Pancreatic Cancer Detection in Intraductal Papillary Mucinous Neoplasm (IPMN)—New Insights
Abstract
Simple Summary
Abstract
1. Introduction
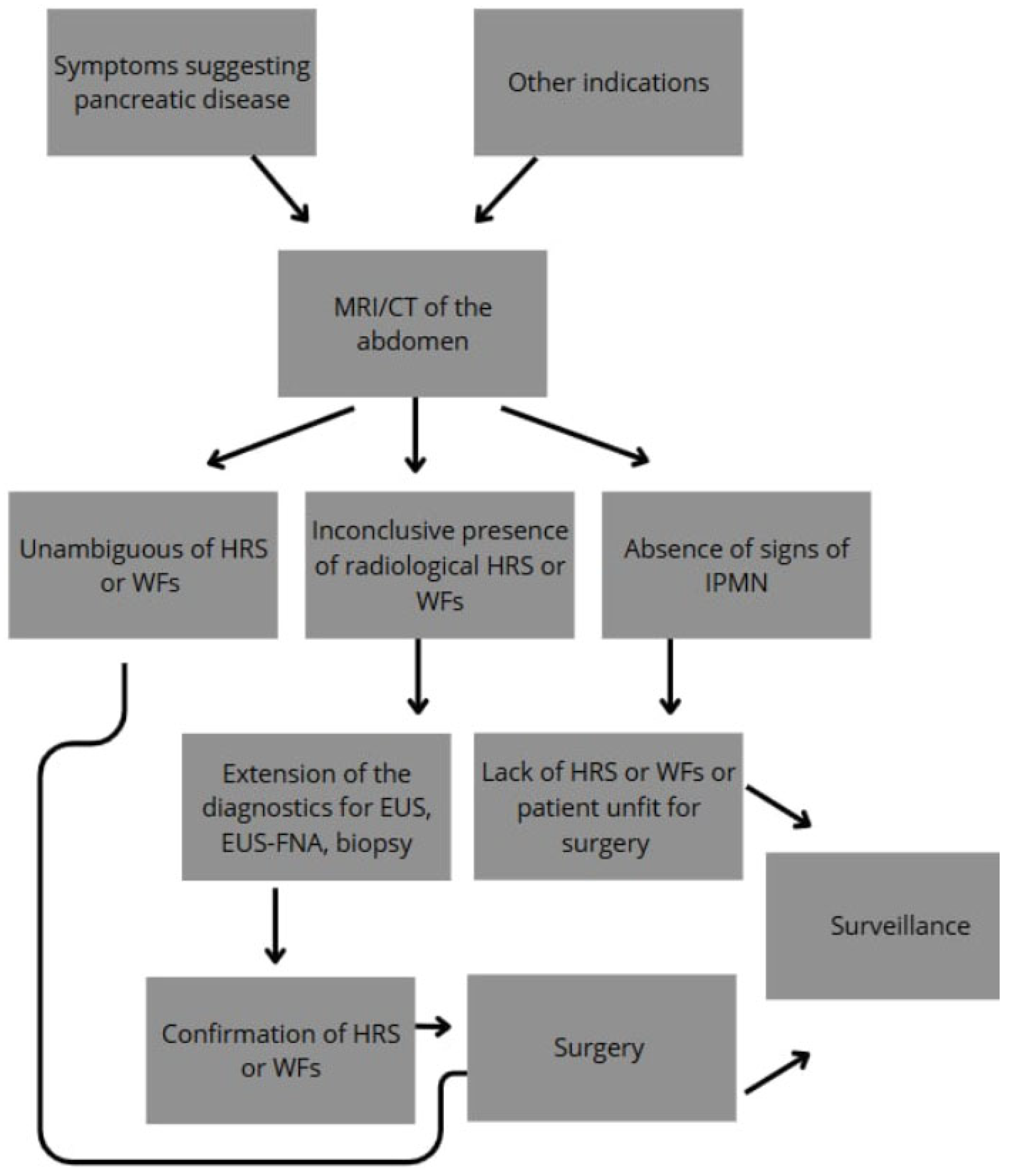
2. Diagnosis and Management
| Worrisome Factors | Sensitivity | Specificity | Guidelines | References | |
|---|---|---|---|---|---|
| Jaundice | 26–83% | 61–97% | IAP | [1,41] | |
| European | |||||
| ACG | |||||
| ACR | |||||
| Enhancing mural nodule or solid component | ≥5 mm | 64.6–100% | 73–87.5% | IAP | [21,42,48] |
| European | |||||
| ACG * | |||||
| ACR * | |||||
| AGA * | |||||
| <5 mm | N/A | N/A | IAP | - | |
| European | |||||
| ACG * | |||||
| ACR * | |||||
| AGA * | |||||
| Main pancreatic duct dilation | ≥10 mm | 28.2–51.7% | 78.7–87.5% | IAP | [42,48,49] |
| European | |||||
| ACR | |||||
| AGA * | |||||
| ≥7 mm | 53.80% | 80.70% | ACR | [50] | |
| AGA * | |||||
| >5 mm | 54.7–74.8% | 58.6–78% | ACG | [49,51] | |
| AGA * | |||||
| ≥5 mm and <10 mm | N/A | N/A | IAP | - | |
| European | |||||
| AGA * | |||||
| Positive cytology | 28.7–64.8% | 84–94% | IAP | [37,38,39,40] | |
| European | |||||
| ACG | |||||
| AGA | |||||
| Acute pancreatitis | 32–42.6% | 86–86.1% | IAP | [41,48] | |
| European | |||||
| ACG | |||||
| New-onset or worsening diabetes | 46% | 83% | IAP | [41] | |
| European | |||||
| ACG | |||||
| Increased serum level of CA 19-9 | >37 U/mL | 41–74% | 85–96% | IAP | [21,41,48] |
| European | |||||
| ACG | |||||
| Cyst diameter | ≥40 mm | N/A | N/A | European | - |
| ≥30 mm | 56.1–64% | 53.7–69% | IAP | [41,51] | |
| ACG | |||||
| ACR | |||||
| AGA | |||||
| Thickened/enhancing cyst walls | 23–38.5% | 89.7–95% | IAP | [41,51] | |
| ACR | |||||
| Abrupt change in caliber of pancreatic duct with distal pancreatic atrophy (IAP) | 19.30% | 95.90% | IAP | [51] | |
| focal dilation of pancreatic duct concerning for MD-IPMN or an obstructing lesion (ACG) | ACG | ||||
| Lymphadenopathy | 5.2–20% | 93–99.6% | IAP | [41,51] | |
| Cystic growth rate | ≥5 mm/year | 56% | 97% | European | [52] |
| >3 mm/year | N/A | N/A | ACG | - | |
| ≥2.5 mm/year | 60.90% | 70.30% | IAP | [51] | |
| Abdominal pain | N/A | N/A | European | - | |
| Non-enhancing mural nodule | N/A | N/A | ACR | - | |
3. MRI Imaging in Detecting High-Risk Malignancy in IPMN
4. Computed Tomography in Detecting Malignancy in IPMN
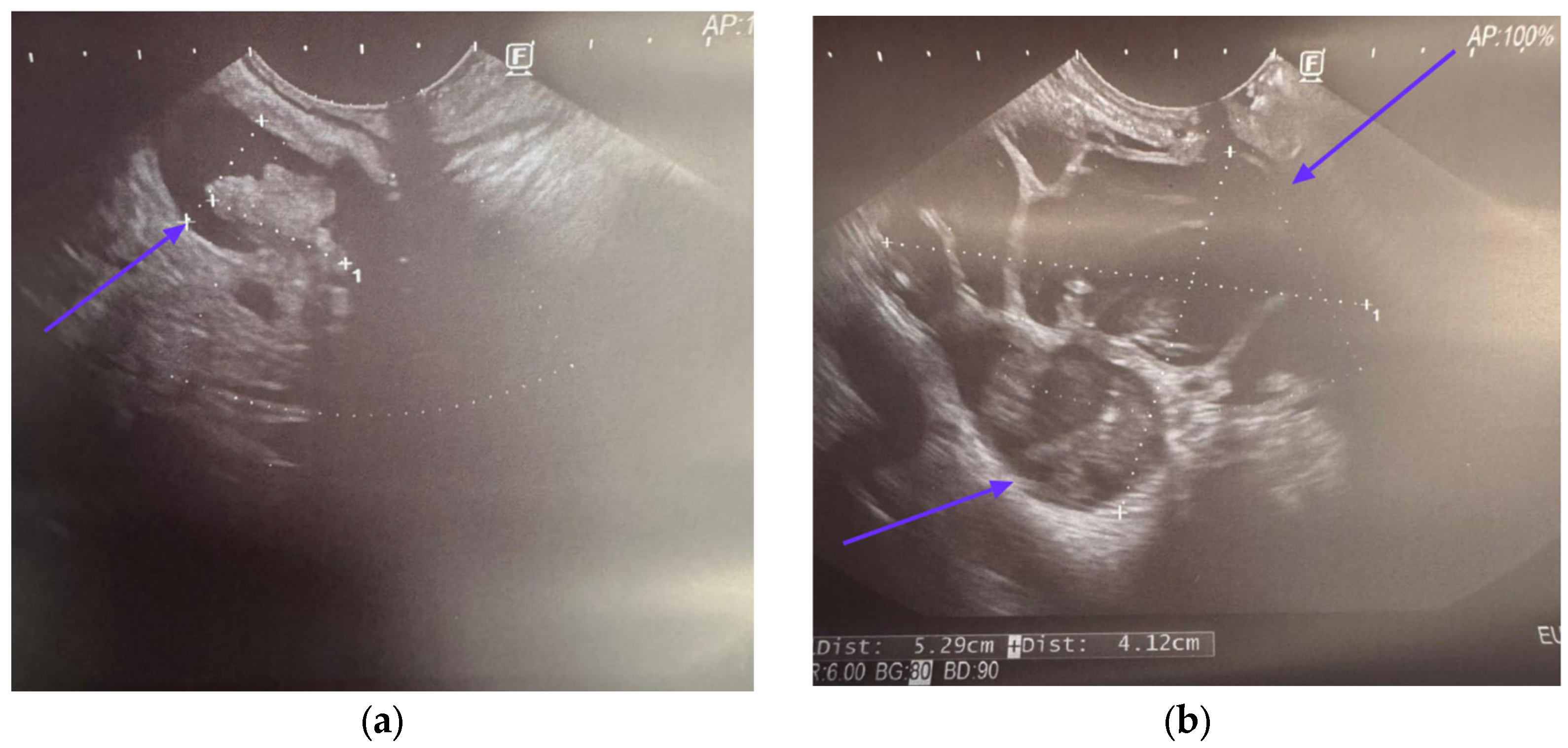
5. Endoscopic Ultrasound in Detecting Malignancy in IPMN
5.1. EUS-Guided Fine-Needle Aspiration
5.1.1. Cyst Fluid Analysis
5.1.2. Cytology
5.2. EUS-Guided Through-the-Needle Biopsy
5.3. EUS-Guided Needle-Based Confocal Laser Endomicroscopy
6. New Emerging Markers for Malignancy in IPMN
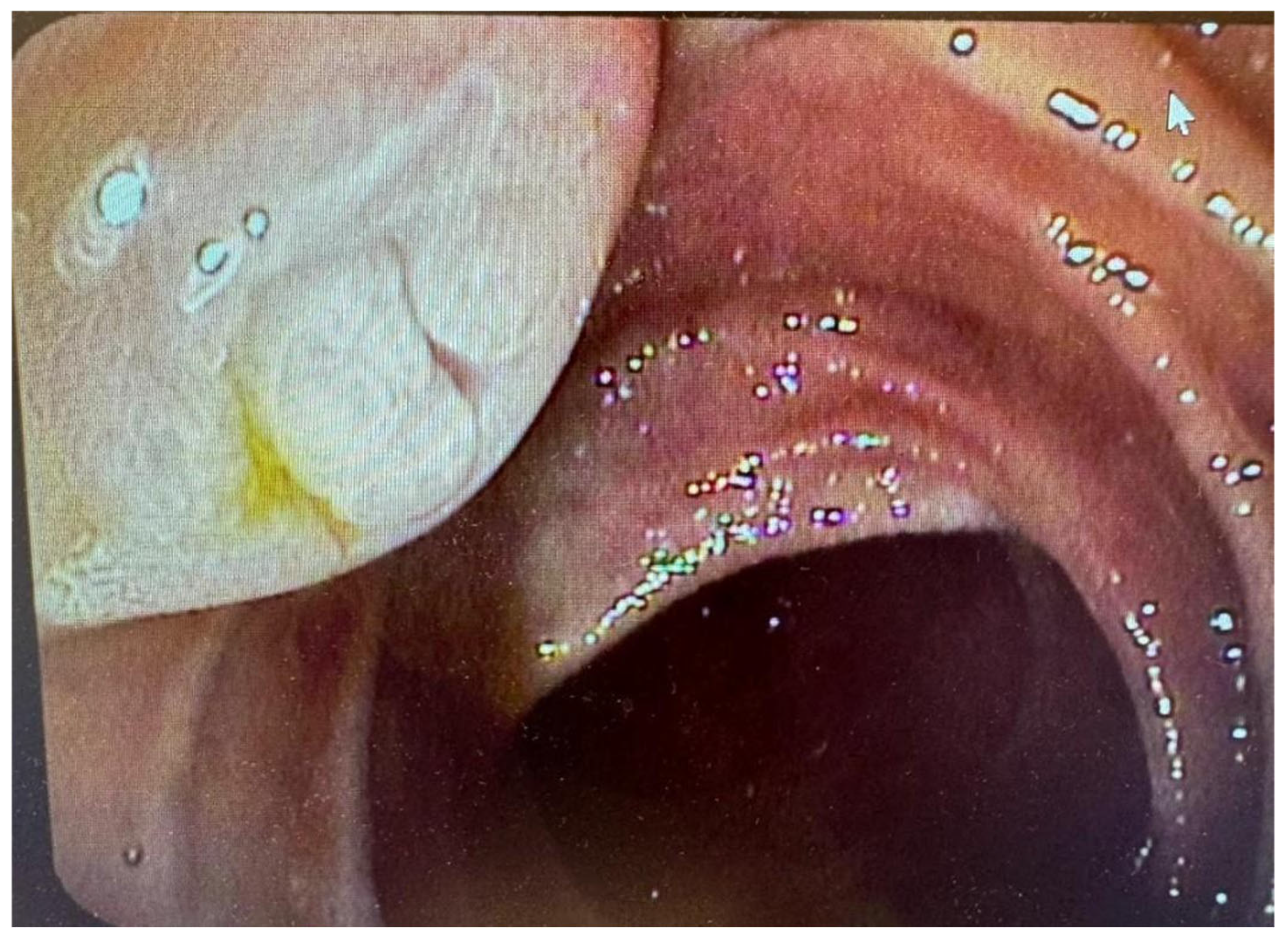
7. Pancreatoscopy
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Winter, K.; Talar-Wojnarowska, R.; Dąbrowski, A.; Degowska, M.; Durlik, M.; Gąsiorowska, A.; Głuszek, S.; Jurkowska, G.; Kaczka, A.; Lampe, P.; et al. Diagnostic and therapeutic recommendations in pancreatic ductal adenocarcinoma. Recommendations of the Working Group of the Polish Pancreatic Club. Prz. Gastroenterol. 2019, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49, Erratum in CA Cancer J. Clin. 2024, 74, 203. https://doi.org/10.3322/caac.21830. [Google Scholar] [CrossRef] [PubMed]
- Kolbeinsson, H.M.; Chandana, S.; Wright, G.P.; Chung, M. Pancreatic Cancer: A Review of Current Treatment and Novel Therapies. J. Investig. Surg. 2023, 36, 2129884. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.; Scheifele, C.; Hinz, U.; Leonhardt, C.-S.; Hank, T.; Koenig, A.-K.; Tjaden, C.; Hackert, T.; Bergmann, F.; Büchler, M.W.; et al. IPMN-associated pancreatic cancer: Survival, prognostic staging and impact of adjuvant chemotherapy. Eur. J. Surg. Oncol. 2022, 48, 1309–1320. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hong, S.-M. Precursor Lesions of Pancreatic Cancer. Oncol. Res. Treat. 2018, 41, 603–610. [Google Scholar] [CrossRef]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef]
- Chang, X.Y.; Wu, Y.; Jiang, Y.; Wang, P.Y.; Chen, J. RNF43 Mutations in IPMN Cases: A Potential Prognostic Factor. Gastroenterol. Res. Pract. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Gardner, T.B.; Park, W.G.; Allen, P.J. Diagnosis and Management of Pancreatic Cysts. Gastroenterology 2024, 167, 454–468. [Google Scholar] [CrossRef]
- Zerboni, G.; Signoretti, M.; Crippa, S.; Falconi, M.; Arcidiacono, P.G.; Capurso, G. Systematic review and meta-analysis: Prevalence of incidentally detected pancreatic cystic lesions in asymptomatic individuals. Pancreatology 2019, 19, 2–9. [Google Scholar] [CrossRef]
- Gonda, T.A.; Cahen, D.L.; Farrell, J.J. Pancreatic Cysts. N. Engl. J. Med. 2024, 391, 832–843. [Google Scholar] [CrossRef]
- Oo, J.; Brown, L.; Loveday, B.P. Intraductal papillary mucinous neoplasm: Overview of management. Aust. J. Gen. Pract. 2024, 53, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Van Erning, F.N.; Mackay, T.M.; Van Der Geest, L.G.M.; Groot Koerkamp, B.; Van Laarhoven, H.W.M.; Bonsing, B.A.; Wilmink, J.W.; Van Santvoort, H.C.; De Vos-Geelen, J.; Van Eijck, C.H.J.; et al. Association of the location of pancreatic ductal adenocarcinoma (head, body, tail) with tumor stage, treatment, and survival: A population-based analysis. Acta Oncol. 2018, 57, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, B.; Szmigiel, P.; Mrowiec, S. Pancreatic intraductal papillary mucinous neoplasms: Current diagnosis and management. World J. Gastrointest. Oncol. 2021, 13, 1880–1895. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Klöppel, G.; Volkan Adsay, N.; Albores-Saavedra, J.; Fukushima, N.; Horii, A.; Hruban, R.H.; Kato, Y.; Klimstra, D.S.; Longnecker, D.S.; et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: A consensus study. Virchows Arch. Int. J. Pathol. 2005, 447, 794–799. [Google Scholar] [CrossRef]
- Ban, S.; Naitoh, Y.; Mino-Kenudson, M.; Sakurai, T.; Kuroda, M.; Koyama, I.; Lauwers, G.Y.; Shimizu, M. Intraductal Papillary Mucinous Neoplasm (IPMN) of the Pancreas: Its Histopathologic Difference Between 2 Major Types. Am. J. Surg. Pathol. 2006, 30, 1561–1569. [Google Scholar] [CrossRef]
