Management of Regional Lymph Nodes in Clinically Node-Negative Cutaneous Squamous Cell Carcinoma of the Head and Neck: A Systematic Review & Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection Criteria and Data Extraction
2.3. Quality Assessment
2.4. Data Analysis
3. Results
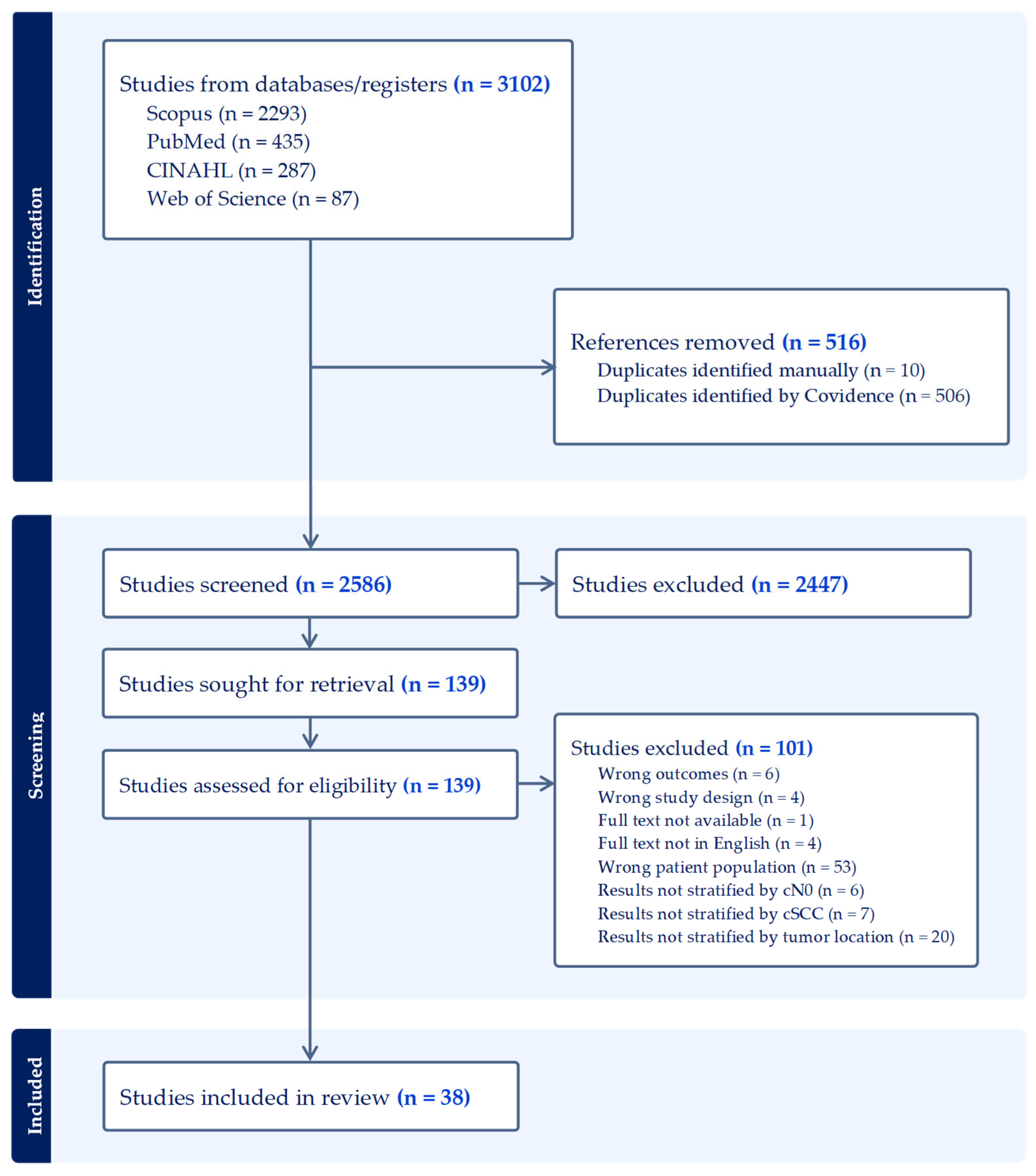
3.1. Literature Search
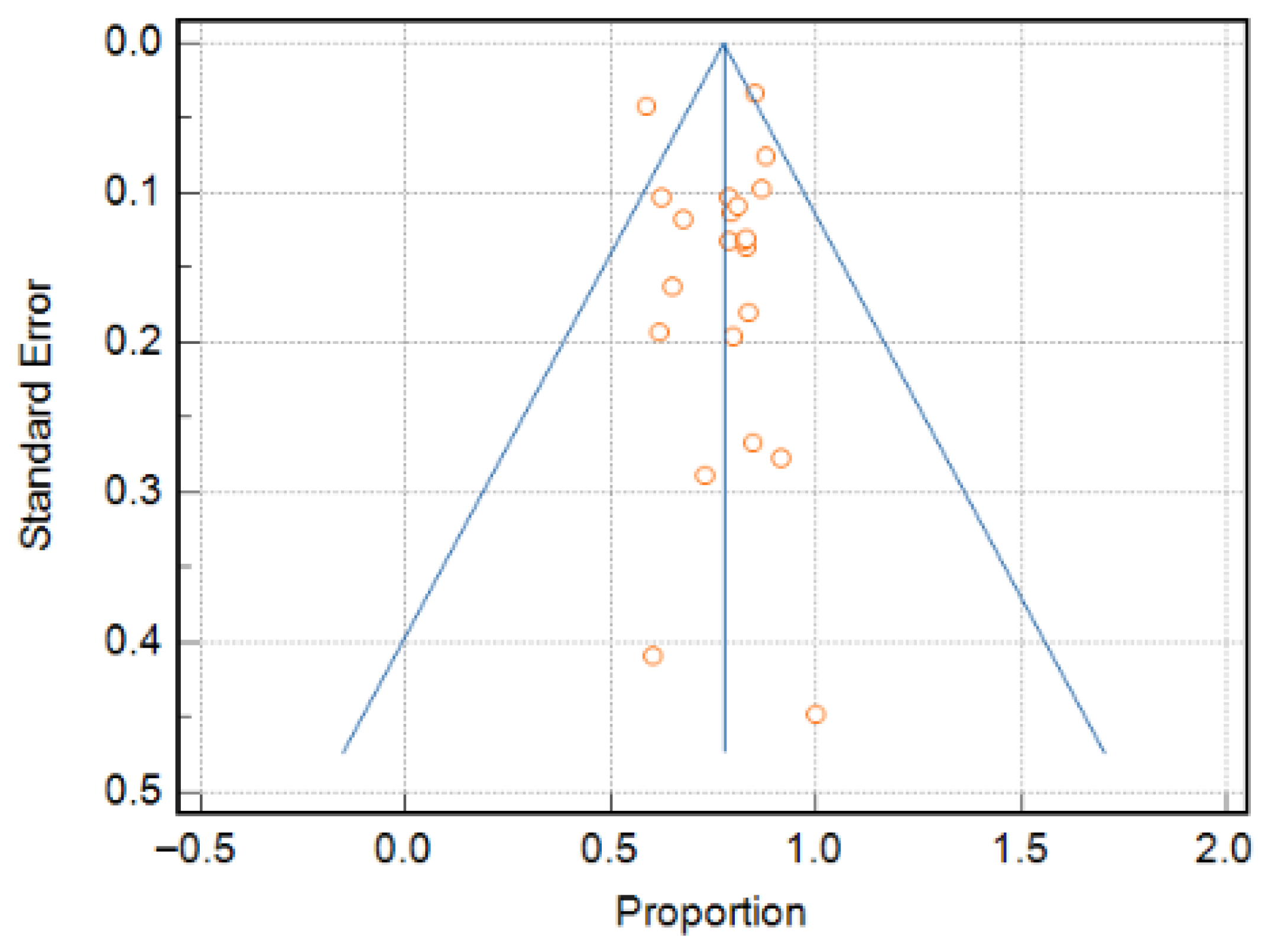
3.2. Quality Assessment
3.3. Patient Demographics and Tumor Characteristics
3.4. Occult Nodal Metastasis
3.5. Clinical Outcomes and Comparison of Group Meta-Proportions
3.6. Clinical Outcomes of Elective Nodal Irradiation
4. Discussion
4.1. Considerations for Sentinel Lymph Node Biopsy
4.2. Observation Versus Sentinel Lymph Node Biopsy
4.3. Considerations for Elective Dissection
4.4. Staging and Risk Stratification
4.5. Limitations
4.6. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| BWH | Brigham and Women’s Hospital |
| CI | Confidence interval |
| cN0 | Clinically node-negative |
| COP | Comparison of proportions |
| cSCC | Cutaneous squamous cell carcinoma |
| DOI | Depth of invasion |
| ED | Elective dissection |
| ENI | Elective nodal irradiation |
| IHC | Immunohistochemistry |
| JBI | Joanna Briggs Institute |
| HNcSCC | Head and neck cutaneous squamous cell carcinoma |
| NCCN | National Comprehensive Cancer Network |
| OLE | Oxford Level of Evidence |
| PNI | Perineural invasion |
| PRISMA | Preferred Reporting Items for Systematic Review and Meta-Analysis |
| SLNB | Sentinel lymph node biopsy |
Appendix A
| Database | Terms | Results, n |
|---|---|---|
| PubMed | (“squamous cell carcinoma of head and neck” OR “cutaneous squamous cell carcinoma” OR “cSCC” OR “skin squamous cell carcinoma”) AND (“clinically node-negative” OR “node-negative” OR “cN0” OR “no nodal metastasis”) AND (“head and neck” OR “head” OR “neck” OR “face” OR “ear” OR “pinna” OR “nose” OR “lip” OR “scalp” OR “cheek” OR “forehead” OR “chin” OR “periorbital” OR “temple” OR “eyelid” OR “jaw” OR “mandible” OR “maxilla” OR “perinasal” OR “oral commissure” OR “external auditory canal” OR “preauricular” OR “postauricular” OR “otolaryngology”) AND ((“SLNB” OR “sentinel lymph node” OR “sentinel node”) OR (“neck dissection” OR “elective neck” OR “cervical lymphadenectomy” OR “END” OR “node dissection”) OR (“surveillance” OR “observation” OR “non-operative”) OR (“radiation” OR “irradiation” OR “radiotherapy”)) AND (“outcomes” OR “mortality” OR “survival” OR “recurrence” OR “metastasis” OR “morbidity” OR “disease-free”) | 388 |
