From TNM 8 to TNM 9: Stage Migration and Histology-Specific Patterns in Lung Cancer
Simple Summary
Abstract
1. Introduction
1.1. Worldwide Prevalence of Lung Cancer
1.2. Key Updates from TNM 8 to TNM 9
- T descriptor
- N descriptor
- N2a—involvement of a single N2 station;
- N2b—involvement of multiple N2 stations. In the 9th TNM edition, it is worth noting that nodal disease is measured by the number of the involved nodal stations, rather than the total number of lymph nodes affected.
- M descriptor
- M1c1—multiple metastatic deposits affecting a single extrathoracic organ;
- M1c2—multiple metastatic deposits affecting multiple extrathoracic organs.
1.3. Role of TNM Classification in Clinical Decision-Making
- Stage groups
2. Materials and Methods
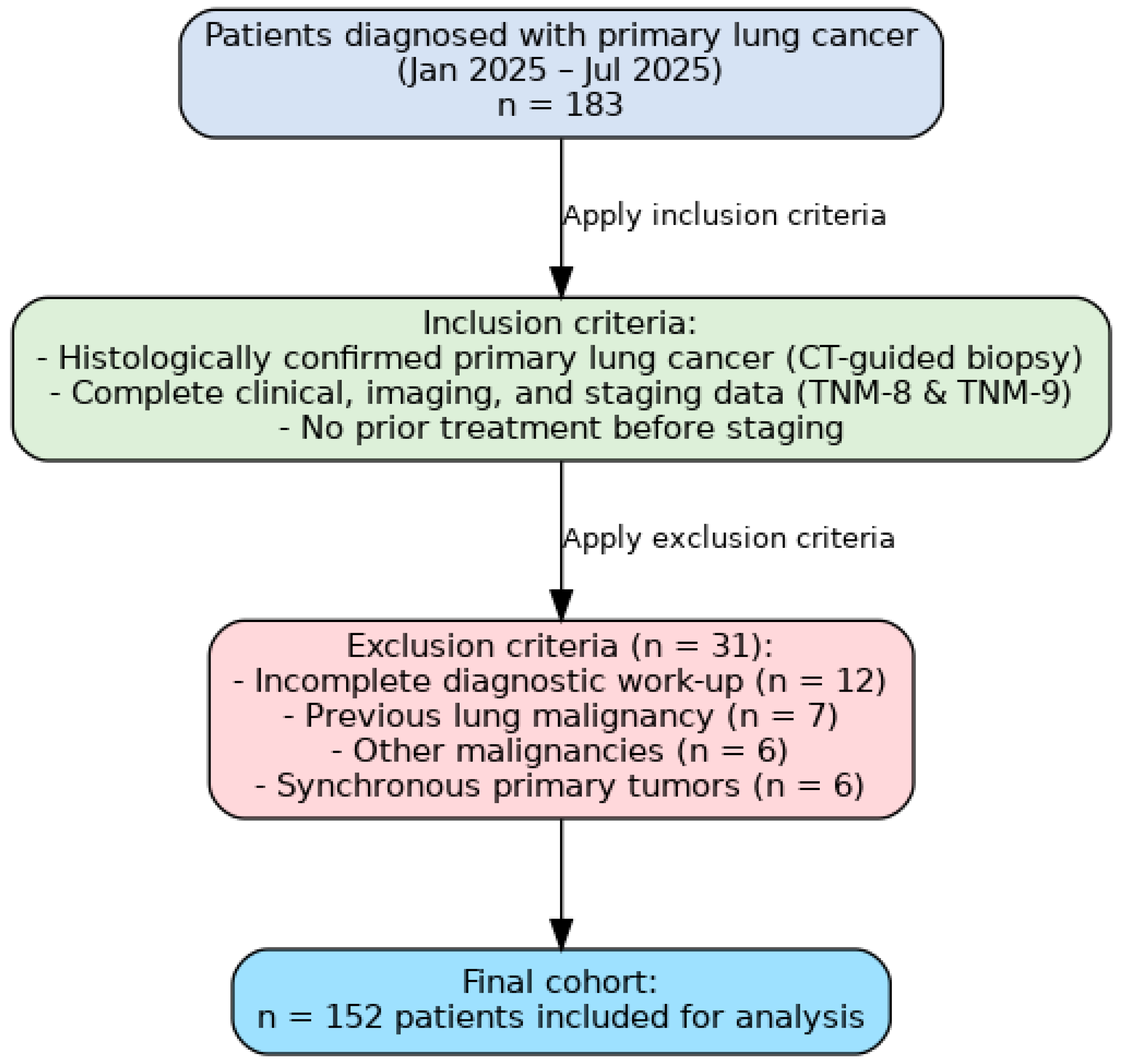
2.1. Study Design and Patient Selection
- Histologically confirmed primary lung cancer, diagnosed through CT-guided transthoracic lung biopsy;
- Complete clinical, imaging, and staging data available for both TNM editions;
- No prior treatment before staging evaluation.
- Incomplete diagnostic work-up;
- Previous history of lung malignancy;
- Presence of other malignancies;
