Eosinophil ETosis and Cancer: Ultrastructural Evidence and Oncological Implications
Simple Summary
Abstract
1. Introduction
2. Methodological Considerations
3. Eosinophils in Tumor Biology: Recruitment, Prognostic Role, and Viral Context
3.1. Tumor-Associated Tissue Eosinophilia (TATE) and Tumor-Associated Blood Eosinophilia (TABE) in Solid Tumors
3.2. Local Mechanisms of Eosinophil Recruitment in Solid Tumors
3.3. Systemic Mechanisms of Eosinophil Recruitment and Regulation in Solid Tumors
3.4. Prognostic Significance of TATE in Human Malignancies
3.5. Dual Role of Eosinophils in Viral and Non-Viral Tumors
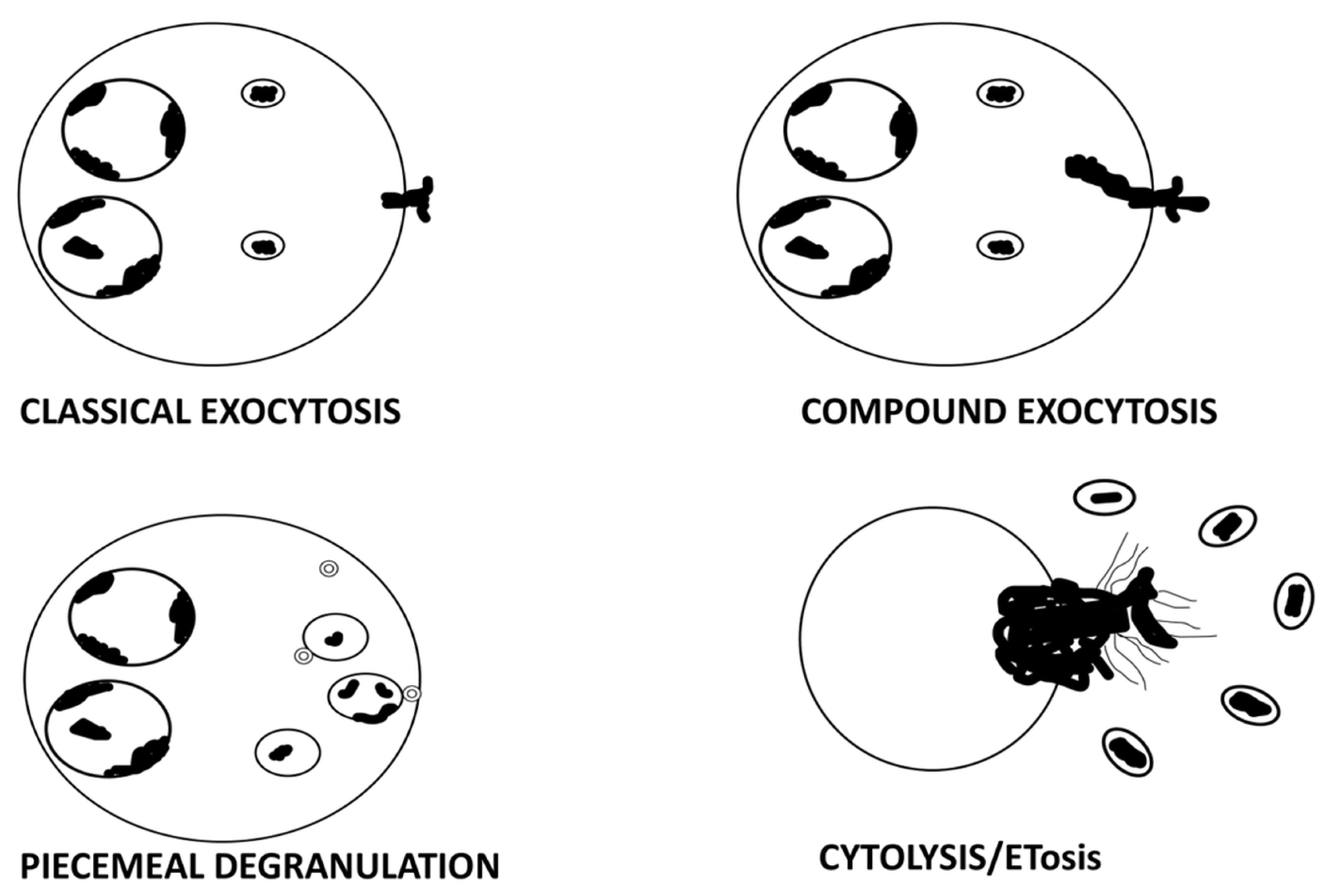
4. ETosis: A Distinct Mechanism of Eosinophil Degranulation
4.1. ETosis: Brief Overview
4.2. ETosis in Non-Neoplastic Eosinophilic Disorders
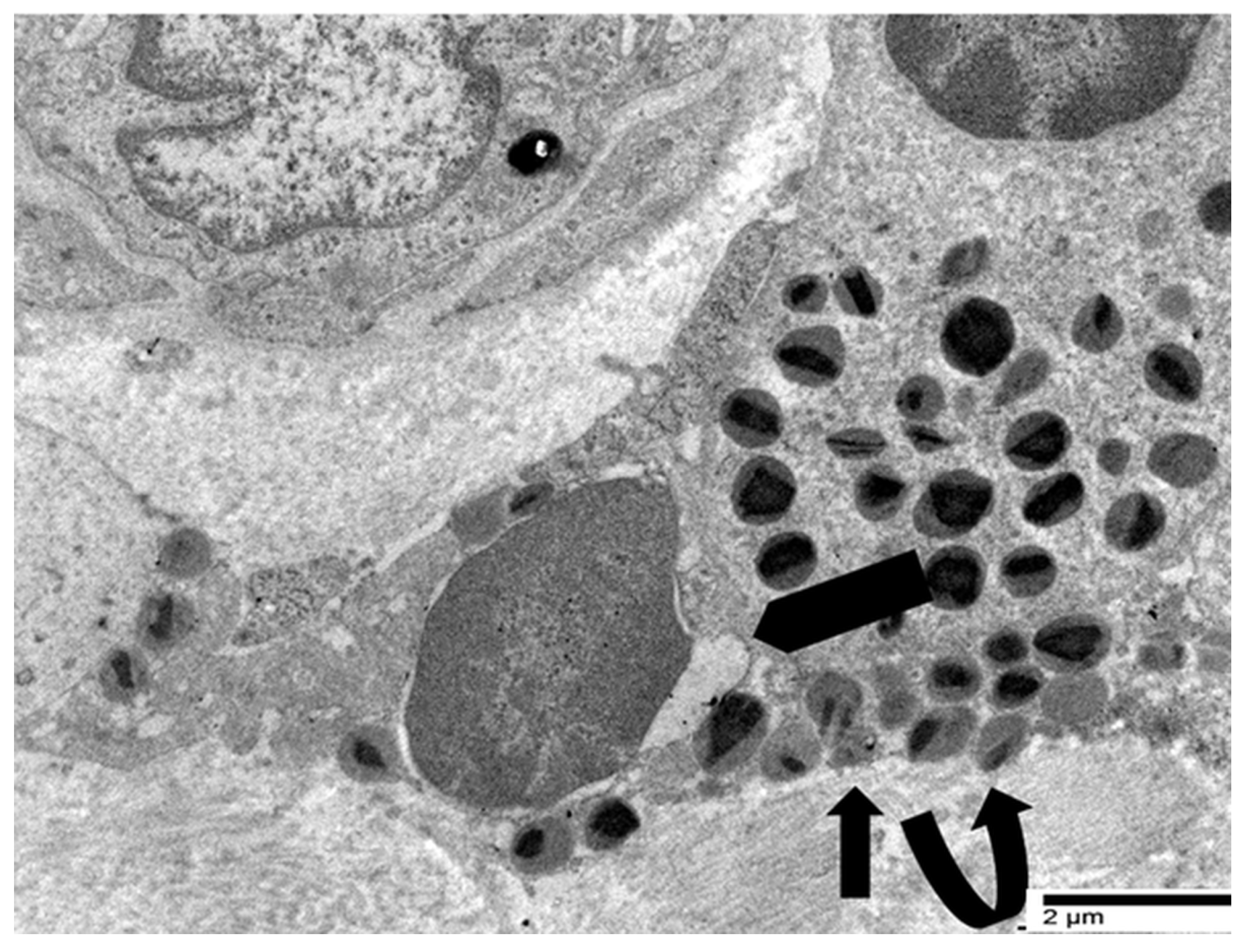
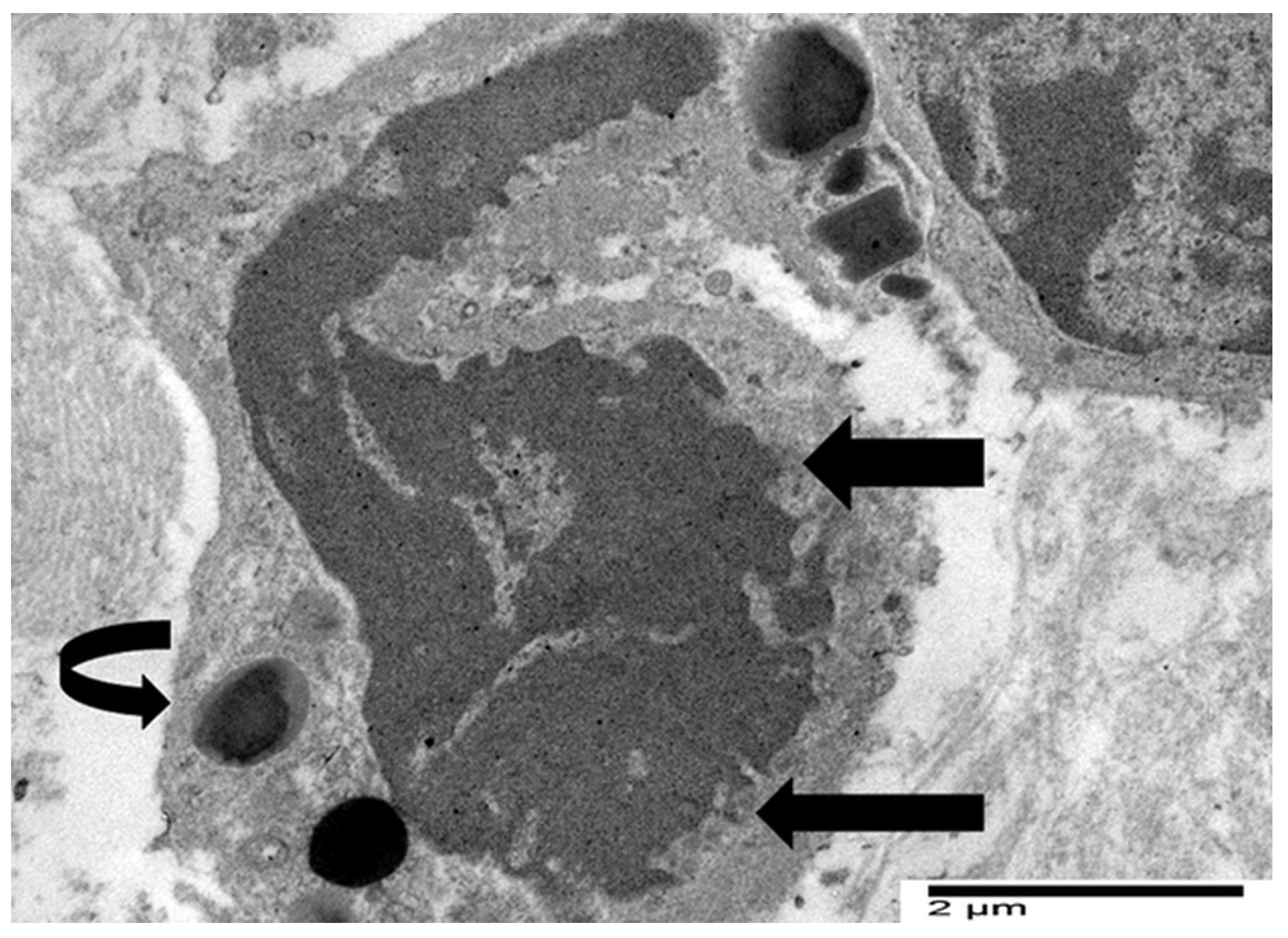
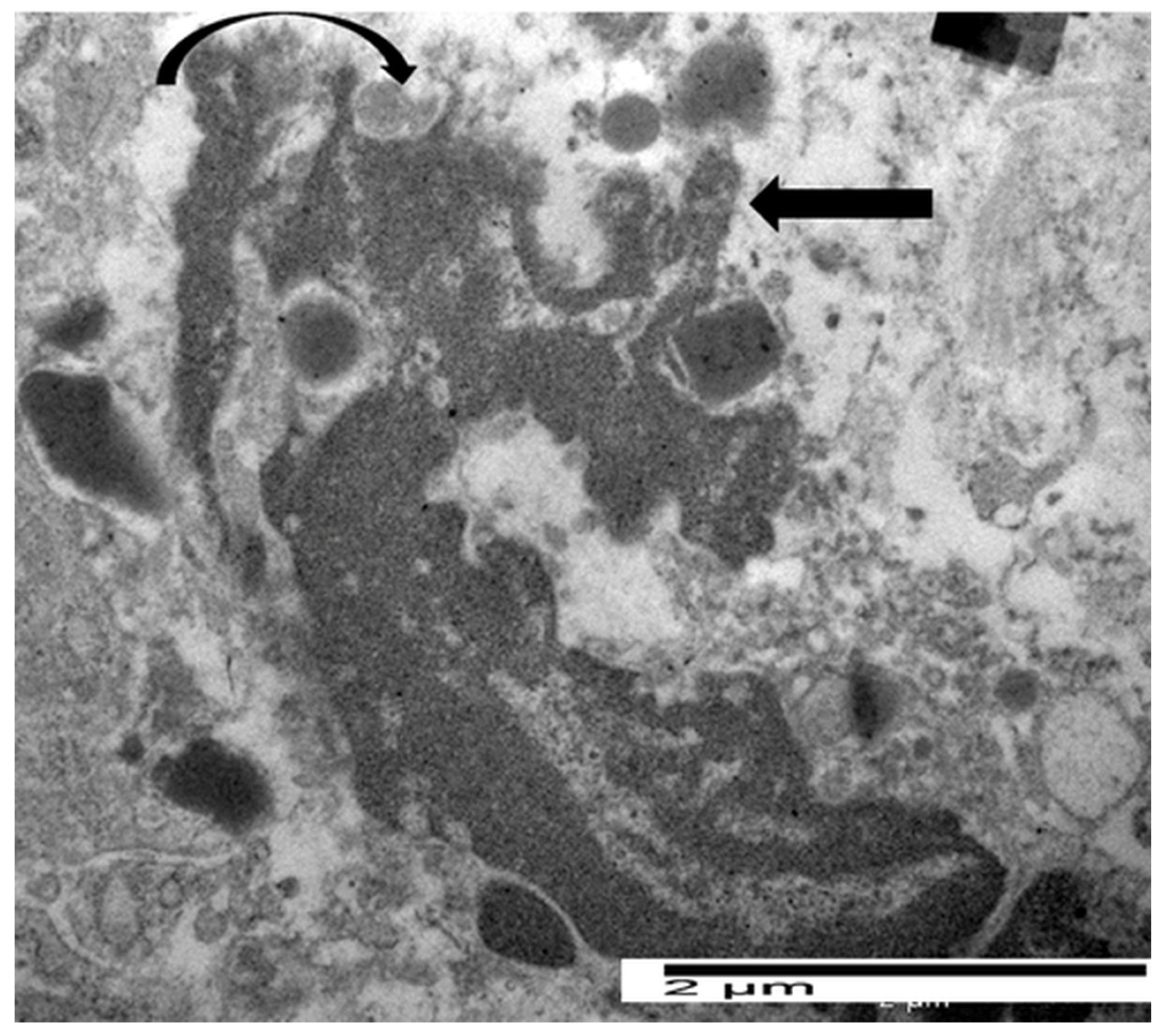
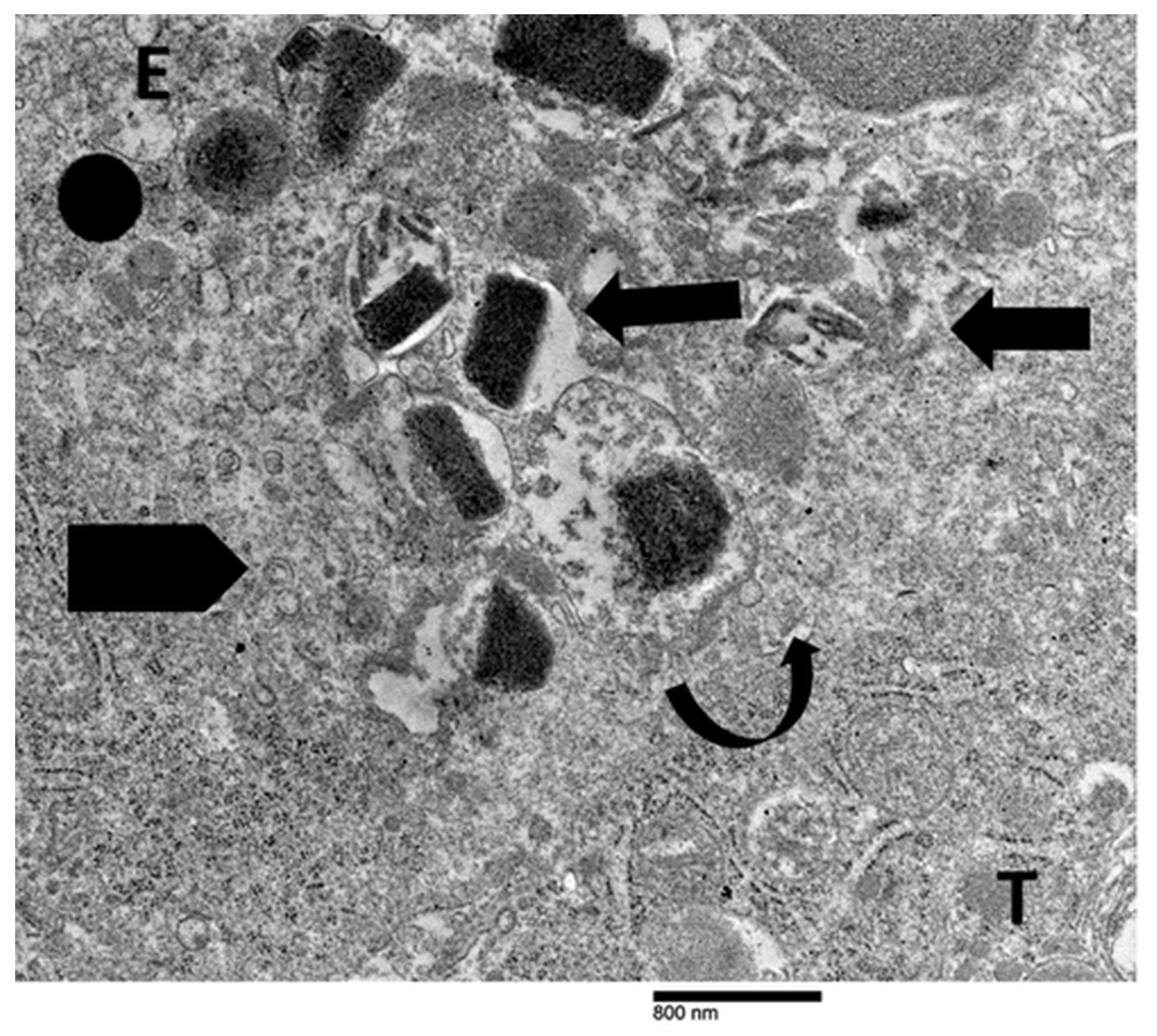
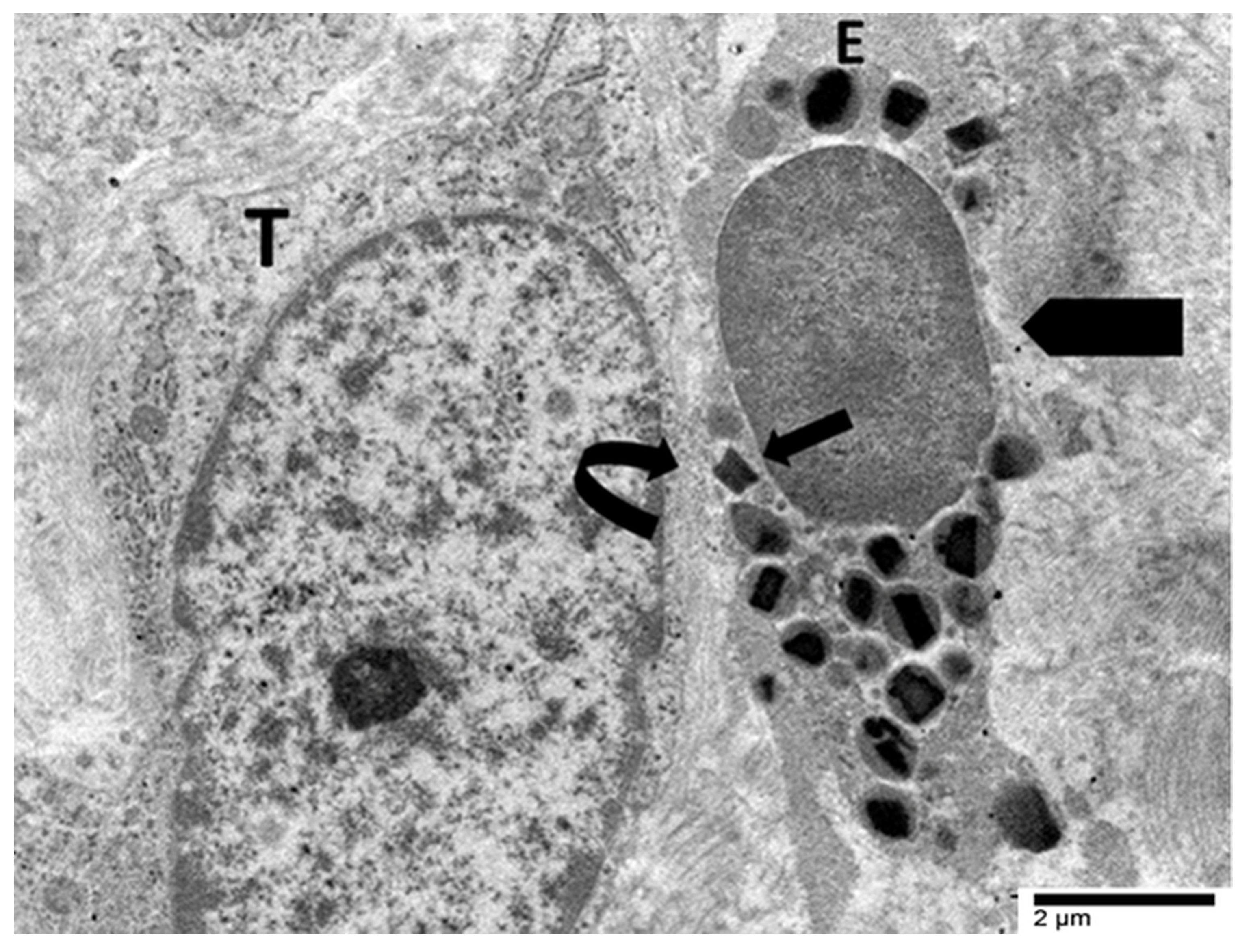
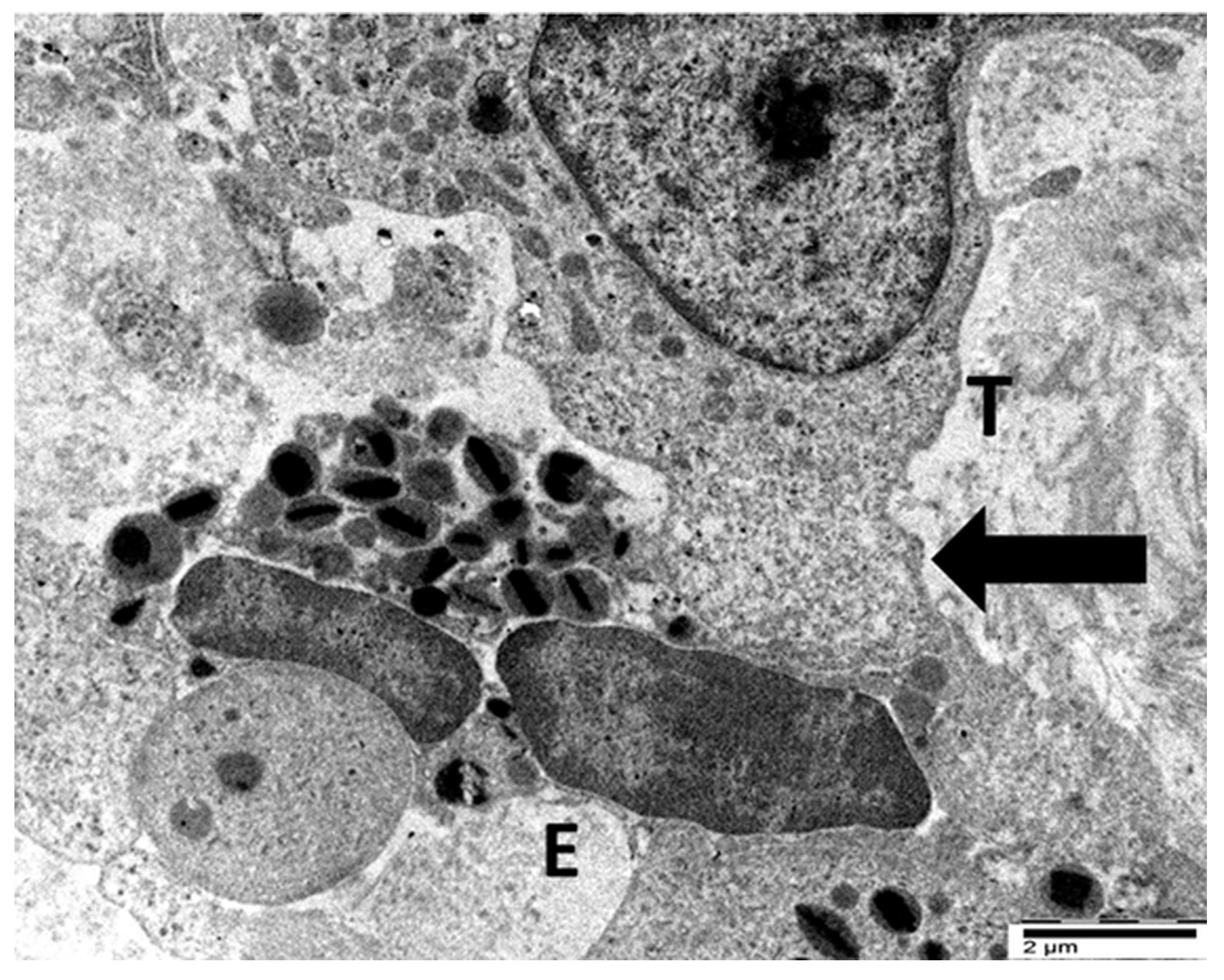
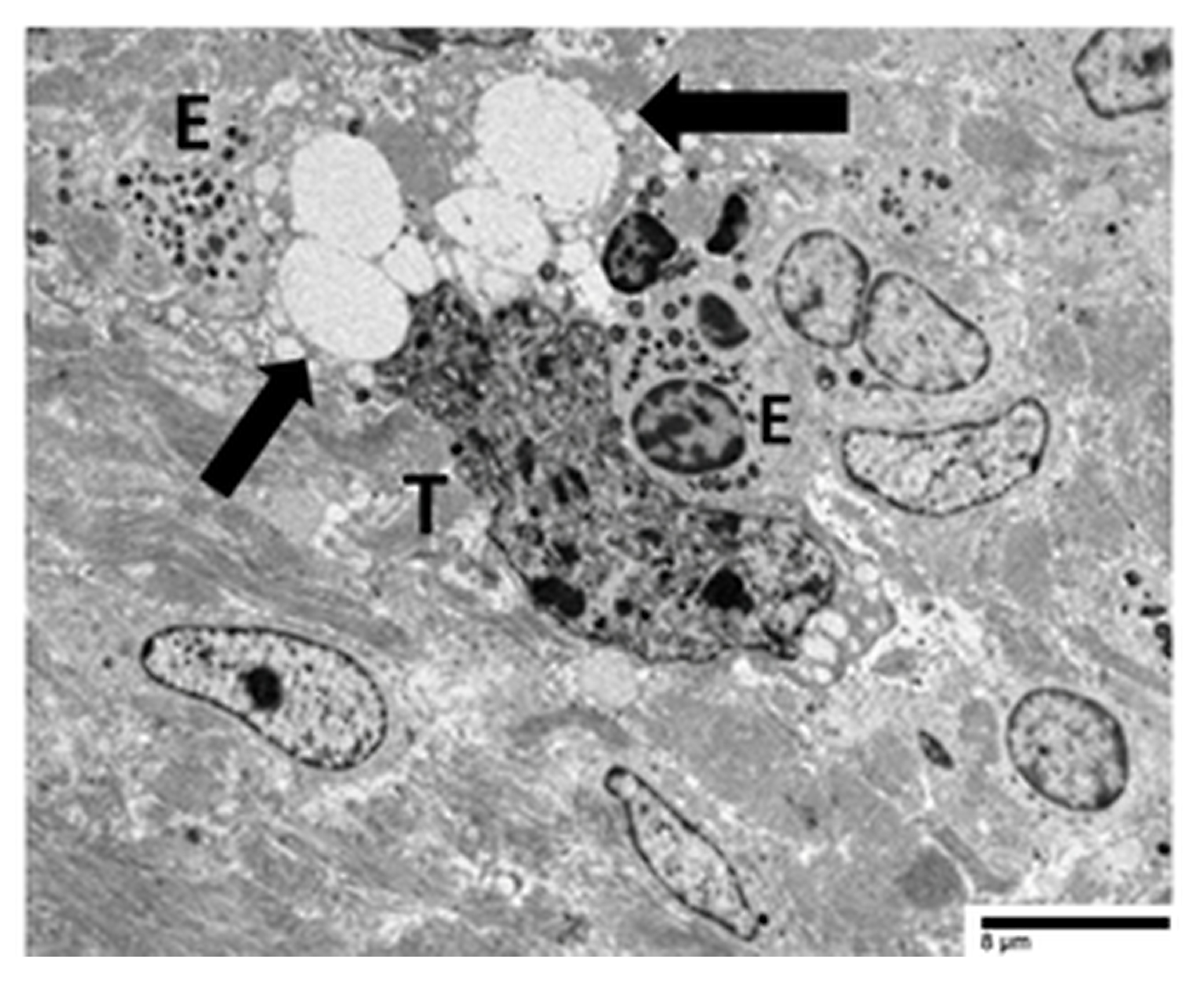
4.3. Our Ultrastructural Observations of Eosinophil ETosis in Gastric Carcinoma
5. Eosinophil-Mediated Tumor Cytotoxicity and Immune Synapse Formation
5.1. Eosinophil-Mediated Tumor Cytotoxicity: From In Vitro Mechanisms to In Vivo Evidence of Immune Synapse Formation
5.2. Ultrastructural Evidence of Eosinophil-Mediated Tumor Cell Injury in Gastric Carcinomas
6. Limitations
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TATE | tumor-associated tissue eosinophilia |
| HPV | Human papillomavirus |
| HTLV-1 | Human T-lymphotropic virus type 1 |
| ETosis | Extracellular trap cell death |
| TEM | Transmission electron microscopy |
| TABE | Tumor associated blood eosinophilia |
| LIAR | Local Immunity and Remodeling |
| ATLL | Adult T-cell leukemia/lymphoma |
| EBV | Epstein–Barr virus |
| FEGs | Free eosinophil granules |
References
- Blanchard, C.; Rothenberg, M.E. Biology of the eosinophil. Adv. Immunol. 2009, 101, 81–121. [Google Scholar] [CrossRef]
- Long, H.; Liao, W.; Wang, L.; Lu, Q. A Player and Coordinator: The Versatile Roles of Eosinophils in the Immune System. Transfus. Med. Hemother. 2016, 43, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.C.N.; Dvorak, A.M.; Weller, P.F. Chapter 3—Eosinophils as secretory cells. In Eosinophil Ultrastructure; Melo, R.C.N., Dvorak, A.M., Weller, P.F., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 61–105. [Google Scholar]
- Ueki, S.; Ohta, N.; Takeda, M.; Konno, Y.; Hirokawa, M. Eosinophilic Otitis Media: The Aftermath of Eosinophil Extracellular Trap Cell Death. Curr. Allergy Asthma Rep. 2017, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.C.N.; Liu, L.; Xenakis, J.J.; Spencer, L.A. Eosinophil-derived cytokines in health and disease: Unraveling novel mechanisms of selective secretion. Allergy 2013, 68, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Spencer, L.A.; Bonjour, K.; Melo, R.C.N.; Weller, P.F. Eosinophil secretion of granule-derived cytokines. Front. Immunol. 2014, 5, 496. [Google Scholar] [CrossRef]
- Melo, R.C.N.; Weller, P.F. Contemporary understanding of the secretory granules in human eosinophils. J. Leukoc. Biol. 2018, 104, 85–93. [Google Scholar] [CrossRef]
- Melo, R.C.N.; Dvorak, A.M.; Weller, P.F. Contributions of electron microscopy to understand secretion of immune mediators by human eosinophils. Microsc. Microanal. 2010, 16, 653–660. [Google Scholar] [CrossRef][Green Version]
