Hepatocellular Carcinoma Growth Kinetics and Outcomes After Transarterial Embolization: A Single-Center Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
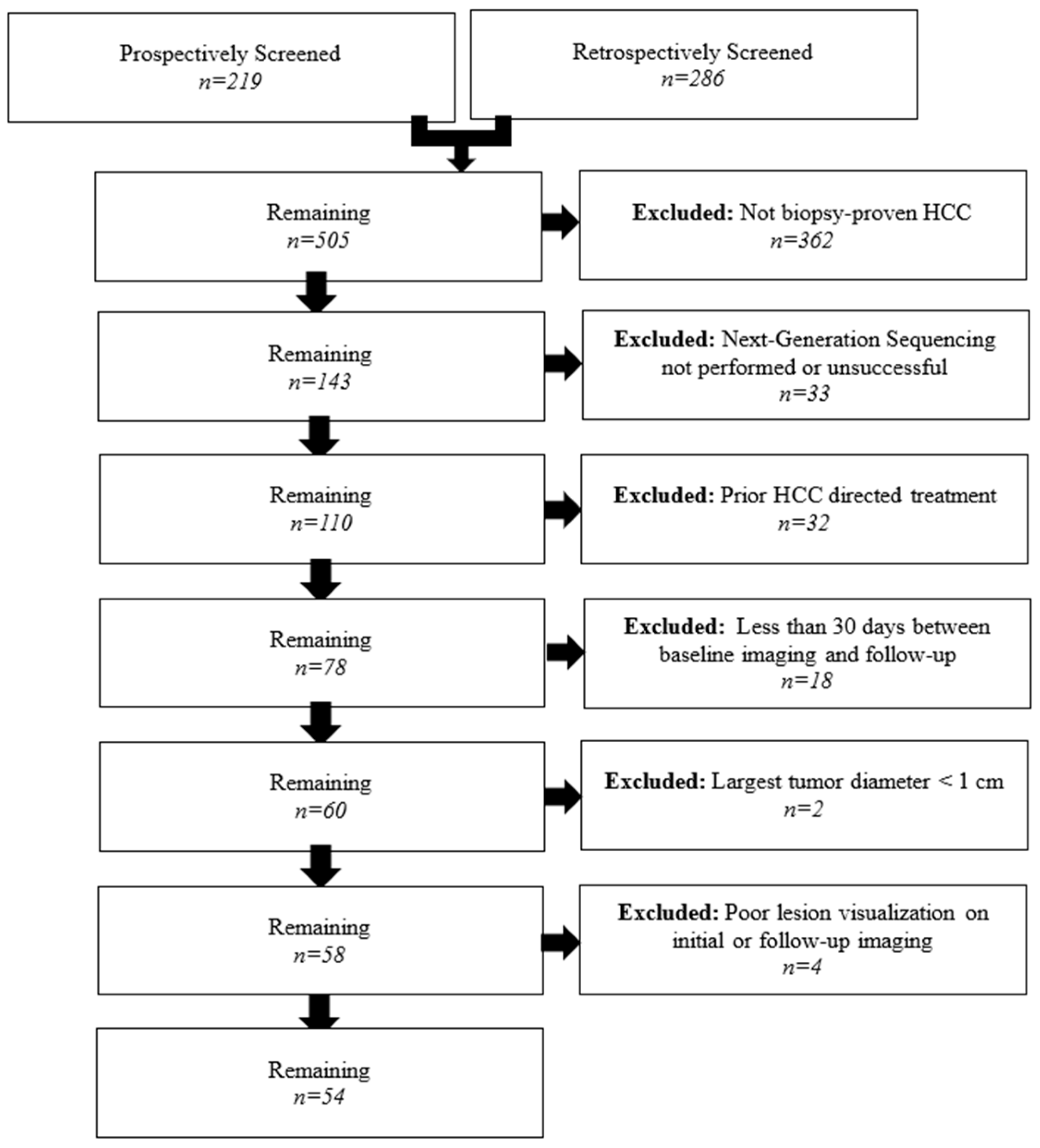
2.1. Patient Selection
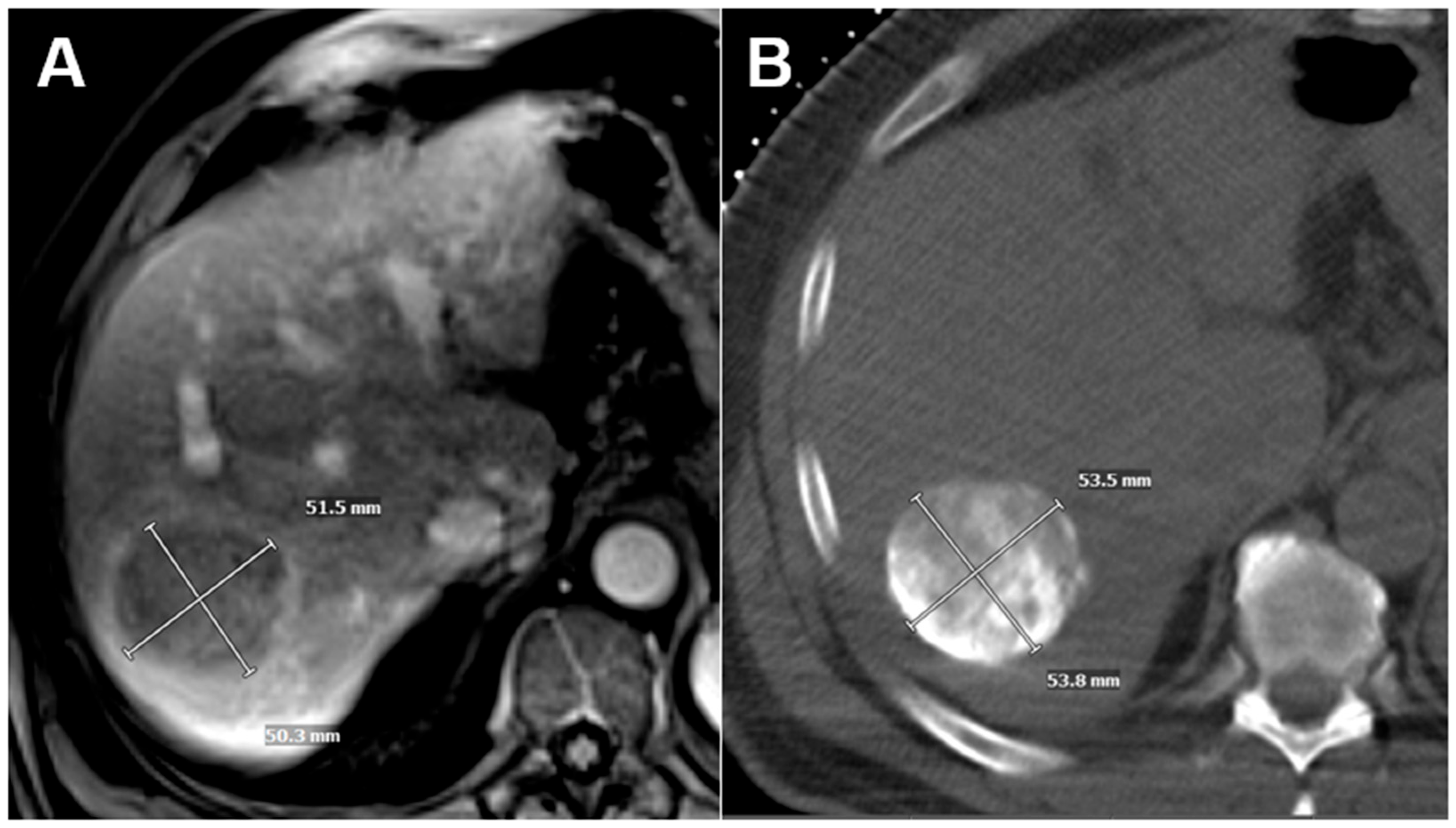
2.2. Tumor Volume Doubling Time Calculation
2.3. Genetic Analysis
2.4. Transarterial Embolization
2.5. Data Collection
2.6. Outcome Assessment
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
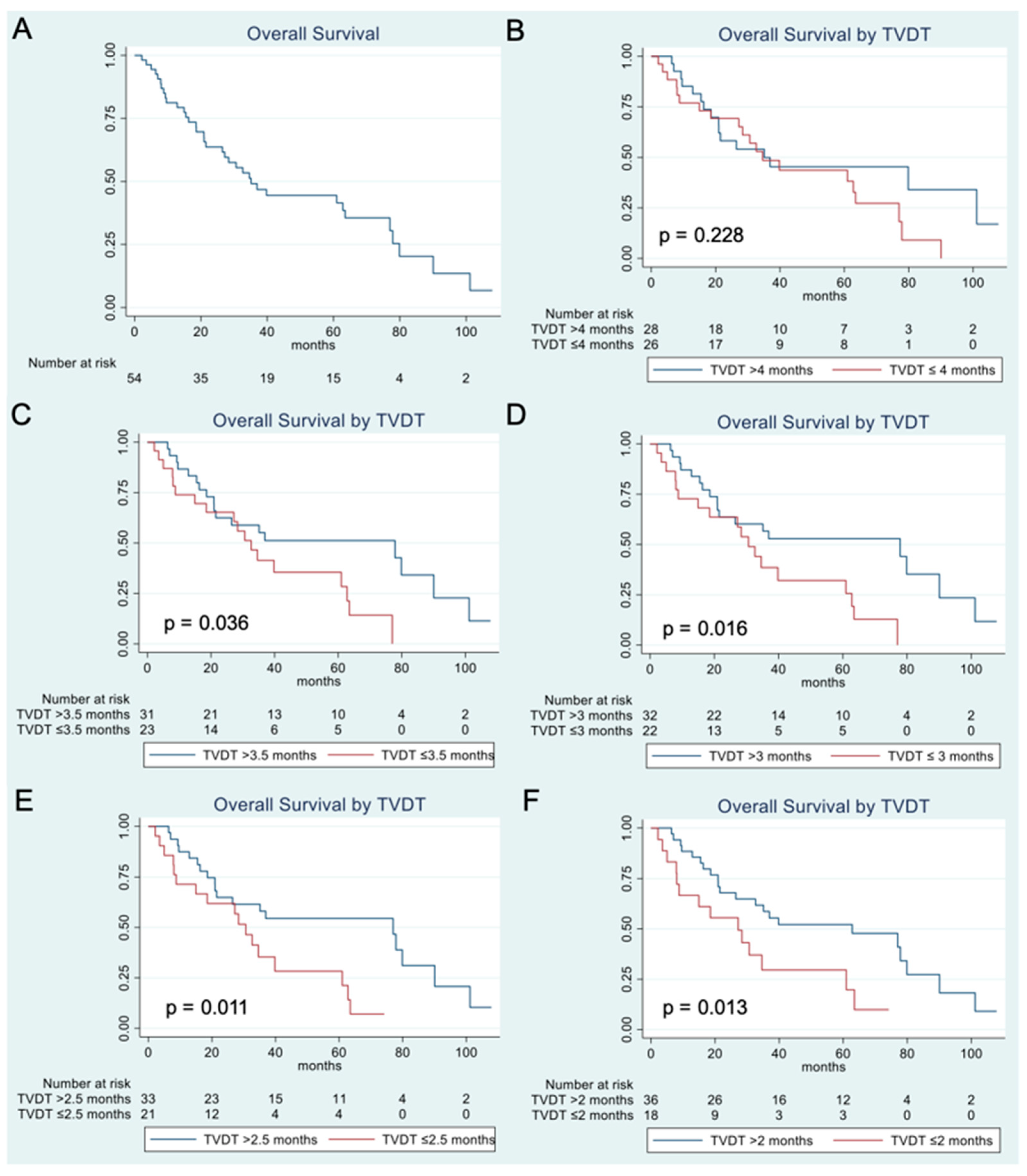
3.2. Overall Survival Analysis
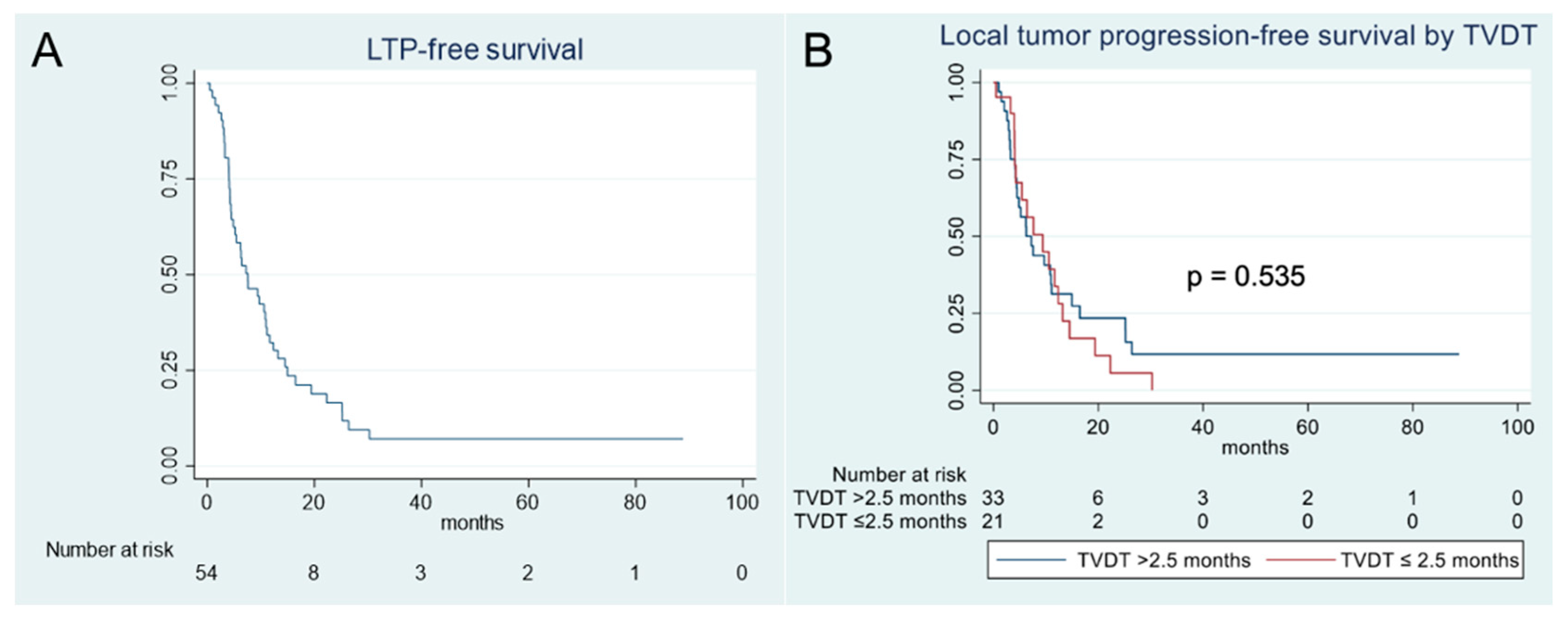
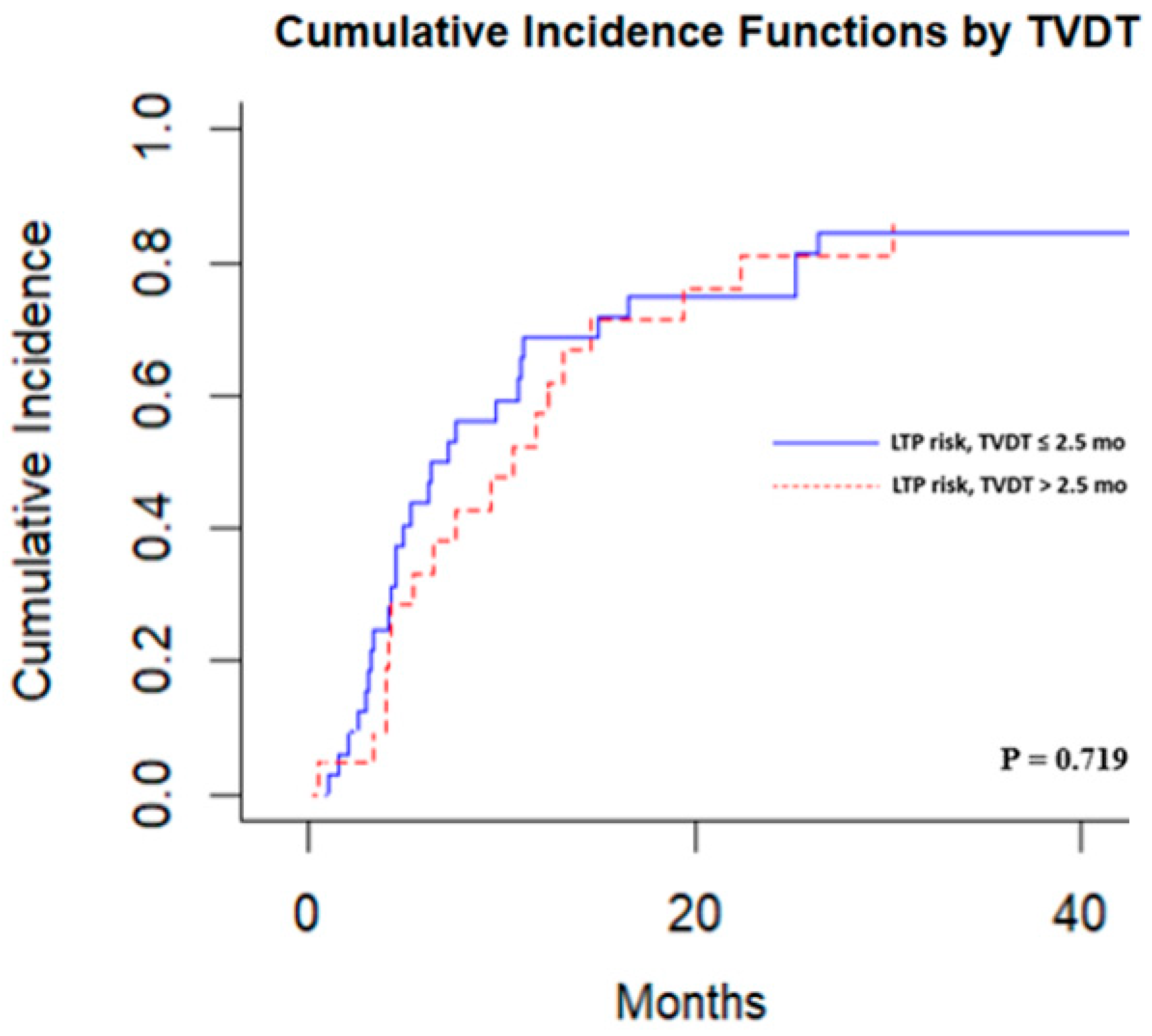
3.3. Local Tumor Progression Analysis
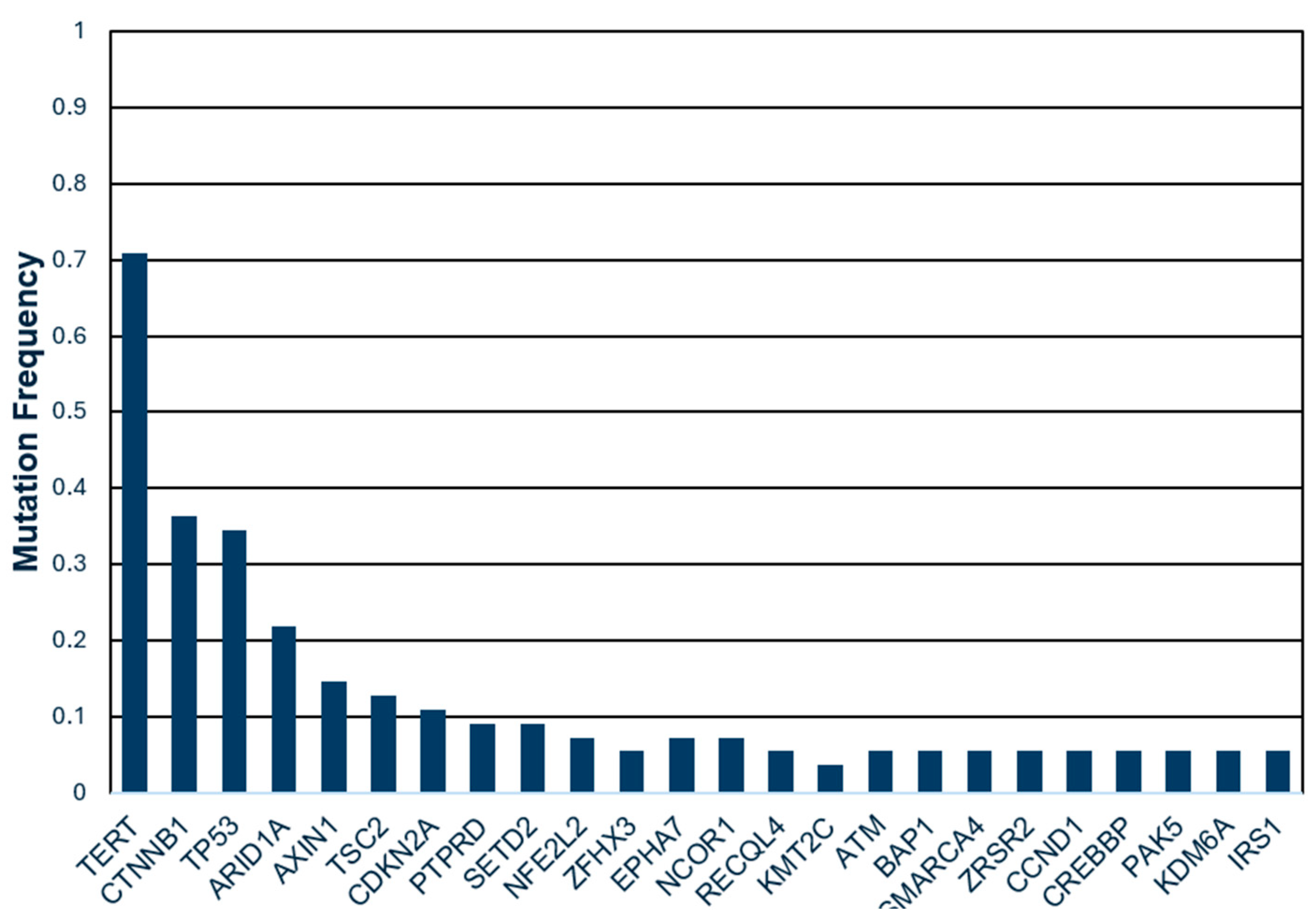
3.4. Genetic Mutations and TVDT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFP | alpha fetoprotein |
| BCLC | Barcelona Clinic Liver Cancer |
| CT | computed tomography |
| ECOG | Eastern Cooperative Oncology Group |
| HCC | hepatocellular carcinoma |
| LTPFS | local tumor progression-free survival |
| MRI | magnetic resonance imaging |
| OS | overall survival |