- Synchronous primary tumors.
2.2. Data Collection and Imaging Evaluation
2.3. Staging Procedures
- N descriptor: subdivision of N2 disease into single-station (N2a) and multi-station (N2b);
- M descriptor: subdivision of M1c into M1c1 (multiple lesions in a single extrathoracic organ) and M1c2 (multiple lesions in multiple extrathoracic organs);
- Corresponding updates in stage grouping.
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
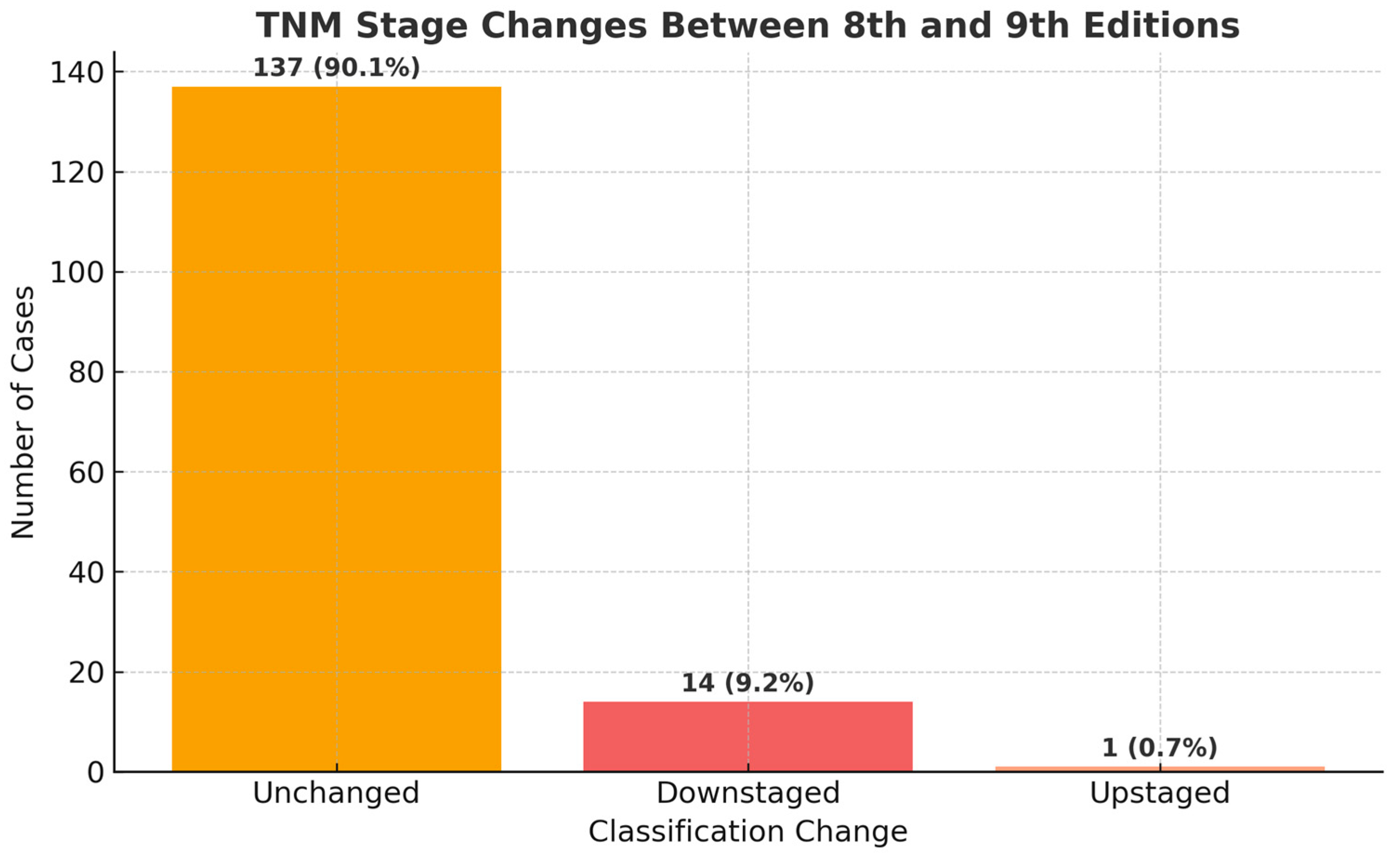
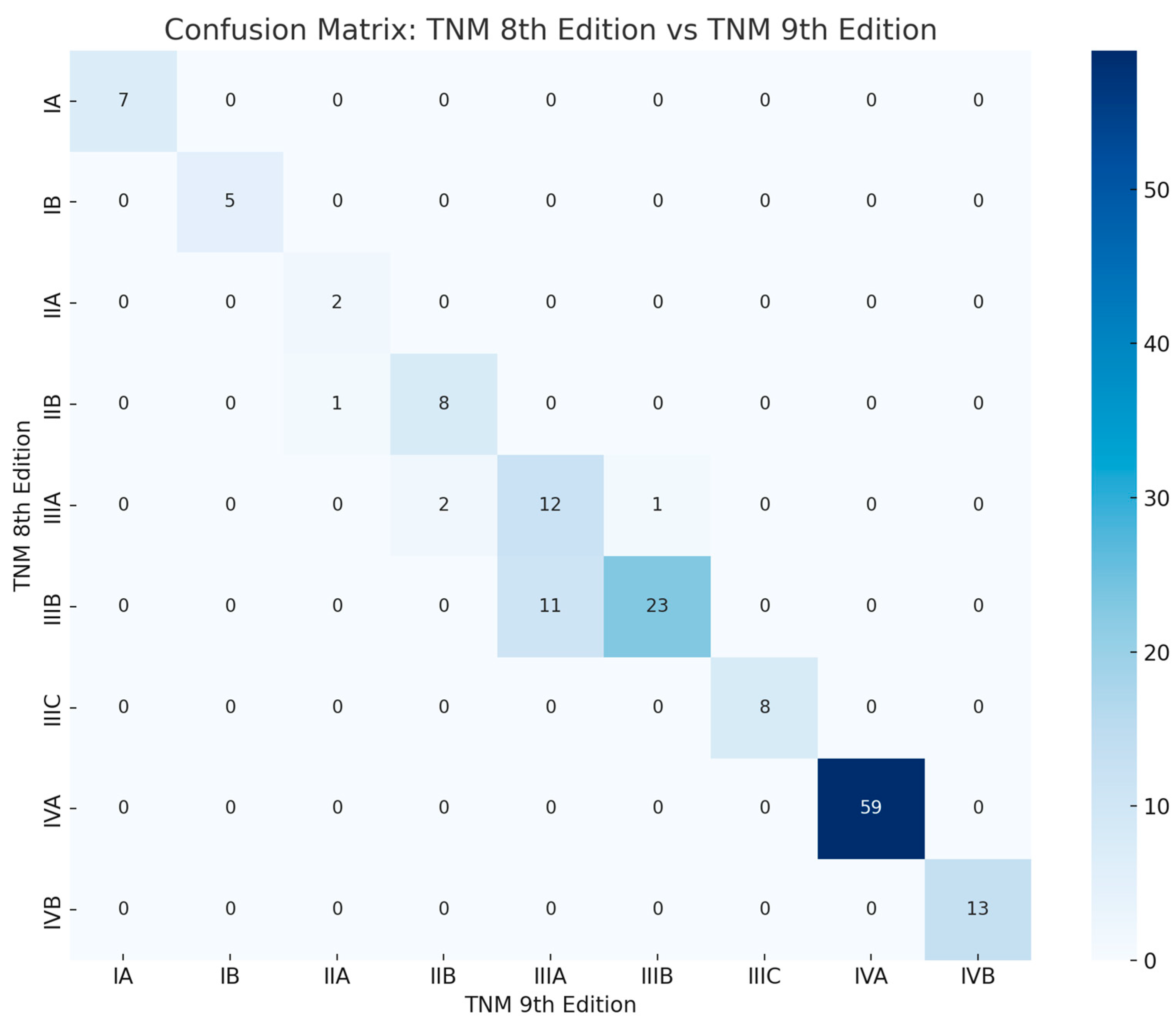
3.1. Comprehensive Alterations in Stage Distribution
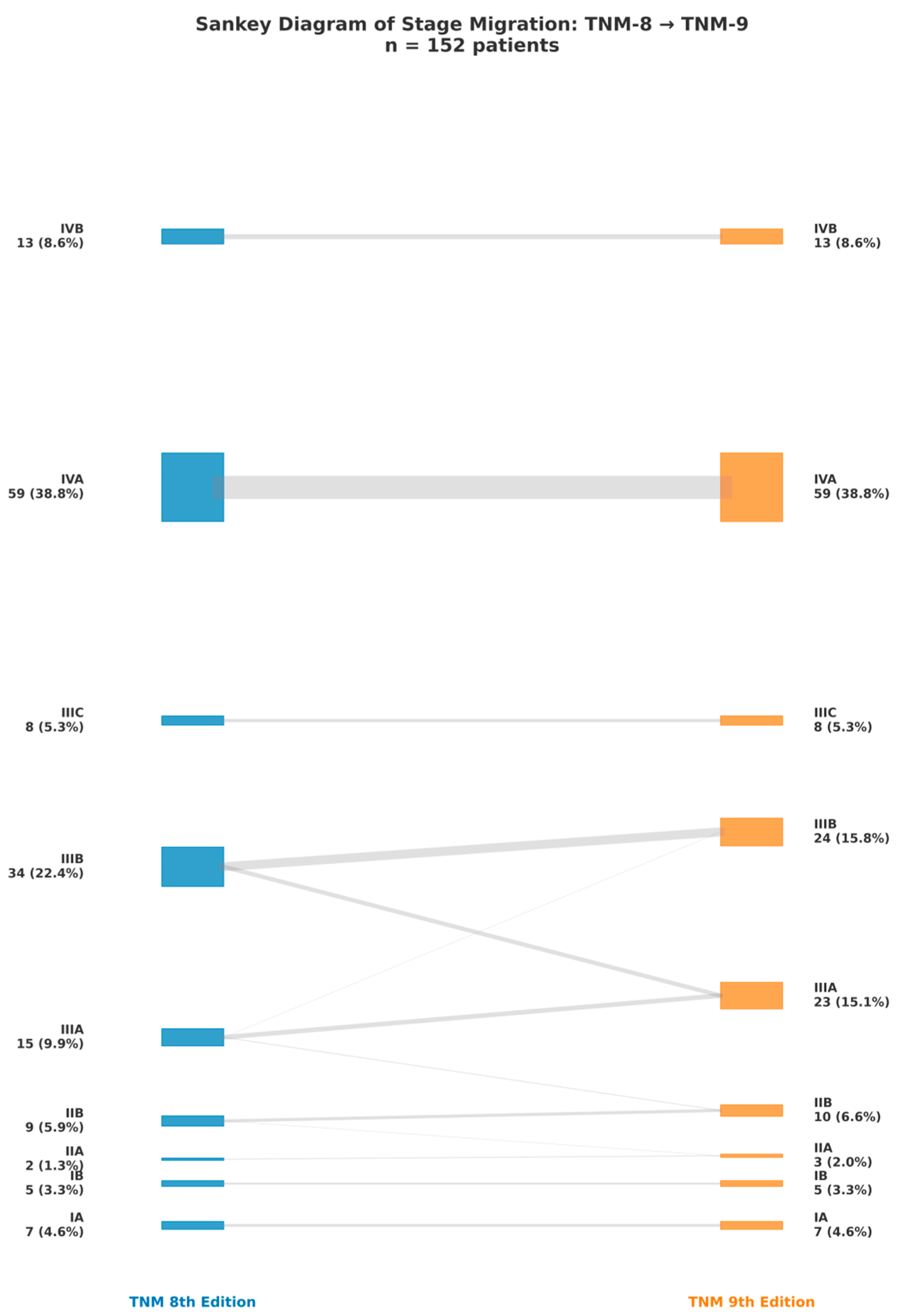
3.2. Comprehensive Reclassification Patterns
3.3. Visual Comparison of Stage Proportions
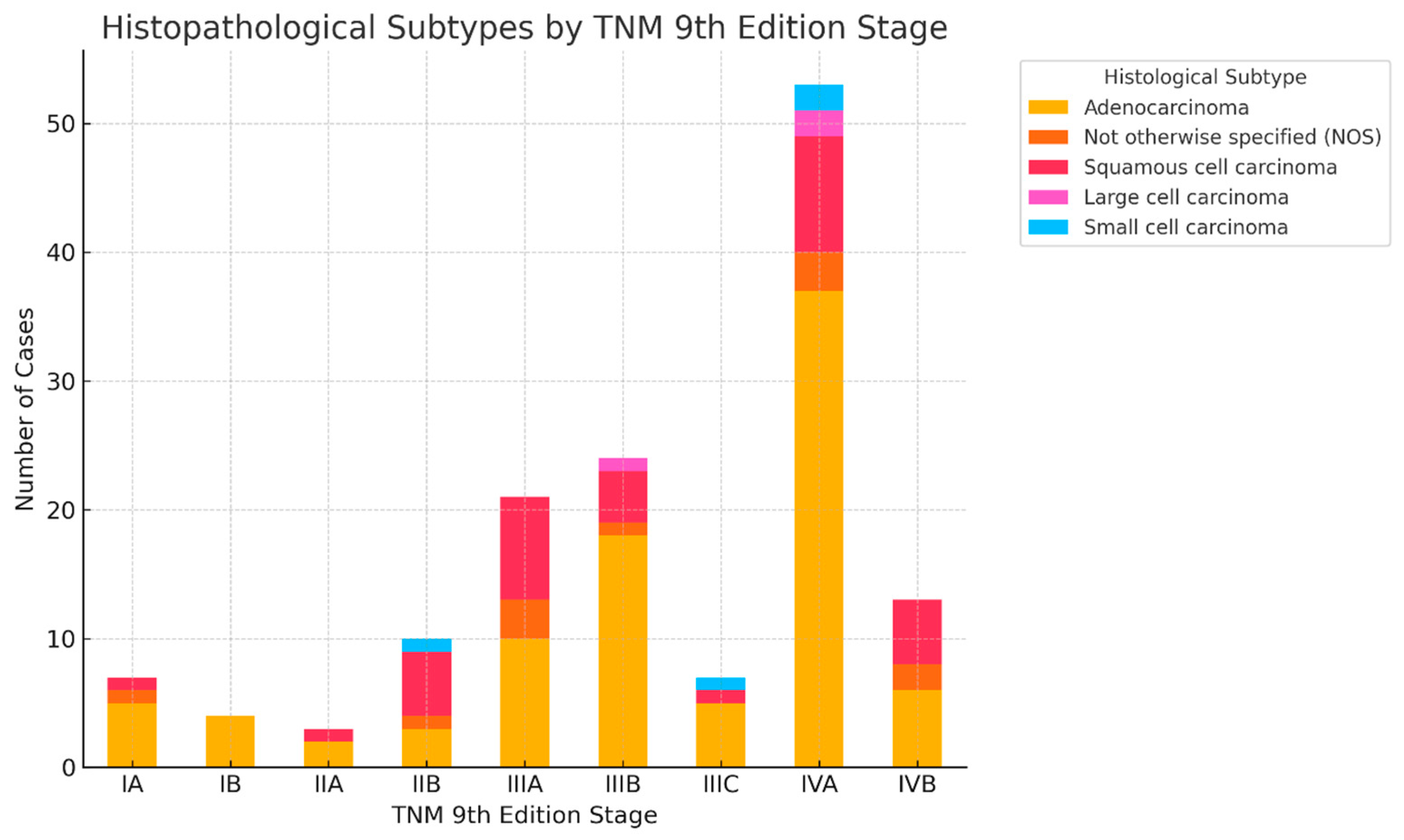
3.4. Distribution of Histological Subtypes Across TNM 9 Stages
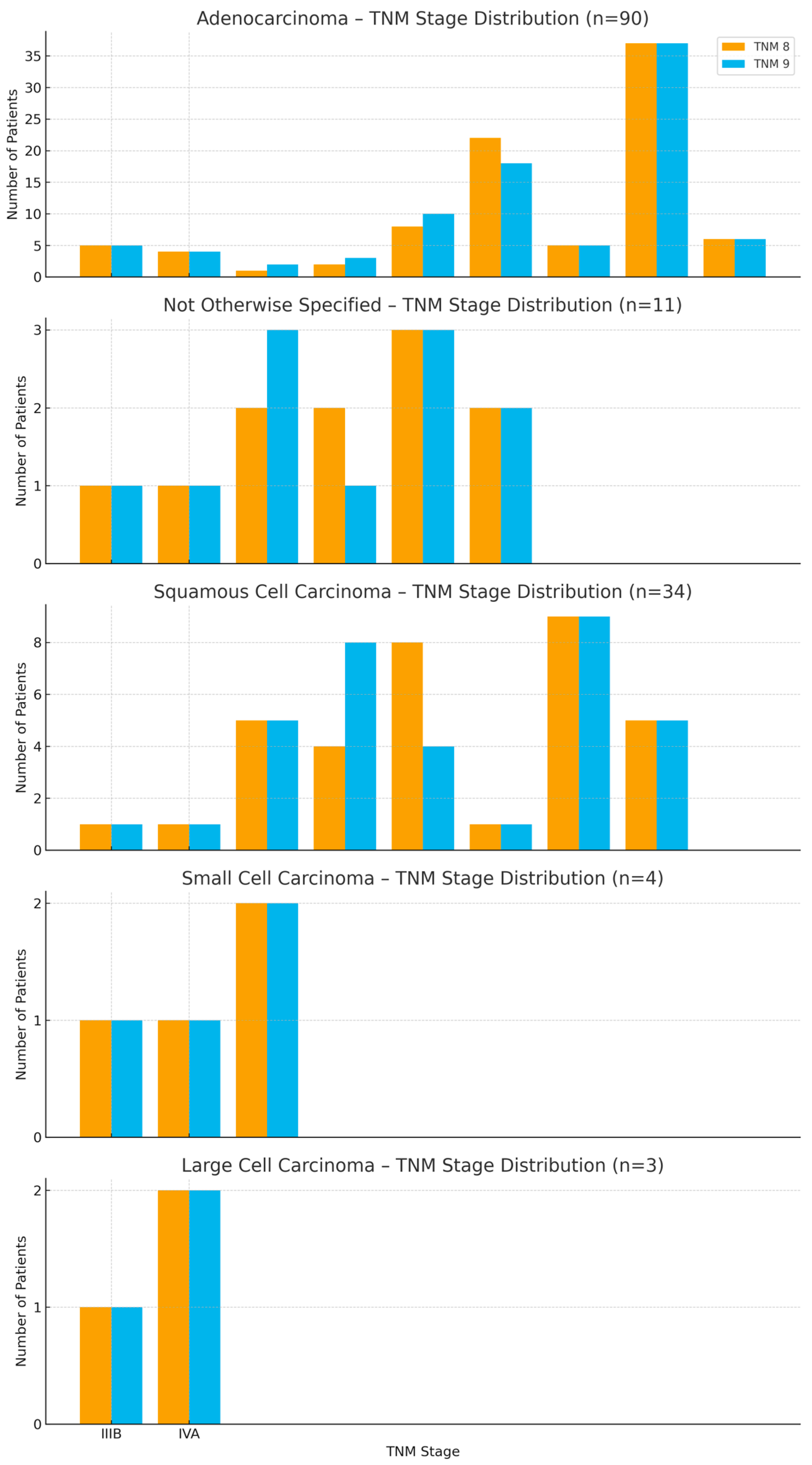
3.5. Staging Transitions by Histology
3.6. Subtype-Specific Staging Comparisons
3.7. Stage Variability Across Histologies
4. Discussion
4.1. Interpretation of Staging Shifts
4.2. Impact on Treatment Eligibility and Prognosis
4.3. Comparison with International Data and Literature