- Melo, R.C.N.; Silva, T.P. Eosinophil activation during immune responses: An ultrastructural view with an emphasis on viral diseases. J. Leukoc. Biol. 2024, 116, 321–334. [Google Scholar] [CrossRef]
- Melo, R.C.N.; Rothenberg, M.E. Imaging eosinophil secretory granules: From storage containers to active, immune responder organelles. J. Allergy Clin. Immunol. 2025, 155, 414–417. [Google Scholar] [CrossRef]
- Ueki, S.; Melo, R.C.N.; Ghiran, I.; Spencer, L.A.; Dvorak, A.M.; Weller, P.F. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood 2013, 121, 2074–2083. [Google Scholar] [CrossRef]
- Ueki, S.; Konno, Y.; Takeda, M.; Moritoki, Y.; Hirokawa, M.; Matsuwaki, Y.; Honda, K.; Ohta, N.; Yamamoto, S.; Takagi, Y.; et al. Eosinophil extracellular trap cell death-derived DNA traps: Their presence in secretions and functional attributes. J. Allergy Clin. Immunol. 2016, 137, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Ueki, S.; Tokunaga, T.; Melo, R.C.N.; Saito, H.; Honda, K.; Fukuchi, M.; Konno, Y.; Takeda, M.; Yamamoto, Y.; Hirokawa, M.; et al. Charcot-Leyden crystal formation is closely associated with eosinophil extracellular trap cell death. Blood 2018, 132, 2183–2187. [Google Scholar] [CrossRef]
- Fukuchi, M.; Miyabe, Y.; Furutani, C.; Saga, T.; Moritoki, Y.; Yamada, T.; Weller, P.F.; Ueki, S. How to detect eosinophil ETosis (EETosis) and extracellular traps. Allergol. Int. 2021, 70, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Neves, V.H.; Palazzi, C.; Bonjour, K.; Ueki, S.; Weller, P.F.; Melo, R.C.N. In Vivo ETosis of Human Eosinophils: The Ultrastructural Signature Captured by TEM in Eosinophilic Diseases. Front. Immunol. 2022, 13, 938691. [Google Scholar] [CrossRef]
- Tomizawa, H.; Arima, M.; Miyabe, Y.; Furutani, C.; Kodama, S.; Ito, K.; Watanabe, K.; Hasegawa, R.; Nishiyama, S.; Hizuka, K.; et al. Characteristics and Regulation of Human Eosinophil ETosis In Vitro. Am. J. Respir. Cell Mol. Biol. 2024, 72, 52–61. [Google Scholar] [CrossRef]
- Lowe, D.; Jorizzo, J.; Hutt, M.S. Tumour-associated eosinophilia: A review. J. Clin. Pathol. 1981, 34, 1343–1348. [Google Scholar] [CrossRef]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Lucarini, V.; Marone, G.; Mattei, F.; Marone, G.; Schiavoni, G. Eosinophils: The unsung heroes in cancer? Oncoimmunology 2017, 7, e1393134. [Google Scholar] [CrossRef]
- Mattei, F.; Andreone, S.; Marone, G.; Gambardella, A.R.; Loffredo, S.; Varricchi, G.; Schiavoni, G. Eosinophils in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1273, 1–28. [Google Scholar] [CrossRef]
- Sabit, H.; Arneth, B.; Abdel-Ghany, S.; Madyan, E.F.; Ghaleb, A.H.; Selvaraj, P.; Shin, D.M.; Bommireddy, R.; Elhashash, A. Beyond Cancer Cells: How the Tumor Microenvironment Drives Cancer Progression. Cells 2024, 13, 1666. [Google Scholar] [CrossRef]
- Caruso, R.A.; Giuffrè, G.; Inferrera, C. Minute and small early gastric carcinoma with special reference to eosinophil infiltration. Histol. Histopathol. 1993, 8, 155–166. [Google Scholar] [PubMed]
- Caruso, R.A.; Bersiga, A.; Rigoli, L.; Inferrera, C. Eosinophil-tumor cell interaction in advanced gastric carcinoma: An electron microscopic approach. Anticancer Res. 2002, 22, 3833–3836. [Google Scholar] [PubMed]
- Caruso, R.A.; Ieni, A.; Fedele, F.; Zuccalà, V.; Riccardo, M.; Parisi, E.; Parisi, A. Degranulation patterns of eosinophils in advanced gastric carcinoma: An electron microscopic study. Ultrastruct. Pathol. 2005, 29, 29–36. [Google Scholar] [CrossRef]
