Prognostic Significance of CK5/6 and GATA3 Expression in Recurrent Urothelial Carcinoma
Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Results
3.1. Survival Analysis
Disease-Free Survival (DFS)
3.2. Univariate Analysis
3.3. Overall Survival (OS)
3.4. Univariate Analysis
3.5. Multivariate Analysis
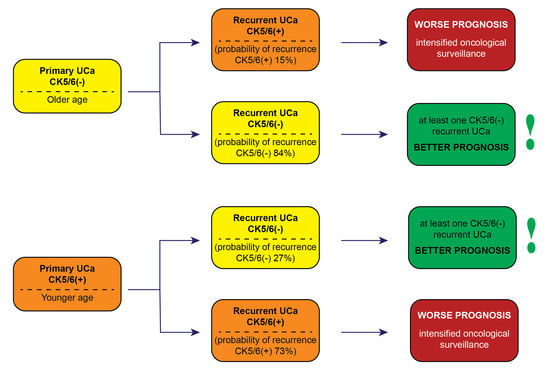
3.6. Summary of Results
4. Discussion
5. Conclusions
- It is advisable to routinely determine CK5/6 and GATA3 in primary tumors and in UCa recurrences due to its prognostic significance. CK5/6 and GATA3 expression in urothelial carcinoma recurrence may differ from CK5/6 and GATA3 expression in primary UCa tumor.
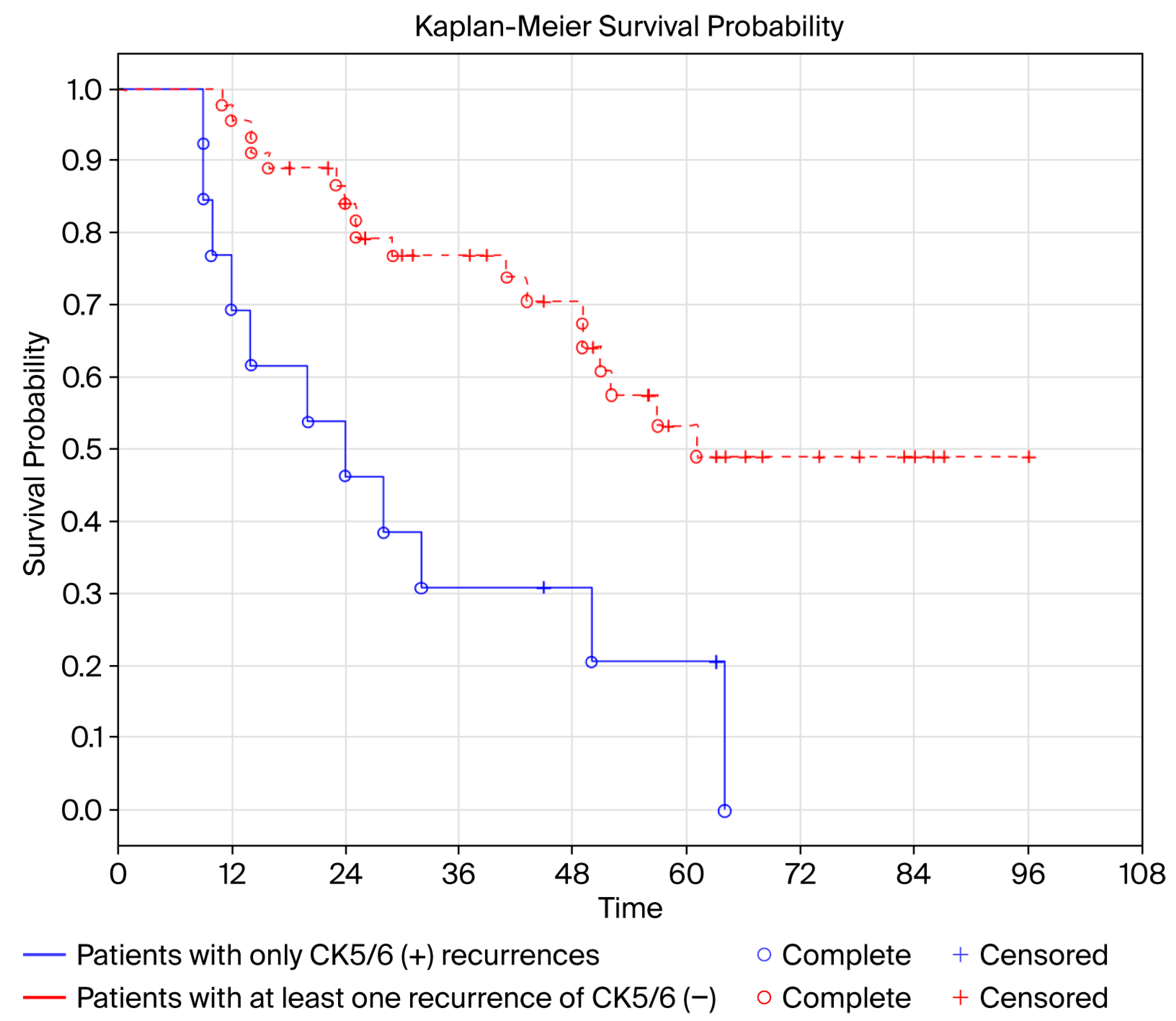
- Intensified oncological surveillance is suggested for patients with recurrent CK5/6(+) UCa. Patients with at least one UCa CK5/6(−) recurrence have better prognosis compared to patients with only CK5/6(+) recurrences.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, X.; Yuan, J.-M.; Skipper, P.L.; Tannenbaum, S.R.; Yu, M.C. Environmental Tobacco Smoke and Bladder Cancer Risk in Never Smokers of Los Angeles County. Cancer Res. 2007, 67, 7540–7545. [Google Scholar] [CrossRef]
- Gontero, P.; Birtle, A.; Capoun, O.; Compérat, E.; Dominguez-Escrig, J.L.; Liedberg, F.; Mariappan, P.; Masson-Lecomte, A.; Mostafid, H.A.; Pradere, B.; et al. European Association of Urology Guidelines on Non–muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—A Summary of the 2024 Guidelines Update. Eur. Urol. 2024, 86, 531–549. [Google Scholar] [CrossRef] [PubMed]
- Witjes, A.J.; Bruins, M.H.; Carrión, A.; Cathomas, R.; Compérat, E.; Efstathiou, J.A.; Fietkau, R.; Gakis, G.; Lorch, A.; Martini, A.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2023 Guidelines. Eur. Urol. 2024, 85, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Czerniak, B.; Dinney, C.; McConkey, D. Origins of Bladder Cancer. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 149–174. [Google Scholar] [CrossRef]
- Halaseh, S.A.; Halaseh, S.; Alali, Y.; Ashour, M.E.; Alharayzah, M.J. A Review of the Etiology and Epidemiology of Bladder Cancer: All You Need To Know. Cureus 2022, 14, e27330. [Google Scholar] [CrossRef]
- Bianchini, F.; Kaaks, R.; Vainio, H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002, 3, 565–574. [Google Scholar] [CrossRef]
- EAU Guidelines. Edn. Presented at the EAU Annual Congress Madrid, Spain 2025; EAU Guidelines Office: Arnhem, The Netherlands, 2025; ISBN 978-94-92671-29-5. [Google Scholar]
