The Impact of Lymph Node Ratio for Children with Wilms Tumors: A National Cancer Database Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Participants
2.3. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leslie, S.W.; Sajjad, H.; Murphy, P.B. Wilms Tumor. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Spreafico, F.; Fernandez, C.V.; Brok, J.; Nakata, K.; Vujanic, G.; Geller, J.I.; Gessler, M.; Maschietto, M.; Behjati, S.; Polanco, A.; et al. Wilms tumour. Nat. Rev. Dis. Primers 2021, 7, 75. [Google Scholar] [CrossRef]
- Laochareonsuk, W.; Laohapansang, M.; Ngerncham, M.; Sangkhathat, S. Predictors of Surgical Complications and Survival in Pediatric Wilms’ Tumor: A 20-Year Retrospective Study from Two Thai Centers. Curr. Oncol. 2025, 32, 413. [Google Scholar] [CrossRef]
- Wilde, J.C.; Aronson, D.C.; Sznajder, B.; Van Tinteren, H.; Powis, M.; Okoye, B.; Cecchetto, G.; Audry, G.; Fuchs, J.; Schweinitz, D.V.; et al. Nephron sparing surgery (NSS) for unilateral wilms tumor (UWT): The SIOP 2001 experience. Pediatr. Blood Cancer 2014, 61, 2175–2179. [Google Scholar] [CrossRef]
- Spreafico, F.; Biasoni, D.; Montini, G. Most appropriate surgical approach in children with Wilms tumour, risk of kidney disease, and related considerations. Pediatr. Nephrol. 2024, 39, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S. Management of Wilms’ tumor: NWTS vs SIOP. J. Indian Assoc. Pediatr. Surg. 2009, 14, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Rabeh, W.; Akel, S.; Eid, T.; Muwakkit, S.; Abboud, M.; Solh, H.E.; Saab, R. Wilms tumor: Successes and challenges in management outside of cooperative clinical trials. Hematol./Oncol. Stem Cell Ther. 2016, 9, 20–25. [Google Scholar] [CrossRef]
- Neagu, M.C.; David, V.L.; Iacob, E.R.; Chiriac, S.D.; Muntean, F.L.; Boia, E.S. Wilms’ Tumor: A Review of Clinical Characteristics, Treatment Advances, and Research Opportunities. Medicina 2025, 61, 491. [Google Scholar] [CrossRef]
- Milford, K.; DeCotiis, K.; Lorenzo, A. Wilms tumor: A review of current surgical controversies. Transl. Androl. Urol. 2020, 9, 2382–2392. [Google Scholar] [CrossRef]
- Jereb, B.; Tournade, M.F.; Lemerle, J.; Voûte, P.A.; Delemarre, J.F.; Ahstrom, L.; Flamant, R.; Gérard-Marchant, R.; Sandstedt, B. Lymph node invasion and prognosis in nephroblastoma. Cancer 1980, 45, 1632–1636. [Google Scholar] [CrossRef]
