Reducing the Time-to-Antibiotic by Adapting a Standard of Procedure for the Treatment of Pediatric Cancer Patients Presenting with Febrile Neutropenia—A Comparative Analysis of Two Patient Cohorts
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
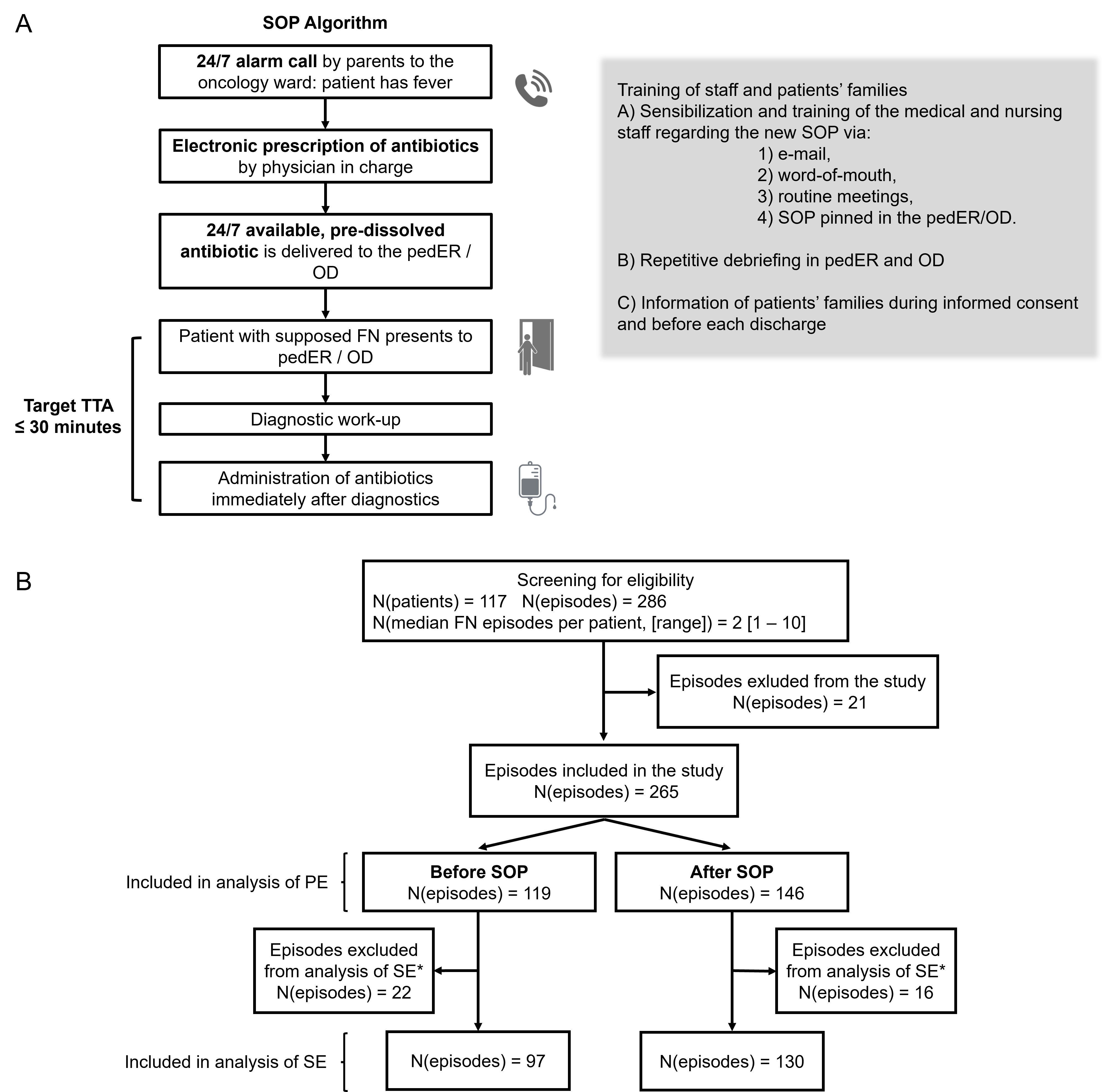
2.1. Study Design
2.2. Local SOP for FN
2.3. Definitions
- Fever = temperature either > 38 °C (100.4 °F) in repetitive measurements for >one hour, or ≥38.5 °C (101.3 °F) in a single measurement.