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, J.; Xu, Y.; Liu, J.; Feng, L.; Yu, J.; Chen, D. Global Burden of Lung Cancer in 2022 and Projections to 2050: Incidence and Mortality Estimates from GLOBOCAN. Cancer Epidemiol. 2024, 93, 102693. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-Small Cell Lung Cancer: Epidemiology, Risk Factors, Treatment, and Survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-Cell Lung Cancer. Nat. Rev. Dis. Primers 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Jiang, Y.; Gong, L.; Wang, Y.; Su, Z.; Li, X.; Wu, H.; Pan, H.; Wang, J.; Meng, Z.; et al. Epidemiological and Demographic Drivers of Lung Cancer Mortality from 1990 to 2019: Results from the Global Burden of Disease Study 2019. Front. Public Health 2023, 11, 1054200. [Google Scholar] [CrossRef]
- Ancusa, V.M.; Trusculescu, A.A.; Constantinescu, A.; Burducescu, A.; Fira-Mladinescu, O.; Manolescu, D.L.; Traila, D.; Wellmann, N.; Oancea, C.I. Temporal Trends and Patient Stratification in Lung Cancer: A Comprehensive Clustering Analysis from Timis County, Romania. Cancers 2025, 17, 2305. [Google Scholar] [CrossRef] [PubMed]