- Caruso, R.A.; Fedele, F.; Zuccalà, V.; Fracassi, M.G.; Venuti, A. Mast cell and eosinophil interaction in gastric carcinomas: Ultrastructural observations. Anticancer Res. 2007, 27, 391–394. [Google Scholar] [PubMed]
- Caruso, R.A.; Parisi, A.; Quattrocchi, E.; Scardigno, M.; Branca, G.; Parisi, C.; Lucianò, R.; Paparo, D.; Fedele, F. Ultrastructural descriptions of heterotypic aggregation between eosinophils and tumor cells in human gastric carcinomas. Ultrastruct. Pathol. 2011, 35, 145–149. [Google Scholar] [CrossRef]
- Caruso, R.A.; Fedele, F.; Parisi, A.; Paparo, D.; Bonanno, A.; Finocchiaro, G.; Branca, G.; Scardigno, M.; Rigoli, L. Chronic allergic-like inflammation in the tumor stroma of human gastric carcinomas: An ultrastructural study. Ultrastruct. Pathol. 2012, 36, 139–144. [Google Scholar] [CrossRef]
- Caruso, R.A.; Branca, G.; Fedele, F.; Parisi, A.; Finocchiaro, G.; Ieni, A.; Rigoli, L. Eosinophil-specific granules in tumor cell cytoplasm: Unusual ultrastructural findings in a case of diffuse-type gastric carcinoma. Ultrastruct. Pathol. 2015, 39, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Irato, E.; Rigoli, L. Eosinophil exocytosis in a poorly differentiated tubular gastric adenocarcinoma: Case report. Ultrastruct. Pathol. 2022, 46, 139–146. [Google Scholar] [CrossRef]
- Caruso, R.; Caruso, V.; Rigoli, L. Ultrastructural evidence of eosinophil clustering and ETosis in association with damage to single tumour cells in a case of poorly cohesive NOS gastric carcinoma. Eur. J. Case Rep. Intern. Med. 2023, 10, 004016. [Google Scholar] [CrossRef]
- Caruso, R.; Caruso, V.; Rigoli, L. Eosinophil cytolysis with or without ETosis in four cases of human gastric cancer: A comparative ultrastructural study. Explor. Target. Antitumor Ther. 2025, 6, 1002309. [Google Scholar] [CrossRef]
- Shomali, W.; Gotlib, J. World Health Organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 129–148. [Google Scholar] [CrossRef]
- Cormier, S.A.; Taranova, A.G.; Bedient, C.; Nguyen, T.; Protheroe, C.; Pero, R.; Dimina, D.; Ochkur, S.I.; O’Neill, K.; Colbert, D.; et al. Pivotal Advance: Eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J. Leukoc. Biol. 2006, 79, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Jacobsen, E.A.; McGarry, M.P.; Schleimer, R.; Lee, N.A. Eosinophils in health and disease: The LIAR hypothesis. Clin. Exp. Allergy 2010, 40, 563–575. [Google Scholar] [CrossRef]
- Kataoka, S.; Konishi, Y.; Nishio, Y.; Fujikawa-Adachi, K.; Tominaga, A. Antitumor activity of eosinophils activated by IL-5 and eotaxin against hepatocellular carcinoma. DNA Cell Biol. 2004, 23, 549–560. [Google Scholar] [CrossRef]
- Korbecki, J.; Kojder, K.; Simińska, D.; Bohatyrewicz, R.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of the Ligands of Receptors CCR1, CCR2, CCR3, and CCR4. Int. J. Mol. Sci. 2020, 21, 8412. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Oon, C.; Diaz, L.; Sandborg, H.; Stempinski, E.S.; Saoi, M.; Morgan, T.K.; López, C.S.; Cross, J.R.; Sherman, M.H. Autotaxin-lysolipid signaling suppresses a CCL11-eosinophil axis to promote pancreatic 627 cancer progression. Nat. Cancer 2024, 5, 283–298. [Google Scholar] [CrossRef]
- Ghaffari, S.; Rezaei, N. Eosinophils in the tumor microenvironment: Implications for cancer immunotherapy. J. Transl. Med. 2023, 21, 551. [Google Scholar] [CrossRef]
- Arrizabalaga, L.; Risson, A.; Ezcurra-Hualde, M.; Aranda, F.; Berraondo, P. Unveiling the multifaceted antitumor effects of interleukin 33. Front. Immunol. 2024, 15, 1425282. [Google Scholar] [CrossRef]