- Basturk, O.; Hong, S.-M.; Wood, L.D.; Adsay, N.V.; Albores-Saavedra, J.; Biankin, A.V.; Brosens, L.A.; Fukushima, N.; Goggins, M.; Hruban, R.H.; et al. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am. J. Surg. Pathol. 2015, 39, 1730–1741. [Google Scholar] [CrossRef]
- Furukawa, T.; Fukushima, N.; Itoi, T.; Ohike, N.; Mitsuhashi, T.; Nakagohri, T.; Notohara, K.; Shimizu, M.; Tajiri, T.; Tanaka, M.; et al. A Consensus Study of the Grading and Typing of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Pancreas 2019, 48, 480–487. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Kanemitsu, S.; Hatori, T.; Maguchi, H.; Shimizu, Y.; Tada, M.; Nakagohri, T.; Hanada, K.; Osanai, M.; Noda, Y.; et al. Pancreatic Ductal Adenocarcinoma Derived From IPMN and Pancreatic Ductal Adenocarcinoma Concomitant with IPMN. Pancreas 2011, 40, 571–580. [Google Scholar] [CrossRef]
- Hecht, E.M.; Khatri, G.; Morgan, D.; Kang, S.; Bhosale, P.R.; Francis, I.R.; Gandhi, N.S.; Hough, D.M.; Huang, C.; Luk, L.; et al. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: Recommendations for Standardized Imaging and Reporting from the Society of Abdominal Radiology IPMN disease focused panel. Abdom. Radiol. 2021, 46, 1586–1606. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Castillo, C.F.-D.; Furukawa, T.; Hijioka, S.; Jang, J.-Y.; Lennon, A.M.; Miyasaka, Y.; Ohno, E.; Salvia, R.; Wolfgang, C.L.; et al. International evidence-based Kyoto guidelines for the management of intraductal papillary mucinous neoplasm of the pancreas. Pancreatology 2024, 24, 255–270. [Google Scholar] [CrossRef]
- Tjaden, C.; Sandini, M.; Mihaljevic, A.L.; Kaiser, J.; Khristenko, E.; Mayer, P.; Hinz, U.; Gaida, M.M.; Berchtold, C.; Diener, M.K.; et al. Risk of the Watch-and-Wait Concept in Surgical Treatment of Intraductal Papillary Mucinous Neoplasm. JAMA Surg. 2021, 156, 818–825. [Google Scholar] [CrossRef]
- Salvia, R.; Burelli, A.; Perri, G.; Marchegiani, G. State-of-the-art surgical treatment of IPMNs. Langenbeck’s Arch. Surg. 2021, 406, 2633–2642. [Google Scholar] [CrossRef]
- de Pretis, N.; Martinelli, L.; Amodio, A.; Caldart, F.; Crucillà, S.; Battan, M.S.; Zorzi, A.; Crinò, S.F.; Bellocchi, M.C.C.; Bernardoni, L.; et al. Branch Duct IPMN-Associated Acute Pancreatitis in a Large Single-Center Cohort Study. Diagnostics 2025, 15, 1676. [Google Scholar] [CrossRef] [PubMed]
- The European Study Group on Cystic Tumours of the Pancreas European Evidence-Based Guidelines on Pancreatic Cystic Neoplasms. Gut 2018, 67, 789–804. [CrossRef] [PubMed]
- Huynh, T.; Ali, K.; Vyas, S.; Dezsi, K.; Strickland, D.; Basinski, T.; Chen, D.-T.; Jiang, K.; Centeno, B.; Malafa, M.; et al. Comparison of imaging modalities for measuring the diameter of intraductal papillary mucinous neoplasms of the pancreas. Pancreatology 2020, 20, 448–453. [Google Scholar] [CrossRef] [PubMed]
- D’ONofrio, M.; Tedesco, G.; Cardobi, N.; De Robertis, R.; Sarno, A.; Capelli, P.; Martini, P.T.; Giannotti, G.; Beleù, A.; Marchegiani, G.; et al. Magnetic resonance (MR) for mural nodule detection studying Intraductal papillary mucinous neoplasms (IPMN) of pancreas: Imaging-pathologic correlation. Pancreatology 2021, 21, 180–187. [Google Scholar] [CrossRef]
- Cui, S.; Tang, T.; Su, Q.; Wang, Y.; Shu, Z.; Yang, W.; Gong, X. Radiomic nomogram based on MRI to predict grade of branching type intraductal papillary mucinous neoplasms of the pancreas: A multicenter study. Cancer Imaging 2021, 21, 26. [Google Scholar] [CrossRef]
- Min, J.H.; Kim, Y.K.; Kim, S.K.; Kim, H.; Ahn, S. Intraductal papillary mucinous neoplasm of the pancreas: Diagnostic performance of the 2017 international consensus guidelines using CT and MRI. Eur. Radiol. 2021, 31, 4774–4784. [Google Scholar] [CrossRef]
- Lee, J.E.; Choi, S.-Y.; Min, J.H.; Yi, B.H.; Lee, M.H.; Kim, S.S.; Hwang, J.A.; Kim, J.H. Determining Malignant Potential of Intraductal Papillary Mucinous Neoplasm of the Pancreas: CT versus MRI by Using Revised 2017 International Consensus Guidelines. Radiology 2019, 293, 134–143. [Google Scholar] [CrossRef]
- Liu, H.; Cui, Y.; Shao, J.; Shao, Z.; Su, F.; Li, Y. The diagnostic role of CT, MRI/MRCP, PET/CT, EUS and DWI in the differentiation of benign and malignant IPMN: A meta-analysis. Clin. Imaging 2021, 72, 183–193. [Google Scholar] [CrossRef]
- Hong, S.B.; Lee, N.K.; Kim, S.; Seo, H.-I.; Park, Y.M.; Noh, B.G.; Kim, D.U.; Han, S.Y.; Kim, T.U. Diagnostic performance of magnetic resonance image for malignant intraductal papillary mucinous neoplasms: The importance of size of enhancing mural nodule within cyst. Jpn. J. Radiol. 2022, 40, 1282–1289. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Ren, S.; Guo, K.; Zhang, Y.; Lin, T.; Wang, Y.; Chen, X.; Wang, Z. Threshold of Main Pancreatic Duct Diameter in Identifying Malignant Intraductal Papillary Mucinous Neoplasm by Magnetic Resonance Imaging. Technol. Cancer Res. Treat. 2023, 22, 15330338231170942. [Google Scholar] [CrossRef]
- Tobaly, D.; Santinha, J.; Sartoris, R.; Burgio, M.D.; Matos, C.; Cros, J.; Couvelard, A.; Rebours, V.; Sauvanet, A.; Ronot, M.; et al. CT-Based Radiomics Analysis to Predict Malignancy in Patients with Intraductal Papillary Mucinous Neoplasm (IPMN) of the Pancreas. Cancers 2020, 12, 3089. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, J.; Midya, A.; Gazit, L.; Attiyeh, M.; Langdon-Embry, L.; Allen, P.J.; Do, R.K.G.; Simpson, A.L. CT radiomics to predict high-risk intraductal papillary mucinous neoplasms of the pancreas. Med. Phys. 2018, 45, 5019–5029. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.; Li, M.; Chu, T.; Duan, H.; Liu, H.; Zhang, J.; Duan, K.; Liu, H.; Wei, F. Comprehensive analysis of clinical data and radiomic features from contrast enhanced CT for differentiating benign and malignant pancreatic intraductal papillary mucinous neoplasms. Sci. Rep. 2024, 14, 17218. [Google Scholar] [CrossRef]