| SCOPUS | PubMed search terms adapted with syntax modifications | 967 |
| CINAHL | PubMed search terms adapted with syntax modifications | 101 |
| Web of Science | PubMed search terms adapted with syntax modifications | 27 |
| Database | Terms | Results, n |
|---|---|---|
| PubMed | (“cutaneous squamous cell carcinoma” OR “cSCC” OR “skin squamous cell carcinoma”) AND (“head and neck” OR “head” OR “neck” OR “face” OR “ear” OR “pinna” OR “nose” OR “lip” OR “scalp” OR “cheek” OR “forehead” OR “chin” OR “periorbital” OR “temple” OR “eyelid” OR “jaw” OR “mandible” OR “maxilla” OR “perinasal” OR “oral commissure” OR “external auditory canal” OR “preauricular” OR “postauricular” OR “otolaryngology”) AND (“occult” OR “micrometastasis” OR “subclinical” OR “undiagnosed nodal involvement” OR “nodal upstaging”) | 47 |
| SCOPUS | PubMed search terms adapted with syntax modifications | 1326 |
| CINAHL | PubMed search terms adapted with syntax modifications | 186 |
| Web of Science | PubMed search terms adapted with syntax modifications | 60 |
References
- Alam, M.; Ratner, D. Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2001, 344, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A Systematic Review of Worldwide Incidence of Nonmelanoma Skin Cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef]
- Rogers, H.W.; Weinstock, M.A.; Harris, A.R.; Hinckley, M.R.; Feldman, S.R.; Fleischer, A.B.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer in the United States, 2006. Arch. Dermatol. 2010, 146, 283–287. [Google Scholar] [CrossRef]
- Schmults, C.D.; Karia, P.S.; Carter, J.B.; Han, J.; Qureshi, A.A. Factors Predictive of Recurrence and Death From Cutaneous Squamous Cell Carcinoma: A 10-Year, Single-Institution Cohort Study. JAMA Dermatol. 2013, 149, 541–547. [Google Scholar] [CrossRef]
- Squamous Cell Skin Cancer. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology, Version 1.2026, 2025.