- Gambardella, A.R.; Antonucci, C.; Zanetti, C.; Noto, F.; Andreone, S.; Vacca, D.; Pellerito, V.; Sicignano, C.; Parrottino, G.; Tirelli, V.; et al. IL-33 stimulates the anticancer activities of eosinophils through extracellular vesicle-driven reprogramming of tumor cells. J. Exp. Clin. Cancer Res. 2024, 43, 209. [Google Scholar] [CrossRef]
- Omero, F.; Speranza, D.; Murdaca, G.; Cavaleri, M.; Marafioti, M.; Cianci, V.; Berretta, M.; Casciaro, M.; Gangemi, S.; Santarpia, M. The role of eosinophils, eosinophil-related cytokines and AI in predicting immunotherapy efficacy in NSCLC. Biomolecules 2025, 15, 491. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Custurone, P.; Li Pomi, F.; Cordiano, R.; Alessandrello, C.; Gangemi, S. IL-31: State of the art for an inflammation-oriented interleukin. Int. J. Mol. Sci. 2022, 23, 6507. [Google Scholar] [CrossRef] [PubMed]
- Artham, S.; Juras, P.K.; Goyal, A.; Chakraborty, P.; Byemerwa, J.; Liu, S.; Wardell, S.E.; Chakraborty, B.; Crowder, D.; Lim, F.; et al. Estrogen signaling suppresses tumor-associated tissue eosinophilia to promote breast tumor growth. Sci. Adv. 2024, 10, eadp2442. [Google Scholar] [CrossRef]
- Iwasaki, K.; Torisu, M.; Fujimura, T. Malignant tumor and eosinophils. I. Prognostic significance in gastric cancer. Cancer 1986, 58, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, A.; Talbot, I.C.; Weeden, S., on behalf of the MRC Upper GI Cancer Working Party. GI Cancer Working. Party. Influence of pathological tumour variables on long-term survival in resectable gastric cancer. Br. J. Cancer 2002, 86, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Pretlow, T.P.; Keith, E.F.; Cryar, A.K.; Bartolucci, A.A.; Pitts, A.M.; Pretlow, T.G.; Kimball, P.M.; Boohaker, E.A. Eosinophil infiltration of human colonic carcinomas as a prognostic indicator. Cancer Res. 1983, 43, 2997–3000. [Google Scholar]
- Nielsen, H.J.; Hansen, U.; Christensen, I.J.; Reimert, C.M.; Brünner, N.; Moesgaard, F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J. Pathol. 1999, 189, 487–495. [Google Scholar] [CrossRef]
- Fernández-Aceñero, M.J.; Galindo-Gallego, M.; Sanz, J.; Aljama, A. Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer 2000, 88, 1544–1548. [Google Scholar] [CrossRef]
- Prizment, A.E.; Vierkant, R.A.; Smyrk, T.C.; Tillmans, L.S.; Lee, J.J.; Sriramarao, P.; Nelson, H.H.; Lynch, C.F.; Thibodeau, S.N.; Church, T.R.; et al. Tumor eosinophil infiltration and improved survival of colorectal cancer patients: Iowa Women’s Health Study. Mod. Pathol. 2016, 29, 516–527. [Google Scholar] [CrossRef]
- Fujii, M.; Yamashita, T.; Ishiguro, R.; Tashiro, M.; Kameyama, K. Significance of epidermal growth factor receptor and tumor associated tissue eosinophilia in the prognosis of patients with nasopharyngeal carcinoma. Auris Nasus Larynx 2002, 29, 175–181. [Google Scholar] [CrossRef]
- Peurala, E.; Tuominen, M.L.E.; Syrjänen, S.; Rautava, J. Eosinophilia is a favorable prognostic marker for oral cavity and lip squamous cell carcinoma. APMIS 2018, 126, 201–207. [Google Scholar] [CrossRef]
- Caponio, V.C.A.; Togni, L.; Zhurakivska, K.; Santarelli, A.; Arena, C.; Rubini, C.; Lo, M.L.; Troiano, G.; Mascitti, M. Prognostic assessment of different methods for eosinophils detection in oral tongue cancer. J. Oral Pathol. Med. 2022, 51, 240–248. [Google Scholar] [CrossRef]
- Ishibashi, S.; Ohashi, Y.; Suzuki, T.; Miyazaki, S.; Moriya, T.; Satomi, S.; Sasano, H. Tumor-associated tissue eosinophilia in human esophageal squamous cell carcinoma. Anticancer Res. 2006, 26, 1419–1424. [Google Scholar]
