Targeting CTC Heterogeneity: Aptamer-Based Liquid Biopsy Predicts Outcome in Lung Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Aptamer Target Proteins in Circulating Tumor Cells
2.2. Isolation of Circulating Tumor Cells
3. Results
3.1. Identification of CTC-Specific Target Proteins for Aptamer LC-17
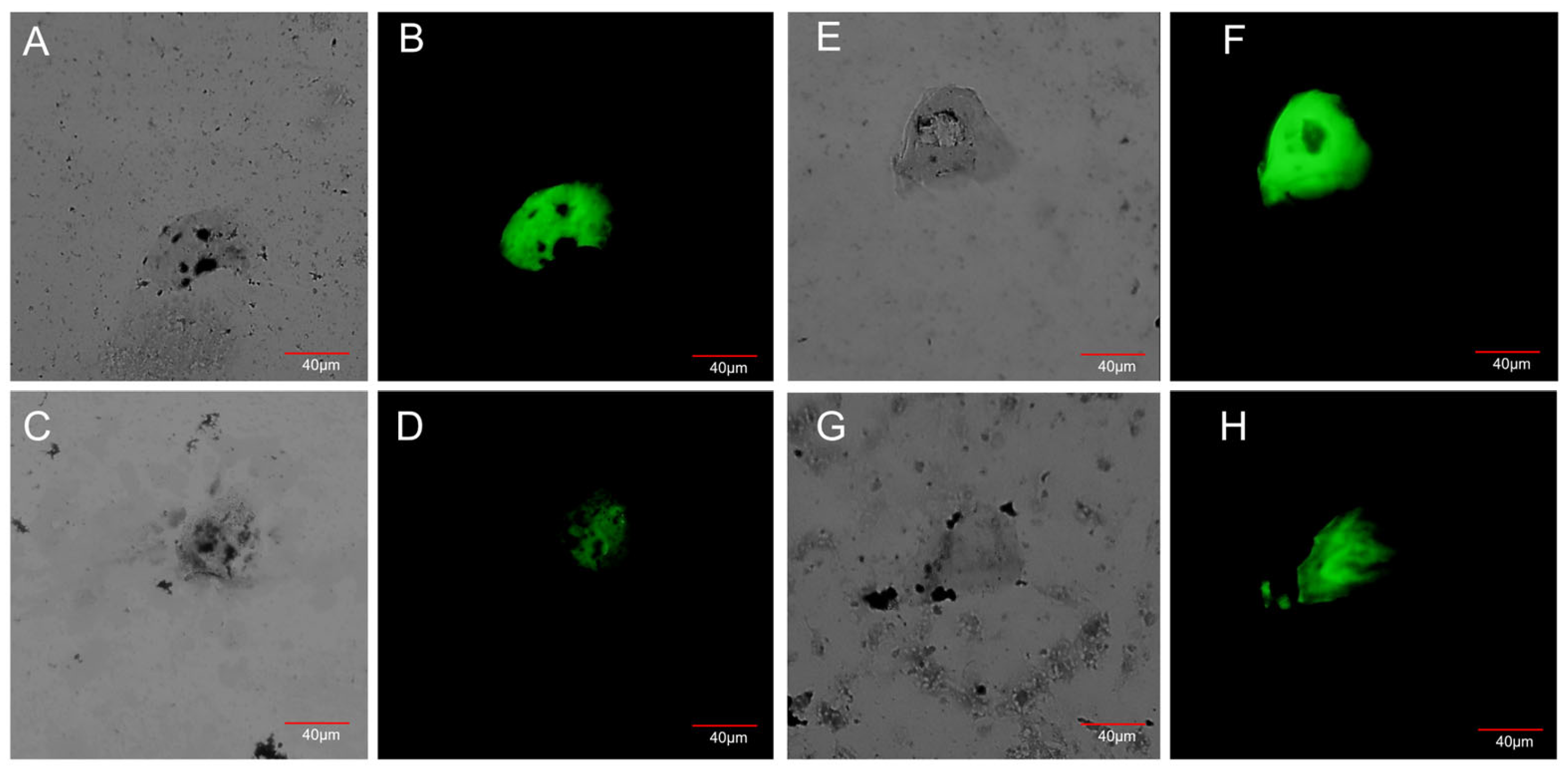
3.2. Detection of CTCs in Blood Samples from NSCLC Patients After Blood Cell Lysis
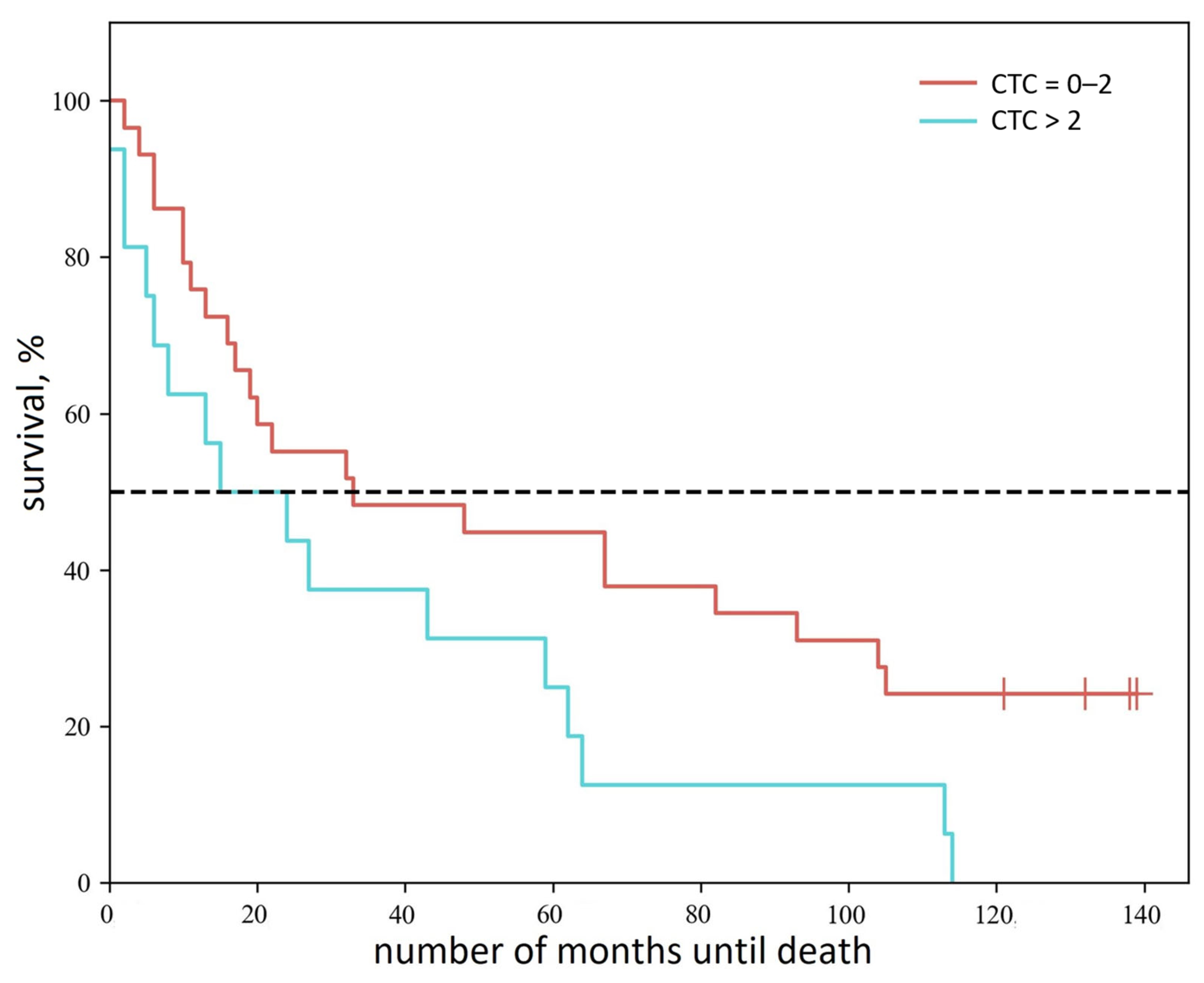
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amini, M.; Looha, M.A.; Zarean, E.; Pourhoseingholi, M.A. Global Pattern of Trends in Incidence, Mortality, and Mortality-to-Incidence Ratio Rates Related to Liver Cancer, 1990–2019: A Longitudinal Analysis Based on the Global Burden of Disease Study. BMC Public Health 2022, 22, 604. [Google Scholar] [CrossRef]
- van de Stolpe, A.; Pantel, K.; Sleijfer, S.; Terstappen, L.W.; Den Toonder, J.M.J. Circulating Tumor Cell Isolation and Diagnostics: Toward Routine Clinical Use. Cancer Res. 2011, 71, 5955–5960. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, J.-B.; Hou, L.-K.; Yu, F.; Zhang, J.; Wu, W.; Tang, X.-M.; Sun, F.; Lu, H.-M.; Deng, J.; et al. Liquid Biopsy in Lung Cancer: Significance in Diagnostics, Prediction, and Treatment Monitoring. Mol. Cancer 2022, 21, 25. [Google Scholar] [CrossRef]
- Dall’Olio, F.G.; Marabelle, A.; Caramella, C.; Garcia, C.; Aldea, M.; Chaput, N.; Robert, C.; Besse, B. Tumour Burden and Efficacy of Immune-Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2022, 19, 75–90. [Google Scholar] [CrossRef]