| TACE | transarterial chemoembolization |
| TAE | transarterial embolization |
| TARE | transarterial radioembolization |
| TVDT | tumor volume doubling time |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fabrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Borad, M.; Brown, D.; Burgoyne, A.; et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 541–565. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J. Hepatol. 2025, 82, 315–374. [Google Scholar] [CrossRef]
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Padia, S.A. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021, 74, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Sher, A.; Abboud, G.; Schwartz, M.; Facciuto, M.; Tabrizian, P.; Knesaurek, K.; Fischman, A.; Patel, R.; Nowakowski, S.; et al. Radiation segmentectomy for curative intent of unresectable very early to early stage hepatocellular carcinoma (RASER): A single-centre, single-arm study. Lancet Gastroenterol. Hepatol. 2022, 7, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.T.; Do, R.K.; Gonen, M.; Covey, A.M.; Getrajdman, G.I.; Sofocleous, C.T.; Jarnagin, W.R.; D’Angelica, M.I.; Allen, P.J.; Erinjeri, J.P.; et al. Randomized Trial of Hepatic Artery Embolization for Hepatocellular Carcinoma Using Doxorubicin-Eluting Microspheres Compared With Embolization With Microspheres Alone. J. Clin. Oncol. 2016, 34, 2046–2053. [Google Scholar] [CrossRef]
- Roth, G.S.; Benhamou, M.; Teyssier, Y.; Seigneurin, A.; Abousalihac, M.; Sengel, C.; Seror, O.; Ghelfi, J.; Ganne-Carrie, N.; Blaise, L.; et al. Comparison of Trans-Arterial Chemoembolization and Bland Embolization for the Treatment of Hepatocellular Carcinoma: A Propensity Score Analysis. Cancers 2021, 13, 812. [Google Scholar] [CrossRef] [PubMed]
- Marelli, L.; Stigliano, R.; Triantos, C.; Senzolo, M.; Cholongitas, E.; Davies, N.; Tibballs, J.; Meyer, T.; Patch, D.W.; Burroughs, A.K. Transarterial therapy for hepatocellular carcinoma: Which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc. Intervent Radiol. 2007, 30, 6–25. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, H.D.; Jun, M.J.; Yun, S.C.; Shim, J.H.; Lee, H.C.; Lee, D.; An, J.; Lim, Y.S.; Chung, Y.H.; et al. Tumor Volume Doubling Time as a Dynamic Prognostic Marker for Patients with Hepatocellular Carcinoma. Dig. Dis. Sci. 2017, 62, 2923–2931. [Google Scholar] [CrossRef]
- Nathani, P.; Gopal, P.; Rich, N.; Yopp, A.; Yokoo, T.; John, B.; Marrero, J.; Parikh, N.; Singal, A.G. Hepatocellular carcinoma tumour volume doubling time: A systematic review and meta-analysis. Gut 2021, 70, 401–407. [Google Scholar] [CrossRef]
- Chen, G.; Xie, X.; Wang, M.; Guo, X.; Zhang, Z.; Zhang, L.; Zhang, B. Prognostic Significance of Tumor Growth Rate (TGR) in Patients with Huge Hepatocellular Carcinoma Undergoing Transcatheter Arterial Chemoembolization. Curr. Oncol. 2022, 29, 423–432. [Google Scholar] [CrossRef]
- Harding, J.J.; Khalil, D.N.; Abou-Alfa, G.K. Biomarkers: What Role Do They Play (If Any) for Diagnosis, Prognosis and Tumor Response Prediction for Hepatocellular Carcinoma? Dig. Dis. Sci. 2019, 64, 918–927. [Google Scholar] [CrossRef]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Karimi, A.; Kelly, L.; Petre, E.; Marinelli, B.; Alexander, E.S.; Sotirchos, V.S.; Erinjeri, J.P.; Covey, A.; Sofocleous, C.T.; et al. TP53 Mutation Predicts Worse Survival and Earlier Local Progression in Patients with Hepatocellular Carcinoma Treated with Transarterial Embolization. Curr. Oncol. 2025, 32, 51. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Wu, Y.; Fan, W.; Guo, J.; Wei, J.; Wang, H.; Tan, J.; Wang, Y.; Yao, W.; Zhao, Y.; et al. Prognostic Value of TP53 Mutation for Transcatheter Arterial Chemoembolization Failure/Refractoriness in HBV-Related Advanced Hepatocellular Carcinoma. Cancer Res. Treat. 2020, 52, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.T.; Mitchell, T.N.; Zehir, A.; Shah, R.H.; Benayed, R.; Syed, A.; Chandramohan, R.; Liu, Z.Y.; Won, H.H.; Scott, S.N.; et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J. Mol. Diagn. 2015, 17, 251–264. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Tustumi, F. Choosing the most appropriate cut-point for continuous variables. Rev. Col. Bras. Cir. 2022, 49, e20223346. [Google Scholar] [CrossRef]
- R-Core-Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021.