- Tacelli, M.; Celsa, C.; Magro, B.; Barchiesi, M.; Barresi, L.; Capurso, G.; Arcidiacono, P.G.; Cammà, C.; Crinò, S.F. Diagnostic performance of endoscopic ultrasound through-the-needle microforceps biopsy of pancreatic cystic lesions: Systematic review with meta-analysis. Dig. Endosc. 2020, 32, 1018–1030. [Google Scholar] [CrossRef]
- Suzuki, R.; Thosani, N.; Annangi, S.; Guha, S.; Bhutani, M.S. Diagnostic yield of EUS-FNA-based cytology distinguishing malignant and benign IPMNs: A systematic review and meta-analysis. Pancreatology 2014, 14, 380–384. [Google Scholar] [CrossRef]
- Wang, Q.-X.; Xiao, J.; Orange, M.; Zhang, H.; Zhu, Y.-Q. EUS-Guided FNA for Diagnosis of Pancreatic Cystic Lesions: A Meta-Analysis. Cell. Physiol. Biochem. 2015, 36, 1197–1209. [Google Scholar] [CrossRef]
- Tanaka, M.; Heckler, M.; Liu, B.; Heger, U.; Hackert, T.; Michalski, C.W. Cytologic Analysis of Pancreatic Juice Increases Specificity of Detection of Malignant IPMN–A Systematic Review. Clin. Gastroenterol. Hepatol. 2019, 17, 2199–2211.e21. [Google Scholar] [CrossRef]
- Heckler, M.; Brieger, L.; Heger, U.; Pausch, T.; Tjaden, C.; Kaiser, J.; Tanaka, M.; Hackert, T.; Michalski, C.W. Predictive performance of factors associated with malignancy in intraductal papillary mucinous neoplasia of the pancreas. BJS Open 2018, 2, 13–24. [Google Scholar] [CrossRef]
- Ohno, E.; Balduzzi, A.; Hijioka, S.; De Pastena, M.; Marchegiani, G.; Kato, H.; Takenaka, M.; Haba, S.; Salvia, R. Association of high-risk stigmata and worrisome features with advanced neoplasia in intraductal papillary mucinous neoplasms (IPMN): A systematic review. Pancreatology 2024, 24, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Tsumura, A.; Hirono, S.; Kawai, M.; Okada, K.-I.; Miyazawa, M.; Kitahata, Y.; Kobayashi, R.; Hayami, S.; Ueno, M.; Yanagisawa, A.; et al. Surgical indication for intraductal papillary mucinous neoplasm without mural nodule ≥5 mm. Surgery 2021, 169, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Liu, F.; Li, J.; Cao, K.; Wang, T.; Zhang, H.; Li, Q.; Meng, Y.; Yu, J.; Feng, X.; et al. Computed tomography nomogram to predict a high-risk intraductal papillary mucinous neoplasm of the pancreas. Abdom. Imaging 2021, 46, 5218–5228. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.K.; Kim, J.H.; Yoo, J.; Kim, J.-E.; Park, S.J.; Han, J.K. Assessment of malignant potential in intraductal papillary mucinous neoplasms of the pancreas using MR findings and texture analysis. Eur. Radiol. 2021, 31, 3394–3404. [Google Scholar] [CrossRef]
- Megibow, A.J.; Baker, M.E.; Morgan, D.E.; Kamel, I.R.; Sahani, D.V.; Newman, E.; Brugge, W.R.; Berland, L.L.; Pandharipande, P.V. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J. Am. Coll. Radiol. 2017, 14, 911–923. [Google Scholar] [CrossRef]
- Vege, S.S.; Ziring, B.; Jain, R.; Moayyedi, P.; Adams, M.A.; Dorn, S.D.; Dudley-Brown, S.L.; Flamm, S.L.; Gellad, Z.F.; Gruss, C.B.; et al. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015, 148, 819–822. [Google Scholar] [CrossRef]
- Hwang, J.A.; Choi, S.-Y.; Lee, J.E.; Kim, S.S.; Lee, S.; Moon, J.Y.; Heo, N.H. Pre-operative nomogram predicting malignant potential in the patients with intraductal papillary mucinous neoplasm of the pancreas: Focused on imaging features based on revised international guideline. Eur. Radiol. 2020, 30, 3711–3722. [Google Scholar] [CrossRef]
- Wu, Y.A.; Oba, A.; Beaty, L.; Colborn, K.L.; Franco, S.R.; Harnke, B.; Meguid, C.; Negrini, D.; Valente, R.; Ahrendt, S.; et al. Ductal Dilatation of ≥5 mm in Intraductal Papillary Mucinous Neoplasm Should Trigger the Consideration for Pancreatectomy: A Meta-Analysis and Systematic Review of Resected Cases. Cancers 2021, 13, 2031. [Google Scholar] [CrossRef]
- Kang, M.J.; Jang, J.; Lee, S.; Park, T.; Lee, S.Y.; Kim, S. Clinicopathological Meaning of Size of Main-Duct Dilatation in Intraductal Papillary Mucinous Neoplasm of Pancreas: Proposal of a Simplified Morphological Classification Based on the Investigation on the Size of Main Pancreatic Duct. World J. Surg. 2015, 39, 2006–2013. [Google Scholar] [CrossRef]
- Kang, J.S.; Park, T.; Han, Y.; Lee, S.; Lim, H.; Kim, H.; Kim, S.H.; Kwon, W.; Kim, S.-W.; Jang, J.-Y. Clinical validation of the 2017 international consensus guidelines on intraductal papillary mucinous neoplasm of the pancreas. Ann. Surg. Treat. Res. 2019, 97, 58–64. [Google Scholar] [CrossRef]
- Kwong, W.T.; Lawson, R.D.; Hunt, G.; Fehmi, S.M.; Proudfoot, J.A.; Xu, R.; Giap, A.; Tang, R.S.; Gonzalez, I.; Krinsky, M.L.; et al. Rapid Growth Rates of Suspected Pancreatic Cyst Branch Duct Intraductal Papillary Mucinous Neoplasms Predict Malignancy. Dig. Dis. Sci. 2015, 60, 2800–2806. [Google Scholar] [CrossRef]
- Gavazzi, F.; Capretti, G.; Giordano, L.; Ridolfi, C.; Spaggiari, P.; Sollai, M.; Carrara, S.; Nappo, G.; Bozzarelli, S.; Zerbi, A. Pancreatic ductal adenocarcinoma and invasive intraductal papillary mucinous tumor: Different prognostic factors for different overall survival. Dig. Liver Dis. 2022, 54, 826–833. [Google Scholar] [CrossRef]
- Zelga, P.; Hernandez-Barco, Y.G.; Qadan, M.M.; Ferrone, C.R.M.; Kambadakone, A.M.; Horick, N.; Jah, A.M.; Warshaw, A.L.M.; Lillemoe, K.D.M.; Balakrishnan, A.M.; et al. Number of Worrisome Features and Risk of Malignancy in Intraductal Papillary Mucinous Neoplasm. J. Am. Coll. Surg. 2022, 234, 1021–1030. [Google Scholar] [CrossRef]
- Idilman, I.S.; Yildiz, A.E.; Karaosmanoglu, A.D.; Ozmen, M.N.; Akata, D.; Karcaaltincaba, M. Proton density fat fraction: Magnetic resonance imaging applications beyond the liver. Diagn. Interv. Radiol. 2022, 28, 83–91. [Google Scholar] [CrossRef]
- Fukui, H.; Hori, M.; Fukuda, Y.; Onishi, H.; Nakamoto, A.; Ota, T.; Ogawa, K.; Ninomiya, K.; Tatsumi, M.; Osuga, K.; et al. Evaluation of fatty pancreas by proton density fat fraction using 3-T magnetic resonance imaging and its association with pancreatic cancer. Eur. J. Radiol. 2019, 118, 25–31. [Google Scholar] [CrossRef]
- Sotozono, H.; Kanki, A.; Yasokawa, K.; Yamamoto, A.; Sanai, H.; Moriya, K.; Tamada, T. Value of 3-T MR imaging in intraductal papillary mucinous neoplasm with a concomitant invasive carcinoma. Eur. Radiol. 2022, 32, 8276–8284. [Google Scholar] [CrossRef] [PubMed]