- Hemenway, G.; Anker, J.F.; Riviere, P.; Rose, B.S.; Galsky, M.D.; Ghatalia, P. Advancements in Urothelial Cancer Care: Optimizing Treatment for Your Patient. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e432054. [Google Scholar] [CrossRef]
- Chavan, S.; Bray, F.; Lortet-Tieulent, J.; Goodman, M.; Jemal, A. International Variations in Bladder Cancer Incidence and Mortality. Eur. Urol. 2014, 66, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, A.; de Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
- Sjödahl, G.; Lauss, M.; Lövgren, K.; Chebil, G.; Gudjonsson, S.; Veerla, S.; Patschan, O.; Aine, M.; Fernö, M.; Ringnér, M.; et al. A Molecular Taxonomy for Urothelial Carcinoma. Clin. Cancer Res. 2012, 18, 3377–3386. [Google Scholar] [CrossRef]
- Choi, W.; Ochoa, A.; McConkey, D.J.; Aine, M.; Höglund, M.; Kim, W.Y.; Real, F.X.; Kiltie, A.E.; Milsom, I.; Dyrskjøt, L.; et al. Genetic Alterations in the Molecular Subtypes of Bladder Cancer: Illustration in the Cancer Genome Atlas Dataset. Eur. Urol. 2017, 72, 354–365. [Google Scholar] [CrossRef]
- Tanaka, H.; Yoshida, S.; Koga, F.; Toda, K.; Yoshimura, R.; Nakajima, Y.; Sugawara, E.; Akashi, T.; Waseda, Y.; Inoue, M.; et al. Impact of Immunohistochemistry-Based Subtypes in Muscle-Invasive Bladder Cancer on Response to Chemoradiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1408–1416. [Google Scholar] [CrossRef]
- Kim, B.; Jang, I.; Kim, K.; Jung, M.; Lee, C.; Park, J.H.; Kim, Y.A.; Moon, K.C. Comprehensive Gene Expression Analyses of Immunohistochemically Defined Subgroups of Muscle-Invasive Urinary Bladder Urothelial Carcinoma. Int. J. Mol. Sci. 2021, 22, 628. [Google Scholar] [CrossRef]
- Dadhania, V.; Zhang, M.; Zhang, L.; Bondaruk, J.; Majewski, T.; Siefker-Radtke, A.; Guo, C.C.; Dinney, C.; Cogdell, D.E.; Zhang, S.; et al. Meta-Analysis of the Luminal and Basal Subtypes of Bladder Cancer and the Identification of Signature Immunohistochemical Markers for Clinical Use. eBioMedicine 2016, 12, 105–117. [Google Scholar] [CrossRef]
- Guo, C.C.; Bondaruk, J.; Yao, H.; Wang, Z.; Zhang, L.; Lee, S.; Lee, J.-G.; Cogdell, D.; Zhang, M.; Yang, G.; et al. Assessment of Luminal and Basal Phenotypes in Bladder Cancer. Sci. Rep. 2020, 10, 9743. [Google Scholar] [CrossRef] [PubMed]
- Ravanini, J.N.; Assato, A.K.; Wakamatsu, A.; Alves, V.A.F. Combined use of immunohistochemical markers of basal and luminal subtypes in urothelial carcinoma of the bladder: Association with clinicopathological features and outcomes. Clinics 2021, 76, e2587. [Google Scholar] [CrossRef] [PubMed]