- Granulocytopenia = ANC < 0.5 × 109/L (<500/μL) on day of admission or a falling trend with expected ANC < 500/μL within the following 48 h (i.e., imminent neutropenia). The terms neutropenia and granulocytopenia are synonyms within this manuscript.
- Febrile neutropenia (FN) = simultaneous presence of fever (1) and neutropenia (2). In our institution, we treat pediatric cancer patients presenting with afebrile neutropenia (or imminent neutropenia) and impaired general condition (e.g., severe fatigue, abdominal pain refractory to non-opioid analgesics, reduced level of consciousness) and/or vital parameters indicating presence of pediatric Systemic Inflammatory Response Syndrome (SIRS: hypotension, tachycardia, hypoxia [11]) as patients with FN according to the corresponding German guideline [4]. Afebrile patients were therefore included in this study to reflect a real-world cohort of FN patients.
- Length of Stay (LOS) = time interval between the admission and the discharge of the patient.
- Sepsis and shock were defined according to the International pediatric sepsis consensus conference [11].
- Time-to-antibiotic (TTA) = lag of time between hospital admission and start of the administration of antibiotics.
- Composite adverse events = presence of ≥ one of the following adverse events: sepsis, admission to ICU, administration of vasopressors, need of respiratory support and death.
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. General Characteristics and Selection of FN Episodes
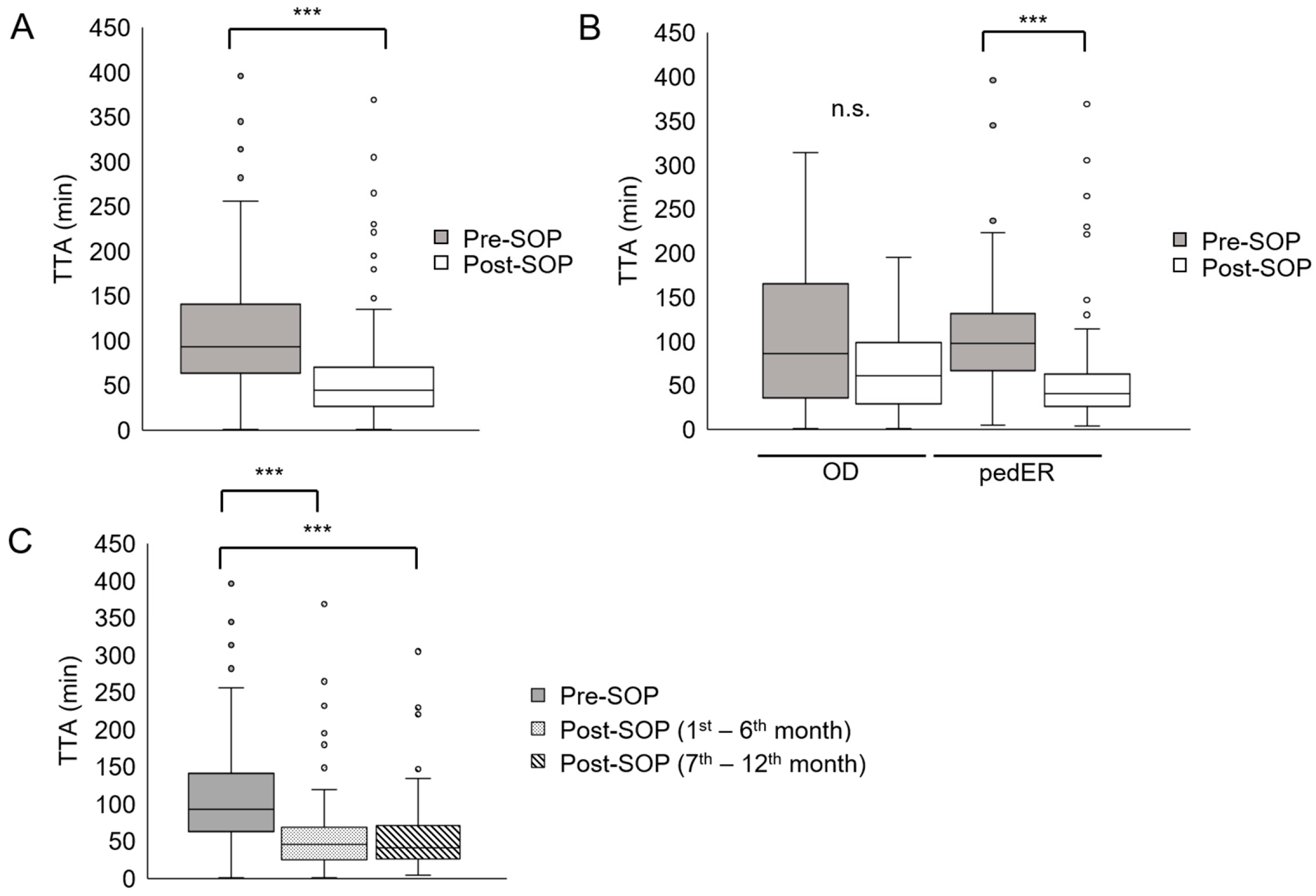
3.2. Reduction in TTA After Adaption of the SOP
3.3. Shorter TTA in Patients with Signs of SIRS or High Fever
3.4. TTA and Adverse Events
3.5. Association Between Laboratory Values and Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse event |
| ANC | Absolute neutrophil count |
| EPR | Electronic patient record |
| FN | Febrile neutropenia |
| FNP | Febrile neutropenia pathway |
| ICU | Intensive care unit |
| IQR | Interquartile range |
| LOS | Length of stay |
| OD | Outpatient department of pediatric hematology and oncology |
| pedER | Pediatric emergency room |
| PE | Primary endpoint |
| post-SOP | After the SOP |
| pre-SOP | Before the SOP |
| QOC | Quality-of-care |
| SE | Secondary endpoint |
| SIRS | Systemic inflammatory response syndrome |
| SOP | Standard operating procedure |
| TTA | Time-to-antibiotic |
References
- Gustinetti, G.; Mikulska, M. Bloodstream infections in neutropenic cancer patients: A practical update. Virulence 2016, 7, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.K.; Fernandez, I.D.; Fisher, S.G.; Asselin, B.L.; Lyman, G.H. Length of Stay and Mortality Associated With Febrile Neutropenia Among Children With Cancer. J. Clin. Oncol. 2005, 23, 7958–7966. [Google Scholar] [CrossRef]