- Kieran, K.; Anderson, J.R.; Dome, J.S.; Ehrlich, P.F.; Ritchey, M.L.; Shamberger, R.C.; Perlman, E.J.; Green, D.M.; Davidoff, A.M. Lymph node involvement in Wilms tumor: Results from National Wilms Tumor Studies 4 and 5. J. Pediatr. Surg. 2012, 47, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, A.F.; Carrasco, A., Jr.; Amini, A.; Aldrink, J.H.; Dasgupta, R.; Gow, K.W.; Glick, R.D.; Ehrlich, P.F.; Cost, N.G. Patterns of lymph node sampling and the impact of lymph node density in favorable histology Wilms tumor: An analysis of the national cancer database. J. Pediatr. Urol. 2018, 14, 161.e1–161.e8. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.P.; Johnson, J.S.; Eguchi, M.M.; Saltzman, A.F.; Cockburn, M.; Cost, N.G. Factors affecting lymph node sampling patterns and the impact on survival of lymph node density in patients with Wilms tumor: A Surveillance, Epidemiology, and End Result (SEER) database review. J. Pediatr. Urol. 2020, 16, 81–88. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Yang, J.; Liu, Q.; Tang, L.; Bu, Q.; Pan, Z.; Lyu, J. The impact of the lymph node density on overall survival in patients with Wilms’ tumor: A SEER analysis. Cancer Manag. Res. 2018, 10, 671–677. [Google Scholar] [CrossRef]
- Khomiak, A.; Ghaffar, S.A.; Rodriguez Franco, S.; Ziogas, I.A.; Cumbler, E.; Gleisner, A.; Del Chiaro, M.; Schulick, R.D.; Mungo, B. The impact of lymph node ratio on survival in gallbladder cancer: A national cancer database analysis. HPB 2024, 26, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.G.; Li, D.W.; Zhuo, C.H.; Cai, G.X.; Cai, S.J. Metastatic lymph node ratio can further stratify prognosis in rectal cancer patients treated with preoperative radiotherapy: A population-based analysis. Tumour Biol. 2014, 35, 6389–6395. [Google Scholar] [CrossRef]
- Liao, X.; Qiu, S.; Zheng, X.; Ai, J.; Jin, X.; Gong, L.; Bao, Y.; Jin, K.; Li, H.; Yang, L.; et al. Lymph Node Density as an Independent Prognostic Factor in Node-Positive Renal-Cell Carcinoma: Results From the Surveillance, Epidemiology, and End Results Program. Clin. Genitourin. Cancer 2019, 17, e968–e980. [Google Scholar] [CrossRef]
- Rosenberg, R.; Friederichs, J.; Schuster, T.; Gertler, R.; Maak, M.; Becker, K.; Grebner, A.; Ulm, K.; Höfler, H.; Nekarda, H.; et al. Prognosis of patients with colorectal cancer is associated with lymph node ratio: A single-center analysis of 3026 patients over a 25-year time period. Ann. Surg. 2008, 248, 968–978. [Google Scholar] [CrossRef]
- Birnbaum, D.J.; Viganò, L.; Russolillo, N.; Langella, S.; Ferrero, A.; Capussotti, L. Lymph node metastases in patients undergoing surgery for a gallbladder cancer. Extension of the lymph node dissection and prognostic value of the lymph node ratio. Ann. Surg. Oncol. 2015, 22, 811–818. [Google Scholar] [CrossRef]
- Cheraghlou, S.; Agogo, G.O.; Girardi, M. Evaluation of Lymph Node Ratio Association With Long-term Patient Survival After Surgery for Node-Positive Merkel Cell Carcinoma. JAMA Dermatol. 2019, 155, 803–811. [Google Scholar] [CrossRef]