- Asamura, H.; Nishimura, K.K.; Giroux, D.J.; Chansky, K.; Hoering, A.; Rusch, V.; Rami-Porta, R.; Asamura, H.; Rusch, V.; Rami-Porta, R.; et al. IASLC Lung Cancer Staging Project: The New Database to Inform Revisions in the Ninth Edition of the TNM Classification of Lung Cancer. J. Thorac. Oncol. 2023, 18, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Woodard, G.A.; Bader, A.S.; Dacic, S.; Grant, M.J.; Park, H.S.; Tanoue, L.T. The Proposed Ninth Edition TNM Classification of Lung Cancer. Chest 2024, 166, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Klug, M.; Kirshenboim, Z.; Truong, M.T.; Sorin, V.; Ofek, E.; Agrawal, R.; Marom, E.M. Proposed Ninth Edition TNM Staging System for Lung Cancer: Guide for Radiologists. RadioGraphics 2024, 44, e240057. [Google Scholar] [CrossRef] [PubMed]
- Rami-Porta, R.; Nishimura, K.K.; Giroux, D.J.; Detterbeck, F.; Cardillo, G.; Edwards, J.G.; Fong, K.M.; Giuliani, M.; Huang, J.; Kernstine, K.H.; et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groups in the Forthcoming (Ninth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2024, 19, 1007–1027. [Google Scholar] [CrossRef] [PubMed]