- Sahn, R.E.; Lang, P.G. Sentinel Lymph Node Biopsy for High-Risk Nonmelanoma Skin Cancers. Dermatol. Surg. 2007, 33, 786. [Google Scholar]
- Amin, M.; Edge, S.; Greene, F.; Byrd, D.; Brookland, R.; Washington, M.; Gershenwald, J.; Compton, C.; Hess, K.; Sullivan, D.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017; ISBN 978-3-319-40617-6. [Google Scholar]
- Kejner, A.E.; Harris, B.N.; Patel, R.; McMullen, C.; Weir, J.; Dahshan, B.A.; Carroll, W.R.; Gillespie, M.B. Management of the Parotid for High-Risk Cutaneous Squamous Cell Carcinoma: A Review from the Salivary Section of the American Head and Neck Society. Am. J. Otolaryngol. 2022, 43, 103374. [Google Scholar] [CrossRef]
- Bello, D.M.; Faries, M.B. The Landmark Series: MSLT-1, MSLT-2 and DeCOG (Management of Lymph Nodes). Ann. Surg. Oncol. 2019, 27, 15–21. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Grekin, R.C.; Garcia, J.; Bucci, M.K.; Margolis, L.W. Radiation Therapy for Cutaneous Squamous Cell Carcinoma Involving the Parotid Area Lymph Nodes: Dose and Volume Considerations. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.P.; Amdur, R.J.; Werning, J.W.; Dziegielewski, P.; Morris, C.G.; Mendenhall, W.M. Elective Neck Management for Squamous Cell Carcinoma Metastatic to the Parotid Area Lymph Nodes. Eur. Arch. Otorhinolaryngol. 2016, 273, 3875–3879. [Google Scholar] [CrossRef]
- Horakova, Z.; Starek, I.; Zapletalova, J.; Salzman, R. Parotid Gland Metastases of Cutaneous Squamous Cell Carcinoma of the Head: Occult Metastases Occurrence and Their Late Manifestation. Int. J. Clin. Pract. 2024, 2024, 5525741. [Google Scholar] [CrossRef]
- Kirke, D.N.; Porceddu, S.; Wallwork, B.D.; Panizza, B.; Coman, W.B. Pathologic Occult Neck Disease in Patients With Metastatic Cutaneous Squamous Cell Carcinoma to the Parotid. Otolaryngol. Head Neck Surg. 2011, 144, 549–551. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.J.; McNeil, E.B.; McMahon, J.D.; Pathak, I.; Lauer, C.S. Incidence of Cervical Node Involvement in Metastatic Cutaneous Malignancy Involving the Parotid Gland. Head Neck 2001, 23, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Pollaers, K.; Davidoss, N.; Hinton-Bayre, A. Management of Occult Neck Disease in Metastatic Squamous Cell Carcinoma to the Parotid Gland. Aust. J. Otolaryngol. 2019, 2, 24. [Google Scholar] [CrossRef]
- Pramana, A.; Browne, L.; Graham, P.H. Metastatic Cutaneous Squamous Cell Carcinoma to Parotid Nodes: The Role of Bolus with Adjuvant Radiotherapy. J. Med. Imaging Radiat. Oncol. 2012, 56, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Martin, J.M.; Khoo, E.; Plank, A.; Grigg, R. Outcomes of Nodal Metastatic Cutaneous Squamous Cell Carcinoma of the Head and Neck Treated in a Regional Center. Head Neck 2015, 37, 1808–1815. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- OCEBM Levels of Evidence. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 17 September 2025).
- JBI Critical Appraisal Checklist; Joanna Briggs Institute: Adelaide, Australia, 2017.
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Identifying and Quantifying Heterogeneity. In Introduction to Meta-Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 107–125. ISBN 978-0-470-74338-6. [Google Scholar]
- Sterne, J.A.; Egger, M. Funnel Plots for Detecting Bias in Meta-Analysis: Guidelines on Choice of Axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for Examining and Interpreting Funnel Plot Asymmetry in Meta-Analyses of Randomised Controlled Trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analysis: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Amit, M.; Liu, C.; Mansour, J.; Gleber-Netto, F.O.; Tam, S.; Baruch, E.N.; Aashiq, M.; El-Naggar, A.K.; Moreno, A.C.; Rosenthal, D.I.; et al. Elective Neck Dissection versus Observation in Patients with Head and Neck Cutaneous Squamous Cell Carcinoma. Cancer 2021, 127, 4413–4420. [Google Scholar] [CrossRef]
- Cannon, R.B.; Dundar, Y.; Thomas, A.; Monroe, M.M.; Buchmann, L.O.; Witt, B.L.; Sowder, A.M.; Hunt, J.P. Elective Neck Dissection for Head and Neck Cutaneous Squamous Cell Carcinoma with Skull Base Invasion. Otolaryngol. Head Neck Surg. 2017, 156, 671–676. [Google Scholar] [CrossRef]
- Civantos, F.J.; Moffat, F.L.; Goodwin, W.J. Lymphatic Mapping and Sentinel Lymphadenectomy for 106 Head and Neck Lesions: Contrasts between Oral Cavity and Cutaneous Malignancy. Laryngoscope 2006, 112, 1–15. [Google Scholar] [CrossRef]
- Demir, H.; Isken, T.; Kus, E.; Ziya Tan, Y.; Isgoren, S.; Daglioz Gorur, G.; Oc, A.; Sen, C.; Cek, D.; Ercin, C.; et al. Sentinel Lymph Node Biopsy with a Gamma Probe in Patients with High-Risk Cutaneous Squamous Cell Carcinoma: Follow-up Results of Sentinel Lymph Node-Negative Patients. Nucl. Med. Commun. 2011, 32, 1216–1222. [Google Scholar] [CrossRef]
- Dür, C.; Salmina, C.; Borner, U.; Giger, R.; Nisa, L. Relevance of Intraparotid Metastases in Head and Neck Skin Squamous Cell Carcinoma. Laryngoscope 2021, 131, 788–793. [Google Scholar] [CrossRef]
- Durham, A.B.; Lowe, L.; Malloy, K.M.; McHugh, J.B.; Bradford, C.R.; Chubb, H.; Johnson, T.M.; McLean, S.A. Sentinel Lymph Node Biopsy for Cutaneous Squamous Cell Carcinoma on the Head and Neck. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Gallegos-Hernández, J.F.; Martínez-Méndez, M.Á.; Ábrego-Vázquez, J.A.; Hernández-Sanjuan, M.; Minauro-Muñoz, G.G.; Ortiz-Maldonado, A.L. Clinical characteristics of malignant tumours originating in the external ear. Cir. Cir. 2015, 83, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Gore, S.M.; Shaw, D.; Martin, R.C.W.; Kelder, W.; Roth, K.; Uren, R.; Gao, K.; Davies, S.; Ashford, B.G.; Ngo, Q.; et al. Prospective Study of Sentinel Node Biopsy for High-Risk Cutaneous Squamous Cell Carcinoma of the Head and Neck. Head Neck 2016, 38 (Suppl. S1), E884–E889. [Google Scholar] [CrossRef]