- Ahn, J.C.; Teng, P.; Chen, P.; Posadas, E.; Tseng, H.; Lu, S.C.; Yang, J.D. Detection of Circulating Tumor Cells and Their Implications as a Biomarker for Diagnosis, Prognostication, and Therapeutic Monitoring in Hepatocellular Carcinoma. Hepatology 2021, 73, 422–436. [Google Scholar] [CrossRef]
- Li, Z.; Xu, K.; Tartarone, A.; Santarpia, M.; Zhu, Y.; Jiang, G. Circulating Tumor Cells Can Predict the Prognosis of Patients with Non-Small Cell Lung Cancer after Resection: A Retrospective Study. Transl. Lung Cancer Res. 2021, 10, 995–1006. [Google Scholar] [CrossRef]
- Magbanua, M.J.M.; Yau, C.; Wolf, D.M.; Lee, J.S.; Chattopadhyay, A.; Scott, J.H.; Bowlby-Yoder, E.; Hwang, E.S.; Alvarado, M.; Ewing, C.A.; et al. Synchronous Detection of Circulating Tumor Cells in Blood and Disseminated Tumor Cells in Bone Marrow Predicts Adverse Outcome in Early Breast Cancer. Clin. Cancer Res. 2019, 25, 5388–5397. [Google Scholar] [CrossRef]
- Magbanua, M.J.M.; Savenkov, O.; Asmus, E.J.; Ballman, K.V.; Scott, J.H.; Park, J.W.; Dickler, M.; Partridge, A.; Carey, L.A.; Winer, E.P.; et al. Clinical Significance of Circulating Tumor Cells in Hormone Receptor–Positive Metastatic Breast Cancer Patients Who Received Letrozole with or Without Bevacizumab. Clin. Cancer Res. 2020, 26, 4911–4920. [Google Scholar] [CrossRef]
- Duan, G.-C.; Zhang, X.-P.; Wang, H.-E.; Wang, Z.-K.; Zhang, H.; Yu, L.; Xue, W.-F.; Xin, Z.-F.; Hu, Z.-H.; Zhao, Q.-T. Circulating Tumor Cells as a Screening and Diagnostic Marker for Early-Stage Non-Small Cell Lung Cancer. OncoTargets Ther. 2020, 13, 1931–1939. [Google Scholar] [CrossRef]