- Argentieri, G.; Valsecchi, C.; Petrella, F.; Jungblut, L.; Frauenfelder, T.; Del Grande, F.; Rizzo, S. Implementation of the 9th TNM for Lung Cancer: Practical Insights for Radiologists. Eur. Radiol. 2025, 35, 4395–4402. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Non-Small Cell Lung Cancer Treatment (PDQ®)—Health Professional Version; National Cancer Institute: Bethesda, MD, USA, 2025. [Google Scholar]
- Constantinescu, A.; Stoicescu, E.R.; Iacob, R.; Chira, C.A.; Cocolea, D.M.; Nicola, A.C.; Mladin, R.; Oancea, C.; Manolescu, D. CT-Guided Transthoracic Core-Needle Biopsy of Pulmonary Nodules: Current Practices, Efficacy, and Safety Considerations. J. Clin. Med. 2024, 13, 7330. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef]
- Erasmus, L.T.; Strange, C.D.; Ahuja, J.; Agrawal, R.; Shroff, G.S.; Marom, E.M.; Truong, M.T. Imaging of Lung Cancer Staging: TNM 9 Updates. Semin. Ultrasound CT MRI 2024, 45, 410–419. [Google Scholar] [CrossRef]
- Magalhães Ferreira, P.; Campos, R.; Valente, C.; Ferreira, J.; Freitas, C.; Sousa, C.; Araújo, D.; Novais Bastos, H.; Magalhães, A.; Fernandes, M.G. Out with the Old—Advancements and Shortcomings of the Updated 9th Edition of the Tumor, Node, Metastasis (TNM) Classification System for Lung Cancer. J. Bras. Pneumol. 2025, 51, e20250022. [Google Scholar] [CrossRef] [PubMed]