- Hintze, J.M.; O’Riordan, I.; Jones, H.; McHugh, A.; Gendre, A.; Timon, C.; Kinsella, J.; Lennon, P.; Walsh, R.M.; Shine, N.; et al. Pattern of Nodal Metastasis of Cutaneous Squamous Cell Carcinoma Involving the Temporal Bone. Laryngoscope Investig. Otolaryngol. 2023, 8, 120–124. [Google Scholar] [CrossRef]
- Hoch, S.; Franke, N.; Katabi, N.; Werner, J.A.; Teymoortash, A. The Value of Elective Parotidectomy in Advanced Squamous Cell Carcinoma of the Skin of the Head. Anticancer Res. 2014, 34, 2433–2436. [Google Scholar] [PubMed]
- Horakova, Z.; Starek, I.; Salzman, R. Elective Parotidectomy and Neck Dissection Are Not Beneficial in Cutaneous Squamous Cell Carcinoma of the Head. Braz. J. Otorhinolaryngol. 2024, 90, 101352. [Google Scholar] [CrossRef]
- Jansen, P.; Petri, M.; Merz, S.F.; Brinker, T.J.; Schadendorf, D.; Stang, A.; Stoffels, I.; Klode, J. The Prognostic Value of Sentinel Lymph Nodes on Distant Metastasis–Free Survival in Patients with High-Risk Squamous Cell Carcinoma. Eur. J. Cancer 2019, 111, 107–115. [Google Scholar] [CrossRef]
- Kadakia, S.; Ducic, Y.; Marra, D.; Saman, M. The Role of Elective Superficial Parotidectomy in the Treatment of Temporal Region Squamous Cell Carcinoma. Oral Maxillofac. Surg. 2016, 20, 143–147. [Google Scholar] [CrossRef]
- Kiyokawa, Y.; Ariizumi, Y.; Ohno, K.; Ito, T.; Kawashima, Y.; Tsunoda, A.; Kishimoto, S.; Asakage, T.; Tsutsumi, T. Indications for and Extent of Elective Neck Dissection for Lymph Node Metastasis from External Auditory Canal Carcinoma. Auris Nasus Larynx 2021, 48, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Kuscu, O.; Bajin, M.D.; Süslü, N.; Hoşal, A.Ş. The Role of Suprahyoid Neck Dissection in the Treatment of Squamous Cell Carcinoma of the Lower Lip: 20 Years’ Experience at a Tertiary Center. J. Cranio-Maxillofac. Surg. 2016, 44, 1404–1407. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Tanaka, R.; Fujisawa, Y.; Nakamura, Y.; Ito, S.; Fujimoto, M. Availability of Sentinel Lymph Node Biopsy for Cutaneous Squamous Cell Carcinoma. J. Dermatol. 2017, 44, 431–437. [Google Scholar] [CrossRef]
- Ma, Y.; Shan, D.; Zhang, H. Imaging and Sentinel Lymph Node Biopsy in High Risk Head and Neck Cutaneous Squamous Cell Carcinoma: A Chinese Cohort Study. Front. Oncol. 2025, 15, 1507137. [Google Scholar] [CrossRef] [PubMed]
- Melo, G.; Guilherme, L.; Palumbo, M.; Rosano, M.; Neves, M.; Callegari, F.; Abrahao, M.; Cervantes, O. Parotidectomy and Neck Dissection in Locally Advanced and Relapsed Cutaneous Squamous Cell Carcinoma of the Head and Neck Region. Braz. J. Otorhinolaryngol. 2022, 88 (Suppl. S4), S152–S162. [Google Scholar] [CrossRef]
- Mooney, C.P.; Martin, R.C.W.; Dirven, R.; Ashford, B.G.; Shannon, K.; Palme, C.E.; Ngo, Q.; Wykes, J.; Davies, S.; Gao, K.; et al. Sentinel Node Biopsy in 105 High-Risk Cutaneous SCCs of the Head and Neck: Results of a Multicenter Prospective Study. Ann. Surg. Oncol. 2019, 26, 4481–4488. [Google Scholar] [CrossRef]
- Moore, B.A.; Weber, R.S.; Prieto, V.; El-Naggar, A.; Holsinger, F.C.; Zhou, X.; Lee, J.J.; Lippman, S.; Clayman, G.L. Lymph Node Metastases from Cutaneous Squamous Cell Carcinoma of the Head and Neck. Laryngoscope 2005, 115, 1561–1567. [Google Scholar] [CrossRef]
- Peiffer, N.; Kutz, J.W.; Myers, L.L.; Isaacson, B.; Sumer, B.D.; Truelson, J.M.; Ahn, C.; Roland, P.S. Patterns of Regional Metastasis in Advanced Stage Cutaneous Squamous Cell Carcinoma of the Auricle. Otolaryngol. Head Neck Surg. 2011, 144, 36–42. [Google Scholar] [CrossRef]
- Pollock, J.; Pollock, A.E.; Hardin, R.; Yousef, M.; Nardo, J.; Polack, E.P. Sentinel Node Lymphoscintigraphy in High-Risk Cutaneous Squamous Cell Carcinoma. Clin. Ski. Cancer 2016, 1, 88–93. [Google Scholar] [CrossRef]
- Pride, R.L.D.; Lopez, J.J.; Brewer, J.D.; Price, D.L.; Otley, C.C.; Roenigk, R.K.; Arpey, C.J.; Baum, C.L. Outcomes of Sentinel Lymph Node Biopsy for Primary Cutaneous Squamous Cell Carcinoma of the Head and Neck. Dermatol. Surg. 2022, 48, 157. [Google Scholar] [CrossRef]
- Ridha, H.; Garioch, J.J.; Tan, E.K.; Heaton, M.J.; Igali, L.; Moncrieff, M.D. Intraoperative Use of Mohs’ Surgery for the Resection of Major Cutaneous Head and Neck Cancer under General Anaesthetic: Initial Experiences, Efficiency and Outcomes. J. Plast. Reconstr. Aesthetic Surg. 2015, 68, 1706–1712. [Google Scholar] [CrossRef]
- Ryu, W.C.; Koh, I.C.; Lee, Y.H.; Cha, J.H.; Kim, S.I.; Kim, C.G. Concordant Surgical Treatment: Non-Melanocytic Skin Cancer of the Head and Neck. Arch. Craniofac. Surg. 2017, 18, 37–43. [Google Scholar] [CrossRef]
- Salgarelli, A.C.; Sartorelli, F.; Cangiano, A.; Pagani, R.; Collini, M. Surgical Treatment of Lip Cancer: Our Experience With 106 Cases. J. Oral Maxillofac. Surg. 2009, 67, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, E.; Sofrin, E.; Bogdanov-Berezovsky, A.; Nash, M.; Segal, N. Lymph Node Metastasis in Cutaneous Head and Neck Squamous Cell Carcinoma. Dermatol. Surg. 2015, 41, 1126. [Google Scholar] [CrossRef] [PubMed]