- Thompson, A.C.; Bradley, P.J.; Griffin, N.R. Tumor-associated tissue eosinophilia and long-term prognosis for carcinoma of the larynx. Am. J. Surg. 1994, 168, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Leisgang, W.; Schuler, G.; Heinzerling, L. Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy. Immunotherapy 2017, 9, 115–121. [Google Scholar] [CrossRef]
- Brand, C.L.; Hunger, R.E.; Seyed, J.S.M. Eosinophilic granulocytes as a potential prognostic marker for cancer progression and therapeutic response in malignant melanoma. Front. Oncol. 2024, 14, 1366081. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, H.; Wang, L.; Ning, Z.; Zhuang, Y.; Gan, J.; Chen, S.; Zhou, D.; Zhu, H.; Tan, D.; et al. Clinical impact of tumor-infiltrating inflammatory cells in primary small cell esophageal carcinoma. Int. J. Mol. Sci. 2014, 15, 9718–9734. [Google Scholar] [CrossRef]
- Ownby, H.E.; Roi, L.D.; Isenberg, R.R.; Brennan, M.J. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer 1983, 52, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Wang, S.; Zhong, K.; Xu, F.; Huang, L.; Chen, W.; Cheng, P. Tumor-associated tissue eosinophilia predicts favorable clinical outcome in solid tumors: A meta-analysis. BMC Cancer 2020, 20, 454. [Google Scholar] [CrossRef]
- van Driel, W.J.; Hogendoorn, P.C.; Jansen, F.W.; Zwinderman, A.H.; Trimbos, J.B.; Fleuren, G.J. Tumor-associated eosinophilic infiltrate of cervical cancer is indicative for a less effective immune response. Hum. Pathol. 1996, 27, 904–911. [Google Scholar] [CrossRef]
- Xie, F.; Liu, L.B.; Shang, W.Q.; Chang, K.K.; Meng, Y.H.; Mei, J.; Yu, J.J.; Li, D.J.; Li, M.Q. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. 2015, 364, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Rimini, M.; Franco, P.; Bertolini, F.; Berardino, B.; Giulia, Z.M.; Stefano, V.; Andrikou, K.; Arcadipane, F.; Napolitano, M.; Buno, L.V.; et al. The prognostic role of baseline eosinophils in HPV-related cancers: A multi-institutional analysis of anal SCC and OPC Patients Treated with Radical CT-RT. J. Gastrointest. Cancer 2023, 54, 662–671. [Google Scholar] [CrossRef]
- Enblad, G.; Sundstrom, C.; Glimelius, B. Infiltration of eosinophils in Hodgkin’s disease involved lymph nodes predicts prognosis. Hematol. Oncol. 1993, 11, 187–193. [Google Scholar] [CrossRef]
- von Wasielewski, R.; Seth, S.; Franklin, J.; Fischer, R.H.K.; Hansmann, M.L.; Diehl, V.; Georgii, A. Tissue eosinophilia correlates strongly with poor prognosis in nodular sclerosing Hodgkin’s disease, allowing for known prognostic factors. Blood 2000, 95, 1207–1213. [Google Scholar] [CrossRef]
- Danielson, D.T.; Aguilera, N.S.; Auerbach, A. Head and neck classic Hodgkin, T and NK lymphomas with eosinophilia. Head Neck Pathol. 2025, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, A.; Ishida, T.; Inagaki, A.; Ishii, T.; Yano, H.; Komatsu, H.; Iida, S.; Yonekura, K.; Takeuchi, S.; Takatsuka, Y.; et al. Clinical significance of a blood eosinophilia in adult T-cell leukemia/lymphoma: A blood eosinophilia is a significant unfavorable prognostic factor. Leuk. Res. 2007, 31, 915–920. [Google Scholar] [CrossRef]
- Gami, B.; Kubba, F.; Ziprin, P. Human papilloma virus and squamous cell carcinoma of the anus. Clin. Med. Insights Oncol. 2014, 8, 113–119. [Google Scholar] [CrossRef]
