Dethroning of Neuroendocrine Tumor as an Orphan Disease: US Incidence, Prevalence, and Survival in the 21st Century
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Variable Definition
2.3. Statistical Analysis
3. Results
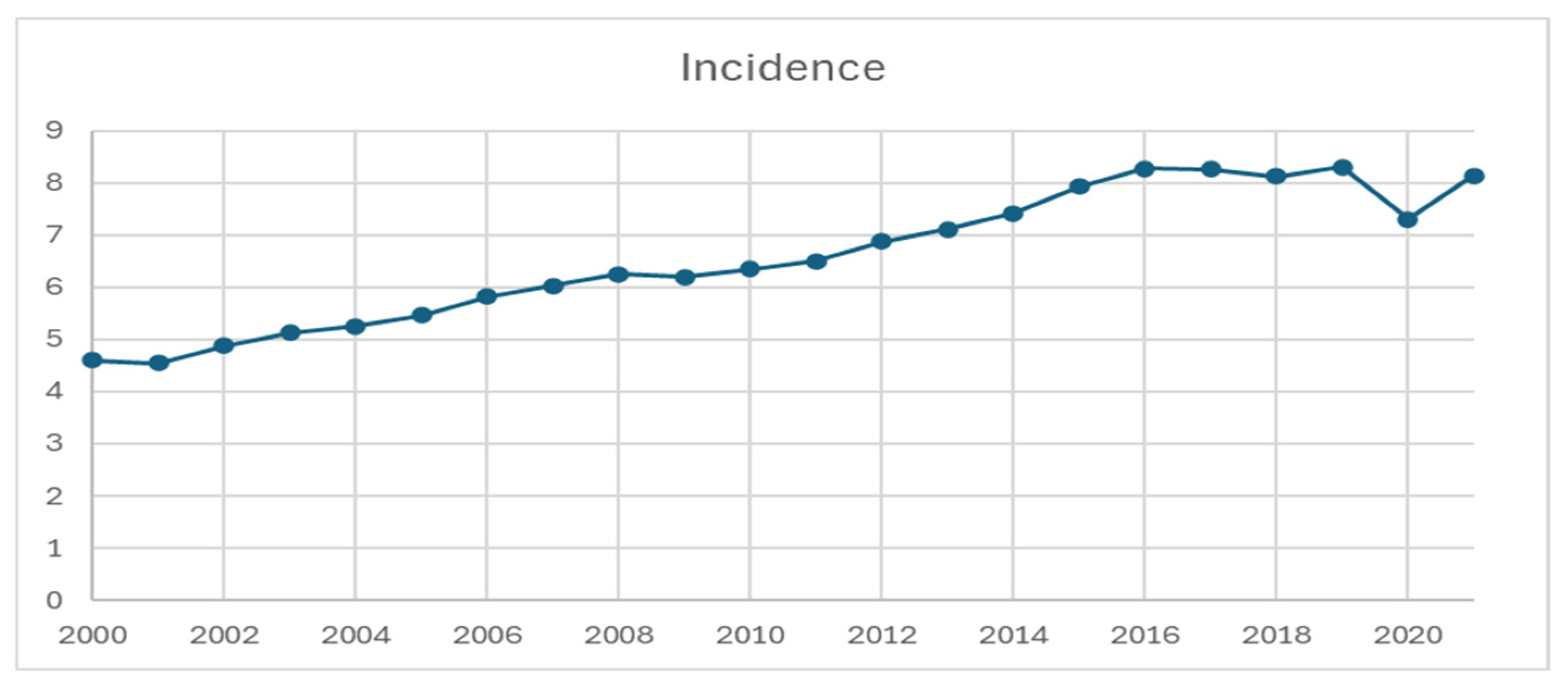
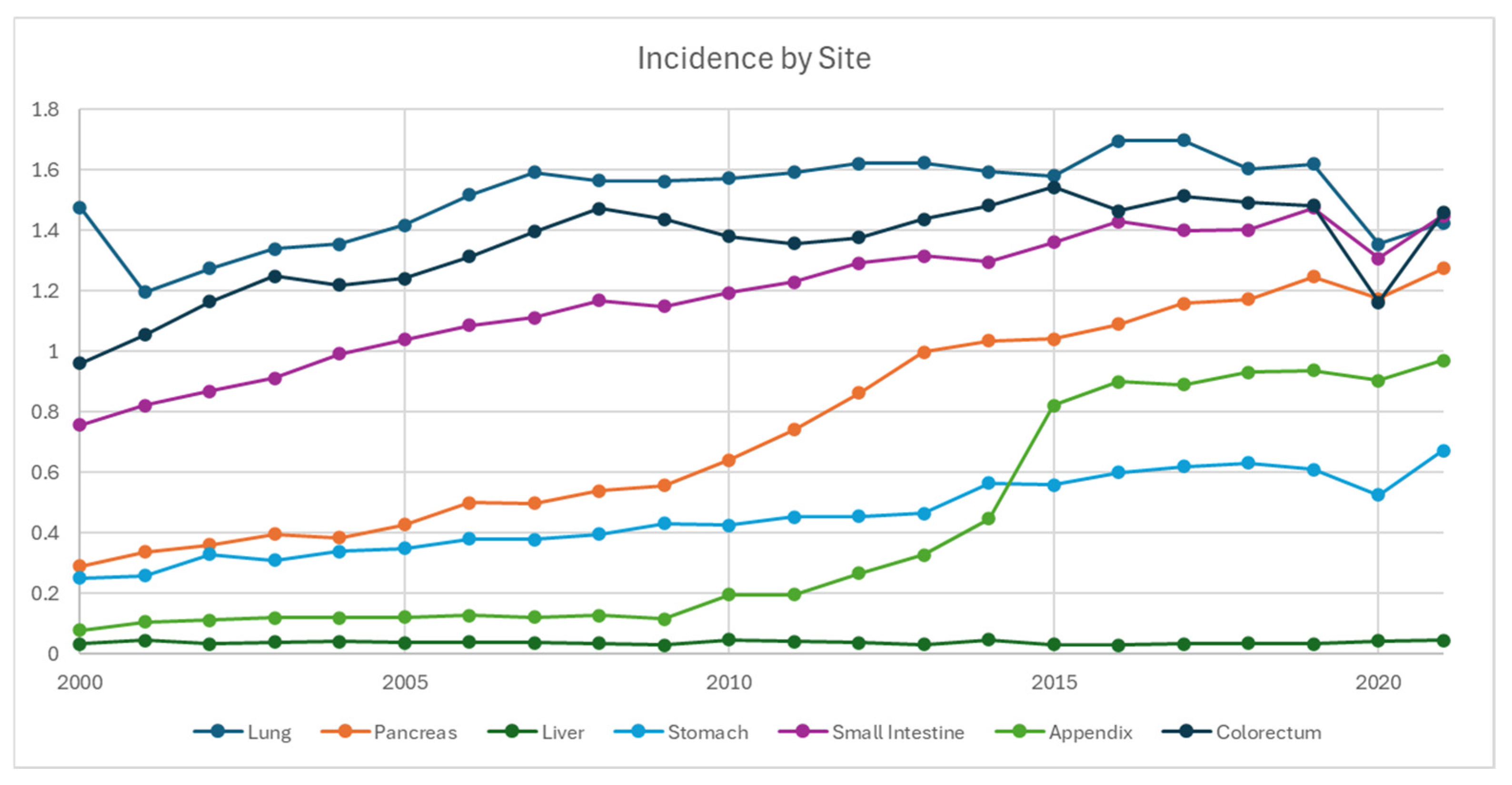
3.1. Incidence
3.2. Prevalence
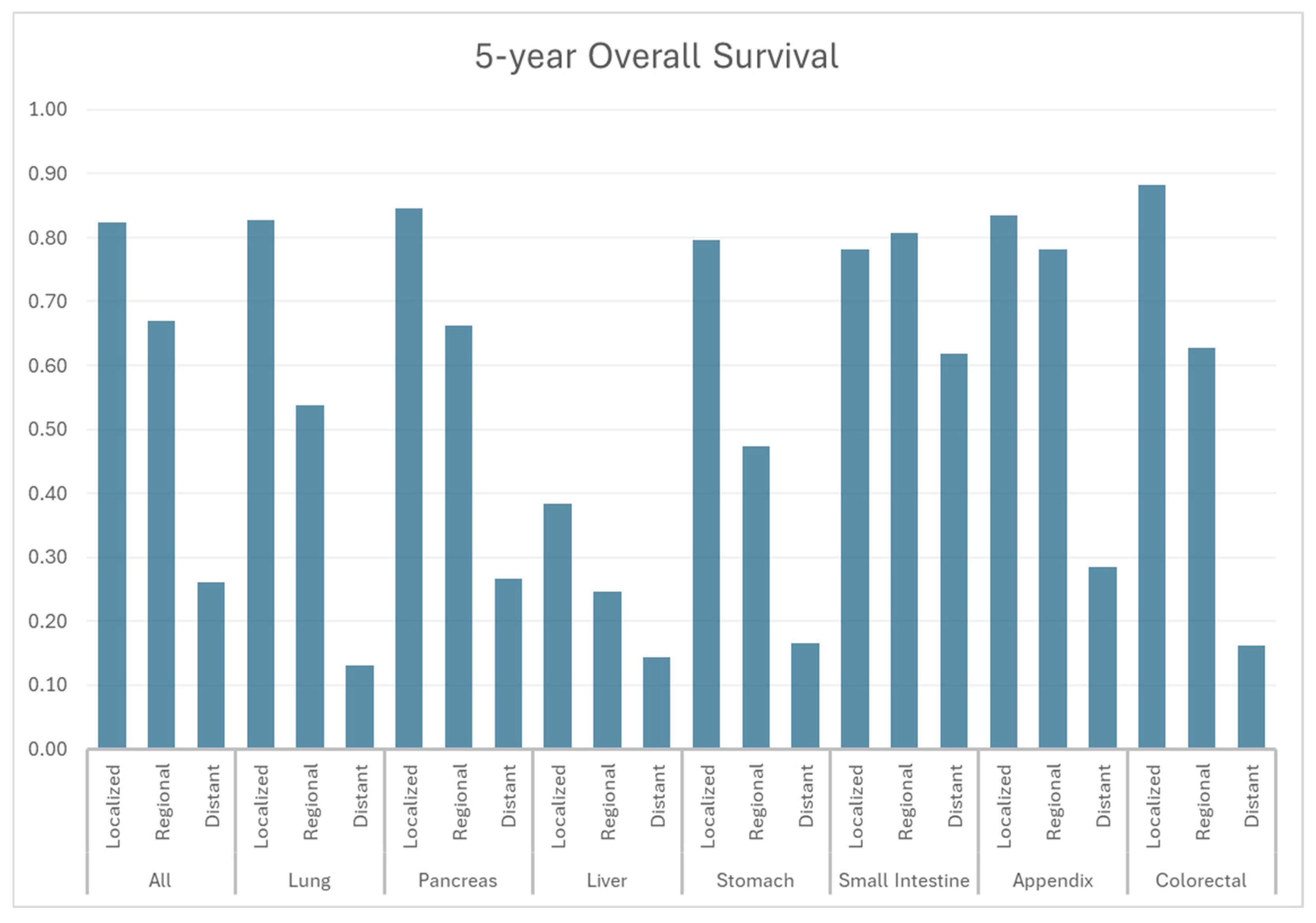
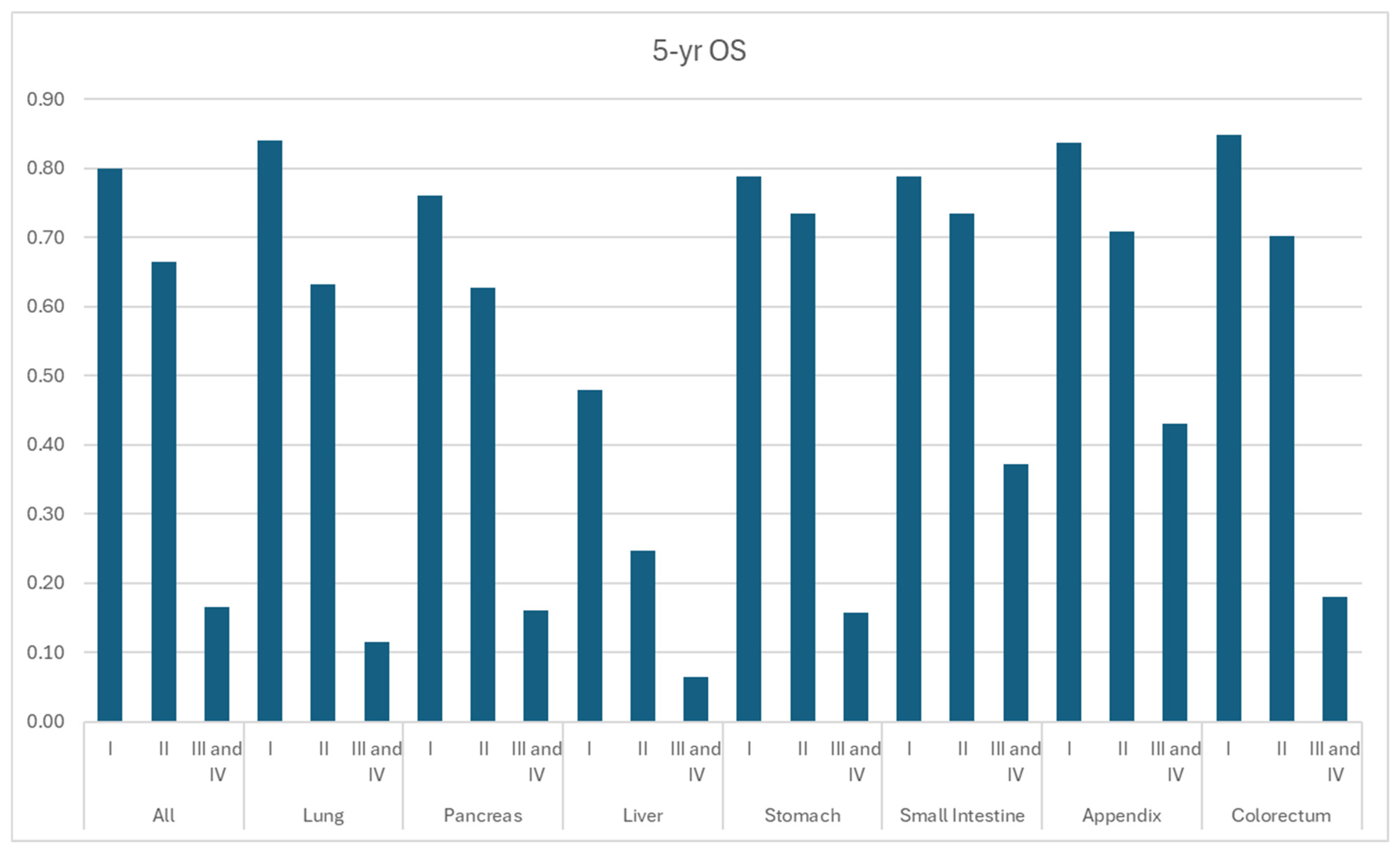
3.3. Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Chauhan, A.; Chan, K.; Halfdanarson, T.R.; Bellizzi, A.M.; Rindi, G.; O’Toole, D.; Ge, P.S.; Jain, D.; Dasari, A.; Anaya, D.A. Critical updates in neuroendocrine tumors: Version 9 American Joint Committee on Cancer staging system for gastroenteropancreatic neuroendocrine tumors. CA Cancer J. Clin. 2024, 74, 359–367. [Google Scholar] [CrossRef]
- Hallet, J.; Law, C.H.L.; Cukier, M.; Saskin, R.; Liu, N.; Singh, S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015, 121, 589–597. [Google Scholar] [CrossRef]
- Dasari, A.; Wallace, K.; Halperin, D.M.; Maxwell, J.; Kunz, P.; Singh, S.; Chasen, B.; Yao, J.C. Epidemiology of Neuroendocrine Neoplasms in the US. JAMA Netw. Open 2025, 8, e2515798. [Google Scholar] [CrossRef]
- Orchard, P.; Preece, R.; Thomas, M.G.; Dixon, S.W.; Wong, N.A.; Chambers, A.C.; Messenger, D.E. Demographic trends in the incidence of malignant appendiceal tumours in England between 1995 and 2016: Population-based analysis. BJS Open 2022, 6, zrac103. [Google Scholar] [CrossRef]
- Zheng, R.; Zhao, H.; An, L.; Zhang, S.; Chen, R.; Wang, S.; Sun, K.; Zeng, H.; Wei, W.; He, J. Incidence and survival of neuroendocrine neoplasms in China with comparison to the United States. Chin. Med. J. 2023, 136, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, T.; Otsuki, K.; Tanaka, Y.; Kanda, S. Epidemiology of neuroendocrine neoplasmas in Japan: Based on analysis of hospital-based cancer registry data, 2009–2015. BMC Endocr. Disord. 2022, 22, 105. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Pavel, M.; Lombard-Bohas, C.; Van Cutsem, E.; Voi, M.; Brandt, U.; He, W.; Chen, D.; Capdevila, J.; De Vries, E.G. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: Overall survival and circulating biomarkers from the randomized, phase III RADIANT-3 study. J. Clin. Oncol. 2016, 34, 3906. [Google Scholar] [CrossRef] [PubMed]
- Faivre, S.; Niccoli, P.; Castellano, D.; Valle, J.W.; Hammel, P.; Raoul, J.-L.; Vinik, A.; Van Cutsem, E.; Bang, Y.-J.; Lee, S.-H. Sunitinib in pancreatic neuroendocrine tumors: Updated progression-free survival and final overall survival from a phase III randomized study. Ann. Oncol. 2017, 28, 339–343. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177Lu-dotatate in the phase III NETTER-1 trial. J. Clin. Oncol. 2018, 36, 2578. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Kunz, P.L.; Catalano, P.J.; Nimeiri, H.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Yao, J.C.; Kulke, M.H.; Hendifar, A.E.; Shanks, J.C. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: A trial of the ECOG-ACRIN Cancer Research Group (E2211). J. Clin. Oncol. 2018, 36, 15. [Google Scholar] [CrossRef]
- Kunz, P.L.; Graham, N.T.; Catalano, P.J.; Nimeiri, H.S.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Martin, B.A.; Yao, J.C.; Kulke, M.H. Randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors (ECOG-ACRIN E2211). J. Clin. Oncol. 2023, 41, 1359–1369. [Google Scholar] [CrossRef]
- Chauhan, A.; Yu, Q.; Ray, N.; Farooqui, Z.; Huang, B.; Durbin, E.B.; Tucker, T.; Evers, M.; Arnold, S.; Anthony, L.B. Global burden of neuroendocrine tumors and changing incidence in Kentucky. Oncotarget 2018, 9, 19245. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Rabe, K.; Rubin, J.; Petersen, G. Pancreatic neuroendocrine tumors (PNETs): Incidence, prognosis and recent trend toward improved survival. Ann. Oncol. 2008, 19, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.E.; Ilaghi, M.; Aslani, A.; Yekta, Z.; Nejadghaderi, S.A. A population-based study on incidence trends of myeloma in the United States over 2000–2020. Sci. Rep. 2023, 13, 20705. [Google Scholar] [CrossRef]