- Gu, X.; He, J.; Ji, G. Combined Use of Circulating Tumor Cells and Salivary mRNA to Detect Non–Small-Cell Lung Cancer. Medicine 2020, 99, e19097. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Shi, J.; Schmidt, B.; Liu, Q.; Shi, G.; Xu, X.; Liu, C.; Gao, Z.; Guo, T.; Shan, B. Circulating Tumor Cells as a Biomarker to Assist Molecular Diagnosis for Early Stage Non-Small Cell Lung Cancer. Cancer Manag. Res. 2020, 12, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Nong, J.; Wang, J.; Yan, Z.; Yi, L.; Gao, X.; Liu, Z.; Zhang, H.; Zhang, S. Isolation of Circulating Tumor Cells and Detection of EGFR Mutations in Patients with Non-small-cell Lung Cancer. Oncol. Lett. 2019, 17, 3799–3807. [Google Scholar] [CrossRef]
- Manjunath, Y.; Upparahalli, S.V.; Suvilesh, K.N.; Avella, D.M.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Li, G.; Kaifi, J.T. Circulating Tumor Cell Clusters Are a Potential Biomarker for Detection of Non-Small Cell Lung Cancer. Lung Cancer 2019, 134, 147–150. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Gao, L.; Jiang, X.; Fu, R.; Zhang, T.; Ren, T.; Hu, P.; Wu, Y.; Zhao, P.; et al. Clinical Significance of Circulating Tumor Cells and Tumor Markers in the Diagnosis of Lung Cancer. Cancer Med. 2019, 8, 3782–3792. [Google Scholar] [CrossRef]