- Swauger, S.E.; Fashho, K.; Hornung, L.N.; Elder, D.A.; Thapaliya, S.; Anton, C.G.; Trout, A.T.; Abu-El-Haija, M. Association of pancreatic fat on imaging with pediatric metabolic co-morbidities. Pediatr. Radiol. 2023, 53, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Cheng, S.; Shi, H.; Lu, M.; Wang, C.; Duan, S.; Xu, Q.; Shi, H. Radiomics Analysis for Predicting Malignant Potential of Intraductal Papillary Mucinous Neoplasms of the Pancreas: Comparison of CT and MRI. Acad. Radiol. 2022, 29, 367–375. [Google Scholar] [CrossRef]
- Attiyeh, M.A.; Chakraborty, J.; Gazit, L.; Langdon-Embry, L.; Gonen, M.; Balachandran, V.P.; D’ANgelica, M.I.; DeMatteo, R.P.; Jarnagin, W.R.; Kingham, T.P.; et al. Preoperative risk prediction for intraductal papillary mucinous neoplasms by quantitative CT image analysis. HPB 2019, 21, 212–218. [Google Scholar] [CrossRef]
- Kim, M.C.; Kim, J.H.; Jeon, S.K.; Kang, H.-J. CT findings and clinical effects of high grade pancreatic intraepithelial neoplasia in patients with intraductal papillary mucinous neoplasms. PLoS ONE 2024, 19, e0298278. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.; Han, Y.; Byun, Y.; Kang, J.S.; Choi, Y.J.; Kim, H.; Jang, J.-Y. Predictive Features of Malignancy in Branch Duct Type Intraductal Papillary Mucinous Neoplasm of the Pancreas: A Meta-Analysis. Cancers 2020, 12, 2618. [Google Scholar] [CrossRef] [PubMed]
- Fouladi, D.F.; Raman, S.P.; Hruban, R.H.; Fishman, E.K.; Kawamoto, S. Invasive Intraductal Papillary Mucinous Neoplasms: CT Features of Colloid Carcinoma Versus Tubular Adenocarcinoma of the Pancreas. Am. J. Roentgenol. 2020, 214, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.T.; Sadler, T.J.; Whitley, S.; Brais, R.; Godfrey, E. The CT fish mouth ampulla sign: A highly specific finding in main duct and mixed intraductal papillary mucinous neoplasms. Br. J. Radiol. 2019, 92, 20190461. [Google Scholar] [CrossRef]
- Correa-Gallego, C.; Miyasaka, Y.; Hozaka, Y.; Nishino, H.; Kawamoto, M.; Vieira, D.L.; Ohtsuka, T.; Wolfgang, C. Surveillance after resection of non-invasive intraductal papillary mucinous neoplasms (IPMN). A systematic review. Pancreatology 2023, 23, 258–265. [Google Scholar] [CrossRef]
- Kin, T.; Shimizu, Y.; Hijioka, S.; Hara, K.; Katanuma, A.; Nakamura, M.; Yamada, R.; Itoi, T.; Ueki, T.; Masamune, A.; et al. A comparative study between computed tomography and endoscopic ultrasound in the detection of a mural nodule in intraductal papillary mucinous neoplasm–Multicenter observational study in Japan. Pancreatology 2023, 23, 550–555. [Google Scholar] [CrossRef]
- Yamashita, Y.; Kawaji, Y.; Shimokawa, T.; Yamazaki, H.; Tamura, T.; Hatamaru, K.; Itonaga, M.; Ashida, R.; Kawai, M.; Kitano, M. Usefulness of Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Diagnosis of Malignancy in Intraductal Papillary Mucinous Neoplasm. Diagnostics 2022, 12, 2141. [Google Scholar] [CrossRef]
- Ohno, E.; Kawashima, H.; Ishikawa, T.; Iida, T.; Suzuki, H.; Uetsuki, K.; Yashika, J.; Yamada, K.; Yoshikawa, M.; Gibo, N.; et al. Can contrast-enhanced harmonic endoscopic ultrasonography accurately diagnose main pancreatic duct involvement in intraductal papillary mucinous neoplasms? Pancreatology 2020, 20, 887–894. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, F.; Zhu, J.; Du, Y.; Jin, Z.; Li, Z. Assessment of morbidity and mortality associated with endoscopic ultrasound-guided fine-needle aspiration for pancreatic cystic lesions: A systematic review and meta-analysis. Dig. Endosc. 2017, 29, 667–675. [Google Scholar] [CrossRef]
- Khoury, T.; Sbeit, W. Cost-effectiveness of rapid on-site evaluation of endoscopic ultrasound fine needle aspiration in gastrointestinal lesions. Cytopathology 2021, 32, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Pflüger, M.J.; Jamouss, K.T.; Afghani, E.; Lim, S.J.; Franco, S.R.; Mayo, H.; Spann, M.; Wang, H.; Singhi, A.; Lennon, A.M.; et al. Predictive ability of pancreatic cyst fluid biomarkers: A systematic review and meta-analysis. Pancreatology 2023, 23, 868–877. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.R.; Garg, R.; Rustagi, T. Pancreatic cyst fluid glucose in differentiating mucinous from nonmucinous pancreatic cysts: A systematic review and meta-analysis. Gastrointest. Endosc. 2021, 94, 698–712.e6. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Takahashi, C.; Snyder, R.A.; Parikh, A.A. Stratifying Intraductal Papillary Mucinous Neoplasms by Cyst Fluid Analysis: Present and Future. Int. J. Mol. Sci. 2020, 21, 1147. [Google Scholar] [CrossRef]
- Park, W.G.-U.; Mascarenhas, R.M.; Palaez-Luna, M.; Smyrk, T.C.; O’Kane, D.; Clain, J.E.; Levy, M.J.; Pearson, R.K.; Petersen, B.T.; Topazian, M.D.; et al. Diagnostic Performance of Cyst Fluid Carcinoembryonic Antigen and Amylase in Histologically Confirmed Pancreatic Cysts. Pancreas 2011, 40, 42–45. [Google Scholar] [CrossRef]
- Van Der Waaij, L.A.; Van Dullemen, H.M.; Porte, R.J. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: A pooled analysis. Gastrointest. Endosc. 2005, 62, 383–389. [Google Scholar] [CrossRef]
- Fischer, C.G.; Wood, L.D. From somatic mutation to early detection: Insights from molecular characterization of pancreatic cancer precursor lesions. J. Pathol. 2018, 246, 395–404. [Google Scholar] [CrossRef]
- Takahashi, K.; Takeda, Y.; Ono, Y.; Isomoto, H.; Mizukami, Y. Current status of molecular diagnostic approaches using liquid biopsy. J. Gastroenterol. 2023, 58, 834–847. [Google Scholar] [CrossRef]
- Wesali, S.; Demir, M.A.; Verbeke, C.S.; Andersson, M.; Bratlie, S.O.; Sadik, R. EUS is accurate in characterizing pancreatic cystic lesions; a prospective comparison with cross-sectional imaging in resected cases. Surg. Endosc. 2020, 35, 6650–6659. [Google Scholar] [CrossRef]
- Pitman, M.B.; Centeno, B.A.; Ali, S.Z.; Genevay, M.; Stelow, E.; Mino-Kenudson, M.; Fernandez-del Castillo, C.; Max Schmidt, C.; Brugge, W.; Layfield, L.; et al. Standardized terminology and nomenclature for pancreatobiliary cytology: The Papanicolaou Society of Cytopathology guidelines. Diagn. Cytopathol. 2014, 42, 338–350. [Google Scholar] [CrossRef]