- Shahmoradi, Z.; Damgacioglu, H.; Clarke, M.A.; Wentzensen, N.; Montealegre, J.; Sonawane, K.; Deshmukh, A.A. Cervical cancer incidence among US women, 2001–2019. JAMA 2022, 328, 2267–2269. [Google Scholar] [CrossRef]
- Chauhan, A.; Yu, Q.; Miller, R.C.; Luque, L.; Weiss, H.; Anthony, L.B. The antidiarrheal efficacy of a proprietary amino acid mixture (enterade) in neuroendocrine tumor (NET) patients. J. Clin. Oncol. 2018, 36, 15. [Google Scholar] [CrossRef]
- Gosain, R.; Gupta, M.; Roy, A.M.; Strosberg, J.; Glaser, K.M.; Iyer, R. Health-related quality of life (HRQoL) in neuroendocrine tumors: A systematic review. Cancers 2022, 14, 1428. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, J.L.; Cella, D.; Phan, A.T.; Choi, S.; Liu, Z.; Yao, J.C. Comparison of health-related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas 2012, 41, 461–466. [Google Scholar] [CrossRef]
- Pearman, T.P.; Beaumont, J.L.; Cella, D.; Neary, M.P.; Yao, J. Health-related quality of life in patients with neuroendocrine tumors: An investigation of treatment type, disease status, and symptom burden. Support. Care Cancer 2016, 24, 3695–3703. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Farooqui, Z.; Silva, S.R.; Murray, L.A.; Hodges, K.B.; Yu, Q.; Myint, Z.W.; Raajesekar, A.K.; Weiss, H.; Arnold, S. Integrating a 92-gene expression analysis for the management of neuroendocrine tumors of unknown primary. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 113. [Google Scholar] [CrossRef][Green Version]
- Panzuto, F.; Barbi, S.; Trama, A.; Fazio, N. The importance of education and training in neuroendocrine neoplasms: Challenges and opportunities for multidisciplinary management. Cancer Treat. Rev. 2025, 139, 102998. [Google Scholar] [CrossRef]