- Rosa, R.G.; Goldani, L.Z. Cohort Study of the Impact of Time to Antibiotic Administration on Mortality in Patients with Febrile Neutropenia. Antimicrob. Agents Chemother. 2014, 58, 3799–3803. [Google Scholar] [CrossRef]
- Lehrnbecher, T.; Robinson, P.D.; Ammann, R.A.; Fisher, B.; Patel, P.; Phillips, R.; Beauchemin, M.P.; Carlesse, F.; Castagnola, E.; Davis, B.L.; et al. Guideline for the Management of Fever and Neutropenia in Pediatric Patients With Cancer and Hematopoietic Cell Transplantation Recipients: 2023 Update. J. Clin. Oncol. 2023, 41, 1774–1785. [Google Scholar] [CrossRef]
- Butts, A.R.; Bachmeier, C.C.; Dressler, E.V.; Liu, M.; Cowden, A.; Talbert, J.; Adams, V.R. Association of time to antibiotics and clinical outcomes in adult hematologic malignancy patients with febrile neutropenia. J. Oncol. Pharm. Pract. 2017, 23, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.L.; Aristizabal, P.; Loera-Reyna, A.; Torres, D.; Ornelas-Sánchez, M.; Nuño-Vázquez, L.; Aguilera, M.; Sánchez, A.; Romano, M.; Rivera-Gómez, R.; et al. The Golden Hour: Sustainability and Clinical Outcomes of Adequate Time to Antibiotic Administration in Children with Cancer and Febrile Neutropenia in Northwestern Mexico. JCO Glob. Oncol. 2021, 7, 659–670. [Google Scholar] [CrossRef]
- Koenig, C.; Schneider, C.; Morgan, J.E.; Ammann, R.A.; Sung, L.; Phillips, B. Interventions aiming to reduce time to antibiotics (TTA) in patients with fever and neutropenia during chemotherapy for cancer (FN), a systematic review. Support. Care Cancer 2019, 28, 2369–2380. [Google Scholar] [CrossRef]
- Keng, M.K.; Thallner, E.A.; Elson, P.; Ajon, C.; Sekeres, J.; Wenzell, C.M.; Seastone, D.J.; Gallagher, E.M.; Weber, C.M.; Earl, M.A.; et al. Reducing Time to Antibiotic Administration for Febrile Neutropenia in the Emergency Department. J. Oncol. Pract. 2015, 11, 450–455. [Google Scholar] [CrossRef]
- Koenig, C.; Kuehni, C.E.; Bodmer, N.; Agyeman, P.K.A.; Ansari, M.; Roessler, J.; von der Weid, N.X.; Ammann, R.A. Time to antibiotics is unrelated to outcome in pediatric patients with fever in neutropenia presenting without severe disease during chemotherapy for cancer. Sci. Rep. 2022, 12, 14028. [Google Scholar] [CrossRef]
- Salstrom, J.L.; Coughlin, R.L.; Pool, K.; Bojan, M.; Mediavilla, C.; Schwent, W.; Rannie, M.; Law, D.; Finnerty, M.; Hilden, J. Pediatric patients who receive antibiotics for fever and neutropenia in less than 60 min have decreased intensive care needs. Pediatr. Blood Cancer 2015, 62, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.; Giroir, B.; Randolph, A. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 2005, 6, 2–8. [Google Scholar] [CrossRef]