- Mego, M.; Karaba, M.; Minarik, G.; Benca, J.; Silvia, J.; Sedlackova, T.; Manasova, D.; Kalavska, K.; Pindak, D.; Cristofanilli, M.; et al. Circulating Tumor Cells with Epithelial–to–Mesenchymal Transition Phenotypes Associated with Inferior Outcomes in Primary Breast Cancer. Anticancer Res. 2019, 39, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.G.; Metcalf, R.L.; Carter, L.; Brady, G.; Blackhall, F.H.; Dive, C. Molecular Analysis of Circulating Tumour Cells—Biology and Biomarkers. Nat. Rev. Clin. Oncol. 2014, 11, 129–144. [Google Scholar] [CrossRef]
- Bork, U.; Rahbari, N.N.; Schölch, S.; Reissfelder, C.; Kahlert, C.; Büchler, M.W.; Weitz, J.; Koch, M. Circulating Tumour Cells and Outcome in Non-Metastatic Colorectal Cancer: A Prospective Study. Br. J. Cancer 2015, 112, 1306–1313. [Google Scholar] [CrossRef]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.J.; Uhr, J.W.; Terstappen, L.W.M.M. Tumor Cells Circulate in the Peripheral Blood of All Major Carcinomas but Not in Healthy Subjects or Patients with Nonmalignant Diseases. Clin. Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef]
- Ciccioli, M.; Kim, K.; Khazan, N.; Khoury, J.D.; Cooke, M.J.; Miller, M.C.; O’Shannessy, D.J.; Pailhes-Jimenez, A.-S.; Moore, R.G. Identification of Circulating Tumor Cells Captured by the FDA-Cleared Parsortix® PC1 System from the Peripheral Blood of Metastatic Breast Cancer Patients Using Immunofluorescence and Cytopathological Evaluations. J. Exp. Clin. Cancer Res. 2024, 43, 240. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Leslie, M.; Kameyama, H.; Volk, D.; Tanaka, T. Aptamer Therapeutics in Cancer: Current and Future. Cancers 2018, 10, 80. [Google Scholar] [CrossRef]