- Demirdöğen, E.; Terzi, O.E.; Aydın Güçlü, Ö.; Ursavaş, A.; Karadağ, M. Differences Between the 8th and 9th Editions of the TNM Staging System in Predicting Mortality in Non-Small Cell Lung Cancer Patients Staged with EBUS. Diagnostics 2025, 15, 1570. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, B.; Park, E.Y.; Yang, B.; Lee, G.K.; Kim, T.S.; Kim, H.Y.; Kim, M.S.; Lee, J.M. Long-Term Survival According to N Stage Diagnosed by Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration in Non-Small Cell Lung Cancer. Chest 2022, 161, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Rami-Porta, R. The TNM Classification of Lung Cancer—A Historic Perspective. J. Thorac. Dis. 2024, 16, 8053–8067. [Google Scholar] [CrossRef] [PubMed]
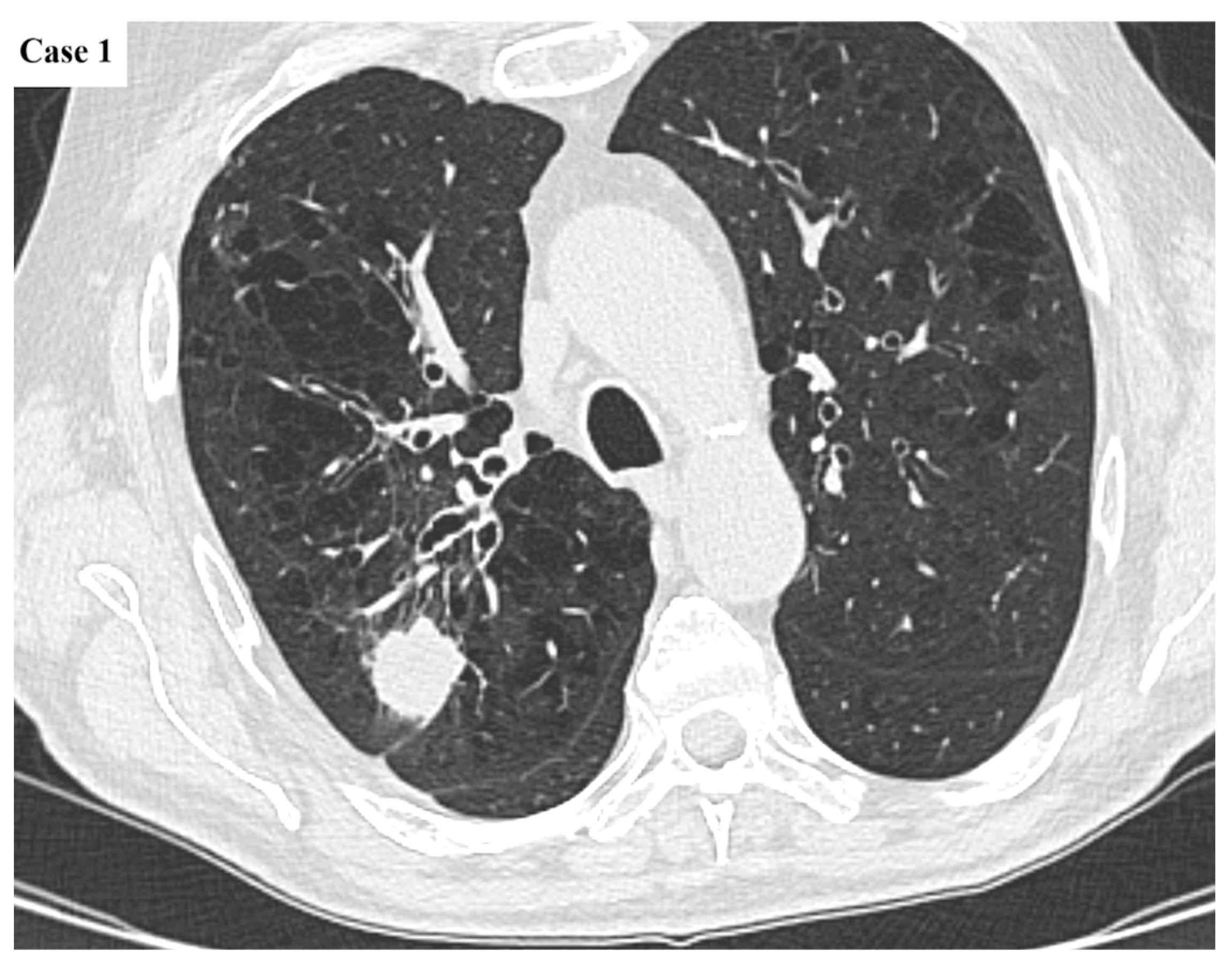
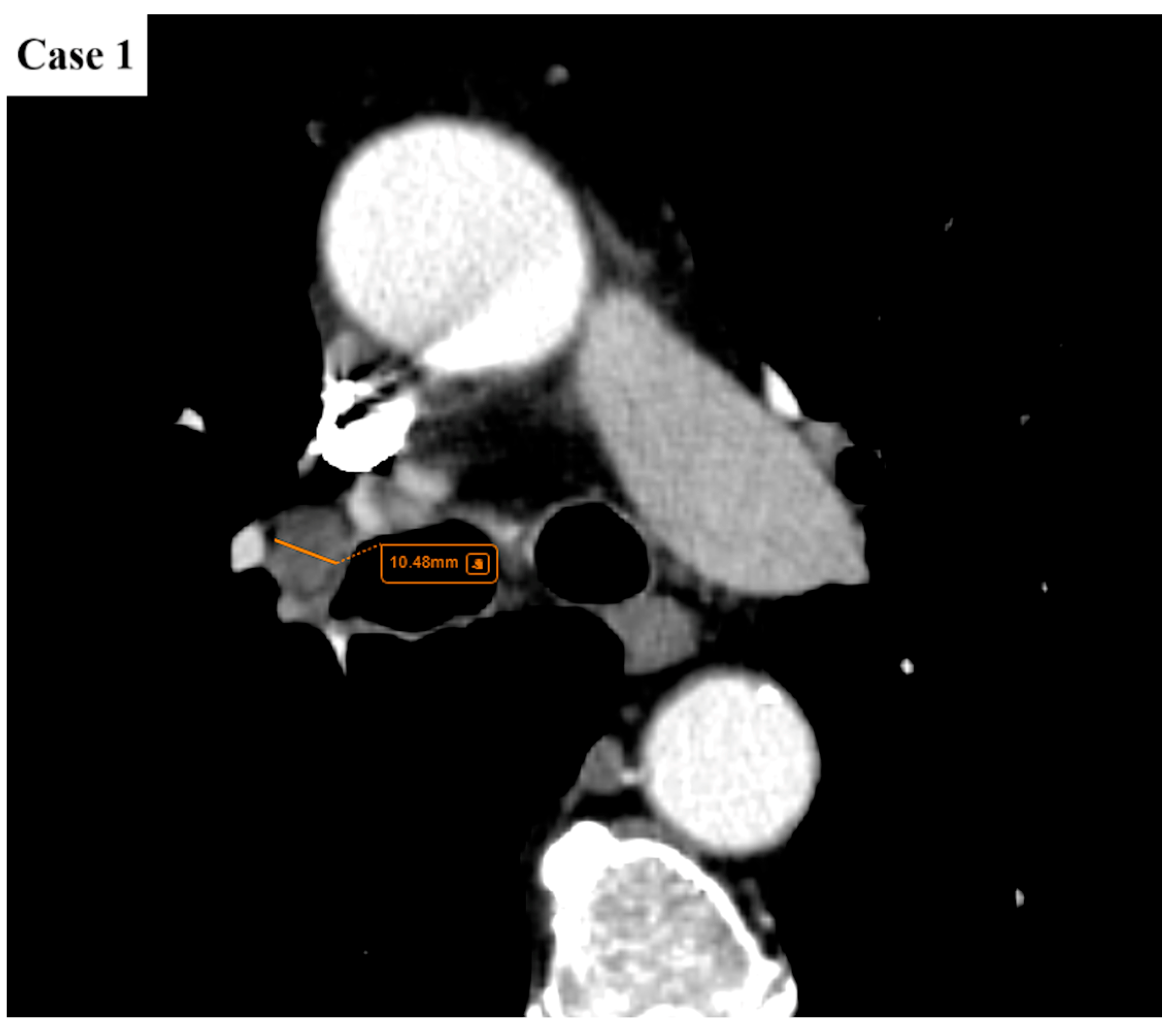
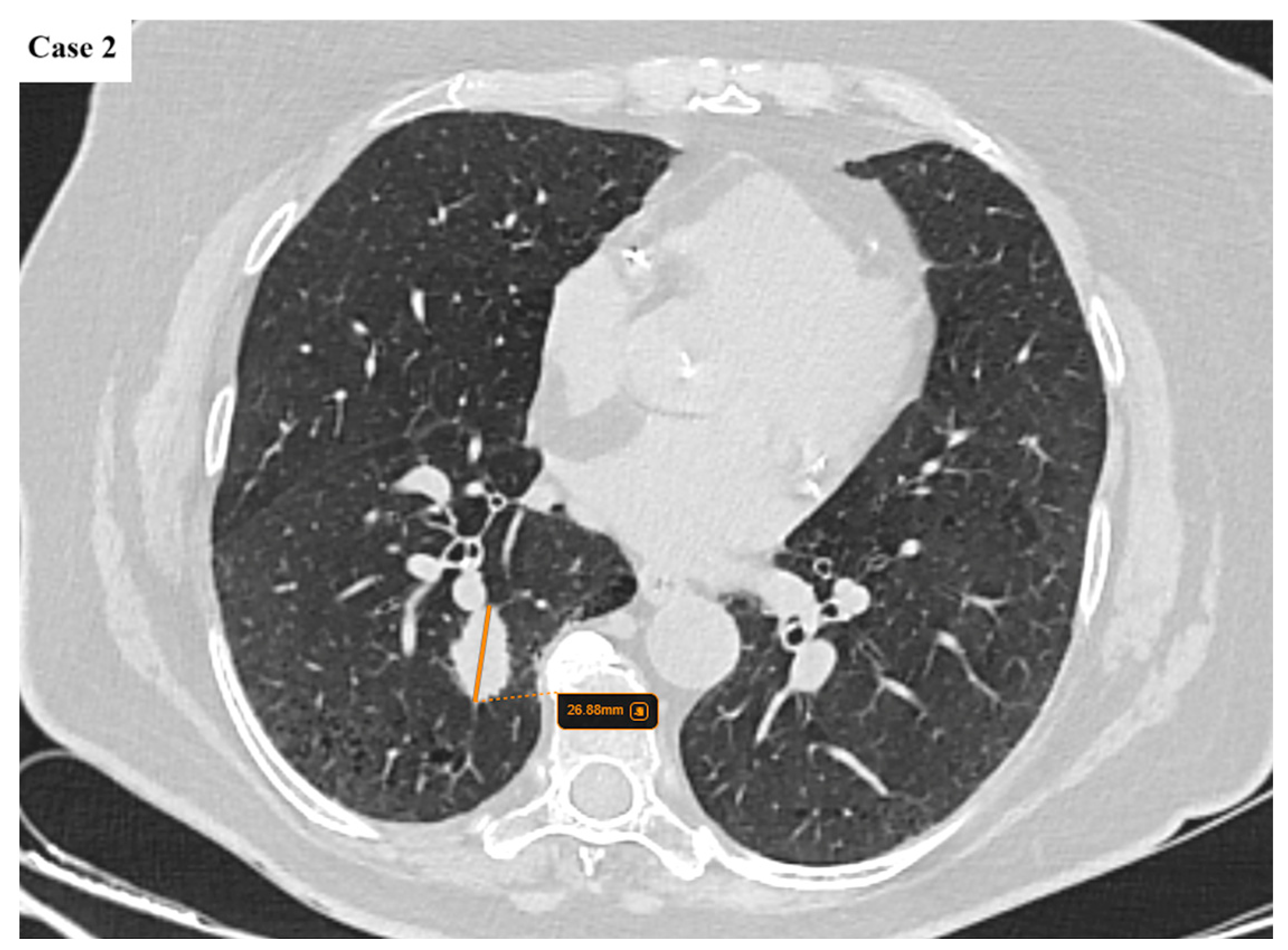
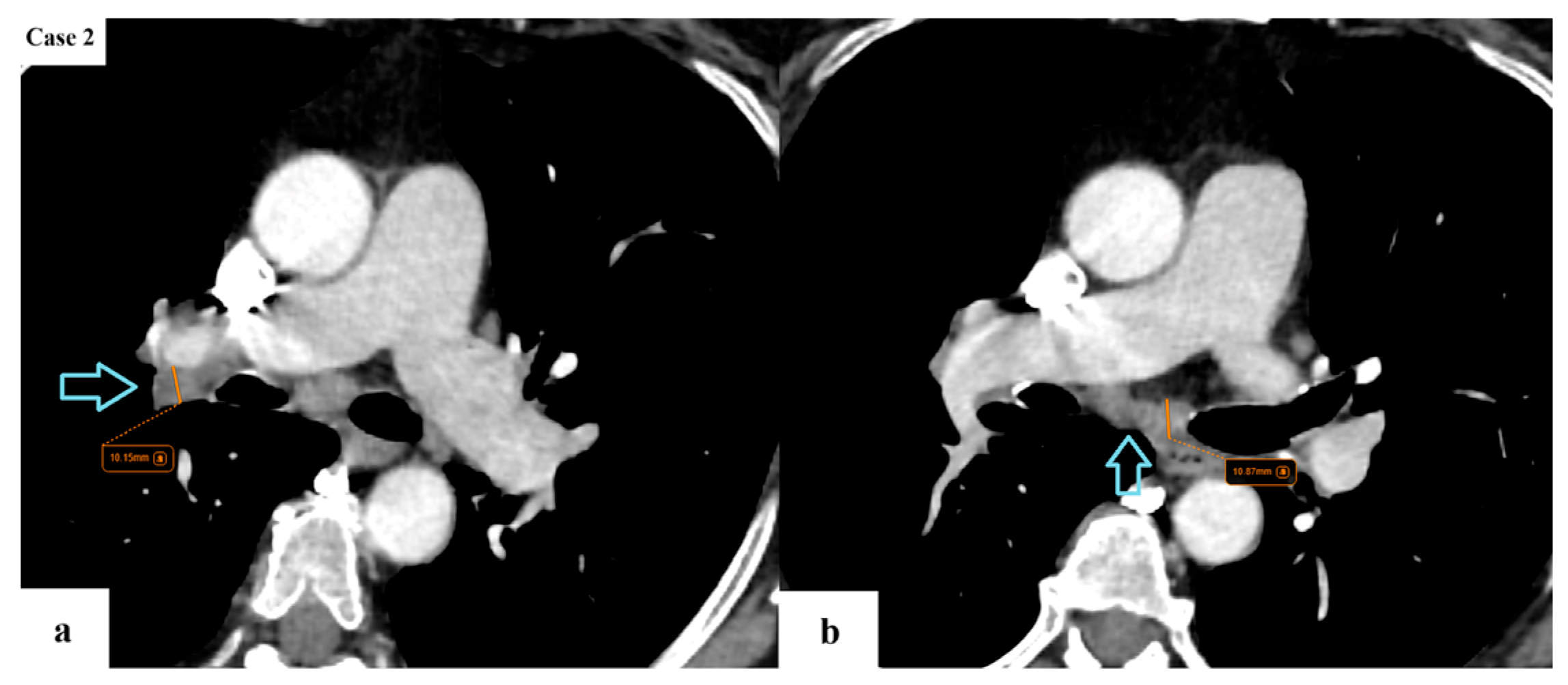
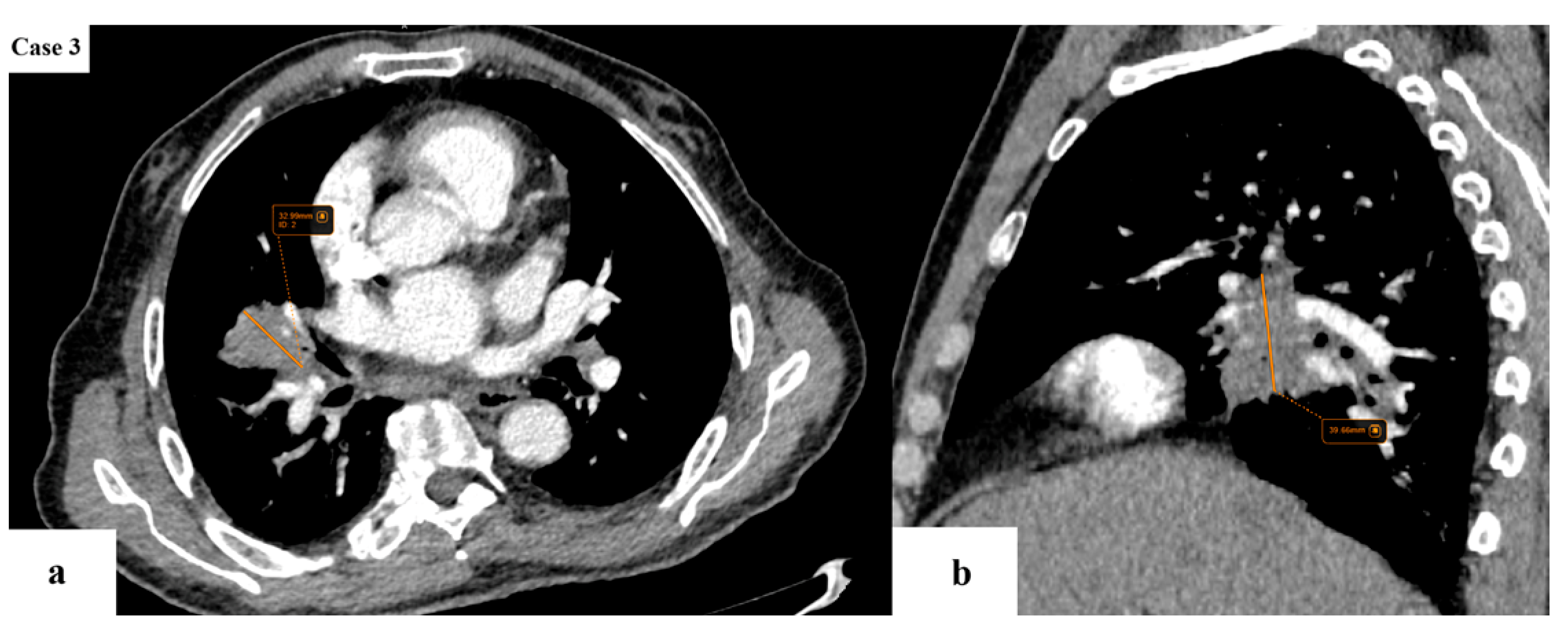
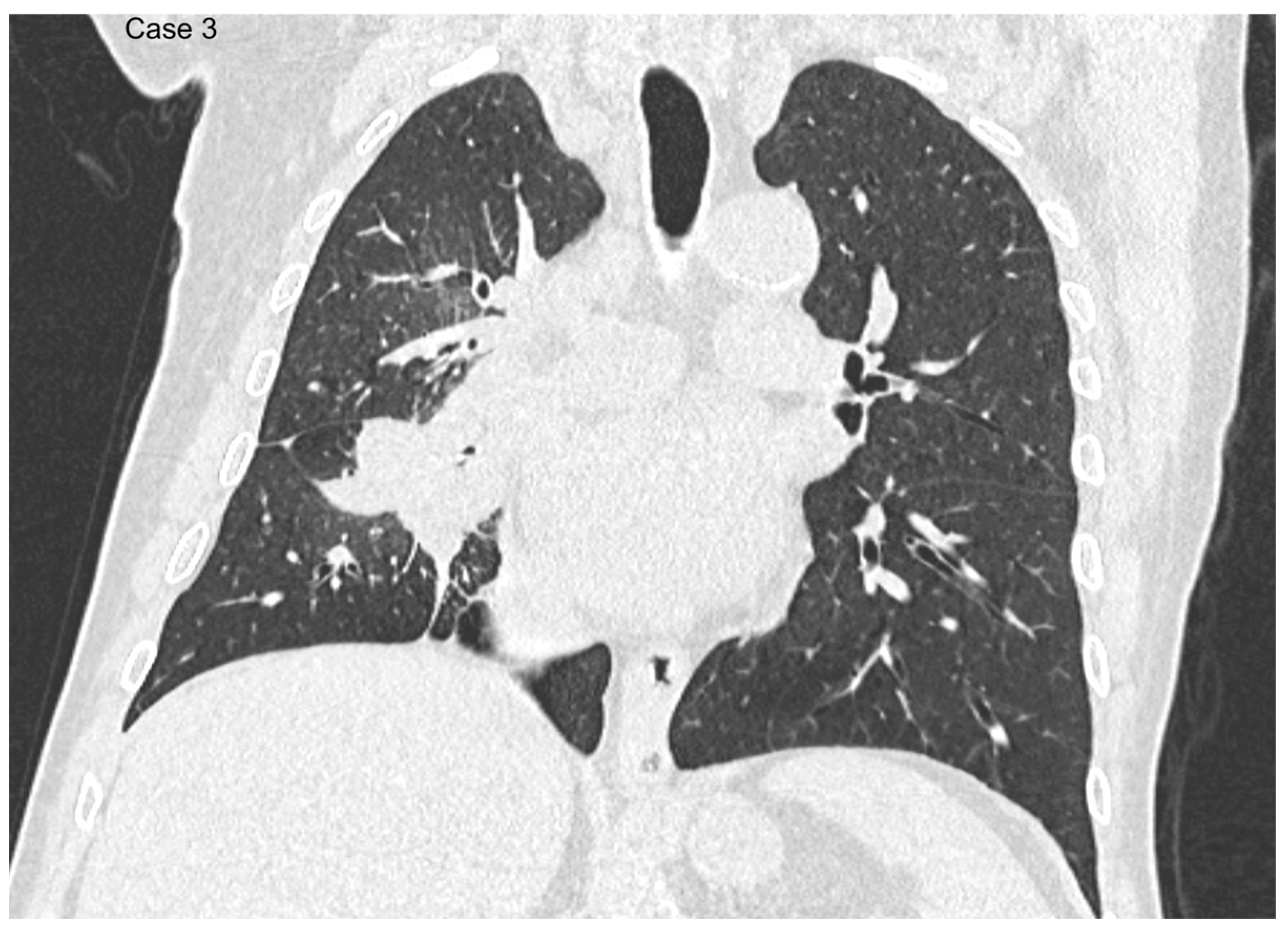