- Neuhauser, H.; Schienkiewitz, A.; Rosario, A.S.; Dortschy, R.; Kurth, B.M. Referenzperzentile für Anthropometrische Maßzahlen und Blutdruck aus der Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland (KiGGS) 2003–2006; Robert-Koch-Institut: Berlin, Germany, 2013. [Google Scholar]
- Cohen, C.; King, A.R.; Lin, C.P.M.; Friedman, G.K.; Monroe, K.; Kutny, M. Protocol for Reducing Time to Antibiotics in Pediatric Patients Presenting to an Emergency Department With Fever and Neutropenia. Pediatr. Emerg. Care 2016, 32, 739–745. [Google Scholar] [CrossRef]
- Campos, L.M.; Pérez-Albert, P.; Ramis, L.F.; Rincón-López, E.M.; Mendoza-Palomar, N.; Soler-Palacin, P.; Aguilera-Alonso, D. Consensus document on the management of febrile neutropenia in paediatric haematology and oncology patients of the Spanish Society of Pediatric Infectious Diseases (SEIP) and the Spanish Society of Pediatric Hematology and Oncology (SEHOP). An. Pediatría 2023, 98, 446–459. [Google Scholar] [CrossRef]
- Santolaya, M.E.; Contardo, V.; Torres, J.P.; López-Medina, E.; Rosanova, M.T.; Álvarez, A.M.; Gutiérrez, V.; Claverie, X.; Rabello, M.; Zubieta, M.; et al. Manejo de los episodios de neutropenia febril en niños con cáncer. Consenso de la Sociedad Latinoamericana de Infectología Pediátrica 2021. Rev. Chil. Infectología 2021, 38, 857–909. [Google Scholar] [CrossRef]
- Daniels, L.M.; Durani, U.; Barreto, J.N.; O’hOro, J.C.; Siddiqui, M.A.; Park, J.G.; Tosh, P.K. Impact of time to antibiotic on hospital stay, intensive care unit admission, and mortality in febrile neutropenia. Support. Care Cancer 2019, 27, 4171–4177. [Google Scholar] [CrossRef]
- Fletcher, M.; Hodgkiss, H.; Zhang, S.; Browning, R.; Hadden, C.; Hoffman, T.; Winick, N.; McCavit, T.L. Prompt administration of antibiotics is associated with improved outcomes in febrile neutropenia in children with cancer. Pediatr. Blood Cancer 2013, 60, 1299–1306. [Google Scholar] [CrossRef]
- Dessie, A.S.; Lanning, M.; Nichols, T.D.; Delgado, E.M.; Hart, L.S.; Agrawal, A.K. Patient Outcomes With Febrile Neutropenia Based on Time to Antibiotics in the Emergency Department. Pediatr. Emerg. Care 2020, 38, e259–e263. [Google Scholar] [CrossRef]
- Ko, B.S.; Ahn, S.; Lee, Y.-S.; Kim, W.Y.; Lim, K.S.; Lee, J.-L. Impact of time to antibiotics on outcomes of chemotherapy-induced febrile neutropenia. Support. Care Cancer 2015, 23, 2799–2804. [Google Scholar] [CrossRef]
