplantation 59 years (with cancer), 53 years (without cancer) | Triple therapy with CNIs, mycophenolate/azathioprine, and prednisolone. Induction with basiliximab or thymoglobulin | CRC in 11% of SOC (≈10/90 cases) CRC ~0.7% prevalence (3/194); survival after SOC diagnosis ~2 years |
| Piselli et al. (2023) [42] | Italy | Cohort (1997–2021) | 11,418 KTRs (63.8% male, 36.2% female); median age at transplantation 50 years; 1646 PTMSs | 1997–2004: predominantly cyclosporine 2005–2012: predominantly tacrolimus 2013–2021: predominant combinations including mTORi | CRC incidence: 0.4 per 1000 person-years in 1997–2004 (7 cases), 0.8 per 1000 person-years in 2005–2012 (29 cases), and 2013–2021 (23 cases). Adjusted IRRs = 1.54; 95% CI: 0.67–3.54 for 2005–2012 and IRR = 1.39; 95% CI: 0.59–3.29 for 2013–2021. SIR = 0.76; 95% CI: 0.58–0.99 |
| Privitera et al. (2021) [43] | Italy | Cross-sectional (matched case–control) | 160 KTRs vs. 594 controls; median colonoscopy after 6.4 years post-transplant | Tacrolimus, mycophenolate, steroids, cyclosporine, and everolimus. Three-drug regimens, with or without induction therapy | 22/160 (13.7%) with advanced colorectal neoplasia, 4/160 (2.5%) with CRC; no increased CRC risk vs. controls (OR = 0.69); higher advanced adenoma risk (OR = 1.65) |
| Pyrza et al. (2022) [44] | Poland | Cross-sectional | 350 KTRs; mean age 48 years; malignancies in 70 patients (20%) | CNIs, azathioprine, prednisone | CRC in 3 cases (0.86%); skin and PTLDs most common; limited CRC-specific data |
| Rosales et al. (2020) [45] | Australia and New Zealand | Retrospective cohort (1980–2016) | 17,628 KTRs (61% male, 39% female); median age at transplantation 45 years; 1061 cancer deaths | CNIs, mTORi | Not mentioned |
| Safaeian et al. (2016) [46] | USA | Retrospective cohort (1987–2010) | 224,098 SOTRs (58.2% kidney); 61.2% male and 38.8% female; median age at transplantation 48 years | Cyclosporine + azathioprine, tacrolimus + mycophenolate, induction therapy, others | 790 CRC cases, overall SIR = 1.12; SIR = 1.69 for proximal colon cancer; SIR = 0.93 for distal colon cancer; SIR = 0.64 for rectal cancer; higher risk in liver and lung recipients (SIR = 2.34; SIR = 1.34, respectively), while non-significant in kidney recipients (SIR = 0.99; 95% CI: 0.89–1.09) |
| Taborelli et al. (2021) [47] | Italy | Retrospective cohort (1997–2017) | 1425 KTRs with cancer vs. 2850 matched controls, 4275 individuals in total; 70 CRC cases | Regimens not fully detailed; mTORi associated with better survival after cancer | CRC in 55 cases (46 in colon, 2 in rectosigmoid junction, 7 in rectum) |
| Teo et al. (2019) [48] | Singapore | Retrospective cohort (2000–2011) | 489 KTRs; mean age 47.1 years; median age at cancer diagnosis 50 years, median time to malignancy 2.6 years; 31 malignancies (6.3%) overall | CNIs, mycophenolate, azathioprine, corticosteroids. mTORi, CNIs + azathioprine, CNIs + mycophenolate | 3/31 CRC cases (9.7%) |
| Unterrainer et al. (2019) [49] | Multi-national | Retrospective cohort (1984–2016) | 272,325 KTRs (Collaborative Transplant Study database), of which 4184 had pre-transplant malignancies | CNIs, mycophenolate, azathioprine, steroids, prophylactic antibody induction therapy (IL2-RA, rATG, other) | No site-specific data for CRC; elevated incidence of CRC recurrence after transplantation (HR = 6.0; 95% CI: 2.7–13.5) |
| Wang et al. (2019) [50] | Taiwan | Retrospective cohort (2000–2008) | 3739 KTRs (59.03% female, 40.97% male) vs. 42,324 dialysis patients; mean age KTRs 61.8 years. | Not specified | CRC incidence 372.9 per 100,000 person-years vs. 232.5 in non-KT; adjusted HR 1.34 (95% CI: 1.11–1.62), higher risk in women and <50 years |
| Zilinska et al. (2017) [51] | Slovakia | Retrospective cohort (2007–2015) | 1421 KTRs; 85 malignancies (6%); median time to cancer 45 months, mean age at diagnosis 54 years | Tacrolimus, cyclosporine A, mTOR inhibitor (alone or with CNIs), mycophenolate, corticosteroids; IL2-RA (Basiliximab/Daclizumab) for induction or rATG for induction | 11/85 were CRC (12.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonforte, F.; Mistretta, A.; Nicosia, V.; Micalizzi, M.C.; Londrigo, D.; Giambra, M.M.; Roscitano, G.; Veroux, P.; Veroux, M. Epidemiological Overview of Colorectal Cancer in Kidney Transplant Recipients: A Systematic Review. Cancers 2025, 17, 3352. https://doi.org/10.3390/cancers17203352
Leonforte F, Mistretta A, Nicosia V, Micalizzi MC, Londrigo D, Giambra MM, Roscitano G, Veroux P, Veroux M. Epidemiological Overview of Colorectal Cancer in Kidney Transplant Recipients: A Systematic Review. Cancers. 2025; 17(20):3352. https://doi.org/10.3390/cancers17203352
Chicago/Turabian StyleLeonforte, Francesco, Antonio Mistretta, Vito Nicosia, Maria Cristina Micalizzi, Davide Londrigo, Martina Maria Giambra, Giuseppe Roscitano, Pierfrancesco Veroux, and Massimiliano Veroux. 2025. "Epidemiological Overview of Colorectal Cancer in Kidney Transplant Recipients: A Systematic Review" Cancers 17, no. 20: 3352. https://doi.org/10.3390/cancers17203352
APA StyleLeonforte, F., Mistretta, A., Nicosia, V., Micalizzi, M. C., Londrigo, D., Giambra, M. M., Roscitano, G., Veroux, P., & Veroux, M. (2025). Epidemiological Overview of Colorectal Cancer in Kidney Transplant Recipients: A Systematic Review. Cancers, 17(20), 3352. https://doi.org/10.3390/cancers17203352