- Sollamo, E.M.J.; Ilmonen, S.K.; Virolainen, M.S.; Suominen, S.H.H. Sentinel Lymph Node Biopsy in cN0 Squamous Cell Carcinoma of the Lip: A Retrospective Study. Head Neck 2016, 38, E1375–E1380. [Google Scholar] [CrossRef]
- Takahashi, A.; Imafuku, S.; Nakayama, J.; Nakaura, J.; Ito, K.; Shibayama, Y. Sentinel Node Biopsy for High-Risk Cutaneous Squamous Cell Carcinoma. Eur. J. Surg. Oncol. (EJSO) 2014, 40, 1256–1262. [Google Scholar] [CrossRef]
- Tartaglione, G.; Potenza, C.; Caggiati, A.; Gabrielli, F.; Russo, A.; Pagan, M. Sentinel Node Radiolocalisation and Predicitive Value in Lip Squamous Cell Carcinoma. La Radiol. Medica 2003, 106, 256–261. [Google Scholar]
- Thanh Pham, T.; Cross, S.; Gebski, V.; Veness, M.J. Squamous Cell Carcinoma of the Lip in Australian Patients: Definitive Radiotherapy Is an Efficacious Option to Surgery in Select Patients. Dermatol. Surg. 2015, 41, 219. [Google Scholar] [CrossRef]
- Tremblay-Abel, V.; Poulin, M.-A.; Blouin, M.-M.; Parent, F.; Perron, É. Sentinel Lymph Node Biopsy in High-Risk Cutaneous Squamous Cell Carcinoma: Analysis of a Large Size Retrospective Series. Dermatol. Surg. 2021, 47, 908. [Google Scholar] [CrossRef]
- Wagner, J.D.; Evdokimow, D.Z.; Weisberger, E.; Moore, D.; Chuang, T.-Y.; Wenck, S.; Coleman, J.J., III. Sentinel Node Biopsy for High-Risk Nonmelanoma Cutaneous Malignancy. Arch. Dermatol. 2004, 140, 75–79. [Google Scholar] [CrossRef]
- Wray, J.; Amdur, R.J.; Morris, C.G.; Werning, J.; Mendenhall, W.M. Efficacy of Elective Nodal Irradiation in Skin Squamous Cell Carcinoma of the Face, Ears, and Scalp. Radiat. Oncol. 2015, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.P.; Sethi, R.K.V.; Emerick, K.S. Sentinel Lymph Node Biopsy for High-Risk Cutaneous Squamous Cell Carcinoma of the Head and Neck. Laryngoscope 2020, 130, 108–114. [Google Scholar] [CrossRef]
- Xiao, Y.; Yuan, S.; Liu, F.; Liu, B.; Zhu, J.; He, W.; Li, W.; Kan, Q. Comparison between Wait-and-See Policy and Elective Neck Dissection in Clinically N0 Cutaneous Squamous Cell Carcinoma of Head and Neck. Medicine 2018, 97, e10782. [Google Scholar] [CrossRef] [PubMed]
- Zanoletti, E.; Danesi, G. The Problem of Nodal Disease in Squamous Cell Carcinoma of the Temporal Bone. Acta. Oto-Laryngol. 2010, 130, 913–916. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Cochran, A.J.; Balda, B.R.; Starz, H.; Bachter, D.; Krag, D.N.; Cruse, C.W.; Pijpers, R.; Morton, D.L. The Augsburg Consensus. Techniques of Lymphatic Mapping, Sentinel Lymphadenectomy, and Completion Lymphadenectomy in Cutaneous Malignancies. Cancer 2000, 89, 236–241. [Google Scholar] [CrossRef]
- Stoffels, I.; Boy, C.; Pöppel, T.; Kuhn, J.; Klötgen, K.; Dissemond, J.; Schadendorf, D.; Klode, J. Association between Sentinel Lymph Node Excision with or without Preoperative SPECT/CT and Metastatic Node Detection and Disease-Free Survival in Melanoma. JAMA 2012, 308, 1007–1014. [Google Scholar] [CrossRef]
- Tejera-Vaquerizo, A.; García-Doval, I.; Llombart, B.; Cañueto, J.; Martorell-Calatayud, A.; Descalzo-Gallego, M.A.; Sanmartín, O. Systematic Review of the Prevalence of Nodal Metastases and the Prognostic Utility of Sentinel Lymph Node Biopsy in Cutaneous Squamous Cell Carcinoma. J. Dermatol. 2018, 45, 781–790. [Google Scholar] [CrossRef]
- Karia, P.S.; Jambusaria-Pahlajani, A.; Harrington, D.P.; Murphy, G.F.; Qureshi, A.A.; Schmults, C.D. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital Tumor Staging for Cutaneous Squamous Cell Carcinoma. J. Clin. Oncol. 2014, 32, 327–334. [Google Scholar] [CrossRef]
- Harris, B.N.; Bayoumi, A.; Rao, S.; Moore, M.G.; Farwell, D.G.; Bewley, A.F. Factors Associated with Recurrence and Regional Adenopathy for Head and Neck Cutaneous Squamous Cell Carcinoma. Otolaryngol. Head Neck Surg. 2017, 156, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.; Rosen, C.; Thielhelm, J.; Bohannon, M.; Fosko, S. 52112 Cutaneous Squamous Cell Carcinoma with Perineural Invasion: Outcomes Differences Between Primary and Recurrent Tumors. J. Am. Acad. Dermatol. 2024, 91, AB172. [Google Scholar] [CrossRef]
- Dean, N.R.; Sweeny, L.; Magnuson, J.S.; Carroll, W.R.; Robinson, D.; Desmond, R.A.; Rosenthal, E.L. Outcomes of Recurrent Head and Neck Cutaneous Squamous Cell Carcinoma. J. Ski. Cancer 2011, 2011, 972497. [Google Scholar] [CrossRef] [PubMed]
| Study Title | Author | Year | Country | Study Design | Management Strategy |
|---|---|---|---|---|---|
| Elective Neck Dissection Versus Observation in Patients With Head and Neck Cutaneous Squamous Cell Carcinoma | Amit | 2021 | USA | Retrospective cohort | Observation, ED |
| Elective Neck Dissection for Head and Neck Cutaneous Squamous Cell Carcinoma with Skull Base Invasion | Cannon | 2017 | USA | Retrospective cohort | Observation, ED |
| Lymphatic Mapping and Sentinel Lymphadenectomy for 106 Head and Neck Lesions: Contrasts Between Oral Cavity and Cutaneous Malignancy | Civantos | 2006 | USA | Prospective case series | SLNB |
| Sentinel lymph node biopsy with a gamma probe in patients with high-risk cutaneous squamous cell carcinoma | Demir | 2011 | Turkey | Prospective case series | 1 |
| Relevance of Intraparotid Metastases in Head and Neck Skin Squamous Cell Carcinoma | Dur | 2020 | Switzerland | Retrospective cohort | 1 |
| Sentinel Lymph Node Biopsy for Cutaneous Squamous Cell Carcinoma on the Head and Neck | Durham | 2016 | USA | Retrospective case series | SLNB |