- Delebarre, M.; Dessein, R.; Lagrée, M.; Mazingue, F.; Sudour-Bonnange, H.; Martinot, A.; Dubos, F. Differential risk of severe infection in febrile neutropenia among children with blood cancer or solid tumor. J. Infect. 2019, 79, 95–100. [Google Scholar] [CrossRef]
- Cennamo, F.; Masetti, R.; Largo, P.; Argentiero, A.; Pession, A.; Esposito, S. Update on Febrile Neutropenia in Pediatric Oncological Patients Undergoing Chemotherapy. Children 2021, 8, 1086. [Google Scholar] [CrossRef]
- Dobrasz, G.; Hatfield, M.; Jones, L.M.; Berdis, J.J.; Miller, E.E.; Entrekin, M.S. Nurse-Driven Protocols for Febrile Pediatric Oncology Patients. J. Emerg. Nurs. 2013, 39, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Lamble, A.; Nguyen, T.; Lindemulder, S.; Spiro, D.M.S.; Nolt, D.; Stork, L. A Clinical Pathway to Reduce Time to Antibiotic Administration in Pediatric Cancer Patients With Fever and Potential Neutropenia. J. Clin. Pathw. 2015, 1, 33–42. [Google Scholar]
- Peyrony, O.; Gerlier, C.; Barla, I.; Ellouze, S.; Legay, L.; Azoulay, E.; Chevret, S.; Fontaine, J.-P. Antibiotic prescribing and outcomes in cancer patients with febrile neutropenia in the emergency department. PLoS ONE 2020, 15, e0229828. [Google Scholar] [CrossRef]
- Dias, A.; Gomez, V.C.; Viola, L.R.; Rodrigues, A.C.P.; Weber, S.P.; Tartaro, L.; Marques, L.d.S.; Boniatti, M.M. Fever is associated with earlier antibiotic onset and reduced mortality in patients with sepsis admitted to the ICU. Sci. Rep. 2021, 11, 23949. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Lehrnbecher, T.; Baltaci, Y.; Dohna-Schwake, C.; Groll, A.; Laws, H.-J.; Potratz, J.; Hufnagel, M.; Bochennek, K. Time to Antibiotics (TTA)—Überlegungen der Arbeitsgruppe Fieber bei Granulozytopenie im Kindes- und Jugendalter (GPOH/DGPI) zu einer Neubewertung. Klin. Pädiatrie 2023, 235, 331–341. [Google Scholar] [CrossRef]
- Reynolds, G.; Lindsay, J. Antibacterial prophylaxis for neutropenic and high-risk hematology patients—Do the benefits outweigh the risk? Transpl. Infect. Dis. 2024, 26, e14255. [Google Scholar] [CrossRef]
- Rondinelli, P.I.P.; Ribeiro, K.d.C.B.; de Camargo, B. A Proposed Score for Predicting Severe Infection Complications in Children With Chemotherapy-induced Febrile Neutropenia. J. Pediatr. Hematol. 2006, 28, 665–670. [Google Scholar] [CrossRef]
- Badiei, Z.; Alami, M.; Khalesi, M.; Farhangi, H.; Banihashem, A.; Razavi, A.; Kianifar, H.; Ghasemi, A. 851 Risk Factors Associated with Life Threatening Infections in Children with Febrile Neutropenia a Data Mining Approach. Arch. Dis. Child. 2012, 97, A245. [Google Scholar] [CrossRef]