- Fernandes, A.; Viveros-Carreño, D.; Hoegl, J.; Ávila, M.; Pareja, R. Human Papillomavirus-Independent Cervical Cancer. Int. J. Gynecol. Cancer 2022, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nicolás, I.; Marimon, L.; Barnadas, E.; Saco, A.; Rodríguez-Carunchio, L.; Fusté, P.; Martí, C.; Rodriguez-Trujillo, A.; Torne, A.; Del, P.M.; et al. HPV-negative tumors of the uterine cervix. Mod. Pathol. 2019, 32, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Saito, M.; Okayama, K.; Okodo, M.; Kurose, N.; Sakamoto, J.; Sasagawa, T. HPV genotyping by molecular mapping of tissue samples in vaginal squamous intraepithelial neoplasia (VaIN) and vaginal squamous cell carcinoma (VaSCC). Cancers 2021, 13, 3260. [Google Scholar] [CrossRef]
- Li, Z.; Liu, P.; Wang, Z.; Zhang, Z.; Chen, Z.; Chu, R.; Li, G.; Han, Q.; Zhao, Y.; Li, L.; et al. Prevalence of human papillomavirus DNA and p16INK4a positivity in vulvar cancer and vulvar intraepithelial neoplasia: A systematic review and meta-analysis. Lancet Oncol. 2023, 24, 403–414. [Google Scholar] [CrossRef]
- Mustasam, A.; Parza, K.; Ionescu, F.; Gullapalli, K.; Paravathaneni, M.; Kim, Y.; Sandstrom, R.E.; Al Assaad, M.; Grass, G.D.; Johnstone, P.; et al. The Prognostic role of HPV or p16INK4a status in penile squamous cell carcinoma: A Meta-Analysis. J. Natl. Compr. Cancer Netw. 2025, 23, e247078. [Google Scholar] [CrossRef]
- Xu, L.; Jin, Y.; Qin, X. Comprehensive analysis of significant genes and immune cell infiltration in HPV-related head and neck squamous cell carcinoma. Int. Immunopharmacol. 2020, 87, 106844. [Google Scholar] [CrossRef]
- Jarrett, A.F.; Armstrong, A.A.; Alexander, E. Epidemiology of EBV and Hodgkin’s lymphoma. Ann. Oncol. 1996, 7 (Suppl. S4), 5–10. [Google Scholar] [CrossRef]
- Teruya-Feldstein, J.; Jaffe, E.S.; Burd, P.R.; Kingma, D.W.; Setsuda, J.E.; Tosato, G. Differential chemokine expression in tissues involved by Hodgkin’s disease: Direct correlation of eotaxin expression and tissue eosinophilia. Blood 1999, 93, 2463–2470. [Google Scholar] [CrossRef] [PubMed]
- Tsotridou, E.; Hatzipantelis, E. Epstein-Barr Infection, Hodgkin’s Lymphoma, and the Immune System: Insights into the Molecular Mechanisms Facilitating Immune Evasion. Cancers 2025, 17, 1481. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.W.; Yoon, D.H.; Suh, C.; Huh, J. Impact of the Epstein–Barr virus positivity on Hodgkin’s lymphoma in a large cohort from a single institute in Korea. Ann. Hematol. 2012, 91, 1403–1412. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Kroemer, G.; El-Deiry, W.S.; Golstein, P.; Peter, M.E.; Vaux, D.; Vandenabeele, P.; Zhivotovsky, B.; Blagosklonny, M.V.; Malorni, W.; Knight, R.A.; et al. Nomenclature Committee on Cell Death. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005, 12 (Suppl. S2), 1463–1467. [Google Scholar] [CrossRef]
- Caruso, R.A.; Branca, G.; Fedele, F.; Irato, E.; Finocchiaro, G.; Parisi, A.; Ieni, A. Mechanisms of coagulative necrosis in malignant epithelial tumors. Oncol. Lett. 2014, 8, 1397–1402. [Google Scholar] [CrossRef]
- Nija, R.J.; Sanju, S.; Sidharthan, N.; Mony, U. Extracellular Trap by blood cells: Clinical implications. Tissue Eng. Regen. Med. 2020, 17, 141–153. [Google Scholar] [CrossRef]
- Yousefi, S.; Gold, J.A.; Andina, N.; Lee, J.J.; Kelly, A.M.; Kozlowski, E.; Schmid, I.; Straumann, A.; Reichenbach, J.; Gleich, G.J.; et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 2008, 14, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Lacy, P.; Ueki, S. Eosinophil Extracellular Traps and Inflammatory Pathologies-Untangling the Web! Front. Immunol. 2018, 9, 2763. [Google Scholar] [CrossRef]