| Clinical characteristics of malignant tumours originating in the external ear | Gallegos-Hernandez | 2015 | Mexico | Retrospective cohort | 1 |
| Prospective study of sentinel node biopsy for high-risk cutaneous squamous cell carcinoma of the head and neck | Gore | 2016 | Australia | Prospective case series | SLNB |
| Pattern of nodal metastasis of cutaneous squamous cell carcinoma involving the temporal bone | Hintze | 2022 | Ireland | Retrospective case series | 1 |
| The value of Elective Parotidectomy in Advanced Squamous Cell Carcinoma of the Skin of the Head | Hoch | 2014 | Germany | Retrospective case series | ED |
| Elective parotidectomy and neck dissection are not beneficial in cutaneous squamous cell carcinoma of the head | Horakova | 2023 | Czech Republic | Retrospective cohort | ED |
| The prognostic value of sentinel lymph nodes on distant metastasis–free survival in patients with high-risk squamous cell carcinoma | Jansen | 2019 | Germany | Ambispective cohort | 1 |
| The role of elective superficial parotidectomy in the treatment of temporal region squamous cell carcinoma | Kadakia | 2015 | USA | Retrospective cohort | ED |
| Indications for and extent of elective neck dissection for lymph node metastasis from external auditory canal carcinoma | Kiyokawa | 2020 | Japan | Retrospective cohort | 1 |
| The role of suprahyoid neck dissection in the treatment of the lower lip: 20 years experience at a Tertiary Center | Kuscu | 2016 | Turkey | Retrospective cohort | 1 |
| Availability of sentinel lymph node biopsy for cutaneous squamous cell carcinoma | Maruyama | 2016 | Japan | Retrospective cohort | 1 |
| Imaging and sentinel lymph node biopsy in high risk head and neck cutaneous squamous cell carcinoma: a Chinese cohort study | Ma | 2025 | China | Retrospective cohort | SLNB |
| Parotidectomy and neck dissection in locally advanced and relapsed cutaneous squamous cell carcinoma of the head and neck region | Melo | 2022 | Brazil | Retrospective cohort | ED |
| Sentinel Node Biopsy in 105 High-Risk Cutaneous SCCs of the Head and Neck: Results of a Multicenter Prospective Study | Mooney | 2019 | Australia | Prospective case series | SLNB |
| Lymph Node Metastases from Cutaneous Squamous Cell Carcinoma of the Head and Neck | Moore | 2005 | USA | Prospective cohort | 1 |
| Patterns of Regional Metastasis in Advanced Stage Cutaneous Squamous Cell Carcinoma of the Auricle | Peiffer | 2011 | USA | Retrospective case series | 1 |
| Sentinel Node Lymphoscintigraphy in High-risk Cutaneous Squamous Cell Carcinoma | Pollock | 2017 | USA | Prospective case series | 1 |
| Outcomes of Sentinel Lymph Node Biopsy for Primary Cutaneous Squamous Cell Carcinoma of the Head and Neck | Pride | 2022 | USA | Retrospective case series | SLNB |
| Intraoperative use of Mohs’ surgery for the resection of major cutaneous head and neck cancer under general anaesthetic: Initial experiences, efficiency and outcomes | Ridha | 2015 | UK | Prospective case series | Observation |
| Concordant Surgical Treatment: Non-melanocytic Skin Cancer of the Head and Neck | Ryu | 2017 | South Korea | Retrospective case series | 1 |
| Sentinel Lymph Node Biopsy for High-Risk Nonmelanoma Skin Cancers | Sahn | 2007 | USA | Retrospective case series | SLNB |
| Surgical Treatment of Lip Cancer: Our Experience With 106 Cases | Salgarelli | 2009 | Italy | Retrospective case series | Observation |
| Lymph Node Metastasis in Cutaneous Head and Neck Squamous Cell Carcinoma | Silberstein | 2015 | Israel | Retrospective case series | Observation |
| Sentinel lymph node biopsy in cN0 squamous cell carcinoma of the lip: A retrospective study | Sollamo | 2015 | Finland | Retrospective case series | SLNB |
| Sentinel node biopsy for high-risk cutaneous squamous cell carcinoma | Takahashi | 2014 | Japan | Retrospective case series | SLNB |
| Sentinel node radiolocalisation and predictive value in lip squamous cell carcinoma | Tartaglione | 2003 | Italy | Prospective case series | SLNB |
| Squamous Cell Carcinoma of the Lip in Australian Patients: Definitive Radiotherapy Is an Efficacious Option to Surgery in Select Patients | Thanh Pham | 2015 | Australia | Retrospective case series | Observation |
| Sentinel Lymph Node Biopsy in High-Risk Cutaneous Squamous Cell Carcinoma: Analysis of a Large Size Retrospective Series | Tremblay-Abel | 2021 | Canada | Retrospective case series | 1 |
| Sentinel Node Biopsy for High-Risk Nonmelanoma Cutaneous Malignancy | Wagner | 2004 | USA | Retrospective cohort | 1 |
| Efficacy of elective nodal irradiation in skin squamous cell carcinoma of the face, ears, and scalp | Wray | 2015 | USA | Retrospective case series | 2 |
| Sentinel Lymph Node Biopsy for High-Risk Cutaneous Squamous Cell Carcinoma of the Head and Neck | Wu | 2020 | USA | Retrospective case series | SLNB |
| Comparison between wait-and-see policy and elective neck dissection in clinically N0 cutaneous squamous cell carcinoma of head and neck | Xiao | 2018 | China | Prospective cohort | Observation, ED |
| The problem of nodal disease in squamous cell carcinoma of the temporal bone | Zanoletti | 2010 | Italy | Retrospective case series | 1 |