- Chauhan, A.; Del Rivero, J.; Ramirez, R.A.; Soares, H.P.; Li, D. Treatment sequencing strategies in advanced neuroendocrine tumors: A review. Cancers 2022, 14, 5248. [Google Scholar] [CrossRef]
- DeGroff, A.; Miller, J.; Sharma, K.; Sun, J.; Helsel, W.; Kammerer, W.; Rockwell, T.; Sheu, A.; Melillo, S.; Uhd, J. COVID-19 impact on screening test volume through the National Breast and Cervical Cancer early detection program, January–June 2020, in the United States. Prev. Med. 2021, 151, 106559. [Google Scholar] [CrossRef]
- Wang, Y.; Alsaraf, Y.; Bandaru, S.S.; Lyons, S.E.; Reap III, L.E.; Yu, Z.; Yu, Q. Epidemiology, survival and new treatment modalities for intrahepatic cholangiocarcinoma (IHCC). J. Gastrointest. Oncol. 2024, 15, 1777. [Google Scholar] [CrossRef]
- Howlader, N.; Bhattacharya, M.; Scoppa, S.; Miller, D.; Noone, A.-M.; Negoita, S.; Cronin, K.; Mariotto, A. Cancer and COVID-19: US cancer incidence rates during the first year of the pandemic. JNCI J. Natl. Cancer Inst. 2024, 116, 208–215. [Google Scholar] [CrossRef] [PubMed]



| d | Rate | Less Than 50 Years | 50–64 Years | 65 Years and Above |
|---|---|---|---|---|
| 2000 Rate | 4.599 | 1.183 | 9.767 | 18.029 |
| 2001 Rate | 4.542 | 1.255 | 9.713 | 17.228 |
| 2002 Rate | 4.876 | 1.26 | 10.875 | 18.462 |
| 2003 Rate | 5.132 | 1.357 | 10.802 | 20.02 |
| 2004 Rate | 5.252 | 1.335 | 11.402 | 20.378 |
| 2005 Rate | 5.459 | 1.372 | 11.979 | 21.119 |
| 2006 Rate | 5.816 | 1.471 | 12.743 | 22.476 |
| 2007 Rate | 6.032 | 1.51 | 13.201 | 23.415 |
| 2008 Rate | 6.247 | 1.591 | 13.765 | 23.985 |
| 2009 Rate | 6.197 | 1.59 | 14.081 | 23.225 |
| 2010 Rate | 6.352 | 1.676 | 13.87 | 24.204 |
| 2011 Rate | 6.496 | 1.689 | 14.059 | 25.044 |
| 2012 Rate | 6.879 | 1.826 | 14.987 | 26.189 |
| 2013 Rate | 7.11 | 1.962 | 15.413 | 26.731 |
| 2014 Rate | 7.409 | 2.112 | 16.027 | 27.51 |
| 2015 Rate | 7.924 | 2.631 | 16.864 | 27.627 |
| 2016 Rate | 8.279 | 2.69 | 17.183 | 29.716 |