- Pitman, M.B.; Layfield, L.J. The Papanicolaou Society of Cytopathology System for Reporting Pancreaticobiliary Cytology; Springer Nature: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Sung, S.; Del Portillo, A.; Gonda, T.A.; Kluger, M.D.; Tiscornia-Wasserman, P.G. Update on risk stratification in the Papanicolaou Society of Cytopathology System for Reporting Pancreaticobiliary Cytology categories: 3-Year, prospective, single-institution experience. Cancer Cytopathol. 2019, 128, 29–35. [Google Scholar] [CrossRef]
- Wright, P.K.; Shelton, D.A.; Holbrook, M.R.; Thiryayi, S.A.; Narine, N.; Slater, D.; Rana, D.N. Outcomes of endoscopic ultrasound-guided pancreatic FNAC diagnosis for solid and cystic lesions at Manchester Royal Infirmary based upon the Papanicolaou Society of Cytopathology pancreaticobiliary terminology classification scheme. Cytopathology 2017, 29, 71–79. [Google Scholar] [CrossRef]
- Pitman, M.B.; Centeno, B.A.; Reid, M.D.; Siddiqui, M.T.; Layfield, L.J.; Perez-Machado, M.; Weynand, B.; Stelow, E.B.; Lozano, M.D.; Fukushima, N.; et al. The World Health Organization Reporting System for Pancreaticobiliary Cytopathology. Acta Cytol. 2023, 67, 304–320. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, A.M.; Bohra, M.; More, N.M.; Naik, L.P. Papanicolaou society of cytopathology system for reporting pancreaticobiliary cytology: Risk stratification and cytology scope—2.5-year study. Cytojournal 2022, 19, 33–35. [Google Scholar] [CrossRef] [PubMed]
- López-Ramírez, A.N.; Villegas-González, L.F.; Serrano-Arévalo, M.L.; Flores-Hernández, L.; Lino-Silva, L.S.; González-Mena, L.E. Reclassification of lesions in biopsies by fine-needle aspiration of pancreas and biliary tree using Papanicolaou classification. J. Gastrointest. Oncol. 2018, 9, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Saieg, M.A.; Munson, V.; Colletti, S.; Nassar, A. The impact of the new proposed Papanicolaou Society of Cytopathology terminology for pancreaticobiliary cytology in endoscopic US-FNA: A single-Institutional experience. Cancer Cytopathol. 2015, 123, 488–494. [Google Scholar] [CrossRef]
- Smith, A.L.; Abdul-Karim, F.W.; Goyal, A. Cytologic categorization of pancreatic neoplastic mucinous cysts with an assessment of the risk of malignancy: A retrospective study based on the Papanicolaou Society of Cytopathology guidelines. Cancer Cytopathol. 2016, 124, 285–293. [Google Scholar] [CrossRef]
- Serinelli, S.; Khurana, K.K. Intraductal papillary mucinous neoplasms of the pancreas: Cytologic-histologic correlation study and evaluation of the cytologic accuracy in identifying high-grade dysplasia/invasive adenocarcinoma. Cytojournal 2024, 21, 6. [Google Scholar] [CrossRef]
- Dhar, J.; Samanta, J.; Nabi, Z.; Aggarwal, M.; Bellocchi, M.C.C.; Facciorusso, A.; Frulloni, L.; Crinò, S.F. Endoscopic Ultrasound-Guided Pancreatic Tissue Sampling: Lesion Assessment, Needles, and Techniques. Medicina 2024, 60, 2021. [Google Scholar] [CrossRef]
- Vilas-Boas, F.; Ribeiro, T.; Macedo, G.; Dhar, J.; Samanta, J.; Sina, S.; Manfrin, E.; Facciorusso, A.; Bellocchi, M.C.C.; De Pretis, N.; et al. Endoscopic Ultrasound-Guided Through-the-Needle Biopsy: A Narrative Review of the Technique and Its Emerging Role in Pancreatic Cyst Diagnosis. Diagnostics 2024, 14, 1587. [Google Scholar] [CrossRef]
- Kovacevic, B.; Klausen, P.; Rift, C.V.; Toxværd, A.; Grossjohann, H.; Karstensen, J.G.; Brink, L.; Hassan, H.; Kalaitzakis, E.; Storkholm, J.; et al. Clinical impact of endoscopic ultrasound-guided through-the-needle microbiopsy in patients with pancreatic cysts. Endoscopy 2021, 53, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Wang, Z.-J.; Pan, C.-Y.; Wu, C.; Li, Z.-S.; Jin, Z.-D.; Wang, K.-X. Comparative Performance of Endoscopic Ultrasound-Based Techniques in Patients With Pancreatic Cystic Lesions: A Network Meta-Analysis. Am. J. Gastroenterol. 2023, 118, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Trindade, A.J.; Yachimski, P.; Benias, P.; Nieto, J.; Manvar, A.; Ho, S.; Esnakula, A.; Gamboa, A.; Sethi, A.; et al. Histologic Analysis of Endoscopic Ultrasound-Guided Through the Needle Microforceps Biopsies Accurately Identifies Mucinous Pancreas Cysts. Clin. Gastroenterol. Hepatol. 2019, 17, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- De Lusong, M.A.A.; Pajes, A.N.N.I. Evaluation of Fine Needle Biopsy (FNB) for Endoscopic Ultrasound (EUS)-guided Tissue Acquisition of Pancreatic Masses to Negate the Need for Rapid On-site Evaluation: A Randomized Control Trial. Acta Medica Philipp. 2024, 58, 51–56. [Google Scholar] [CrossRef]
- Facciorusso, A.; Crinò, S.F.; Ramai, D.; Madhu, D.; Fugazza, A.; Carrara, S.; Spadaccini, M.; Mangiavillano, B.; Gkolfakis, P.; Mohan, B.P.; et al. Comparative diagnostic performance of different techniques for EUS-guided fine-needle biopsy sampling of solid pancreatic masses: A network meta-analysis. Gastrointest. Endosc. 2023, 97, 839–848.e5. [Google Scholar] [CrossRef]
- Mishra, A.; Hunold, T.M.; Peddu, D.K.; Philips, G.M.; Wamsteker, E.-J.; Kwon, R.S.; Schulman, A.R.; Shi, J.; Carpenter, E.S.; Machicado, J.D. Histologic Diagnosis of Pancreatic Cystic Lesions with Endoscopic Ultrasound Fine Needle Biopsy and Impact on Management Decisions. Dig. Dis. Sci. 2025, 70, 2873–2881. [Google Scholar] [CrossRef]
- Barresi, L.; Tarantino, I.; Traina, M.; Granata, A.; Curcio, G.; Azzopardi, N.; Baccarini, P.; Liotta, R.; Fornelli, A.; Maimone, A.; et al. Endoscopic ultrasound-guided fine needle aspiration and biopsy using a 22-gauge needle with side fenestration in pancreatic cystic lesions. Dig. Liver Dis. 2014, 46, 45–50. [Google Scholar] [CrossRef]
- Crinò, S.F.; Bellocchi, M.C.C.; Di Mitri, R.; Inzani, F.; Rimbaș, M.; Lisotti, A.; Manfredi, G.; Teoh, A.Y.B.; Mangiavillano, B.; Sendino, O.; et al. Wet-suction versus slow-pull technique for endoscopic ultrasound-guided fine-needle biopsy: A multicenter, randomized, crossover trial. Endoscopy 2023, 55, 225–234. [Google Scholar] [CrossRef]