| Study ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total | OLE | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Civantos | Y | Y | Y | Y | N | Y | Y | Y | N | Y | N/A | 8 | 4 | JBI Questions for case series 1. Were there clear criteria for inclusion in the case series? 2. Was the condition measured in a standard, reliable way for all participants included in the case series? 3. Were valid methods used for identification of the condition for all participants included in the case series? 4. Did the case series have consecutive inclusion of participants? 5. Did the case series have complete inclusion of participants? 6. Was there clear reporting of the demographics of the participants in the study? 7. Was there clear reporting of clinical information of the participants? 8. Were the outcomes or follow-up results of cases clearly reported? 9. Was there clear reporting of the presenting site(s)/clinic(s) demographic information? 10. Was statistical analysis appropriate? | |
| Demir | Y | Y | Y | Y | N | Y | Y | Y | N | Y | N/A | 8 | 4 | ||
| Dur | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N/A | 9 | 4 | ||
| Durham | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N/A | 9 | 4 | ||
| Gallegos-Hernandez | Y | Y | Y | Y | Y | N | N | Y | N | N | N/A | 6 | 4 | ||
| Gore | Y | Y | Y | Y | Y | Y | N | Y | N | Y | N/A | 8 | 4 | ||
| Hintze | Y | Y | Y | Y | Y | N | Y | Y | N | Y | N/A | 8 | 4 | ||
| Hoch | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N/A | 8 | 4 | ||
| Horakova | Y | Y | Y | Y | N | Y | Y | Y | N | N | N/A | 7 | 4 | ||
| Mooney | Y | N | Y | Y | N | Y | Y | Y | N | Y | N/A | 7 | 4 | ||
| Peiffer | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N/A | 9 | 4 | ||
| Pollock | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N/A | 9 | 4 | ||
| Pride | Y | Y | Y | Y | N | Y | Y | Y | N | N | N/A | 7 | 4 | ||
| Ridha | Y | Y | Y | Y | Y | N | Y | Y | N | Y | N/A | 8 | 4 | ||
| Ryu | Y | Y | Y | Y | Y | Y | Y | N | N | N | N/A | 7 | 4 | ||
| Sahn | Y | Y | N | Y | N | Y | Y | Y | N | N | N/A | 6 | 4 | ||
| Salgarelli | N | Y | Y | Y | Y | N | Y | Y | N | N | N/A | 6 | 4 | ||
| Silberstein | Y | Y | N | Y | Y | Y | Y | Y | N | Y | N/A | 8 | 4 | ||
| Sollamo | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N/A | 9 | 4 | ||
| Takahashi | Y | Y | Y | Y | N | Y | Y | Y | N | Y | N/A | 8 | 4 | ||
| Tartaglione | Y | Y | Y | Y | N | Y | N | Y | N | N | N/A | 6 | 4 | ||
| Thanh Pham | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N/A | 9 | 4 | ||
| Tremblay-Abel | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N/A | 9 | 4 | ||
| Wagner | Y | Y | Y | Y | N | Y | Y | Y | N | Y | N/A | 8 | 4 | ||
| Wray | Y | Y | Y | Y | N | Y | Y | Y | N | Y | N/A | 8 | 4 | ||
| Wu | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N/A | 9 | 4 | ||
| Zanoletti | Y | Y | Y | Y | Y | N | Y | Y | N | Y | N/A | 8 | 4 | ||
| Amit | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11 | 3 | JBI Questions for cohort studies 1. Were the two groups similar and recruited from the same population? 2. Were the exposures measured similarly to assign people to both exposed and unexposed groups? 3. Was the exposure measured in a valid and reliable way? 4. Were confounding factors identified? 5. Were strategies to deal with confounding factors stated? 6. Were the groups/participants free of the outcome at the start of the study (or moment of exposure)? 7. Were the outcomes measured in a valid and reliable way? 8. Was the follow up time reported and sufficient to be long enough for outcomes to occur? 9. Was follow up complete, and if not, were the reasons to loss to follow-up described and explored? 10. Were strategies to address incomplete follow up utilized? 11. Was appropriate statistical analysis used? | |
| Cannon | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11 | 3 | ||
| Jansen | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | 10 | 3 | ||
| Kadakia | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11 | 3 | ||
| Kiyokawa | Y | Y | Y | Y | N | Y | Y | Y | Y | N | Y | 9 | 3 | ||
| Kuscu | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | 9 | 3 | ||
| Maruyama | Y | Y | Y | N | N | Y | Y | Y | N | N | Y | 7 | 3 | ||
| Ma | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | 10 | 3 | ||
| Melo | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11 | 3 | ||
| Moore | Y | Y | Y | Y | Y | Y | Y | Y | N | N | Y | 9 | 3 | ||
| Xiao | Y | Y | Y | Y | N | Y | Y | Y | N | N | Y | 8 | 3 | ||
| Observation | 95% CI | SLNB | 95% CI | ED | 95% CI | |
|---|---|---|---|---|---|---|
| Sample, n | 1799 | N/A | 494 | N/A | 411 | N/A |
| Tumors, n | 1952 | N/A | 499 | N/A | 411 | N/A |
| Age, years (SD) | 69.3 (0.9) | 67.5–71.0 | 68.3 (1.3) | 65.7–70.8 | 68.3 (2.1) | 64.2–72.4 |
| Sex, % male | 75.5 | 57.9–89.5 | 80.9 | 77.2–84.3 | 78.0 | 67.1–87.2 |
| Follow-up period, months (SE) | 26.5 (1.2) | 24.2–28.8 | 26.8 (2.2) | 22.5–31.2 | 37.6 (7.8) | 22.3–52.9 |
| Tumor Location, % | ||||||
| Ear and periauricular 1 | 1.6 | 0.6–10.5 | 7.6 | 2.7–14.8 | 9.2 | 1.3–23.3 |
| Face 2 | 2.8 | 0.4–14.9 | 6.5 | 1.9–13.7 | 3.9 | 0.5–10.6 |
| Forehead 3 | 3.2 | 0.002–12.1 | 0.7 | 0.2–1.9 | 1.3 | 0.1–4.0 |
| Nose | 1.9 | 0.06–8.6 | 3.9 | 1.5–7.4 | 4.0 | 0.5–10.6 |
| Orbital 4 | 1.6 | 0.0006–6.4 | 0.9 | 0.3–2.2 | 2.4 | 0.04–8.3 |
| Perioral 5 | 35.4 * | 3.5–78.3 | 25.7 | 8.9–47.6 | 0.6 | 0.09–2.0 |
| Scalp | 1.8 | 0.05–8.3 | 8.7 | 2.5–18.1 | 1.3 | 0.1–4.0 |
| Neck | 0.9 | 0.05–4.3 | 3.4 | 2.0–5.4 | 0.4 | 0.02–1.6 |
| Temple | 1.0 | 0.1–5.4 | 1.1 | 0.4–2.5 | 13.3 * | 2.8–62.9 |
| Unspecified | 27.9 * | 4.9–94.6 | 30.1 * | 5.7–63.2 | 41.7 * | 0.6–94.0 |
| Tumor Characteristics, % | ||||||