- Serag Eldien, M.M.; Abdou, A.G.; Elghrabawy, G.R.A.; Alhanafy, A.M.; Mahmoud, S.F. Stratification of urothelial bladder carcinoma depending on immunohistochemical expression of GATA3 and CK5/6. J. Immunoass. Immunochem. 2021, 42, 662–678. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Zanelli, M.; Palicelli, A.; Ascani, S.; Zizzo, M.; Cocco, G.; Björnebo, L.; Lantz, A.; Landriscina, M.; Conteduca, V.; et al. Are We Ready to Implement Molecular Subtyping of Bladder Cancer in Clinical Practice? Part 2: Subtypes and Divergent Differentiation. Int. J. Mol. Sci. 2022, 23, 7844. [Google Scholar] [CrossRef]
- Choi, W.; Porten, S.; Kim, S.; Willis, D.; Plimack, E.R.; Hoffman-Censits, J.; Roth, B.; Cheng, T.; Tran, M.; Lee, I.-L.; et al. Identification of Distinct Basal and Luminal Subtypes of Muscle-Invasive Bladder Cancer with Different Sensitivities to Frontline Chemotherapy. Cancer Cell 2014, 25, 152–165. [Google Scholar] [CrossRef]
- Terlević, R.; Ulamec, M.; Štimac, G.; Murgić, J.; Krušlin, B. Molecular classification of muscle-invasive bladder cancer based on a simplified immunohistochemical panel using GATA3, CK5/6 and p16. Biomol. Biomed. 2023, 23, 968–975. [Google Scholar] [CrossRef]
- Baras, A.S.; Gandhi, N.; Munari, E.; Faraj, S.; Shultz, L.; Marchionni, L.; Schoenberg, M.; Hahn, N.; Hoque, M.; Berman, D.; et al. Identification and Validation of Protein Biomarkers of Response to Neoadjuvant Platinum Chemotherapy in Muscle Invasive Urothelial Carcinoma. PLoS ONE 2015, 10, e0131245. [Google Scholar]
- Bejrananda, T.; Kanjanapradit, K.; Saetang, J.; Sangkhathat, S. Impact of immunohistochemistry-based subtyping of GATA3, CK20, CK5/6, and CK14 expression on survival after radical cystectomy for muscle-invasive bladder cancer. Sci. Rep. 2021, 11, 21186. [Google Scholar] [CrossRef] [PubMed]
- Razzaghdoust, A.; Ghajari, M.; Basiri, A.; Torbati, P.M.; Jafari, A.; Fattahi, M.-R.; Salahi, M.; Mofid, B. Association of immunohistochemical markers of tumor subtype with response to neoadjuvant chemotherapy and survival in patients with muscle-invasive bladder cancer. Investig. Clin. Urol. 2021, 62, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Kollberg, P.; Chebil, G.; Eriksson, P.; Sjödahl, G.; Liedberg, F. Molecular subtypes applied to a population-based modern cystectomy series do not predict cancer-specific survival. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.A.; Hussain, Z.F.; Irfan, M.; Edhi, M.M.; Kanwal, S.; Faridi, N.; Khan, A. Cytokeratin 5/6 expression in bladder cancer: Association with clinicopathologic parameters and prognosis. BMC Res. Notes 2018, 11, 207. [Google Scholar] [CrossRef]
- Olkhov-Mitsel, E.; Hodgson, A.; Liu, S.K.; Vesprini, D.; Xu, B.; Downes, M.R. Three-antibody classifier for muscle invasive urothelial carcinoma and its correlation with p53 expression. J. Clin. Pathol. 2022, 75, 766–771. [Google Scholar] [CrossRef]