- Chen, T.; Zou, X.; Li, Y.; Peng, L.; Song, Z.; Chao, H.; Fu, B.; Zeng, T. Evaluation of the association between lymph node ratio and long-term survival in patients after surgery for lymph node-positive bladder cancer: A SEER population-based study with external validation. BMC Cancer 2025, 25, 135. [Google Scholar] [CrossRef]
- Iocca, O.; Copelli, C.; Campo, F.; Petruzzi, G.; Pellini, R.; Ramieri, G.; Di Maio, P. Lymph node ratio (LNR) and lymph node yield (LNY) in head and neck cancer: A systematic review and meta-analysis. J. Craniomaxillofac. Surg. 2025, 53, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Khomiak, A.; Ghaffar, S.A.; Rodriguez Franco, S.; Ziogas, I.A.; Cumbler, E.; Gleisner, A.L.; Del Chiaro, M.; Schulick, R.D.; Mungo, B. Prognostic Significance of Lymph Node Ratio in Intrahepatic and Extrahepatic Cholangiocarcinomas. Cancers 2025, 17, 220. [Google Scholar] [CrossRef]
- Boffa, D.J.; Rosen, J.E.; Mallin, K.; Loomis, A.; Gay, G.; Palis, B.; Thoburn, K.; Gress, D.; McKellar, D.P.; Shulman, L.N.; et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol. 2017, 3, 1722–1728. [Google Scholar] [CrossRef]
- Ziogas, I.A.; Hills-Dunlap, J.L.; Corkum, K.S.; Cost, N.G.; Gosain, A.; Roach, J.P. Current Management Strategies and Outcomes in Children With Adrenocortical Carcinoma. J. Surg. Res. 2024, 301, 110–117. [Google Scholar] [CrossRef]
- Ziogas, I.A.; Roach, J.P.; Acker, S.N.; Corkum, K.S.; Diaz-Miron, J.L.; Kulungowski, A.M.; Gosain, A.; Hills-Dunlap, J.L. Association of Sociodemographic Factors with Surgical Management of Hepatoblastoma and Hepatocellular Carcinoma in Children. J. Pediatr. 2024, 269, 113963. [Google Scholar] [CrossRef]
- Evageliou, N.; Renfro, L.A.; Geller, J.; Perlman, E.; Kalapurakal, J.; Paulino, A.; Dix, D.; Eklund, M.J.; Murphy, A.J.; Romao, R.L.P.; et al. Prognostic impact of lymph node involvement and loss of heterozygosity of 1p or 16q in stage III favorable histology Wilms tumor: A report from Children’s Oncology Group Studies AREN03B2 and AREN0532. Cancer 2024, 130, 792–802. [Google Scholar] [CrossRef]
- Groenendijk, A.; Spreafico, F.; de Krijger, R.R.; Drost, J.; Brok, J.; Perotti, D.; van Tinteren, H.; Venkatramani, R.; Godziński, J.; Rübe, C.; et al. Prognostic Factors for Wilms Tumor Recurrence: A Review of the Literature. Cancers 2021, 13, 3142. [Google Scholar] [CrossRef]
- Honeyman, J.N.; Rich, B.S.; McEvoy, M.P.; Knowles, M.A.; Heller, G.; Riachy, E.; Kobos, R.; Shukla, N.; Wolden, S.L.; Steinherz, P.G.; et al. Factors associated with relapse and survival in Wilms tumor: A multivariate analysis. J. Pediatr. Surg. 2012, 47, 1228–1233. [Google Scholar] [CrossRef]