| AJCC stage T1–T2 | 73.3 | 48.9–91.9 | 33.2 * | 2.4–77.3 | 56.4 * | 27.7–82.9 |
| AJCC stage T3–T4 | 25.4 | 5.6–53.2 | 66.8 | 22.8–97.7 | 43.6 * | 17.1–72.3 |
| Primary Tumor | 83.4 | 53.8–99.1 | 92.5 | 81.8–98.7 | 52.9 * | 13.9–89.9 |
| Recurrent Tumor | 16.6 | 0.9–46.2 | 6.3 | 0.7–17.0 | 47.1 * | 10.1–86.1 |
| Perineural Invasion | 53.3 * | 0.3–100.0 | 33.0 | 23.4–43.2 | 48.7 * | 12.8–85.4 |
| Lymphovascular Invasion | – | 8.9 | 5.6–13.2 | 16.3 | 11.3–22.3 | |
| Adjuvant Therapy, % | ||||||
| Chemoradiation | 0.2 | 0.0006–0.8 | 3.0 | 0.7–6.7 | 10.7 | 3.1–22.0 |
| Radiation | 28.7 * | 2.9–66.9 | 21.6 | 5.5–44.5 | 43.8 * | 11.1–79.9 |
| Chemotherapy | 0.7 | 0.02–3.2 | 3.0 | 0.7–6.7 | 5.4 | 0.05–18.8 |
| Intervention | Subgroup | Sample, n | Occult Rate, % | I2 | 95% CI |
|---|---|---|---|---|---|
| All Interventions | Overall | 1673 | 13.9 | 75.4 | 10.5–17.7 |
| High risk only | 977 | 12.5 | 72.4 | 8.5–17.0 | |
| SLNB | All SLNB | 707 | 8.8 | 30.0 | 6.8–11.1 |
| High risk | 631 | 8.4 | 30.0 | 6.3–10.8 | |
| Unspecified risk | 76 | 12.6 | 31.4 | 6.2–22.0 | |
| Elective Dissection | All ED 1 | 966 | 17.3 | 82.1 | 11.6–23.7 |
| High risk 1 | 346 | 18.8 | 75.5 | 10.3–29.1 | |
| Unspecified risk 1 | 620 | 16.3 | 84.1 | 9.2–25.0 | |
| Parotidectomy only | 166 | 20.2 | 28.9 | 14.4–27.0 |
| Outcome, % | Observation | 95% CI | SLNB | 95% CI | ED 1 | 95% CI |
|---|---|---|---|---|---|---|
| Local Recurrence | 8.2 | 4.2–14.2 | 11.2 | 8.2–14.8 | 21.8 | 11.9–34.9 |
| Regional Recurrence | 6.7 | 3.2–11.4 | 6.8 | 4.5–9.7 | 7.6 | 5.1–10.8 |
| Distant Metastasis | 6.7 | 1.1–34.5 | 4.5 | 2.5–7.3 | 9.5 | 2.9–19.4 |
| Overall Recurrence | 16.9 | 4.9–34.2 | 8.3 | 2.1–18.1 | 23.7 | 15.1–33.7 |
| Disease-Specific Death | 5.0 | 0.7–12.9 | 5.6 | 3.5–8.3 | 6.7 | 0.1–22.9 |
| 5-year DFS | 69.0 | 45.7–88.0 | – | – | – | – |
| Overall Mortality | 29.9 | 27.1–32.8 | 6.1 | 4.0–8.9 | 31.4 | 3.4–71.1 |
| Obs. (n = 1799) | SLNB (n = 494) | ED 1 (n = 411) | Obs. vs. SLNB | Obs. vs. ED 1 | SLNB vs. ED 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient Characteristics | ∆ | 95% CI | p | ∆ | 95% CI | p | ∆ | 95% CI | p | |||
| Mean age, yr. (SE) | 69.3 (0.9) | 68.3 (1.3) | 68.3 (2.1) | 1.0 | 0.9–1.1 | <0.0001 | 0.9 | 0.8–1.1 | <0.0001 | 0.04 | −0.2–0.3 | 0.7178 |
| Sex, % male | 75.5 | 80.9 | 78.0 | 5.4 | 1.2–9.3 | 0.0132 | 0.03 | −2.3–6.9 | 0.2969 | 2.9 | −2.5–8.4 | 0.2922 |
| Mean follow up period, mo. (SE) | 26.5 (1.2) | 26.8 (2.2) | 37.6 (7.8) | 0.3 | 0.2–0.5 | 0.0001 | 11.1 | 10.7–11.4 | <0.0001 | 10.7 | 10.0–11.5 | <0.0001 |
| Tumor characteristics, % | ||||||||||||
| AJCC stage T1–T2 | 73.3 | 33.2 | 56.4 | 40.2 | 33.6–46.2 | <0.0001 | 16.9 | 11.1–22.9 | <0.0001 | 23.2 | 14.9–31.1 | <0.0001 |
| AJCC stage T3–T4 | 25.4 | 66.8 | 43.6 | 41.4 | 34.9–47.4 | <0.0001 | 18.2 | 12.4–24.1 | <0.0001 | 23.2 | 14.9–31.1 | <0.0001 |
| Primary Tumor | 83.4 | 92.5 | 52.9 | 9.1 | 3.4–12.8 | 0.0039 | 30.4 | 24.9–35.9 | <0.0001 | 39.5 | 32.0–45.7 | <0.0001 |
| Recurrent Tumor | 16.6 | 6.3 | 47.1 | 10.3 | 7.1–13.0 | <0.0001 | 32.4 | 26.9–37.9 | <0.0001 | 42.7 | 36.8–48.3 | <0.0001 |
| Perineural Invasion | 53.3 | 33.0 | 48.7 | 20.3 | 9.4–30.9 | 0.0002 | 4.6 | −7.3–16.3 | 0.4534 | 15.7 | 7.6–23.7 | 0.0001 |
| Lymphovascular Invasion | – | 8.9 | 16.3 | – | – | – | – | – | – | 7.4 | 1.1–14.1 | 0.0202 |
| Outcomes, % | ||||||||||||
| Overall Recurrence | 16.9 | 8.3 | 23.7 | 8.6 | 4.6–11.8 | 0.0002 | 6.8 | 1.6–12.7 | 0.0084 | 15.4 | 9.3–21.7 | <0.0001 |
| Local Recurrence | 8.2 | 11.2 | 21.8 | 3.0 | −3.6–8.0 | 0.3373 | 13.6 | 2.8–26.9 | 0.0103 | 10.6 | 0.9–23.8 | 0.0287 |
| Regional Recurrence | 6.7 | 6.8 | 7.6 | 0.1 | −2.3–3.2 | 0.9573 | 0.9 | −1.7–4.3 | 0.5147 | 0.9 | −2.9–4.7 | 0.6444 |
| Distant Metastasis | 6.7 | 4.5 | 9.5 | 2.2 | −2.0–8.0 | 0.3306 | 2.9 | −3.7–9.1 | 0.3662 | 5.1 | 0.5–10.7 | 0.0273 |
| Disease-Specific Death | 5.0 | 5.6 | 6.7 | 0.5 | −1.8–3.5 | 0.6798 | 1.6 | −1.0–5.1 | 0.2455 | 1.1 | −2.4–4.9 | 0.5399 |
| 5-year DFS | 69.0 | – | – | – | – | – | – | – | – | – | – | – |
| Overall Mortality | 29.9 | 6.1 | 31.4 | 23.8 | 19.9–27.3 | <0.0001 | 1.5 | −4.9–8.4 | 0.6570 | 25.3 | 19.0–32.0 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberts, K.A.; Chen, K.; Wahle, B.M.; Nguyen, S.A.; Moore, M.G.; Yesensky, J.A. Management of Regional Lymph Nodes in Clinically Node-Negative Cutaneous Squamous Cell Carcinoma of the Head and Neck: A Systematic Review & Meta-Analysis. Cancers 2025, 17, 3335. https://doi.org/10.3390/cancers17203335
Roberts KA, Chen K, Wahle BM, Nguyen SA, Moore MG, Yesensky JA. Management of Regional Lymph Nodes in Clinically Node-Negative Cutaneous Squamous Cell Carcinoma of the Head and Neck: A Systematic Review & Meta-Analysis. Cancers. 2025; 17(20):3335. https://doi.org/10.3390/cancers17203335
Chicago/Turabian StyleRoberts, Kaitlyn A., Kaiwen Chen, Benjamin M. Wahle, Shaun A. Nguyen, Michael G. Moore, and Jessica A. Yesensky. 2025. "Management of Regional Lymph Nodes in Clinically Node-Negative Cutaneous Squamous Cell Carcinoma of the Head and Neck: A Systematic Review & Meta-Analysis" Cancers 17, no. 20: 3335. https://doi.org/10.3390/cancers17203335
APA StyleRoberts, K. A., Chen, K., Wahle, B. M., Nguyen, S. A., Moore, M. G., & Yesensky, J. A. (2025). Management of Regional Lymph Nodes in Clinically Node-Negative Cutaneous Squamous Cell Carcinoma of the Head and Neck: A Systematic Review & Meta-Analysis. Cancers, 17(20), 3335. https://doi.org/10.3390/cancers17203335