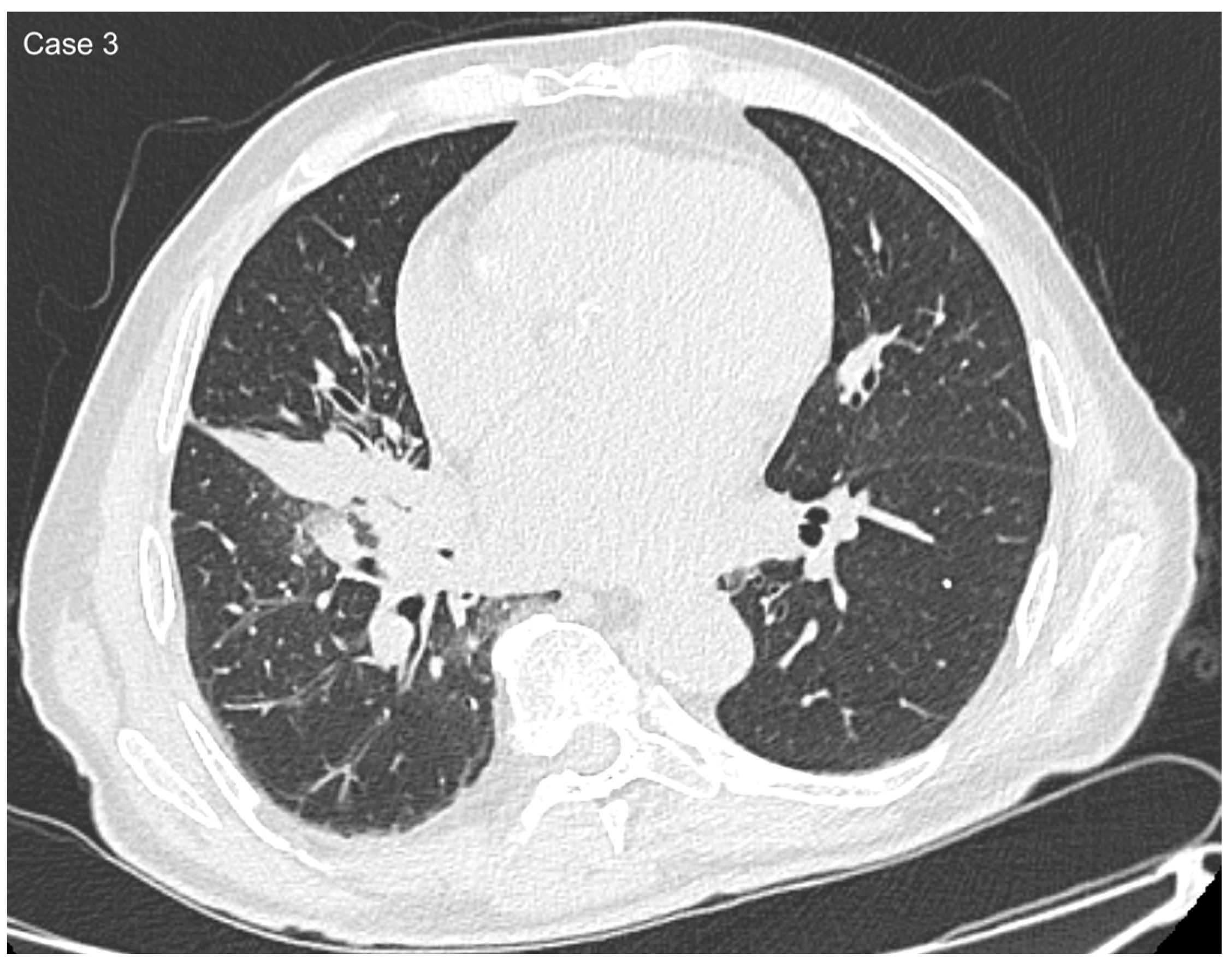
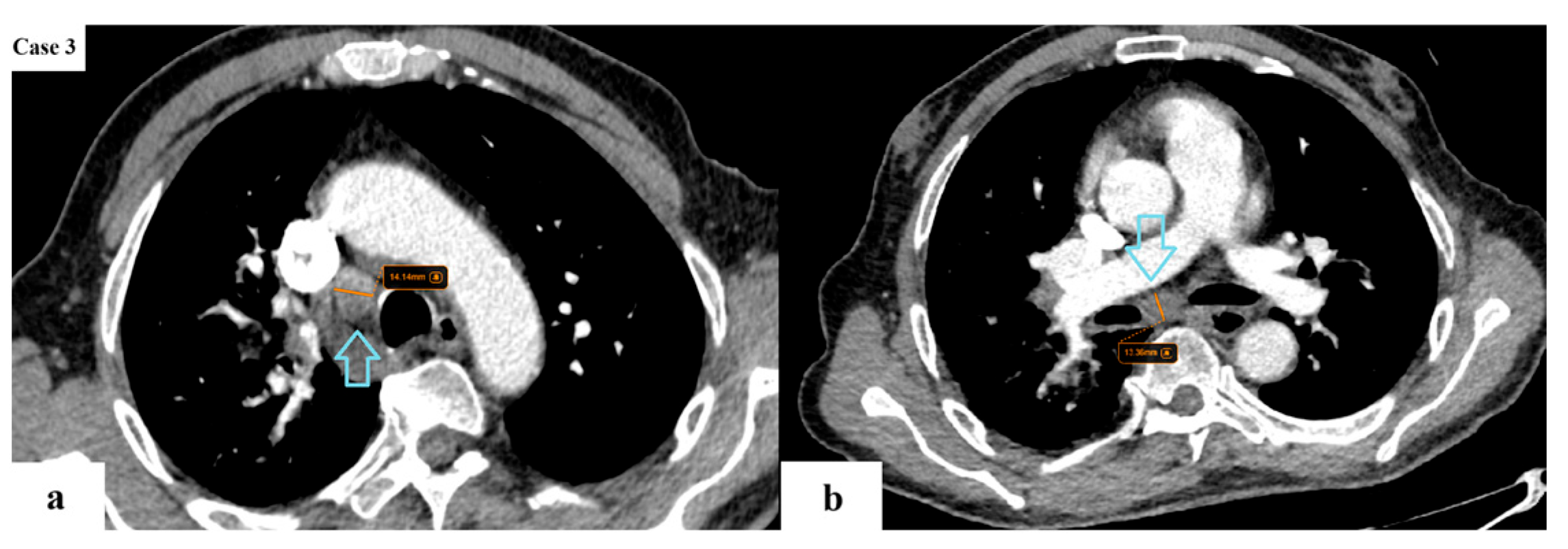
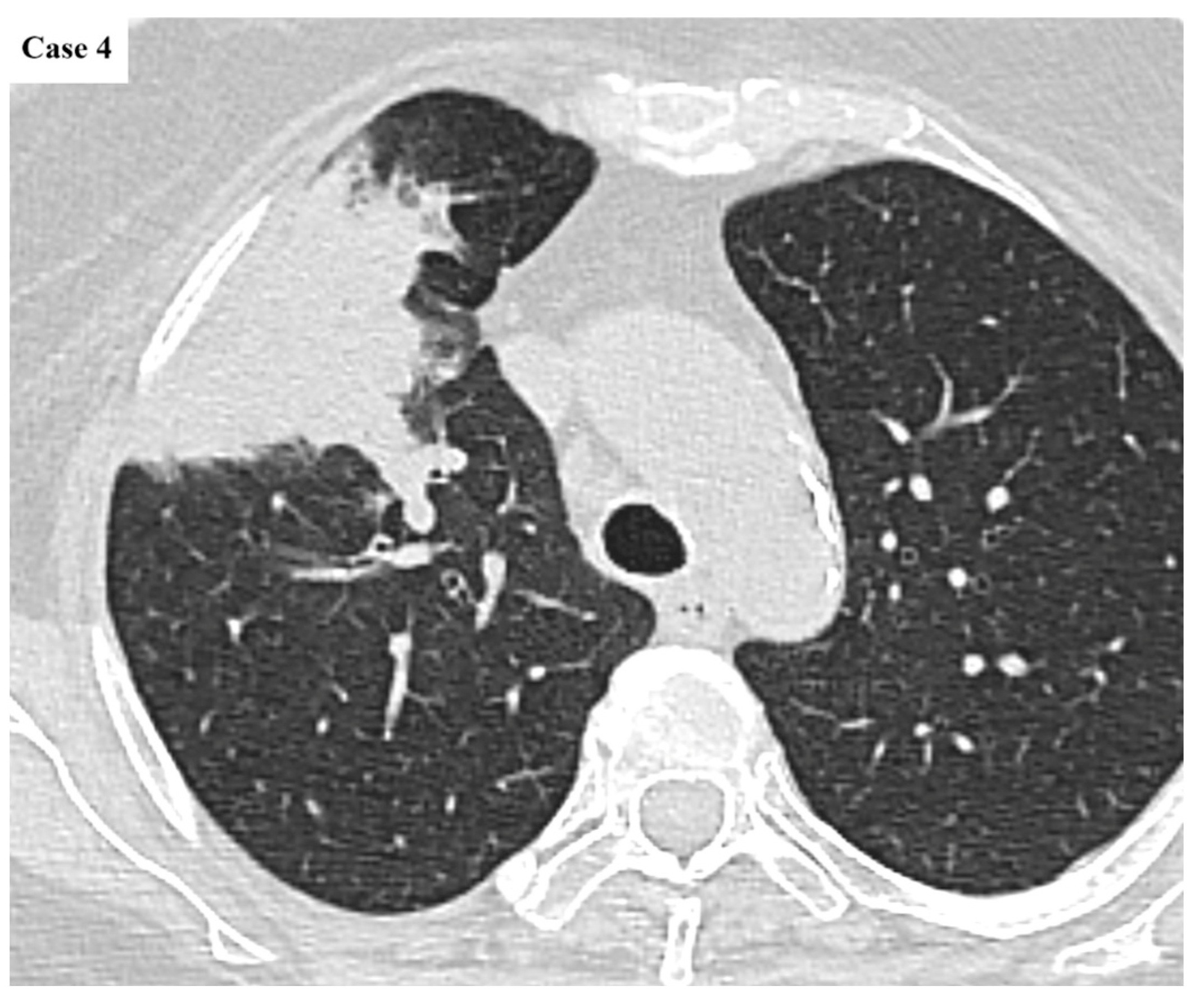
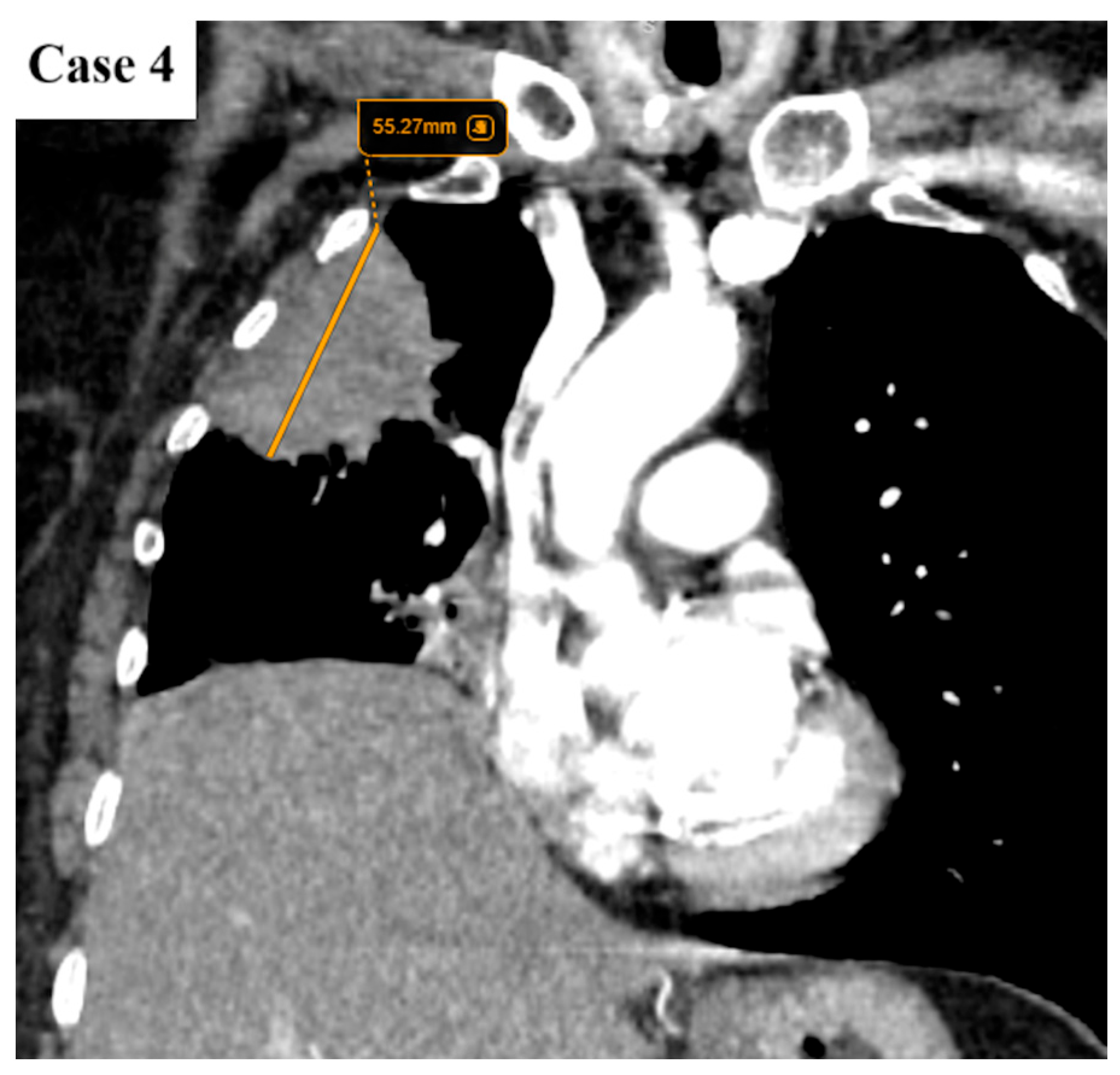


| Age (years) | Mean 66.4 ± 9.2 (range 40–87) |
| Sex | Male: 107 (70.4%) Female: 45 (29.6%) |
| Smoking status | Current: 71 (46.7%) Former: 49 (32.2%) Never: 32 (21.1%) |
| BMI (kg/m2) | Median 25.3 (IQR 22.3–28.5) |
| Pack-years | Median 30 (IQR 20–50) |
| Stage at diagnosis (TNM 8) | IA: 5 (3.3%) IB: 6 (3.9%) IIA: 7 (4.6%) IIB: 9 (5.9%) IIIA: 23 (15.1%) IIIB: 29 (19.1%) IIIC: 12 (7.9%) IVA: 46 (30.3%) IVB: 15 (9.9%) |
| Histology | Adenocarcinoma: 84 (55.3%) Squamous cell carcinoma: 39 (25.7%) Small cell: 12 (7.9%) Large cell: 7 (4.6%) Other/unspecified: 10 (6.6%) |
| Tumor size (cm) | Median 4.2 (IQR 2.6–5.9) |
| Tumor location | Upper lobe: 80 (52.6%) Lower lobe: 66 (43.4%) Diffuse involvement: 6 (3.9%) |
| Imaging features | Spiculated margins: 88% Central necrosis: 38.4% Cavity: 10.4% Lymphangitic spread: 43.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantinescu, A.; Căprariu, R.-N.; Stoicescu, E.-R.; Iacob, R.; Mânzatu, M.; Drimus, J.C.; Roșian, A.-S.; Ionescu, A.; Oancea, C.; Manolescu, D. From TNM 8 to TNM 9: Stage Migration and Histology-Specific Patterns in Lung Cancer. Cancers 2025, 17, 3290. https://doi.org/10.3390/cancers17203290