- Ohuchi, S. Cell-SELEX Technology. BioRes. Open Access 2012, 1, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, X.; Gao, H.; Chen, J.; Jiang, H. Progress in Aptamer Research and Future Applications. ChemistryOpen 2025, 14, e202400463. [Google Scholar] [CrossRef]
- Zamay, G.S.; Kolovskaya, O.S.; Zamay, T.N.; Glazyrin, Y.E.; Krat, A.V.; Zubkova, O.; Spivak, E.; Wehbe, M.; Gargaun, A.; Muharemagic, D.; et al. Aptamers Selected to Postoperative Lung Adenocarcinoma Detect Circulating Tumor Cells in Human Blood. Mol. Ther. 2015, 23, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Zamay, G.S.; Ivanchenko, T.I.; Zamay, T.N.; Grigorieva, V.L.; Glazyrin, Y.E.; Kolovskaya, O.S.; Garanzha, I.V.; Barinov, A.A.; Krat, A.V.; Mironov, G.G.; et al. DNA Aptamers for the Characterization of Histological Structure of Lung Adenocarcinoma. Mol. Ther. Nucleic Acids 2017, 6, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Evangelisti, C.; Paganelli, F.; Giuntini, G.; Mattioli, E.; Cappellini, A.; Ramazzotti, G.; Faenza, I.; Maltarello, M.; Martelli, A.; Scotlandi, K.; et al. Lamin A and Prelamin A Counteract Migration of Osteosarcoma Cells. Cells 2020, 9, 774. [Google Scholar] [CrossRef]
- Chen, Z.; Fang, Z.; Ma, J. Regulatory Mechanisms and Clinical Significance of Vimentin in Breast Cancer. Biomed. Pharmacother. 2021, 133, 111068. [Google Scholar] [CrossRef]