- Arima, M.; Ito, K.; Abe, T.; Oguma, T.; Asano, K.; Mukherjee, M.; Ueki, S. Eosinophilic mucus diseases. Allergol. Int. 2024, 73, 362–374. [Google Scholar] [CrossRef]
- Neves, V.H.; Palazzi, C.; Malta, K.K.; Bonjour, K.; Kneip, F.; Dias, F.F.; Neves, J.S.; Weller, P.F.; Melo, R.C.N. Extracellular sombrero vesicles are hallmarks of eosinophilic cytolytic degranulation in tissue sites of human diseases. J. Leukoc. Biol. 2024, 116, 398–408. [Google Scholar] [CrossRef]
- Neves, J.S.; Perez, S.A.; Spencer, L.A.; Melo, R.C.N.; Reynolds, L.; Ghiran, I.; Mahmudi-Azer, S.; Odemuyiwa, S.O.; Dvorak, A.M.; Moqbel, R.; et al. Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc. Natl. Acad. Sci. USA 2008, 105, 18478–18483. [Google Scholar] [CrossRef]
- Muniz, V.S.; Baptista-Dos-Reis, R.; Neves, J.S. Functional extracellular eosinophil granules: A bomb caught in a trap. Int. Arch. Allergy Immunol. 2013, 162, 276–282. [Google Scholar] [CrossRef]
- Legrand, F.; Driss, V.; Delbeke, M.; Loiseau, S.; Hermann, E.; Dombrowicz, D.; Capron, M. Human eosinophils exert TNF-α and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J. Immunol. 2010, 185, 7443–7451. [Google Scholar] [CrossRef] [PubMed]
- Gatault, S.; Delbeke, M.; Driss, V.; Sarazin, A.; Dendooven, A.; Kahn, J.E.; Lefèvre, G.; Capron, M. IL-18 is involved in eosinophil-mediated tumoricidal activity against a colon carcinoma cell line by upregulating LFA-1 and ICAM-1. J. Immunol. 2015, 195, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Andreone, S.; Spadaro, F.; Buccione, C.; Mancini, J.; Tinari, A.; Sestili, P.; Gambardella, A.R.; Lucarini, V.; Ziccheddu, G.; Parolini, I.; et al. IL-33 promotes CD11b/CD18-mediated adhesion of eosinophils to cancer cells and synapse-polarized degranulation leading to tumor cell killing. Cancers 2019, 11, 1664. [Google Scholar] [CrossRef]
- Ghadially, F.N. Ultrastructural Pathology of the Cell and Matrix, 4th ed.; Butterworth-Heinemann: Boston, MA, USA, 1997; pp. 136–145. [Google Scholar]
- Pavelka, M.; Roth, J. Functional Ultrastructure. An Atlas of Tissue Biology and Pathology; Springer: Vienna, Austria, 2005; pp. 22–23. [Google Scholar]
- Caruso, R.A.; Fabiano, V.; Rigoli, L.; Inferrera, A. Focal parietal cell differentiation in a well-differentiated (intestinal-type) early gastric cancer. Ultrastruct. Pathol. 2000, 24, 417–422. [Google Scholar] [CrossRef]
- Caruso, R.A.; Basile, F.; Fedele, F.; Zuccalà, V.; Crisafulli, C.; Fracassi, M.G.; Quattrocchi, E.; Venuti, A.; Fabiano, V. Gastric hepatoid adenocarcinoma with autophagy-related necrosis-like tumor cell death: Report of a case. Ultrastruct. Pathol. 2006, 30, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.A.; Napoli, P.; Nania, A.; Parisi, A.; Fedele, F.; Zuccalà, V. Mitochondrion-rich differentiated adenocarcinomas of the stomach: Clinicopathological, immunohistochemical and electron microscopy study of nine cases. Virchows Arch. 2010, 456, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.A.; Fedele, F.; Consolo, P.; Luigiano, C.; Venuti, A.; Cavallari, V. Abnormal nuclear structures (micronuclei, nucleoplasmic bridges, and nuclear buds) in a pleomorphic giant cell carcinoma of the stomach. Ultrastruct. Pathol. 2008, 32, 11–15. [Google Scholar] [CrossRef]
- Caruso, R.A.; Basile, G.; Crisafulli, C.; Pizzi, G.; Finocchiaro, G.; Fedele, F.; Paparo, D.; Parisi, A. Granulomatous inflammatory reaction in human gastric adenocarcinomas: A light and electron microscopy study. Ultrastruct. Pathol. 2009, 33, 269–273. [Google Scholar] [CrossRef]