| 2017 Rate | 8.266 | 2.771 | 17.103 | 29.248 |
| 2018 Rate | 8.117 | 2.763 | 16.901 | 28.348 |
| 2019 Rate | 8.296 | 2.779 | 17.588 | 28.856 |
| 2020 Rate | 7.307 | 2.594 | 14.912 | 25.265 |
| 2021 Rate | 8.128 | 2.954 | 16.831 | 27.429 |
| Year | Prevalence | Count | Population | Duration (Year) |
|---|---|---|---|---|
| 2001 | 0.00369% | 4044 | 116,872,166 | 1 |
| 2002 | 0.00671% | 7509 | 118,156,034 | 2 |
| 2003 | 0.00979% | 11,191 | 119,330,102 | 3 |
| 2004 | 0.01287% | 14,975 | 120,448,064 | 4 |
| 2005 | 0.01582% | 18,748 | 121,440,491 | 5 |
| 2006 | 0.0187% | 22,589 | 122,471,714 | 6 |
| 2007 | 0.02178% | 26,811 | 123,703,952 | 7 |
| 2008 | 0.02475% | 31,157 | 125,057,830 | 8 |
| 2009 | 0.02764% | 35,589 | 126,480,118 | 9 |
| 2010 | 0.03029% | 39,922 | 127,859,227 | 10 |
| 2011 | 0.03306% | 44,536 | 129,189,098 | 11 |
| 2012 | 0.03582% | 49,310 | 130,486,503 | 12 |
| 2013 | 0.03864% | 54,341 | 131,716,649 | 13 |
| 2014 | 0.04159% | 59,668 | 132,945,252 | 14 |
| 2015 | 0.04451% | 65,270 | 134,221,529 | 15 |
| 2016 | 0.04796% | 71,654 | 135,444,427 | 16 |
| 2017 | 0.05157% | 78,382 | 136,518,227 | 17 |
| 2018 | 0.05499% | 84,963 | 137,401,622 | 18 |
| 2019 | 0.05829% | 91,468 | 138,133,638 | 19 |
| 2020 | 0.06174% | 98,379 | 138,704,580 | 20 |
| 2021 | 0.06392% | 103,221 | 138,877,544 | 21 |
| Site | Stage | mOS (Months) | 1-yr OS | 3-yr OS | 5-yr OS |
|---|---|---|---|---|---|
| All | All | NR | 77.4% | 66.1% | 59.3% |
| Localized | NR | 94.4% | 88.2% | 82.3% | |
| Regional | NR | 86.3% | 74.7% | 66.9% | |
| Distant | 13.72 | 52.6% | 34.0% | 26.1% | |
| Lung | All | 56.0 | 67.7% | 54.6% | 49.1% |
| Localized | NR | 94.9% | 88.5% | 82.7% | |
| Regional | NR | 79.3% | 61.6% | 53.8% | |
| Distant | 6.87 | 35.1% | 17.0% | 13.0% | |
| Pancreas | All | NR | 79.6% | 65.5% | 56.4% |
| Localized | NR | 95.3% | 89.9% | 84.6% | |
| Regional | NR | 87.2% | 74.7% | 66.2% | |
| Distant | 20.96 | 60.9% | 38.8% | 26.7% | |