- Berr, A.L.; Wiese, K.; Dos Santos, G.; Koch, C.M.; Anekalla, K.R.; Kidd, M.; Davis, J.M.; Cheng, Y.; Hu, Y.-S.; Ridge, K.M. Vimentin Is Required for Tumor Progression and Metastasis in a Mouse Model of Non–Small Cell Lung Cancer. Oncogene 2023, 42, 2074–2087. [Google Scholar] [CrossRef]
- Adyns, L.; Proost, P.; Struyf, S. Role of Defensins in Tumor Biology. Int. J. Mol. Sci. 2023, 24, 5268. [Google Scholar] [CrossRef]
- Lopes, D.; Maiato, H. The Tubulin Code in Mitosis and Cancer. Cells 2020, 9, 2356. [Google Scholar] [CrossRef]
- Kim, Y.; Jang, H.H. The Role of Peroxiredoxin Family in Cancer Signaling. J. Cancer Prev. 2019, 24, 65–71. [Google Scholar] [CrossRef]
- Peng, L.; Xiong, Y.; Wang, R.; Xiang, L.; Zhou, H.; Fu, Z. The Critical Role of Peroxiredoxin-2 in Colon Cancer Stem Cells. Aging 2021, 13, 11170–11187. [Google Scholar] [CrossRef] [PubMed]

| Aptamer Name | Aptamer Sequence |
|---|---|
| LC-17 | 5′-CTC CTC TGA CTG TAA CCA CGC TTT TGT CTT TAG CCG AAT TTT ACT AAG CCG GGC TGA TCA GCA TAG GTA GTC CAG AAG CC-3′ |
| LC-18 | 5′-CTC CTC TGA CTG TAA CCA CGT GCC CGA ACG CGA GTT GAG TTC CGA GAG CTC CGA CTT CTT GCA TAG GTA GTC CAG AAG CC-3′ |
| Target Protein | CTCs | Lung Cancer Cell Culture | Healthy Donor Blood |
|---|---|---|---|
| Neutrophil defensin 1 | Present | Present | Absent |
| Peroxiredoxin-2 | Present | Absent | Absent |
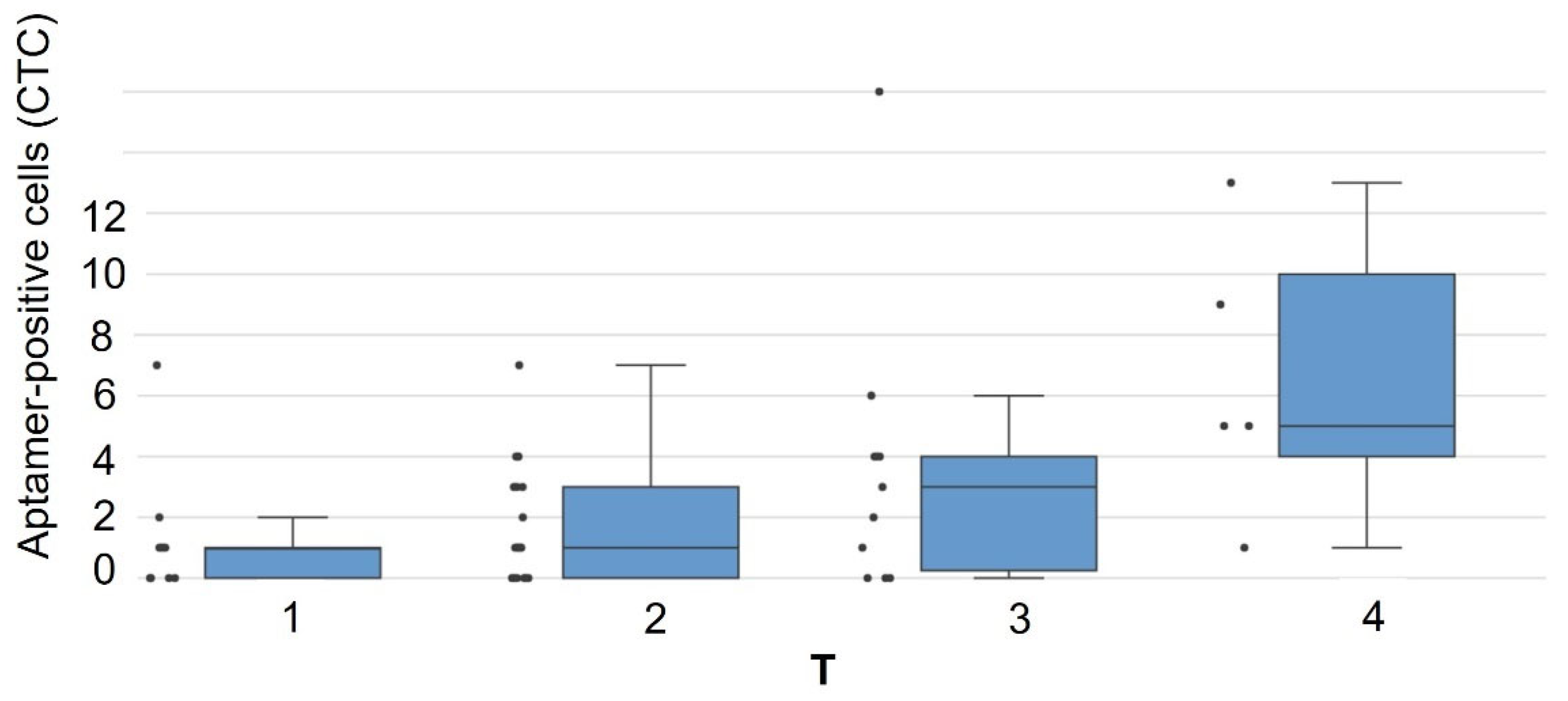
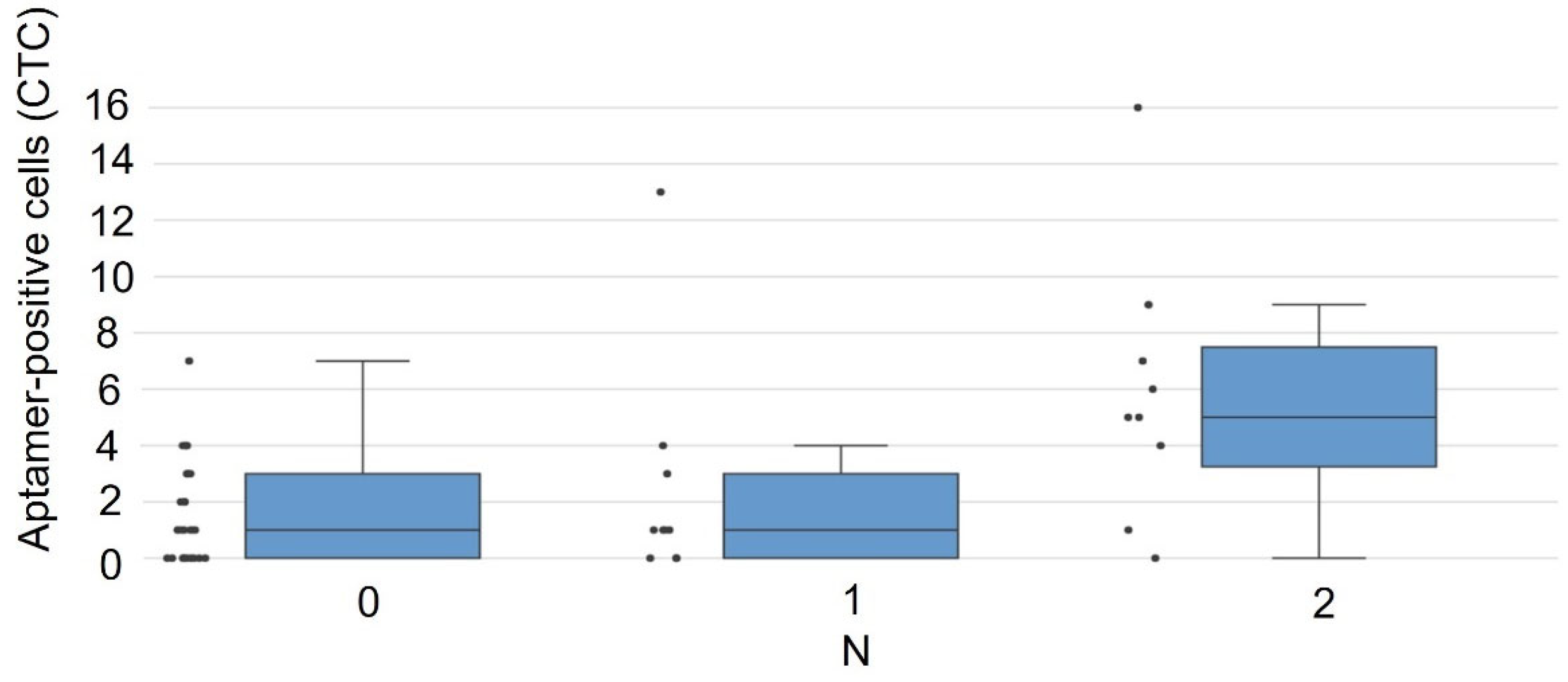
| № | Histological Type of LC | Stage | TNM | Patient Survival Duration, Months | Number of CTCs, Units in 4 mL of Blood |