- Caruso, R.A.; Angelico, G.; Irato, E.; de Sarro, R.; Tuccari, G.; Ieni, A. Autophagy in advanced low- and high-grade tubular adenocarcinomas of the stomach: An ultrastructural investigation. Ultrastruct. Pathol. 2018, 42, 10–17. [Google Scholar] [CrossRef] [PubMed]


| Neoplasm | Virus Identification | Type of Eosinophilia | Clinical Outcome | Reference |
|---|---|---|---|---|
| Cervical squamous cell carcinoma | HPV | TATE | TATE increases with cancer progression | Xie et al. [60] |
| Cervical squamous cell carcinoma | Not performed | TATE | TATE predicts worse overall survival | Van Driel et al. [59] † |
| Anal squamous cell carcinoma | HPV | TABE | TABE is a negative independent prognostic factor for disease-free survival | Rimini et al. [61] |
| Oropharyngeal cancer | HPV | TABE | TABE is associated with worse disease-free survival | Rimini et al. [61] |
| Adult T-cell leukemia/lymphoma | HTLV-1 | TABE | TABE is an independent unfavorable prognostic factor | Utsunomiya et al. [65] |
| Feature | Apoptosis | Neutrophil ETosis | Eosinophil ETosis |
|---|---|---|---|
| Type of cell death | Programmed, non-inflammatory | Programmed, pro-inflammatory | Programmed, pro-inflammatory |
| Nuclear organization | Condensation and fragmentation | Delobulation and decondensation | Delobulation and decondensation |
| Nuclear envelope integrity | Preserved until late stages | Disrupted | Disrupted |
| Plasma membrane rupture | No | Yes | Yes |
| Cytoplasmic granules | Intact, not involved | Disintegrated; enzymes released into nucleus | Intact; associated with extracellular traps |
| Content of extracellular traps | Absent | DNA + elastase, myeloperoxidase, other granule-derived enzymes | DNA + morphologically intact specific granules |
| Model | Eosinophil-Tumor Cell Interaction | Eosinophil Cytoxicity | Tumor Cell Outcome | References |
|---|---|---|---|---|
| In vitro assays (colo-205 carcinoma cell line | Mediated by CD11a/C and IL-18 | Degranulation and mediator release (eosinophil cationic protein, eosinophil-derived neurotoxin, TNF-alpha, granzyme A | Induction of apoptosis | Legrand et al. [88]; Gatault et al. [89] |
| In vivo murine melanoma | Mediated by IL-33 | Synapse-polarized degranulation and mediator release (eosinophil cationic protein, eosinophil peroxidase, granzyme B | Induction of apoptosis | Andreone et al. [90] |
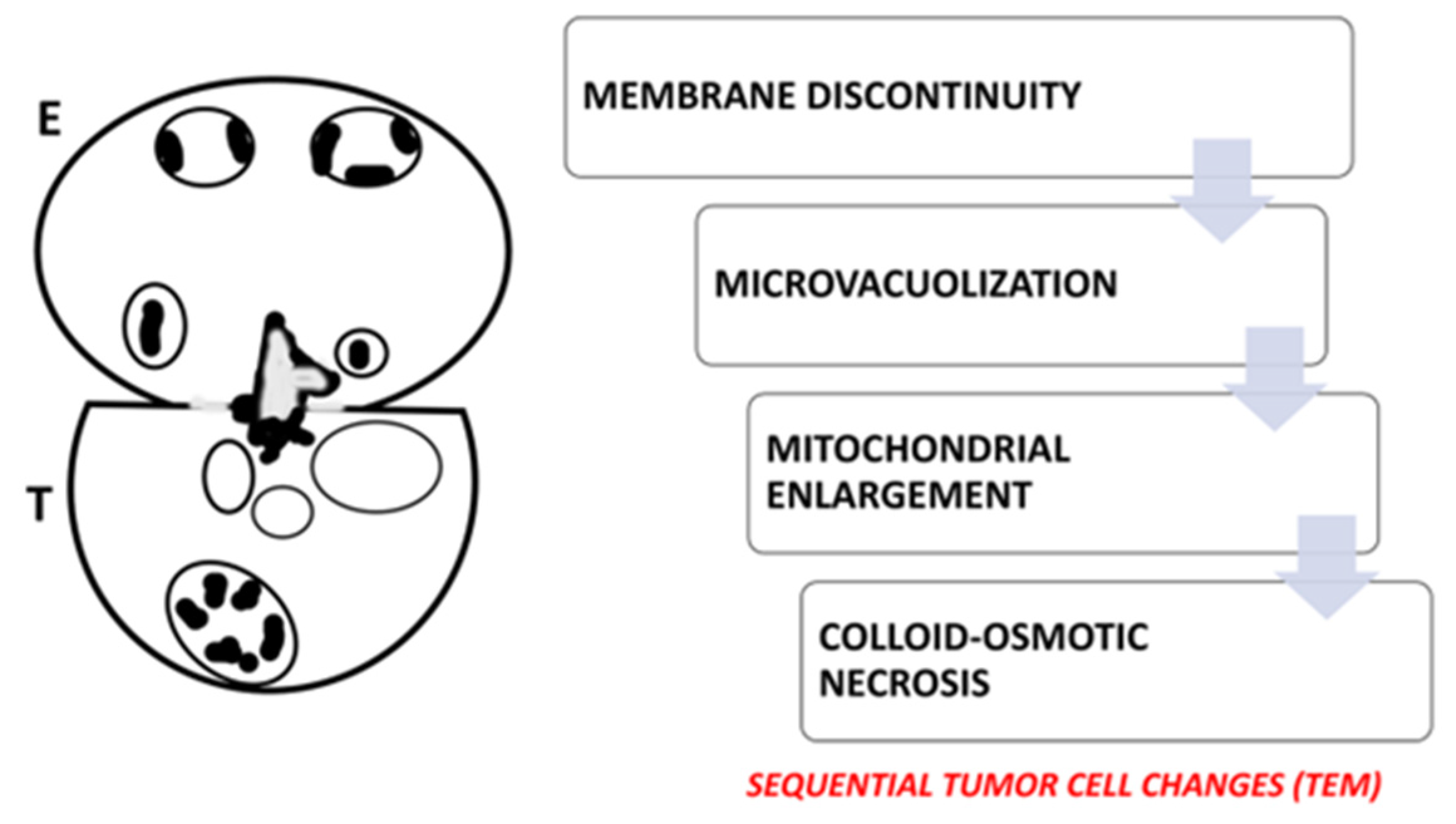
| Human gastric cancer TEM | Synapse-like interaction | Compound exocytosis or ETosis | Induction of colloid-osmotic death | Caruso et al. [28]; Caruso et al. [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, R.; Caruso, V.; Rigoli, L. Eosinophil ETosis and Cancer: Ultrastructural Evidence and Oncological Implications. Cancers 2025, 17, 3250. https://doi.org/10.3390/cancers17193250
Caruso R, Caruso V, Rigoli L. Eosinophil ETosis and Cancer: Ultrastructural Evidence and Oncological Implications. Cancers. 2025; 17(19):3250. https://doi.org/10.3390/cancers17193250
Chicago/Turabian StyleCaruso, Rosario, Valerio Caruso, and Luciana Rigoli. 2025. "Eosinophil ETosis and Cancer: Ultrastructural Evidence and Oncological Implications" Cancers 17, no. 19: 3250. https://doi.org/10.3390/cancers17193250
APA StyleCaruso, R., Caruso, V., & Rigoli, L. (2025). Eosinophil ETosis and Cancer: Ultrastructural Evidence and Oncological Implications. Cancers, 17(19), 3250. https://doi.org/10.3390/cancers17193250