- Zhuge, Y.; Cheung, M.C.; Yang, R.; Koniaris, L.G.; Neville, H.L.; Sola, J.E. Improved Survival with Lymph Node Sampling in Wilms Tumor. J. Surg. Res. 2011, 167, e199–e203. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.C.; Sykes-Martin, K.D.; Tobillo, R.; Ali, N.; Wynne, J.F.; Eaton, B.R.; Paulino, A.C.; Kalapurakal, J.A.; Esiashvili, N. Impact of Age on Overall Survival Among Children With Wilms Tumor: A Population-based Registry Analysis. Am. J. Clin. Oncol. 2023, 46, 213–218. [Google Scholar] [CrossRef] [PubMed]


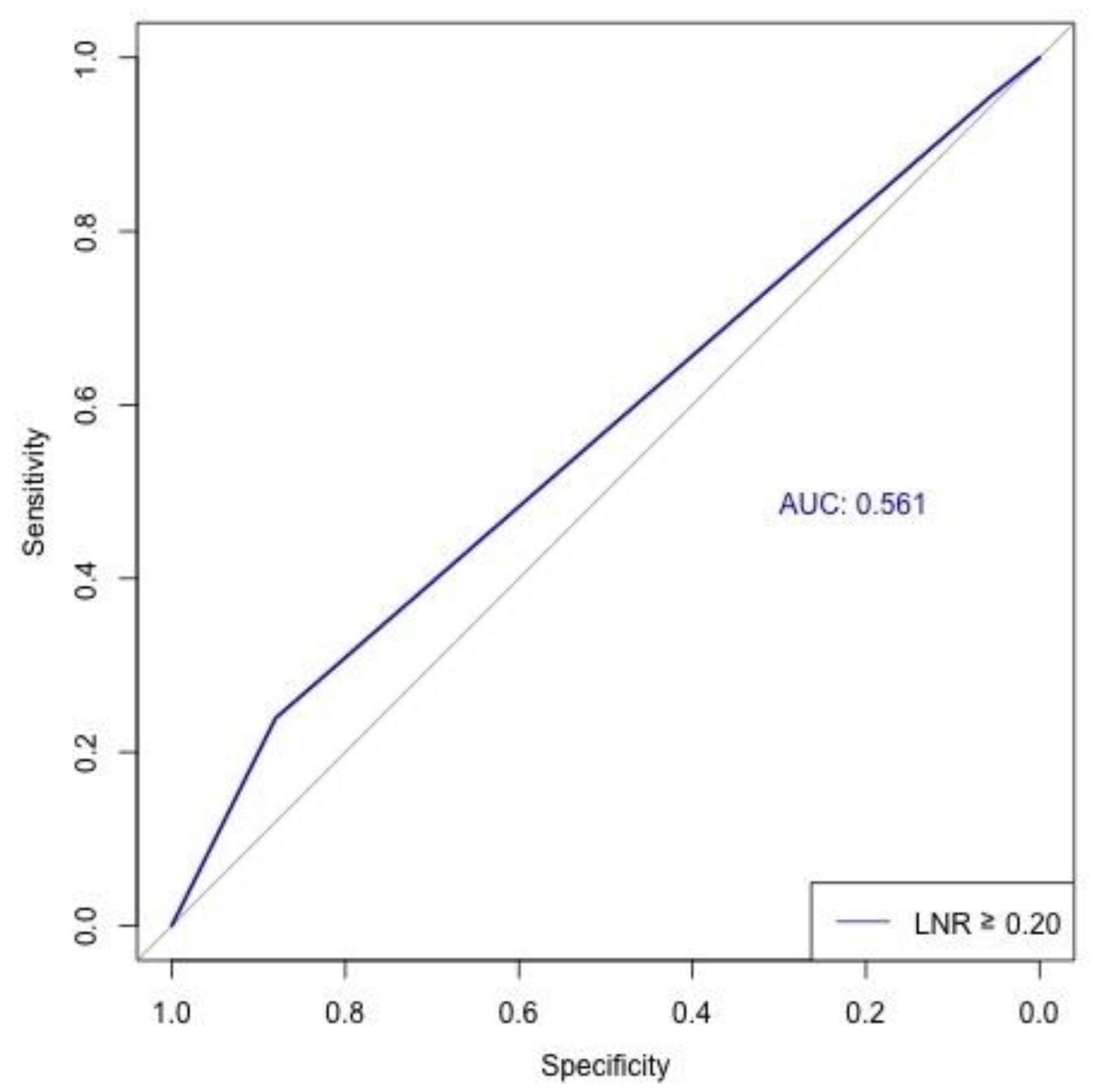

| Variable | LNR 0 (n = 1811) | LNR < 0.2 (n = 120) | LNR ≥ 0.2 (n = 275) | Total (n = 2206) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 3.0 (1.0–4.0) | 3.0 (2.0–5.0) | 3.0 (2.0–5.0) | 3.0 (1.0–5.0) | <0.001 |
| Sex | 0.57 | ||||
| Male | 886 (48.9%) | 54 (45.0%) | 128 (46.6%) | 1068 (48.4%) | |
| Female | 925 (51.1%) | 66 (55.0%) | 147 (53.4%) | 1138 (51.6%) | |
| Race/ethnicity | 0.62 | ||||
| White | 1111 (61.4%) | 81 (67.5%) | 163 (59.3%) | 1355 (61.4%) | |
| Black | 346 (19.1%) | 17 (14.2%) | 54 (19.6%) | 417 (18.9%) | |
| Hispanic | 261 (14.4%) | 16 (13.3%) | 47 (17.1%) | 324 (14.7%) | |
| Other | 93 (5.1%) | 6 (5.0%) | 11 (4.0%) | 110 (5.0%) | |
| Insurance status | 0.36 | ||||
| Not insured | 32 (1.8%) | 4 (3.3%) | 3 (1.1%) | 39 (1.8%) | |
| Private insurance | 991 (54.7%) | 67 (55.8%) | 164 (59.6%) | 1222 (55.4%) | |
| Medicaid | 711 (39.3%) | 41 (34.2%) | 97 (35.3%) | 849 (38.5%) | |
| Other | 77 (4.3%) | 8 (6.7%) | 11 (4.0%) | 96 (4.4%) | |
| Charlson–Deyo score | 0.83 | ||||
| 0 | 1729 (95.5%) | 116 (96.7%) | 263 (95.6%) | 2108 (95.6%) | |
| ≥1 | 82 (4.5%) | 4 (3.3%) | 12 (4.4%) | 98 (4.4%) | |
| Tumor size (cm) | 10.2 (7.7–13.0) | 12.0 (9.3–14.0) | 11.5 (8.9–14.0) | 10.5 (8.0–13.0) | <0.001 |
| Number of positive lymph nodes | 0.0 (0.0–0.0) | 1.0 (1.0–1.0) | 2.0 (1.0–4.0) | 0.0 (0.0–0.0) | <0.001 |
| Number of lymph nodes examined | 4.0 (2.0–8.0) | 11.5 (7.5–16.5) | 5.0 (3.0–7.0) | 5.0 (2.0–8.0) | <0.001 |
| Type of operation | 0.28 | ||||
| Partial or subtotal nephrectomy | 80 (4.4%) | 1 (0.8%) | 8 (2.9%) | 89 (4.0%) | |
| Complete/total/simple nephrectomy | 332 (18.3%) | 19 (15.8%) | 47 (17.1%) | 398 (18.0%) | |
| Radical nephrectomy | 1338 (73.9%) | 93 (77.5%) | 209 (76.0%) | 1640 (74.3%) | |
| Nephrectomy and en bloc organ resection | 61 (3.4%) | 7 (5.8%) | 11 (4.0%) | 79 (3.6%) | |
| Surgical margin status | <0.001 | ||||
| Negative | 1518 (83.8%) | 88 (73.3%) | 195 (70.9%) | 1801 (81.6%) | |
| Positive | 230 (12.7%) | 26 (21.7%) | 69 (25.1%) | 325 (14.7%) | |