Constantinescu A, Căprariu R-N, Stoicescu E-R, Iacob R, Mânzatu M, Drimus JC, Roșian A-S, Ionescu A, Oancea C, Manolescu D. From TNM 8 to TNM 9: Stage Migration and Histology-Specific Patterns in Lung Cancer. Cancers. 2025; 17(20):3290. https://doi.org/10.3390/cancers17203290
Chicago/Turabian StyleConstantinescu, Amalia, Radu-Nicolae Căprariu, Emil-Robert Stoicescu, Roxana Iacob, Marius Mânzatu, Janet Camelia Drimus, Alessia-Stephania Roșian, Alexandre Ionescu, Cristian Oancea, and Diana Manolescu. 2025. "From TNM 8 to TNM 9: Stage Migration and Histology-Specific Patterns in Lung Cancer" Cancers 17, no. 20: 3290. https://doi.org/10.3390/cancers17203290
APA StyleConstantinescu, A., Căprariu, R.-N., Stoicescu, E.-R., Iacob, R., Mânzatu, M., Drimus, J. C., Roșian, A.-S., Ionescu, A., Oancea, C., & Manolescu, D. (2025). From TNM 8 to TNM 9: Stage Migration and Histology-Specific Patterns in Lung Cancer. Cancers, 17(20), 3290. https://doi.org/10.3390/cancers17203290