- Barbara, L.; Benzi, G.; Gaiani, S.; Fusconi, F.; Zironi, G.; Siringo, S.; Rigamonti, A.; Barbara, C.; Grigioni, W.; Mazziotti, A.; et al. Natural history of small untreated hepatocellular carcinoma in cirrhosis: A multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology 1992, 16, 132–137. [Google Scholar] [CrossRef]
- An, C.; Choi, Y.A.; Choi, D.; Paik, Y.H.; Ahn, S.H.; Kim, M.J.; Paik, S.W.; Han, K.H.; Park, M.S. Growth rate of early-stage hepatocellular carcinoma in patients with chronic liver disease. Clin. Mol. Hepatol. 2015, 21, 279–286. [Google Scholar] [CrossRef]
- Rich, N.E.; John, B.V.; Parikh, N.D.; Rowe, I.; Mehta, N.; Khatri, G.; Thomas, S.M.; Anis, M.; Mendiratta-Lala, M.; Hernandez, C.; et al. Hepatocellular Carcinoma Demonstrates Heterogeneous Growth Patterns in a Multicenter Cohort of Patients With Cirrhosis. Hepatology 2020, 72, 1654–1665. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Ma, L.; Gou, J.; Feng, Y.; Lou, Y.; Zuo, L.; Wang, T.; Liang, Y.; Zhang, Y.; et al. Identification of transarterial chemoembolization candidates in advanced hepatocellular carcinoma patients classified solely by performance status 1: A multicenter retrospective study. Sci. Rep. 2025, 15, 18792. [Google Scholar] [CrossRef]
- Jin, B.; Wang, D.; Lewandowski, R.J.; Riaz, A.; Ryu, R.K.; Sato, K.T.; Larson, A.C.; Salem, R.; Omary, R.A. Chemoembolization endpoints: Effect on survival among patients with hepatocellular carcinoma. AJR Am. J. Roentgenol. 2011, 196, 919–928. [Google Scholar] [CrossRef]
- Jeong, S.O.; Kim, E.B.; Jeong, S.W.; Jang, J.Y.; Lee, S.H.; Kim, S.G.; Cha, S.W.; Kim, Y.S.; Cho, Y.D.; Kim, H.S.; et al. Predictive Factors for Complete Response and Recurrence after Transarterial Chemoembolization in Hepatocellular Carcinoma. Gut Liver 2017, 11, 409–416. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Yoon, H.K.; Ko, G.Y.; Shin, J.H.; Gwon, D.I.; Ko, H.K.; Chu, H.H.; Kim, S.H.; Kim, G.H.; et al. Transarterial chemoembolization for advanced hepatocellular carcinoma without macrovascular invasion or extrahepatic metastasis: Analysis of factors prognostic of clinical outcomes. Front. Oncol. 2023, 13, 1072922. [Google Scholar] [CrossRef]
- Peng, S.Y.; Chen, W.J.; Lai, P.L.; Jeng, Y.M.; Sheu, J.C.; Hsu, H.C. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: Significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int. J. Cancer 2004, 112, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cui, G.; Xu, H.; Chun, J.; Yang, D.; Zhang, Z.; Yang, L.; Wang, J.; Wan, M.; Calvisi, D.F.; et al. Loss of TP53 cooperates with c-MET overexpression to drive hepatocarcinogenesis. Cell Death Dis. 2023, 14, 476. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Yim, S.Y.; Jeong, Y.S.; Li, Q.X.; Kang, S.H.; Sohn, B.H.; Kumar, S.V.; Shin, J.H.; Choi, Y.R.; Shim, J.J.; et al. Consensus subtypes of hepatocellular carcinoma associated with clinical outcomes and genomic phenotypes. Hepatology 2022, 76, 1634–1648. [Google Scholar] [CrossRef] [PubMed]
- Boyault, S.; Rickman, D.S.; de Reynies, A.; Balabaud, C.; Rebouissou, S.; Jeannot, E.; Herault, A.; Saric, J.; Belghiti, J.; Franco, D.; et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology 2007, 45, 42–52. [Google Scholar] [CrossRef]
- Ruchalski, K.; Braschi-Amirfarzan, M.; Douek, M.; Sai, V.; Gutierrez, A.; Dewan, R.; Goldin, J. A Primer on RECIST 1.1 for Oncologic Imaging in Clinical Drug Trials. Radiol. Imaging Cancer 2021, 3, e210008. [Google Scholar] [CrossRef] [PubMed]
- Mehrara, E.; Forssell-Aronsson, E.; Ahlman, H.; Bernhardt, P. Specific growth rate versus doubling time for quantitative characterization of tumor growth rate. Cancer Res. 2007, 67, 3970–3975. [Google Scholar] [CrossRef] [PubMed]
| All Patients n = 54 | Rapidly Growing Tumors TVDT ≤ 2.5 Months n = 21 | Slowly Growing Tumors (TVDT > 2.5 Months) n = 33 | p-Value | |