| Liver | All | 17.00 | 56.0% | 36.1% | 28.2% |
| Localized | 27.58 | 64.9% | 48.6% | 38.4% | |
| Regional | 12.69 | 51.4% | 34.0% | 24.6% | |
| Distant | 6.36 | 39.2% | 21.9% | 14.4% | |
| Stomach | All | NR | 85.9% | 76.1% | 69.0% |
| Localized | NR | 94.2% | 86.4% | 79.6% | |
| Regional | 53.50 | 76.5% | 58.0% | 47.4% | |
| Distant | 9.66 | 43.7% | 22.2% | 16.5% | |
| Small Intestine | All | NR | 91.4% | 82.8% | 74.4% |
| Localized | NR | 92.4% | 85.2% | 78.2% | |
| Regional | NR | 94.0% | 88.0% | 80.8% | |
| Distant | NR | 87.5% | 73.9% | 61.8% | |
| Appendix | All | NR | 91.0% | 84.4% | 75.9% |
| Localized | NR | 94.4% | 90.5% | 83.5% | |
| Regional | NR | 93.4% | 89.1% | 78.1% | |
| Distant | 29.47 | 72.8% | 42.4% | 28.5% | |
| Colorectal | All | NR | 84.8% | 77.4% | 72.1% |
| Localized | NR | 97.1% | 93.1% | 88.2% | |
| Regional | NR | 83.8% | 70.8% | 62.7% | |
| Distant | 8.49 | 41.6% | 23.0% | 16.2% |
| Site | Grade | mOS (Months) | 1-yr OS | 3-yr OS | 5-yr OS |
|---|---|---|---|---|---|
| All | I | NR | 93.8% | 86.9% | 80.0% |
| II | NR | 87.8% | 75.8% | 66.5% | |
| III and IV | 9.51 | 43.3% | 21.6% | 16.6% | |
| Lung | I | NR | 95.6% | 89.7% | 84.1% |
| II | NR | 84.8% | 71.2% | 63.3% | |
| III and IV | 8.29 | 38.7% | 15.7% | 11.5% | |
| Pancreas | I | NR | 92.8% | 83.6% | 76.0% |
| II | NR | 87.7% | 72.6% | 62.8% | |
| III and IV | 8.88 | 41.3% | 21.6% | 16.0% | |
| Liver | I | NR | 85.6% | 59.5% | 48.0% |
| II | 14.16 | 54.7% | 33.6% | 24.6% | |
| III and IV | 4.55 | 29.4% | 11.7% | 6.4% | |
| Stomach | I | NR | 94.0% | 86.4% | 78.9% |
| II | NR | 91.0% | 81.3% | 73.5% | |
| III and IV | 9.84 | 44.2% | 20.8% | 15.8% | |
| Small Intestine | I | NR | 93.8% | 86.7% | 78.9% |
| II | NR | 92.6% | 83.6% | 73.4% | |
| III and IV | 29.21 | 64.7% | 47.3% | 37.2% | |
| Appendix | I | NR | 94.6% | 91.0% | 83.8% |
| II | NR | 92.7% | 82.3% | 70.9% | |
| III and IV | 46.74 | 76.3% | 57.1% | 43.1% | |
| Colorectum | I | NR | 95.0% | 90.1% | 84.8% |