- Bernardo, C.; Monteiro, F.L.; Direito, I.; Amado, F.; Afreixo, V.; Santos, L.L.; Helguero, L.A. Association Between Estrogen Receptors and GATA3 in Bladder Cancer: A Systematic Review and Meta-Analysis of Their Clinicopathological Significance. Front. Endocrinol. 2021, 12, 684140. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Falagario, U.G.; Zanelli, M.; Palicelli, A.; Zizzo, M.; Ascani, S.; Tortorella, S.; Mancini, V.; Cormio, A.; Carrieri, G.; et al. Clinicopathological Features and Survival Analysis in Molecular Subtypes of Muscle-Invasive Bladder Cancer. Int. J. Mol. Sci. 2023, 24, 6610. [Google Scholar] [CrossRef]
- Guo, C.C.; Dadhania, V.; Zhang, L.; Majewski, T.; Bondaruk, J.; Sykulski, M.; Wronowska, W.; Gambin, A.; Wang, Y.; Zhang, S.; et al. Gene Expression Profile of the Clinically Aggressive Micropapillary Variant of Bladder Cancer. Eur. Urol. 2016, 70, 611–620. [Google Scholar] [CrossRef]
- Peña, K.B.; Riu, F.; Gumà, J.; Martínez-Madueño, F.; Miranda, M.J.; Vidal, A.; Grifoll, M.; Badia, J.; Rodriguez-Balada, M.; Parada, D. Immunohistochemical Algorithm for the Classification of Muscle-Invasive Urinary Bladder Carcinoma with Lymph Node Metastasis: An Institutional Study. J. Clin. Med. 2022, 11, 7430. [Google Scholar] [CrossRef]
- Sjodahl, G.; Eriksson, P.; Lovgren, K.; Marzouka, N.A.; Bernardo, C.; Nordentoft, I.; Dyrskjøt, L.; Liedberg, F.; Höglund, M. Discordant molecular subtype classification in the basal-squamous subtype of bladder tumors and matched lymph-node metastases. Mod. Pathol. 2018, 31, 1869–1881. [Google Scholar] [CrossRef] [PubMed]
- Olah, C.; Shmorhun, O.; Klamminger, G.G.; Rawitzer, J.; Sichward, L.; Hadaschik, B.; Al-Nader, M.; Krafft, U.; Niedworok, C.; Váradi, M.; et al. Immunohistochemistry-based molecular subtypes of urothelial carcinoma derive different survival benefit from platinum chemotherapy. J. Pathol. Clin. Res. 2025, 11, e70017. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef]
- Damrauer, J.S.; Hoadley, K.A.; Chism, D.D.; Fan, C.; Tiganelli, C.J.; Wobker, S.E.; Yeh, J.J.; Milowsky, M.I.; Iyer, G.; Parker, J.S.; et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc. Natl. Acad. Sci. USA 2014, 111, 3110–3115. [Google Scholar] [CrossRef]
- Helal, D.S.; Darwish, S.A.; Awad, R.A.; Ali, D.A.; El-Guindy, D.M. Immunohistochemical based molecular subtypes of muscle-invasive bladder cancer: Association with HER2 and EGFR alterations, neoadjuvant chemotherapy response and survival. Diagn. Pathol. 2023, 18, 11. [Google Scholar] [CrossRef]