|---|---|---|---|---|
| Age, years (median, IQR) | 69 (59–73) | 62 (57–71) | 70 (63–73) | 0.14 |
| Sex | 0.29 | |||
| Male | 47 (87%) | 17 (81.0%) | 30 (90.9%) | |
| Female | 7 (13%) | 4 (19%) | 3 (9.1%) | |
| Child Pugh score | 0.64 | |||
| A | 50 (92.6%) | 19 (90.5%) | 31 (93.9%) | |
| B | 4 (7.4%) | 2 (9.5%) | 2 (6.1%) | |
| ECOG score | 0.52 | |||
| 0 | 37 (68.5%) | 15 (71.4%) | 22 (69.7%) | |
| 1 or 2 | 17 (31.5%) | 6 (28.6%) | 11 (33.3%) | |
| Genetic mutations | ||||
| TERT | 39 (72.2%) | 16 (76.2%) | 23 (69.7%) | 0.60 |
| CTNNB | 20 (37%) | 7 (33.3%) | 13 (39.4%) | 0.65 |
| TP53 | 19 (35.2%) | 7 (33.3%) | 12 (36.4%) | 0.82 |
| ARID1A | 12 (22.2%) | 4 (19.1%) | 8 (24.2%) | 0.65 |
| AXIN1 | 8 (14.8%) | 4 (12.1%) | 4 (19.1%) | 0.49 |
| TSC2 | 7 (13%) | 4 (19.1%) | 3 (9.1%) | 0.29 |
| CDKN2A | 6 (11.1%) | 2 (9.5%) | 4 (12.2%) | 0.77 |
| Liver Disease Etiology 1 | ||||
| NASH | 15 (27.8%) | 6 (28.6%) | 9 (27.3%) | 1.0 |
| Alcohol | 13 (24.1%) | 5 (23.8%) | 8 (24.2%) | 1.0 |
| HBV | 4 (7.4%) | 0 | 4 (12.1%) | 0.29 |
| HCV | 20 (37%) | 10 (47.6%) | 10 (30.3%) | 0.25 |
| Other | 3 (5.6%) | 0 | 3 (9.1%) | 0.28 |
| AFP at baseline 2 | 0.69 | |||
| ≤200 ng/mL | 43 (79.6%) | 16 (76.2%) | 27 (81.8%) | |
| >200 ng/mL | 9 (16.7%) | 4 (19.0%) | 5 (15.2%) | |
| Extrahepatic disease | 6 (11.1%) | 2 (9.5%) | 4 (12.1%) | 0.77 |
| Macrovascular invasion | 8 (14.8%) | 5 (23.8%) | 3 (9.1%) | 0.14 |
| Infiltrative pattern | 2 (3.7%) | 2 (9.5%) | 0 | 0.07 |
| More than five tumors | 8 (14.8%) | 4 (19.1%) | 4 (12.1%) | 0.49 |
| Bilobar disease | 23 (42.6%) | 12 (57.1%) | 11 (33.3%) | 0.09 |
| Size of index tumor, mm (median, IQR) | 52 (33–72) | 52 (37–72) | 52 (31–71) | 0.45 |
| Overall Survival Stratified by TVDT n = Number of Patients in Each Group, Months (95%CI) | |||
|---|---|---|---|
| TVDT Threshold | Rapid Tumor Growth | Slow Tumor Growth | p-Value |
| 4 months | n = 26, | n = 28, | 0.228 |
| 34.6 months (18.5–63.5) | 35.1 months (18.6–101.2) | ||
| 3.5 months | n = 23, | n = 31, | 0.036 |
| 32.6 months (14.9–61.0) | 77.9 months (20.9–90.1) | ||
| 3 months | n = 22, | n = 32, | 0.016 |
| 30.6 months (8.8–61.0) | 77.9 months (21.0–90.1) | ||
| 2.5 months | n = 21, | n = 33, | 0.011 |
| 30.6 months (8.8–39.8) | 77 months (21.0–90.1) | ||
| 2 months | n = 18, | n = 36, | 0.013 |
| 27.2 months (8.0–61.0) | 62.8 months (21.5–80.0) | ||
| 1.5 months | n = 12, | n = 42, | 0.062 |
| 27.2 months (3.5–61.0) | 39.8 months (21.5–80.0) | ||
| Factor | HR (95% CI) | p-Value |
|---|---|---|
| Age | 1.01 (0.98–1.04) | 0.655 |
| Sex | 0.180 | |
| Male | Reference | |
| Female | 1.84 (0.75–4.51) | |
| Child Pugh score | 0.054 | |
| A | Reference | |
| B | 3.33 (0.98–11.26) | |
| ECOG status | 0.003 | |
| 0 | Reference | |
| 1 and 2 | 2.94 (1.43–6.06) | |
| Genetic mutations | ||
| TERT | 0.78 (0.38–1.60) | 0.504 |
| CTNNB | 0.71 (0.35–1.44) | 0.345 |
| TP53 | 2.06 (1.04–4.07) | 0.039 |
| ARID1A | 1.42 (0.68–2.99) | 0.352 |
| AXIN1 | 1.67 (0.72–3.86) | 0.233 |
| TSC2 | 2.99 (1.19–7.46) | 0.019 |
| CDKN2A | 0.98 (0.34–2.80) | 0.968 |
| AFP at baseline | 0.004 | |
| ≤200 ng/mL | Reference | |
| >200 ng/mL | 3.39 (1.49–7.73) | |
| TVDT | 0.013 | |
| >2.5 months | Reference | |
| ≤2.5 months | 2.47 (1.21–5.05) | |
| Presence of extrahepatic disease | 1.55 (0.62–3.83) | 0.346 |
| Macrovascular invasion | 1.92 (0.78–4.72) | 0.158 |
| Number of tumors | 0.364 | |
| ≤5 tumors | Reference | |
| >5 tumors | 1.52 (0.62–3.72) | |
| Tumor distribution | 0.278 | |
| Unilobar | Reference | |
| Bilobar | 1.44 (0.74–2.80) | |
| Size of index tumor | 1.002 (0.92–1.09) | 0.971 |
| Factor | HR (95% CI) | p-Value |
|---|---|---|
| TVDT | 0.036 | |
| >2.5 months | Reference | |
| ≤2.5 months | 2.21 (1.05–4.66) | |
| ECOG status | 0.006 | |
| 0 | Reference | |