| Unknown | 63 (3.5%) | 6 (5.0%) | 11 (4.0%) | 80 (3.6%) | |
| Receipt of chemotherapy | 1625 (89.7%) | 114 (95.0%) | 255 (92.7%) | 1994 (90.4%) | 0.06 |
| Receipt of radiation | 710 (39.2%) | 116 (96.7%) | 254 (92.4%) | 1080 (49.0%) | <0.001 |
| Variable | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| LNR | |||
| 0 | Reference | - | - |
| <0.2 | 0.76 | 0.27–2.15 | 0.61 |
| ≥0.2 | 1.75 | 1.03–2.97 | 0.04 |
| Age (years) | 1.11 | 1.05–1.17 | <0.001 |
| Sex | |||
| Male | Reference | - | - |
| Female | 1.33 | 0.88–2.03 | 0.18 |
| Race/ethnicity | |||
| White | Reference | - | - |
| Black | 1.17 | 0.71–1.93 | 0.55 |
| Hispanic | 0.96 | 0.52–1.77 | 0.90 |
| Other | 0.49 | 0.12–2.02 | 0.33 |
| Tumor size (cm) | 1.03 | 1.00–1.06 | 0.03 |
| Chemotherapy | 0.92 | 0.42–2.01 | 0.84 |
| Radiation | 1.43 | 0.89–2.31 | 0.14 |
| Variable | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| LNR | 2.60 | 1.27–5.32 | 0.01 |
| Age (years) | 1.11 | 1.05–1.18 | <0.001 |
| Sex | |||
| Male | Reference | - | - |
| Female | 1.32 | 0.87–2.01 | 0.19 |
| Race/ethnicity | |||
| White | Reference | - | - |
| Black | 1.16 | 0.70–1.92 | 0.57 |
| Hispanic | 0.97 | 0.53–1.78 | 0.91 |
| Other | 0.51 | 0.12–2.09 | 0.35 |
| Tumor size (cm) | 1.03 | 1.00–1.06 | 0.03 |
| Chemotherapy | 0.95 | 0.43–2.07 | 0.89 |
| Radiation | 1.38 | 0.87–2.19 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziogas, I.A.; Khomiak, A.; Olson, K.E.; Moris, D.P.; Robbins, A.J.; Stevens, J.; Acker, S.N.; Roach, J.P.; Corkum, K.S.; Cost, N.G. The Impact of Lymph Node Ratio for Children with Wilms Tumors: A National Cancer Database Analysis. Cancers 2025, 17, 3276. https://doi.org/10.3390/cancers17193276
Ziogas IA, Khomiak A, Olson KE, Moris DP, Robbins AJ, Stevens J, Acker SN, Roach JP, Corkum KS, Cost NG. The Impact of Lymph Node Ratio for Children with Wilms Tumors: A National Cancer Database Analysis. Cancers. 2025; 17(19):3276. https://doi.org/10.3390/cancers17193276
Chicago/Turabian StyleZiogas, Ioannis A., Andrii Khomiak, Kaitlin E. Olson, Dimitrios P. Moris, Alexandria J. Robbins, Jenny Stevens, Shannon N. Acker, Jonathan P. Roach, Kristine S. Corkum, and Nicholas G. Cost. 2025. "The Impact of Lymph Node Ratio for Children with Wilms Tumors: A National Cancer Database Analysis" Cancers 17, no. 19: 3276. https://doi.org/10.3390/cancers17193276
APA StyleZiogas, I. A., Khomiak, A., Olson, K. E., Moris, D. P., Robbins, A. J., Stevens, J., Acker, S. N., Roach, J. P., Corkum, K. S., & Cost, N. G. (2025). The Impact of Lymph Node Ratio for Children with Wilms Tumors: A National Cancer Database Analysis. Cancers, 17(19), 3276. https://doi.org/10.3390/cancers17193276