- El Kholy, M.A.M.; Ali, M.Y.; Aboelsaad, A.Y.; Abdel Gawad, A.M. GATA3 and CK5/6 Immunohistochemical Expression in Urothelial Carcinoma: Diagnostic, Biological and Prognostic Significance. Int. J. Med. Arts 2021, 3, 1306–1315. [Google Scholar] [CrossRef]
- Wang, C.-C.; Tsai, Y.-C.; Jeng, Y.-M. Biological significance of GATA3, cytokeratin 20, cytokeratin 5/6 and p53 expression in muscle-invasive bladder cancer. PLoS ONE 2019, 14, e0221785. [Google Scholar] [CrossRef]
- Weyerer, V.; Stoehr, R.; Bertz, S.; Lange, F.; Geppert, C.I.; Wach, S.; Taubert, H.; Sikic, D.; Wullich, B.; Hartmann, A.; et al. Prognostic impact of molecular muscle-invasive bladder cancer subtyping approaches and correlations with variant histology in a population-based mono-institutional cystectomy cohort. World J. Urol. 2021, 39, 4011–4019. [Google Scholar] [CrossRef]
- Miyamoto, H.; Izumi, K.; Yao, J.L.; Li, Y.; Yang, Q.; McMahon, L.A.; Gonzalez-Roibon, N.; Hicks, D.G.; Tacha, D.; Netto, G.J. GATA binding protein 3 is down-regulated in bladder cancer yet strong expression is an independent predictor of poor prognosis in invasive tumor. Hum. Pathol. 2012, 43, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, K.H.; Siddiqui, M.T.; Cohen, C. GATA3 immunohistochemical expression in invasive urothelial carcinoma. Urol. Oncol. 2016, 34, 432.e9–432.e13. [Google Scholar] [CrossRef]
- Mitra, A.P. Molecular substratification of bladder cancer: Moving towards individualized patient management. Ther. Adv. Urol. 2016, 8, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.H.; Abdel Hady, H.H.; Assem, A.; Mohamed, M.E.; Hamada, E. Identification of Molecular Subgroups of Muscle-Invasive Urothelial Bladder Cancer and Their Impact on Treatment Outcome. Asian Pac. J. Cancer Prev. 2024, 25, 3953–3965. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.; Larrinaga, G.; Errarte, P.; Martín, A.M.; Dotor, A.; Esquinas, C.; Nunes-Xavier, C.E.; Pulido, R.; López, J.I.; Angulo, J.C. The coexpression of fibroblast activation protein (FAP) and basal-type markers (CK 5/6 and CD44) predicts prognosis in high-grade invasive urothelial carcinoma of the bladder. Hum. Pathol. 2019, 91, 61–68. [Google Scholar] [CrossRef]
- Labban, M.; Najdi, J.; Mukherji, D.; Abou-Kheir, W.; Tabbarah, A.; El-Hajj, A. Triple—Marker immunohistochemical assessment of muscle—Invasive bladder cancer: Is there prognostic significance? Cancer Rep. 2021, 4, e1313. [Google Scholar] [CrossRef] [PubMed]
- Elzohery, N.; Ismael, N.S.; Khairy, R.A.; Soliman, S.A.M. Expression of GATA3 and Cytokeratin 14 in Urinary Bladder Carcinoma (Histopathological and Immunohistochemical Study). Open Access Maced. J. Med. Sci. 2021, 9, 858–864. [Google Scholar] [CrossRef]