| 1 and 2 | 3.15 (1.39–7.13) | |
| AFP | 0.013 | |
| ≤200 ng/mL | Reference | |
| >200 ng/mL | 3.23 (1.28–8.15) | |
| TSC2 | 2.23 (0.83–6.02) | 0.112 |
| TP53 | 1.40 (0.65–3.03) | 0.391 |
| Factor | HR (95% CI) | p-Value |
|---|---|---|
| Age | 1.02 (0.99–1.05) | 0.288 |
| Sex | 0.405 | |
| Male | Reference | |
| Female | 1.59 (0.53–4.72) | |
| Child Pugh score | 0.689 | |
| A | Reference | |
| B | 0.79 (0.24–2.54) | |
| ECOG status | 0.036 | |
| 0 | Reference | |
| 1 and 2 | 2.11 (1.05–4.25) | |
| Genetic mutations | ||
| TERT | 1.73 (0.87–3.48) | 0.120 |
| CTNNB | 1.10 (0.62–1.94) | 0.747 |
| TP53 | 2.06 (1.01–4.21) | 0.046 |
| ARID1A | 1.56 (0.78–3.14) | 0.210 |
| AXIN1 | 1.19 (0.47–3.03) | 0.716 |
| TSC2 | 1.62 (0.80–3.29) | 0.184 |
| CDKN2A | 1.60 (0.80–3.19) | 0.183 |
| AFP at baseline | 0.121 | |
| ≤200 ng/mL | Reference | |
| >200 ng/mL | 2.74 (0.77–9.78) | |
| TVDT | 0.704 | |
| >2.5 months | Reference | |
| ≤2.5 months | 0.89 (0.50–1.59) | |
| Presence of extrahepatic disease | 1.18 (0.39–3.57 | 0.767 |
| Macrovascular invasion | 1.49 (0.58–3.82) | 0.412 |
| Number of tumors | <0.001 | |
| ≤5 tumors | Reference | |
| >5 tumors | 3.73 (1.98–7.01) | |
| Tumor distribution | 0.197 | |
| Unilobar | Reference | |
| Bilobar | 1.47 (0.82–2.66) | |
| Size of index tumor | 1.00 (0.94–1.07) | 0.933 |
| Factor | HR (95% CI) | p-Value |
|---|---|---|
| ECOG score | 0.351 | |
| 0 | Reference | |
| 1 and 2 | 1.48 (0.65–3.41) | |
| TP53 | 1.61 (0.69–3.78) | 0.271 |
| Number of tumors | 0.002 | |
| ≤5 tumors | Reference | |
| >5 tumors | 3.11 (1.49–6.48) |
| Local Progression Free Survival Stratified by TVDT n = Number of Patients in Each Group, Median Months (95%CI) | |||
|---|---|---|---|
| TVDT Threshold | Rapid Tumor Growth | Slow Tumor Growth | p-Value |
| 4 months | n = 26, | n = 28, | 0.922 |
| 9.4 months (4.1–13.2) | 6.3 months (4.2–11.0) | ||
| 3.5 months | n = 23, | n = 31, | 0.239 |
| 7.6 months (4.5–15.0) | 7.6 months (4.0–12.4) | ||
| 3 months | n = 22, | n = 32, | 0.367 |
| 7.6 months (4.0–12.4) | 7.2 months (4.4–11.1) | ||
| 2.5 months | n = 21, | n = 33, | 0.535 |
| 9.4 months (4.1–12.4) | 7.2 months (4.2–11.1) | ||
| 2 months | n = 18, | n = 36, | 0.149 |
| 6.4 months (4.0–12.4) | 7.6 months (4.5–11.1) | ||
| 1.5 months | n = 12, | n = 42, | |
| 7.6 months (4.0–13.2) | 7.2 months (4.4–11.0) | 0.511 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Blume, H.; Petre, E.N.; Xenos, D.; Kobus, Z.; Alexander, E.S.; Sotirchos, V.S.; Geevarghese, R.; Covey, A.; Erinjeri, J.P.; et al. Hepatocellular Carcinoma Growth Kinetics and Outcomes After Transarterial Embolization: A Single-Center Analysis. Cancers 2025, 17, 3346. https://doi.org/10.3390/cancers17203346
Zhao K, Blume H, Petre EN, Xenos D, Kobus Z, Alexander ES, Sotirchos VS, Geevarghese R, Covey A, Erinjeri JP, et al. Hepatocellular Carcinoma Growth Kinetics and Outcomes After Transarterial Embolization: A Single-Center Analysis. Cancers. 2025; 17(20):3346. https://doi.org/10.3390/cancers17203346
Chicago/Turabian StyleZhao, Ken, Harrison Blume, Elena N. Petre, Dimitrios Xenos, Zuzanna Kobus, Erica S. Alexander, Vlasios S. Sotirchos, Ruben Geevarghese, Anne Covey, Joseph P. Erinjeri, and et al. 2025. "Hepatocellular Carcinoma Growth Kinetics and Outcomes After Transarterial Embolization: A Single-Center Analysis" Cancers 17, no. 20: 3346. https://doi.org/10.3390/cancers17203346
APA StyleZhao, K., Blume, H., Petre, E. N., Xenos, D., Kobus, Z., Alexander, E. S., Sotirchos, V. S., Geevarghese, R., Covey, A., Erinjeri, J. P., Ziv, E., Sofocleous, C. T., Harding, J. J., Soares, K., Sigel, C., Vakiani, E., & Yarmohammadi, H. (2025). Hepatocellular Carcinoma Growth Kinetics and Outcomes After Transarterial Embolization: A Single-Center Analysis. Cancers, 17(20), 3346. https://doi.org/10.3390/cancers17203346