- Santolaya, M.E.; Cofre, J.; Beresi, V. C-Reactive Protein: A Valuable Aid for the Management of Febrile Children with Cancer and Neutropenia. Clin. Infect. Dis. 1994, 18, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Santolaya, M.E.; Alvarez, A.M.; Becker, A.; Cofré, J.; Enríquez, N.; O’rYan, M.; Payá, E.; Pilorget, J.; Salgado, C.; Tordecilla, J.; et al. Prospective, Multicenter Evaluation of Risk Factors Associated With Invasive Bacterial Infection in Children With Cancer, Neutropenia, and Fever. J. Clin. Oncol. 2001, 19, 3415–3421. [Google Scholar] [CrossRef]
- Pakakasama, S.; Surayuthpreecha, K.; Pandee, U.; Anurathapan, U.; Maleewan, V.; Udomsubpayakul, U.; Butthep, P.; Santanirand, P.; Sirachainan, N.; Hongeng, S. Clinical practice guidelines for children with cancer presenting with fever to the emergency room. Pediatr. Int. 2011, 53, 902–905. [Google Scholar] [CrossRef] [PubMed]
| Variable | N | % | N | % |
|---|---|---|---|---|
| Pre-SOP | Post-SOP | |||
| Patients | 47 | 70 | ||
| FN episodes § | 119 | 44.9 | 146 | 55.1 |
| Age, years (median; range) | 11.1; 0.8–18.9 | 9.2; 0.5–18.6 | ||
| Gender §§ | ||||
| Male * | 75 | 63.0 | 56 | 38.4 |
| Female * | 44 | 37.0 | 90 | 61.6 |
| Diagnosis §§ | ||||
| Hematologic malignancy | 59 | 49.6 | 73 | 50.0 |
| ALL | 36 | 30.3 | 40 | 27.4 |
| AML | 7 | 5.9 | 12 | 8.2 |
| Lymphoma | 14 | 11.8 | 13 | 8.9 |
| Others | 2 | 1.7 | 8 | 5.5 |
| Solid tumor | 60 | 50.4 | 73 | 50.0 |
| Ewing | 16 | 13.4 | 19 | 13.0 |
| Germ cell tumor | 5 | 4.2 | 1 | 0.7 |
| Nephroblastoma | 8 | 6.7 | 4 | 2.7 |
| Neuroblastoma | 12 | 10.1 | 14 | 9.6 |
| Osteosarcoma | 13 | 10.9 | 3 | 2.1 |
| CNS tumor | 1 | 0.7 | 4 | 2.7 |
| Others | 5 | 4.2 | 28 | 19.2 |
| All | Pre-SOP | Post-SOP | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| TTA ≤ 30 min | 7 (119) * | 5.9 | 48 (146) | 32.9 | p < 0.0001 |
| TTA 31–60 min | 20 (119) | 16.8 | 48 (146) | 32.9 | p < 0.01 |
| TTA 61–90 min | 29 (119) | 24.4 | 23 (146) | 15.8 | n.s. |
| TTA 91–120 min | 25 (119) | 21.0 | 12 (146) | 8.2 | p < 0.01 |
| TTA > 120 min | 38 (119) | 31.9 | 15 (146) | 10.3 | p < 0.0001 |
| TTA ≤ 60 min | 27 (119) | 22.7 | 96 (146) | 65.8 | p < 0.0001 |
| OD | |||||
| TTA ≤ 30 min | 3 (26) | 11.5 | 9 (38) | 23.7 | n.s. |
| TTA 31–60 min | 7 (26) | 26.9 | 10 (38) | 26.3 | n.s. |
| TTA 61–90 min | 5 (26) | 19.2 | 8 (38) | 21.1 | n.s. |
| TTA 91–120 min | 2 (26) | 7.7 | 7 (38) | 18.4 | n.s. |
| TTA > 120 min | 9 (26) | 34.6 | 4 (38) | 10.5 | p < 0.01 |
| TTA ≤ 60 min | 10 (26) | 38.4 | 19 (38) | 50.0 | n.s. |
| pedER | |||||
| TTA ≤ 30 min | 4 (93) | 4.3 | 39 (108) | 36.1 | p < 0.0001 |