| II | NR | 89.0% | 78.7% | 70.2% | |
| III and IV | 8.87 | 41.1% | 22.2% | 18.0% |
| Overall (n = 120,827) | Distant GI NET (n = 8497) | Distant Pancreatic NET (n = 5533) | Distant Pulmonary NET (n = 10,657) | |||||
|---|---|---|---|---|---|---|---|---|
| HR | p-Value | HR | p-Value | HR | p-Value | HR | p-Value | |
| Year | ||||||||
| 2000–2004 | ref | ref | ref | ref | ref | ref | ref | ref |
| 2005–2009 | 0.95 (0.93–0.98) | <0.001 | 1.00 (0.92–1.08) | 0.996 | 0.97 (0.87–1.08) | 0.591 | 0.98 (0.92–1.05) | 0.660 |
| 2010–2014 | 0.97 (0.94–0.99) | 0.017 | 1.07 (0.98–1.16) | 0.123 | 0.90 (0.81–1.00) | 0.050 | 0.96 (0.90–1.03) | 0.292 |
| 2015–2021 | 0.92 (0.89–0.95) | <0.001 | 0.99 (0.90–1.08) | 0.798 | 0.88 (0.80–0.98) | 0.019 | 0.89 (0.93–0.95) | 0.001 |
| Grade | ||||||||
| I: Well differentiated | ref | ref | ref | ref | ref | ref | ref | ref |
| II: Moderately differentiated | 1.33 (1.28–1.39) | <0.001 | 1.52 (1.37–1.69) | <0.001 | 1.20 (1.05–1.37) | 0.009 | 2.23 (1.90–2.61) | <0.001 |
| III and IV: Poorly differentiated and undifferentiated; anaplastic | 3.71 (3.59–3.83) | <0.001 | 7.02 (6.45–7.63) | <0.001 | 3.19 (2.82–3.61) | <0.001 | 5.36 (4.73–6.06) | <0.001 |
| Race | ||||||||
| White | ref | ref | ref | ref | ref | ref | ref | ref |
| American Indian/Alaska Native | 1.20 (1.08–1.34) | 0.001 | 1.09 (0.76–1.57) | 0.649 | 1.10 (0.73–1.66) | 0.643 | 1.02 (0.73–1.45) | 0.874 |
| Asian or Pacific Islander | 0.94 (0.91–0.99) | 0.010 | 1.23 (1.07–1.41) | 0.003 | 1.07 (0.94–1.22) | 0.298 | 0.96 (0.86–1.08) | 0.503 |
| Black | 1.15 (1.12–1.18) | <0.001 | 1.14 (1.05–1.23) | 0.002 | 1.15 (1.04–1.28) | 0.007 | 1.08 (1.01–1.17) | 0.029 |
| Age | ||||||||
| ≤30 | ref | ref | ref | ref | ref | ref | ref | ref |
| 31–60 | 2.30 (2.0–2.56) | <0.001 | 1.10 (0.81–1.49) | 0.536 | 0.93 (0.74–1.18) | 0.555 | 2.29 (1.52–3.46) | <0.001 |
| ≥61 | 5.38 (4.84–5.98) | <0.001 | 2.00 (1.48–2.70) | <0.001 | 1.48 (1.17–1.87) | 0.001 | 3.04 (2.02–4.59) | <0.001 |