- Facciorusso, A.; Arvanitakis, M.; Crinò, S.F.; Fabbri, C.; Fornelli, A.; Leeds, J.; Archibugi, L.; Carrara, S.; Dhar, J.; Gkolfakis, P.; et al. Endoscopic ultrasound-guided tissue sampling: European Society of Gastrointestinal Endoscopy (ESGE) Technical and Technology Review. Endoscopy 2025, 57, 390–418. [Google Scholar] [CrossRef]
- Saftoiu, A.; Balaban, V.; Cazacu, I.; Pinte, L.; Jinga, M.; Bhutani, M. EUS-through-the-needle microbiopsy forceps in pancreatic cystic lesions: A systematic review. Endosc. Ultrasound 2021, 10, 19. [Google Scholar] [CrossRef]
- Krishna, S.; Abdelbaki, A.; Hart, P.A.; Machicado, J.D. Endoscopic Ultrasound-Guided Needle-Based Confocal Endomicroscopy as a Diagnostic Imaging Biomarker for Intraductal Papillary Mucinous Neoplasms. Cancers 2024, 16, 1238. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Chao, W.-L.; Cao, T.; Culp, S.; Napoléon, B.; El-Dika, S.; Machicado, J.D.; Pannala, R.; Mok, S.; Luthra, A.K.; et al. Improving Pancreatic Cyst Management: Artificial Intelligence-Powered Prediction of Advanced Neoplasms through Endoscopic Ultrasound-Guided Confocal Endomicroscopy. Biomimetics 2023, 8, 496. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M. Needle-based confocal laser endomicroscopy. Endosc. Ultrasound 2015, 4, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.G.; Hart, P.A.; DeWitt, J.M.; DiMaio, C.J.; Kongkam, P.; Napoleon, B.; Othman, M.O.; Tan, D.M.Y.; Strobel, S.G.; Stanich, P.P.; et al. EUS-guided confocal laser endomicroscopy: Prediction of dysplasia in intraductal papillary mucinous neoplasms (with video). Gastrointest. Endosc. 2020, 91, 551–563.e5. [Google Scholar] [CrossRef]
- Krishna, S.G.; Abdelbaki, A.; Li, Z.; Culp, S.; Xiong, X.; Napoleon, B.; Mok, S.; Bertani, H.; Feng, Y.; Kongkam, P.; et al. Towards automating risk stratification of intraductal papillary mucinous Neoplasms: Artificial intelligence advances beyond human expertise with confocal laser endomicroscopy. Pancreatology 2025, 25, 658–666. [Google Scholar] [CrossRef]
- Machicado, J.D.; Napoleon, B.; Lennon, A.M.; El-Dika, S.; Pereira, S.P.; Tan, D.; Pannala, R.; Girotra, M.; Kongkam, P.; Bertani, H.; et al. Accuracy and agreement of a large panel of endosonographers for endomicroscopy-guided virtual biopsy of pancreatic cystic lesions. Pancreatology 2022, 22, 994–1002. [Google Scholar] [CrossRef]
- Cheesman, A.R.; Zhu, H.; Liao, X.; Szporn, A.H.; Kumta, N.A.; Nagula, S.; DiMaio, C.J. Impact of EUS-guided microforceps biopsy sampling and needle-based confocal laser endomicroscopy on the diagnostic yield and clinical management of pancreatic cystic lesions. Gastrointest. Endosc. 2020, 91, 1095–1104. [Google Scholar] [CrossRef]
- Saghir, S.M.; Dhindsa, B.S.; Daid, S.G.S.; Mashiana, H.S.; Dhaliwal, A.; Cross, C.; Singh, S.; Bhat, I.; Ohning, G.V.; Adler, D.G. Efficacy of EUS-guided needle-based confocal laser endomicroscopy in the diagnosis of pancreatic lesions: A systematic review and meta-analysis. Endosc. Ultrasound 2022, 11, 275–282. [Google Scholar] [CrossRef]
- Karia, K.; Kahaleh, M. A Review of Probe-Based Confocal Laser Endomicroscopy for Pancreaticobiliary Disease. Clin. Endosc. 2016, 49, 462–466. [Google Scholar] [CrossRef]
- Maker, A.V.; Katabi, N.; Gonen, M.; DeMatteo, R.P.; D’angelica, M.I.; Fong, Y.; Jarnagin, W.R.; Brennan, M.F.; Allen, P.J. Pancreatic Cyst Fluid and Serum Mucin Levels Predict Dysplasia in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann. Surg. Oncol. 2011, 18, 199–206. [Google Scholar] [CrossRef]
- Stiles, Z.E.; Khan, S.; Patton, K.T.; Jaggi, M.; Behrman, S.W.; Chauhan, S.C. Transmembrane mucin MUC13 distinguishes intraductal papillary mucinous neoplasms from non-mucinous cysts and is associated with high-risk lesions. HPB 2019, 21, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Maker, A.V.; Hu, V.; Kadkol, S.S.; Hong, L.; Brugge, W.; Winter, J.; Yeo, C.J.; Hackert, T.; Büchler, M.; Lawlor, R.T.; et al. Cyst Fluid Biosignature to Predict Intraductal Papillary Mucinous Neoplasms of the Pancreas with High Malignant Potential. J. Am. Coll. Surg. 2019, 228, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Matthaei, H.; Wylie, D.; Lloyd, M.B.; Dal Molin, M.; Kemppainen, J.; Mayo, S.C.; Wolfgang, C.L.; Schulick, R.D.; Langfield, L.; Andruss, B.F.; et al. miRNA biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts. Clin Cancer Res. 2012, 18, 4713–4724. [Google Scholar] [CrossRef] [PubMed]
- Utomo, W.; Looijenga, L.; Bruno, M.; Hansen, B.; Gillis, A.; Biermann, K.; Peppelenbosch, M.; Fuhler, G.; Braat, H. A MicroRNA Panel in Pancreatic Cyst Fluid for the Risk Stratification of Pancreatic Cysts in a Prospective Cohort. Mol. Ther. Nucleic Acids 2016, 5, e350. [Google Scholar] [CrossRef]
- Shirakami, Y.; Iwashita, T.; Uemura, S.; Imai, H.; Murase, K.; Shimizu, M. Micro-RNA Analysis of Pancreatic Cyst Fluid for Diagnosing Malignant Transformation of Intraductal Papillary Mucinous Neoplasm by Comparing Intraductal Papillary Mucinous Adenoma and Carcinoma. J. Clin. Med. 2021, 10, 2249. [Google Scholar] [CrossRef]
- Boroughs, L.K.; DeBerardinis, R.J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 2015, 17, 351–359. [Google Scholar] [CrossRef]
- Di Gangi, I.M.; Mazza, T.; Fontana, A.; Copetti, M.; Fusilli, C.; Ippolito, A.; Mattivi, F.; Latiano, A.; Andriulli, A.; Vrhovsek, U.; et al. Metabolomic profile in pancreatic cancer patients: A consensus-based approach to identify highly discriminating metabolites. Oncotarget 2016, 7, 5815–5829. [Google Scholar] [CrossRef]
- Mayers, J.R.; Wu, C.; Clish, C.B.; Kraft, P.; Torrence, M.E.; Fiske, B.P.; Yuan, C.; Bao, Y.; Townsend, M.K.; Tworoger, S.S.; et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat. Med. 2014, 20, 1193–1198. [Google Scholar] [CrossRef]
- Mehta, K.Y.; Wu, H.-J.; Menon, S.S.; Fallah, Y.; Zhong, X.; Rizk, N.; Unger, K.; Mapstone, M.; Fiandaca, M.S.; Federoff, H.J.; et al. Metabolomic biomarkers of pancreatic cancer: A meta-analysis study. Oncotarget 2017, 8, 68899–68915. [Google Scholar] [CrossRef]