|---|---|---|---|---|---|
| 1 | Squamous cell lung cancer | IB | T2N0M0 | 105 | 0 |
| 2 | Pulmonary adenocarcinoma | IV | T3N1M1 | 10 | 0 |
| 3 | Pulmonary adenocarcinoma | IA | T1N0M0 | 93 | 0 |
| 4 | Squamous cell lung cancer | IIB | T3N0M0 | 17 | 0 |
| 5 | Squamous cell lung cancer | IIIB | T3N2M0 | 2 | 0 |
| 6 | Squamous cell lung cancer | IIB | T2N1M0 | 67 | 0 |
| 7 | Pulmonary adenocarcinoma | IIA | T2N0M0 | 6 | 0 |
| 8 | Squamous cell lung cancer | IIB | T2N1M0 | 33 | 0 |
| 9 | Pulmonary adenocarcinoma | IIA | T2N0M0 | Alive | 0 |
| 10 | Pulmonary adenocarcinoma | IA | T1N0M0 | Alive | 0 |
| 11 | Pulmonary adenocarcinoma | IIA | T2N0M0 | 16 | 0 |
| 12 | Pulmonary adenocarcinoma | IIIB | T4N2M0 | 82 | 0 |
| 13 | Squamous cell lung cancer | IA | T1N0M0 | 20 | 1 |
| 14 | Small cell lung cancer | IV | T2N0M1 | 19 | 1 |
| 15 | Pulmonary adenocarcinoma | IV | T2N2M1 | Alive | 1 |
| 16 | Pulmonary adenocarcinoma | IB | T2N0M0 | 105 | 1 |
| 17 | Pulmonary adenocarcinoma | IIA | T2N0M0 | 22 | 1 |
| 18 | Pulmonary adenocarcinoma | IIA | T2N0M0 | 32 | 1 |
| 19 | Adenosquamous lung carcinoma | IIA | T2N0M0 | 48 | 6 |
| 20 | Pulmonary adenocarcinoma | IIA | T2N0M0 | 6 | 1 |
| 21 | Squamous cell lung cancer | IA | T1N0M0 | 11 | 1 |
| 22 | Pulmonary adenocarcinoma | IA | T1N0M0 | Alive | 1 |
| 23 | Pulmonary adenocarcinoma | IIA | T2N0M0 | Alive | 1 |
| 24 | Pulmonary adenocarcinoma | IIIA | T2N2M0 | 67 | 1 |
| 25 | Pulmonary adenocarcinoma | IIA | T2N0M0 | 4 | 1 |
| 26 | Pulmonary adenocarcinoma | IA | T1N0M0 | Alive | 2 |