| Stage | ||||||||
| Localized | ref | ref | NA | NA | NA | NA | NA | NA |
| Regional | 1.68 (1.63–1.73) | <0.001 | NA | NA | NA | NA | NA | NA |
| Distant | 4.46 (4.35–4.57) | <0.001 | NA | NA | NA | NA | NA | NA |
| Site | ||||||||
| Lung | ref | ref | NA | NA | NA | NA | NA | NA |
| Appendix | 0.55 (0.51–0.59) | <0.001 | NA | NA | NA | NA | NA | NA |
| Colorectal | 0.54 (0.52–0.56) | <0.001 | NA | NA | NA | NA | NA | NA |
| Liver | 1.75 (1.59–1.92) | <0.001 | NA | NA | NA | NA | NA | NA |
| Pancreas | 0.90 (0.87–0.93) | <0.001 | NA | NA | NA | NA | NA | NA |
| Small bowel | 0.61 (0.59–0.63) | <0.001 | NA | NA | NA | NA | NA | NA |
| Stomach | 0.83 (0.80–0.86) | <0.001 | NA | NA | NA | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Cao, F.; Hosein, P.; Huang, B.; Pinheiro, P.S.; Wang, Y.; Del Rivero, J.; Lopes, G.; Chauhan, A. Dethroning of Neuroendocrine Tumor as an Orphan Disease: US Incidence, Prevalence, and Survival in the 21st Century. Cancers 2025, 17, 3323. https://doi.org/10.3390/cancers17203323
Yu Q, Cao F, Hosein P, Huang B, Pinheiro PS, Wang Y, Del Rivero J, Lopes G, Chauhan A. Dethroning of Neuroendocrine Tumor as an Orphan Disease: US Incidence, Prevalence, and Survival in the 21st Century. Cancers. 2025; 17(20):3323. https://doi.org/10.3390/cancers17203323
Chicago/Turabian StyleYu, Qian, Fan Cao, Peter Hosein, Bin Huang, Paulo S. Pinheiro, Yating Wang, Jaydira Del Rivero, Gilberto Lopes, and Aman Chauhan. 2025. "Dethroning of Neuroendocrine Tumor as an Orphan Disease: US Incidence, Prevalence, and Survival in the 21st Century" Cancers 17, no. 20: 3323. https://doi.org/10.3390/cancers17203323
APA StyleYu, Q., Cao, F., Hosein, P., Huang, B., Pinheiro, P. S., Wang, Y., Del Rivero, J., Lopes, G., & Chauhan, A. (2025). Dethroning of Neuroendocrine Tumor as an Orphan Disease: US Incidence, Prevalence, and Survival in the 21st Century. Cancers, 17(20), 3323. https://doi.org/10.3390/cancers17203323