- Gaiser, R.A.; Pessia, A.; Ateeb, Z.; Davanian, H.; Fernández Moro, C.; Alkharaan, H.; Healy, K.; Ghazi, S.; Arnelo, U.; Valente, R.; et al. Integrated targeted metabolomic and lipidomic analysis: A novel approach to classifying early cystic precursors to invasive pancreatic cancer. Sci. Rep. 2019, 9, 10208. [Google Scholar] [CrossRef]
- Das, K.K.; Xiao, H.; Geng, X.; Fernandez-Del-Castillo, C.; Morales-Oyarvide, V.; Daglilar, E.; Forcione, D.G.; Bounds, B.C.; Brugge, W.R.; Pitman, M.B.; et al. mAb Das-1 is specific for high-risk and malignant intraductal papillary mucinous neoplasm (IPMN). Gut 2014, 63, 1626.1–1634. [Google Scholar] [CrossRef]
- Das, K.K.; Geng, X.; Brown, J.W.; Morales-Oyarvide, V.; Huynh, T.; Pergolini, I.; Pitman, M.B.; Ferrone, C.; Al Efishat, M.; Haviland, D.; et al. Cross Validation of the Monoclonal Antibody Das-1 in Identification of High-Risk Mucinous Pancreatic Cystic Lesions. Gastroenterology 2019, 157, 720–730.e2. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Repici, A.; Koçollari, A.; Auriemma, F.; Bianchetti, M.; Mangiavillano, B. Pancreatoscopy: An update. World J. Gastrointest. Endosc. 2019, 11, 22–30. [Google Scholar] [CrossRef] [PubMed]
- de Jong, D.M.; Stassen, P.M.C.; Koerkamp, B.G.; Ellrichmann, M.; Karagyozov, P.I.; Anderloni, A.; Kylänpää, L.; Webster, G.J.M.; van Driel, L.M.J.W.; Bruno, M.J.; et al. The role of pancreatoscopy in the diagnostic work-up of intraductal papillary mucinous neoplasms: A systematic review and meta-analysis. Endoscopy 2023, 55, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Vehviläinen, S.; Fagerström, N.; Valente, R.; Seppänen, H.; Udd, M.; Lindström, O.; Mustonen, H.; Swahn, F.; Arnelo, U.; Kylänpää, L. Single-operator peroral pancreatoscopy in the preoperative diagnostics of suspected main duct intraductal papillary mucinous neoplasms: Efficacy and novel insights on complications. Surg. Endosc. 2022, 36, 7431–7443. [Google Scholar] [CrossRef]
- Miura, T.; Igarashi, Y.; Okano, N.; Miki, K.; Okubo, Y. Endoscopic diagnosis of intraductal papillary-mucinous neoplasm of the pancreas by means of peroral pancreatoscopy using a small-diameter videoscope and narrow-band imaging. Dig. Endosc. 2010, 22, 119–123. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Okano, N.; Ito, K.; Takuma, K.; Hara, S.; Iwasaki, S.; Yoshimoto, K.; Yamada, Y.; Watanabe, K.; Kimura, Y.; et al. Peroral Pancreatoscopy with Videoscopy and Narrow-Band Imaging in Intraductal Papillary Mucinous Neoplasms with Dilatation of the Main Pancreatic Duct. Clin. Endosc. 2022, 55, 270–278. [Google Scholar] [CrossRef]
- Koshita, S.; Noda, Y.; Kanno, Y.; Ogawa, T.; Kusunose, H.; Sakai, T.; Yonamine, K.; Miyamoto, K.; Kozakai, F.; Okano, H.; et al. Digital peroral pancreatoscopy to determine surgery for patients who have intraductal papillary mucinous neoplasms of the pancreas with mural nodules. Endosc. Int. Open 2024, 12, E1401–E1410. [Google Scholar] [CrossRef]
- Kodama, T.; Koshitani, T. Chapter 25—Pancreatoscopy. In ERCP; Elsevier: Amsterdam, The Netherlands, 2013; pp. 234–242.e1. [Google Scholar] [CrossRef]
| BD-IPMN | Branch duct IPMN |
| MD-IPMN | Main duct IPMN |
| MT-IPMN | Mixed type IPMN |
| Gastric |
| Intestinal |
| Pancreatobiliary |
| Oncocytic |
| LG-IPMN | Low-grade IPMN |
| HG-IPMN | High-grade IPMN |
| IC-IPMN | Invasive carcinoma IPMN |
| Technique | Sensitivity | Specificity | AUC | PPV | NPV | References |
|---|---|---|---|---|---|---|
| MRI | 73.4–90.8% | 64.4–94.8% | 81.1–90.3% | 71.00% | 82.40% | [27,28,29,30,31] |
| MRCP | 38.3–94.1% | 62.5–93.1% | N/A | N/A | N/A | [27,31,32,33] |
| CT | 62–90.4% | 57–86% | 71–90.4% | 48–80% | 58–90% | [29,30,31,34,35,36] |
| EUS | 60% | 80% | 79% | N/A | N/A | [31] |
| EUS-FNA Cytology | 28.7–64.8% | 84–94% | 84–94% | N/A | N/A | [37,38,39,40] |
| EUS-TTNB | 69.50% | N/A | N/A | N/A | N/A | [37] |
| Guidelines | Indications for Surgery | Indications for Surveillance |
|---|---|---|
| AGA, 2015 [47] | Presence of a solid component, a dilated MPD, or concerning features on EUS or EUS-FNA. | Cyst < 3 cm without solid component or dilated MPD should undergo MRI surveillance in 1 year and then every 2 years and a total of 5 years if there is no change in size or characteristics. |
| ACR, 2017 [46] | Presence of HRS or WFs, according to ACR, should prompt EUS-FNA and surgery. HRS: obstructive jaundice with a cyst in the head of pancreas, enhancing solid component within a cyst, MPD caliber ≥ 10 mm in the absence of obstruction. WFs: cyst ≥ 3 cm, thickened/enhancing cyst wall, non-enhancing mural nodule, MPD caliber ≥ 7 mm. | Cyst > 3 cm without any additional HRS or WFs; 9–10 years follow-up based on initial size. |
| European, 2018 [25] | Absolute: jaundice, enhancing mural nodule > 5 mm or solid component, MPD diameter > 10 mm, positive cytology. Relative: MPD diameter 5–9.9 mm, cyst diameter > 40 mm, cyst growth rate > 5 mm/year, symptoms, enhancing mural nodules < 5 mm, new onset of DM, acute pancreatitis, CA 19-9 > 37 U/mL. | Patients without absolute or relative indications for surgery should be placed under surveillance. A 6-month follow-up in the first year, then yearly follow-ups are recommended. For patients with relative indications for surgery, the “elderly”, and those affected by severe comorbidity, a 6-month follow-up is recommended. |
| ACG, 2018 [7] | Any of the following symptoms: jaundice, acute pancreatitis, and elevated serum level CA 19-9. Any of the following imaging findings: presence of mural nodule or solid component, dilation of MPD > 5 mm, focal dilation of the MPD, cyst diameter ≥ 3 cm, and positive cytology. The decision whether or not to resect a cystic lesion is best determined by a pancreatic team that integrates multiple different factors, such as patient comorbidities and life expectancy. | Pancreatic cyst surveillance should be offered to surgically fit patients with asymptomatic IPMNs or MCNs. The cyst surveillance strategy is stratified based on cyst size. Cyst < 1 cm: MRI every 2 years for 4 years. Cyst 1–2 cm: MRI every 1 year for 3 years. Cyst 2–3 cm: MRI or EUS every 6–12 months for 3 years. Cyst > 3 cm: MRI or EUS every 6 months for 3 years, consider referral to multidisciplinary group. Surveillance should be stopped after 5 years if there are no high-risk features and the size of the cyst is stable. |