- Rebola, J.; Aguiar, P.; Blanca, A.; Montironi, R.; Cimadamore, A.; Cheng, L.; Henriques, V.; Lobato-Faria, P.; Lopez-Beltran, A. Predicting outcomes in non-muscle invasive (Ta/T1) bladder cancer: The role of molecular grade based on luminal/basal phenotype. Virchows Arch. 2019, 475, 445–455. [Google Scholar] [CrossRef]
- Breyer, J.; Wirtz, R.M.; Otto, W.; Erben, P.; Kriegmair, M.C.; Stoehr, R.; Eckstein, M.; Eidt, S.; Denzinger, S.; Burger, M.; et al. In stage pT1 non-muscle-invasive bladder cancer (NMIBC), high KRT20 and low KRT5 mRNA expression identify the luminal subtype and predict recurrence and survival. Virchows Arch. 2017, 470, 267–274. [Google Scholar] [CrossRef]
- Hedegaard, J.; Lamy, P.; Nordentoft, I.; Algaba, F.; Høyer, S.; Ulhøi, B.P.; Vang, S.; Reinert, T.; Hermann, G.G.; Mogensen, K.; et al. Comprehensive Transcriptional Analysis of Early-Stage Urothelial Carcinoma. Cancer Cell 2016, 30, 27–42. [Google Scholar] [CrossRef]
- Jung, M.; Lee, J.H.; Kim, B.; Park, J.H.; Moon, K.C. Transcriptional Analysis of Immunohistochemically Defined Subgroups of Non-Muscle-Invasive Papillary High-Grade Upper Tract Urothelial Carcinoma. Int. J. Mol. Sci. 2019, 20, 570. [Google Scholar] [CrossRef]
- Jung, M.; Kim, B.; Moon, K.C. Immunohistochemistry of cytokeratin 5/6, 44 and CK20 as prognostic biomarkers of non-muscle—Invasive papillary upper tract urothelial carcinoma. Histopathology 2019, 74, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Koll, F.J.; Schwarz, A.; Köllermann, J.; Banek, S.; Kluth, L.; Wittler, C.; Bankov, K.; Döring, C.; Becker, N.; Chun, F.K.H.; et al. CK5/6 and GATA3 Defined Phenotypes of Muscle-Invasive Bladder Cancer: Impact in Adjuvant Chemotherapy and Molecular Subtyping of Negative Cases. Front. Med. 2022, 9, 875142. [Google Scholar] [CrossRef] [PubMed]
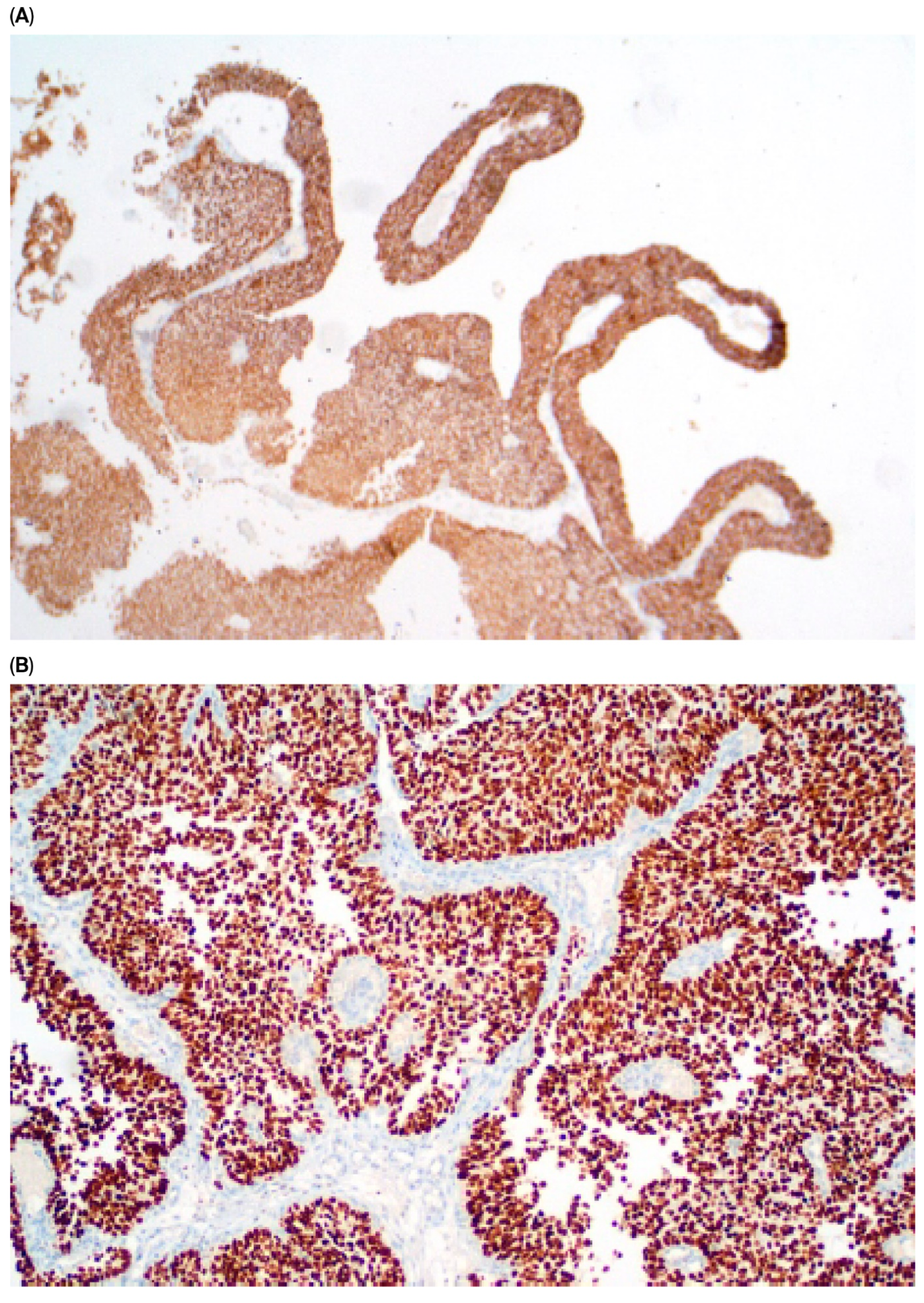
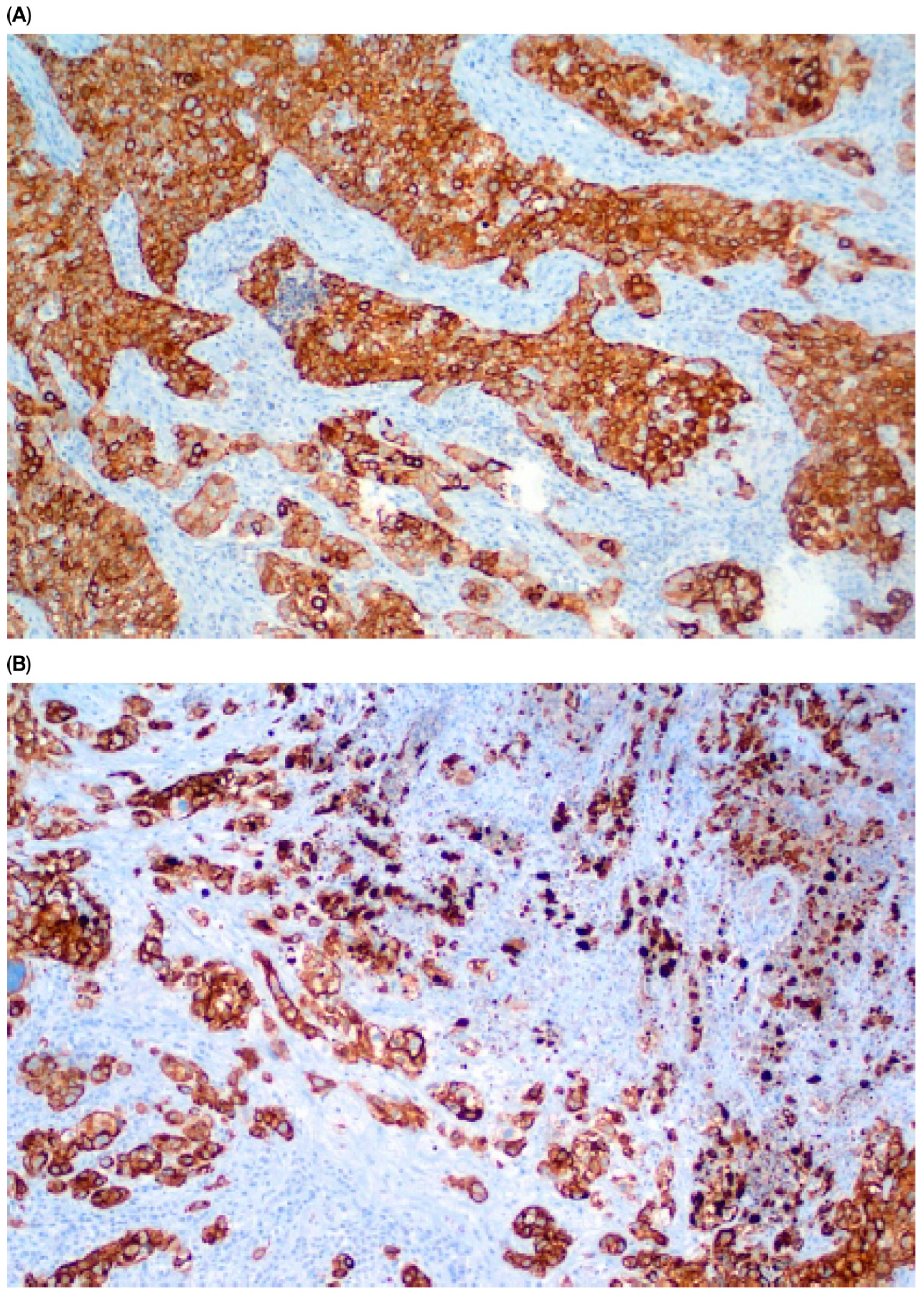
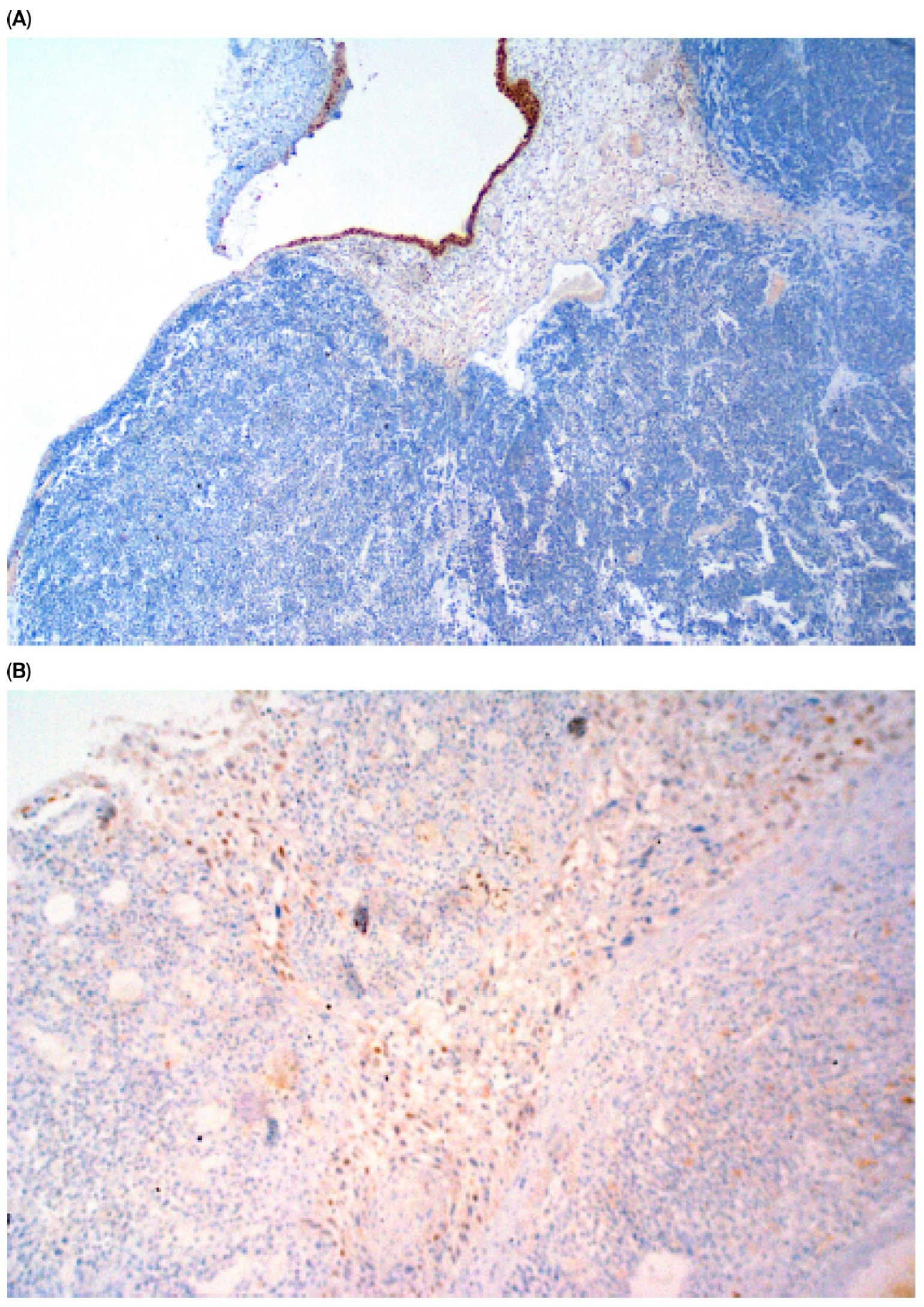

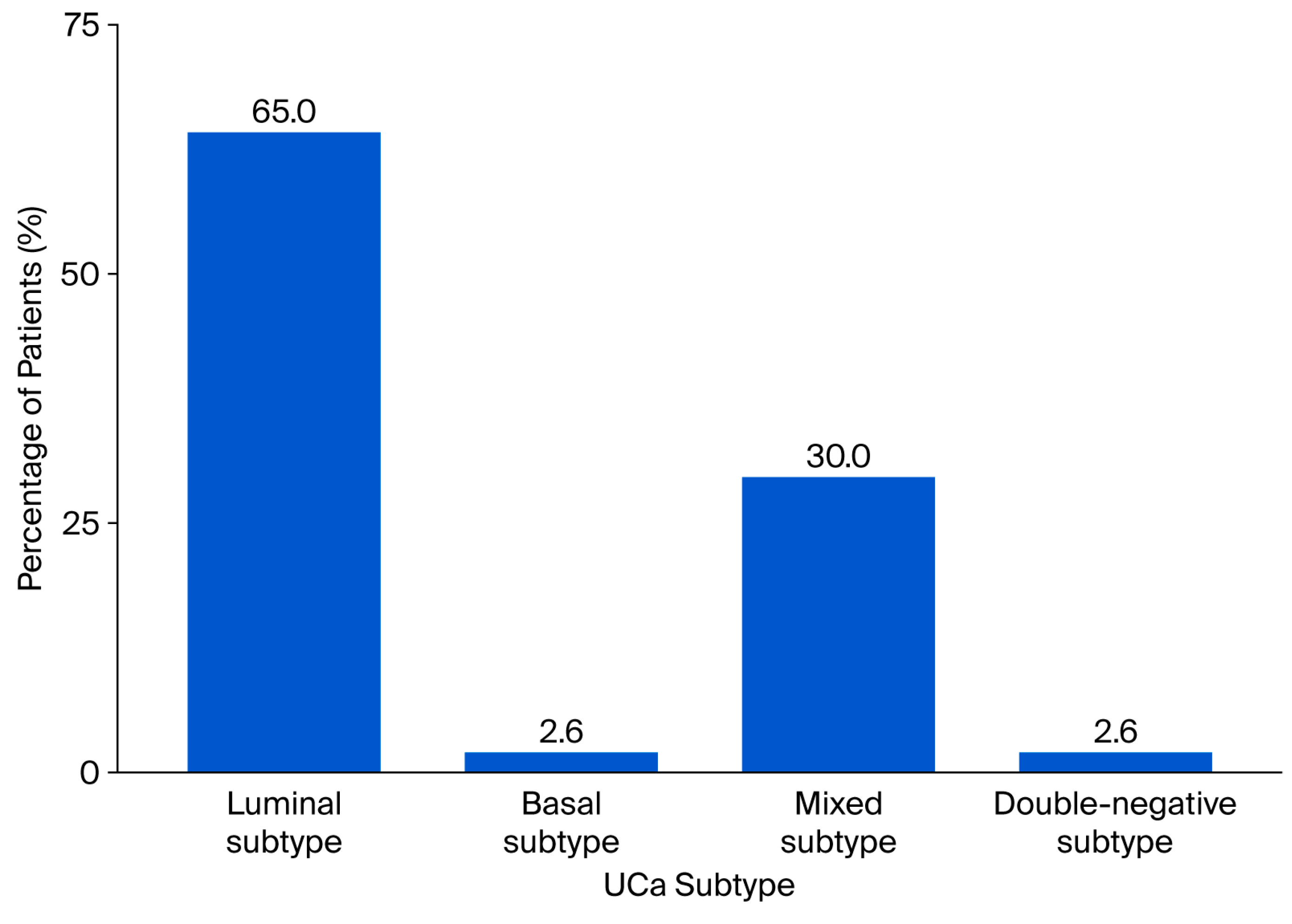
| Parameter | n (%) |
|---|---|
| Number of patients n (%) | 77 (100%) |
| Age (years) | |
| median = 68 | |
| ≤68 | 39 (50.7%) |
| >68 | 38 (49.3%) |
| Sex | |
| Female n (%) | 17 (22.1%) |
| Male n (%) | 60 (77.9%) |
| Tumor stage T * | |
| Ta | 17 (22.1%) |
| T1 | 17 (22.1%) |
| T2 | 16 (20.8%) |
| T3 | 5 (6.5%) |
| Tx ** | 22 (28.5%) |
| Grade *** | |
| G1 | 7 (9.3%) |
| G2 | 23 (30.7%) |
| G3 | 45 (60.0%) |
| Subtype of UCa in the primary tumor | |
| Subtype 1: GATA 3+,CK 5/6-, luminal subtype | 50 (64.9%) |
| Subtype 2: GATA-3−, CK 5/6+, basal subtype | 2 (2.5%) |