| TTA 31–60 min | 13 (93) | 14.0 | 41 (108) | 35.2 | p < 0.001 |
| TTA 61–90 min | 25 (93) | 25.8 | 12 (108) | 13.9 | p < 0.01 |
| TTA 91–120 min | 22 (93) | 24.7 | 5 (108) | 4.6 | p < 0.0001 |
| TTA > 120 min | 29 (93) | 31.2 | 11 (108) | 10.2 | p < 0.001 |
| TTA ≤ 60 min | 17 (93) | 17.3 | 80 (108) | 74.1 | p < 0.0001 |
| Findings | N (%) | TTA (min), Median (IQR) | p-Value | |
|---|---|---|---|---|
| Tachycardia | present | 114 (43.0) | 52 (30–96) | p < 0.01 |
| absent | 151 (57.0) | 75 (41.5–118) | ||
| Hypotension | present | 3 (1.1) | 50 (39.5–62.5) | n.s. |
| absent | 262 (98.9) | 61 (33.75–109.25) | ||
| Temperature (°C) | <38° | 90 (34.0) | 81 (45–127) | |
| 38.0–38.9° | 139 (52.5) | 61 (32–100) | p < 0.05 | |
| ≥39.0° | 36 (13.5) | 45 (28–86) |
| Pre-SOP (N = 97) | Post-SOP (N = 130) | |||
|---|---|---|---|---|
| Days | Days | |||
| LOS (days), median (IQR) | 6 (4–8) | 7 (4–10) | ||
| N | % | N | % | |
| Sepsis | 13 | 13.4 | 12 | 9.2 |
| ICU admission | 3 | 3.1 | 5 | 3.8 |
| Vasopressors/inotropic agents | 4 | 4.1 | 4 | 3.1 |
| Respiratory support | 8 | 8.2 | 7 | 5.4 |
| Composite AEs§ | 17 | 17.5 | 16 | 12.3 |
| Mortality | 1 | 1.0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malvestiti, S.; Strahm, B.; Flotho, C.; Hufnagel, M.; Feuchtinger, T.; Puzik, A. Reducing the Time-to-Antibiotic by Adapting a Standard of Procedure for the Treatment of Pediatric Cancer Patients Presenting with Febrile Neutropenia—A Comparative Analysis of Two Patient Cohorts. Cancers 2025, 17, 3280. https://doi.org/10.3390/cancers17203280
Malvestiti S, Strahm B, Flotho C, Hufnagel M, Feuchtinger T, Puzik A. Reducing the Time-to-Antibiotic by Adapting a Standard of Procedure for the Treatment of Pediatric Cancer Patients Presenting with Febrile Neutropenia—A Comparative Analysis of Two Patient Cohorts. Cancers. 2025; 17(20):3280. https://doi.org/10.3390/cancers17203280
Chicago/Turabian StyleMalvestiti, Stefano, Brigitte Strahm, Christian Flotho, Markus Hufnagel, Tobias Feuchtinger, and Alexander Puzik. 2025. "Reducing the Time-to-Antibiotic by Adapting a Standard of Procedure for the Treatment of Pediatric Cancer Patients Presenting with Febrile Neutropenia—A Comparative Analysis of Two Patient Cohorts" Cancers 17, no. 20: 3280. https://doi.org/10.3390/cancers17203280
APA StyleMalvestiti, S., Strahm, B., Flotho, C., Hufnagel, M., Feuchtinger, T., & Puzik, A. (2025). Reducing the Time-to-Antibiotic by Adapting a Standard of Procedure for the Treatment of Pediatric Cancer Patients Presenting with Febrile Neutropenia—A Comparative Analysis of Two Patient Cohorts. Cancers, 17(20), 3280. https://doi.org/10.3390/cancers17203280