| IAP, 2024 [21] | Presence of any HRS or WF. HRS: obstructive jaundice in a patient with a cystic lesion of the head of the pancreas, enhancing mural nodule ≥ 5 mm or solid component, MPD ≥ 10 mm, suspicious or positive results of cytology. WFs: acute pancreatitis, increased serum level of CA 19-9, new onset or acute exacerbation of DM within the past year, cyst ≥ 30 mm, enhancing mural nodule < 5 mm, thickened/enhancing cyst walls, MPD ≥ 5 mm and <10 mm, abrupt change in caliber of the pancreatic duct with distal pancreatic atrophy, lymphadenopathy, cystic growth rate ≥ 2.5 mm/year. | Absence of HRS and WFs. Surveillance scheme depends on initial cyst size: Cyst < 20 mm: 6 months once, then every 18 months. Cyst 20–30 mm: 6 months twice, then every 12 months. Cyst > 30 mm: every 6 months. Surveillance may be stopped after 5 years, if the cyst is stable. |
| Technique | Additional Features Assessed | Sensitivity | Specificity | Accuracy | PPV | NPV | AUC | References |
|---|---|---|---|---|---|---|---|---|
| CT | HRS, WFs (2017 ICG Fukuoka) | 79.5–86% | 67.8–74% | 73.7–78% | 71.40% | 76.60% | - | [29,30] |
| CT + radiomics | - | 68–82% | 57–84% | 64–78% | 48–80% | 58–90% | 0.71–0.84 | [34,35,36] |
| HRS, WFs (2017 ICG Fukuoka, 2018 European) | 69–80% | 65–72% | 67–76% | 72–78% | 61–75% | 0.75–0.83 | [34] | |
| Age, cyst size, presence of solid component, symptoms, gender | 19–93% | 35–100% | 50–80% | 36–97% | 78–95% | 0.74–0.81 | [35,61] | |
| Type of IPMN, cyst size, cystic solid, CA199, CA125, bilirubin, alkaline phosphase, gamma-ggtt, diabetes | 90.40% | 74% | 80% | - | - | 0.904 | [36] |
| Type of Different Detection PCLs | Sensitivity | Specificity | AUC | Accuracy | References |
|---|---|---|---|---|---|
| Detection of HGD or IC in IPMN | 87–90% | 73–100% | 0.95 | 83% | [105] |
| Detection of HGD or IC in BD-IPMN by humans without revised Fukuoka HRS and WFs | 58.20% | 58.80% | 0.59 | 58.50% | [106] |
| Detection of HGD or IC in BD-IPMN by humans with revised Fukuoka HRS and WFs | 72% | 56.80% | 0.64 | 62.60% | |
| Detection of HGD or IC in BD-IPMN by AI-algorithm without revised Fukuoka HRS and WFs | 87% | 54.10% | 0.7 | 66.70% | |
| Detection of HGD or IC in BD-IPMN by an AI algorithm with revised Fukuoka HRS and WFs | 78.30% | 78.40% | 0.85 | 78.30% | |
| Differentiating mucinous from non-mucinous lesions | 95.20% | 94.20% | N/A | 94.80% | [107] |
| Differentiating IPMN from other PCLs | 84.40% | 88% | N/A | 86.20% |
| Technique | Advantages | Disadvantages |
|---|---|---|
| MRI | Effective in detection mural nodules > 5 mm Detecting PDFF No radiation | Less effective in detecting MPD dilation Relatively long-lasting examination Less available than CT |
| CT | Shorter examination than MRI More available than MRI | Radiation Lower image resolution |
| Radiomics—MRI and CT | Analysis of quantitative features normally not available for humans AI algorithm integrates image features with clinical data | Still in research phase, not yet available in clinical practice |
| EUS | High resolution in detecting mural nodules and vascularity visualization Enables acquisition of cyst fluid and cytology No radiation | Training required High dependence on experience of the operators |
| EUS-FNA, cyst fluid analysis | Routinely assessed markers useful for differentiating mucinous and non-mucinous cysts | Routinely assessed markers ineffective in differentiating malignancy Complications: pancreatitis, pain, infection Training required |
| EUS-FNA, cytology | Highly specific in detecting malignancy | Insufficient sensitivity in detecting malignancy |
| EUS-TTNB | Enabling acquisition of macroscopically visible tissue samples Differentiating mucinous from non-mucinous as well as malignant cysts | Training required Complications: bleeding, pancreatitis, infections |
| EUS-FNB | Better preserved tissue architecture | Training required Complications similar to EUS-FNA |
| EUS-nCLE | Enables in vivo imaging similar to histopathology Detection of malignancy in IPMN as well as mucinous cysts | High cost Training required Not widely available Complications: pancreatitis, bleeding, abdominal pain |
| New markers: mucins, miRNA, metabolic profile, Das-1 antibody | Biomarkers present strong association with malignancy risk of IPMN, available to assess from cyst fluid or blood | Not widely available High cost |
| Pancreatoscopy | Enables direct visualization of MPD Effective in the detection malignancy of IPMN Biopsy under POP more efficient than EUS-FNA biopsy | Training required Complications: more common pancreatitis than EUS-TTNB and EUS-nCLE High cost Not widely available |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawłowski, W.; Stefański, M.; Włodarczyk, B.; Durko, Ł.; Małecka-Wojciesko, E. Pancreatic Cancer Detection in Intraductal Papillary Mucinous Neoplasm (IPMN)—New Insights. Cancers 2025, 17, 3341. https://doi.org/10.3390/cancers17203341
Pawłowski W, Stefański M, Włodarczyk B, Durko Ł, Małecka-Wojciesko E. Pancreatic Cancer Detection in Intraductal Papillary Mucinous Neoplasm (IPMN)—New Insights. Cancers. 2025; 17(20):3341. https://doi.org/10.3390/cancers17203341
Chicago/Turabian StylePawłowski, Wojciech, Mateusz Stefański, Barbara Włodarczyk, Łukasz Durko, and Ewa Małecka-Wojciesko. 2025. "Pancreatic Cancer Detection in Intraductal Papillary Mucinous Neoplasm (IPMN)—New Insights" Cancers 17, no. 20: 3341. https://doi.org/10.3390/cancers17203341
APA StylePawłowski, W., Stefański, M., Włodarczyk, B., Durko, Ł., & Małecka-Wojciesko, E. (2025). Pancreatic Cancer Detection in Intraductal Papillary Mucinous Neoplasm (IPMN)—New Insights. Cancers, 17(20), 3341. https://doi.org/10.3390/cancers17203341