| Subtype 3: GATA3+, CK 5/6+, mixed subtype | 23 (30.0%) |
| Subtype 4: GATA3−, CK5/6-, double-negative subtype | 2 (2.5%) |
| Surgery | |
| YES | 35 (45.5%) |
| NO | 42 (54.5%) |
| Chemotherapy | |
| YES | 46 (59.7%) |
| NO | 31 (40.3%) |
| Radiotherapy **** | |
| YES | 18 (23.4%) |
| NO | 57 (74.0%) |
| Number of Patients with Subsequent Recurrences | Number of Positive and Negative Immunohistochemical Staining Reactions | |||
|---|---|---|---|---|
| GATA3(+) | GATA3(−) | CK5/6(+) | CK5/6(−) | |
| first recurrence n = 57 | 54 | 3 | 18 | 39 |
| second recurrence n = 40 | 38 | 2 | 9 | 31 |
| third recurrence n = 22 | 20 | 2 | 6 | 16 |
| fourth recurrence n = 15 | 14 | 1 | 5 | 10 |
| fifth recurrence n = 9 | 9 | 3 | 6 | |
| sixth recurrence n = 2 | 2 | 1 | 1 | |
| seventh recurrence n = 2 | 2 | 2 | ||
| n = 147 (100%) * | 139 (94.6%) | 8 (5.4%) | 42 (28.6%) | 105 (71.4%) |
| UCa Recurrences | Grade (G) n (%) * | ||
|---|---|---|---|
| n | G1 | G2 | G3 |
| first recurrence n = 56 (100%) | 13 (23.2%) | 12 (21.4%) | 31 (55.4%) |
| second recurrence n = 31 (100%) | 8 (25.8%) | 9 (29.0%) | 14 (45.2%) |
| third recurrence n = 14 (100%) | 5 (35.7%) | 6 (42.8%) | 3 (21.4%) |
| fourth recurrence n = 11 (100%) | 6 (54.5%) | 1 (9.1%) | 4 (36.4%) |
| fifth recurrence n = 6 (100%) | 2 (33.3%) | 1 (16.7%) | 3 (50.0%) |
| sixth recurrence n = 2 (100%) | 2 (100.0%) | ||
| Variable | Primary Tumor CK5/6(−) | Primary Tumor CK5/6(+) | p |
|---|---|---|---|
| Medium ± SD * (Min–Max) | Medium ± SD * (Min–Max) | ||
| Age | 70.15 ± 8.04 (50.00–86.00) | 66.20 ± 8.25 (49.00–86.00) | 0.046 |
| probability of recurrence CK5/6(−) | 0.84 ± 0.26 (0.00–1.00) | 0.27 ± 0.41 (0.00–1.00) | 0.000005 |
| probability of recurrence CK5/6(+) | 0.15 ± 0.26 (0.00–1.00) | 0.73 ± 0.41 (0.00–1.00) | 0.000005 |
| DFS-1 * | |||
|---|---|---|---|
| Independent Variable | n | Hazard Ratio (95%CI) | p |
| Grade ** (/1 grade) | 75 | 1.335 (0.831–2.146) | 0.232 |
| T *** (/1 grade) | 55 | 1.379 (0.985–1.931) | 0.061 |
| GATA3(+) expression primary tumor (yes vs. no) | 77 | 0.327 (0.097–1.100) | 0.071 |
| CK5/6(+) expression primary tumor (yes vs. no) | 77 | 1.396 (0.735–2.651) | 0.307 |
| Chemotherapy (yes vs. no) | 77 | 0.741 (0.383–1.433) | 0.373 |
| Radiotherapy (yes vs. no) | 77 | 0.671 (0.318–1.415) | 0.294 |
| Sex (M vs. F) | 77 | 1.363 (0.570–3.256) | 0.486 |
| Age (/1 year of life) | 77 | 1.004 (0.964–1.044) | 0.855 |
| Surgery (yes vs. no) | 77 | 2.483 (1.309–4.711) | 0.005 |
| Probability of recurrence CK5/6(+) | 58 | 1.383 (0.663–2.883) | 0.386 |
| Probability of recurrence GATA 3(+) | 58 | 0.374 (0.131–1.073) | 0.067 |
| Neoadjuvant chemotherapy | 71 | 0.498 (0.256–0.971) | 0.04 |
| OS * | |||
|---|---|---|---|
| Independent Variable | n | Hazard Ratio (95%CI) | p |
| Grade ** (/1 grade) | 75 | 1.607 (0.944–2.735) | 0.080 |
| T *** (/1 grade) | 55 | 1.032 (0.720–1.490) | 0.862 |
| GATA3(+) expression in primary tumor (yes vs. no) | 72 | 0.731 (0.0221–2.422) | 0.609 |
| CK5/6(+) expression in primary tumor (yes vs. no) | 72 | 1.772 (0.913–3.440) | 0.098 |
| Chemotherapy (yes vs. no) | 77 | 1.420 (0.667–3.023) | 0.363 |
| Radiotherapy (yes vs. no) | 77 | 1.081 (0.554–2.109) | 0.819 |
| Sex (M vs. F) | 77 | 1.258 (0.551–2.872) | 0.585 |
| Age (/1 year of life) | 77 | 1.004 (0.964–1.046) | 0.833 |
| Surgery (yes vs. no) | 77 | 1.470 (0.769–2.809) | 0.243 |
| Probability of recurrence CK5/6(+) | 58 | 3.586 (1.499–8.571) | 0.004 |
| Probability of recurrence GATA3(+) | 58 | 0.260 (0.079–0.853) | 0.026 |
| Neoadjuvant chemotherapy | 71 | 0.982 (0.488–2.018) | 0.982 |
| Independent Variable (n = 43) | OS * | |
|---|---|---|
| Hazard Ratio (95%CI) | p | |
| Age (/1 year of life) | 0.960 (0.897–1.02) | 0.174 |
| Surgery (yes vs. no) | 1.059 (0.379–2.965) | 0.911 |
| Grade ** (/1 grade) | 2.279 (0.919–5.659) | 0.075 |
| T *** (/1 grade) | 0.765 (0.421–1.391) | 0.380 |
| Probability of recurrence GATA3(+) | 0.0784 (0.010–0.609) | 0.015 |
| Probability of recurrence CK5/6(+) | 2.859 (1.028–7.949) | 0.044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenda-Petrykowska, M.; Sulżyc-Bielicka, V.; Dobrzycki, W.; Safranow, K.; Świtała, J.; Kostrzewa-Nowak, D.; Bielicki, P. Prognostic Significance of CK5/6 and GATA3 Expression in Recurrent Urothelial Carcinoma. Cancers 2025, 17, 3267. https://doi.org/10.3390/cancers17193267
Lenda-Petrykowska M, Sulżyc-Bielicka V, Dobrzycki W, Safranow K, Świtała J, Kostrzewa-Nowak D, Bielicki P. Prognostic Significance of CK5/6 and GATA3 Expression in Recurrent Urothelial Carcinoma. Cancers. 2025; 17(19):3267. https://doi.org/10.3390/cancers17193267
Chicago/Turabian StyleLenda-Petrykowska, Marzena, Violetta Sulżyc-Bielicka, Wojciech Dobrzycki, Krzysztof Safranow, Jerzy Świtała, Dorota Kostrzewa-Nowak, and Paweł Bielicki. 2025. "Prognostic Significance of CK5/6 and GATA3 Expression in Recurrent Urothelial Carcinoma" Cancers 17, no. 19: 3267. https://doi.org/10.3390/cancers17193267
APA StyleLenda-Petrykowska, M., Sulżyc-Bielicka, V., Dobrzycki, W., Safranow, K., Świtała, J., Kostrzewa-Nowak, D., & Bielicki, P. (2025). Prognostic Significance of CK5/6 and GATA3 Expression in Recurrent Urothelial Carcinoma. Cancers, 17(19), 3267. https://doi.org/10.3390/cancers17193267