| 27 | Squamous cell lung cancer | III A | T3N0M0 | 133 | 2 |
| 28 | Squamous cell lung cancer | III A | T3N0M0 | 10 | 2 |
| 29 | Pulmonary adenocarcinoma | IIB | T2N2M0 | 13 | 3 |
| 30 | Squamous cell lung cancer | IIIB | T3N2M0 | 9 | 3 |
| 31 | Pulmonary adenocarcinoma | IIA | T2N0M0 | 1 | 3 |
| 32 | Squamous cell lung cancer | IIIB | T3N2M0 | 113 | 3 |
| 33 | Squamous cell lung cancer | IIB | T2N1M0 | 5 | 4 |
| 34 | Squamous cell lung cancer | IIA | T2N0M0 | 27 | 4 |
| 35 | Squamous cell lung cancer | IIIA | T3N0M0 | 59 | 4 |
| 36 | Pulmonary adenocarcinoma | IB | T2N0M0 | 43 | 4 |
| 37 | Pulmonary adenocarcinoma | IIIB | T4N2M0 | 113 | 4 |
| 38 | Squamous cell lung cancer | IV | T4N2M1 | 3 | 5 |
| 39 | Squamous cell lung cancer | IIIA | T2N2M0 | 6 | 5 |
| 40 | Pulmonary adenocarcinoma | IA | T1N0M0 | 2 | 6 |
| 41 | Pulmonary adenocarcinoma | IIIA | T2N2M0 | 62 | 7 |
| 42 | Squamous cell lung cancer | IIIB | T4N2M0 | 64 | 7 |
| 43 | Squamous cell lung cancer | IIIB | T4N1M0 | 15 | 9 |
| LC-17 | LC-18 |
|---|---|
| Neutrophil defensin | Vimentin |
| Tubulin | Lamin A/C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krat, A.V.; Zamay, G.S.; Veprintsev, D.V.; Kirichenko, D.A.; Kolovskaya, O.S.; Zamay, T.N.; Glazyrin, Y.E.; Minic, Z.; Sidorov, S.A.; Komissarova, V.A.; et al. Targeting CTC Heterogeneity: Aptamer-Based Liquid Biopsy Predicts Outcome in Lung Cancer. Cancers 2025, 17, 3244. https://doi.org/10.3390/cancers17193244
Krat AV, Zamay GS, Veprintsev DV, Kirichenko DA, Kolovskaya OS, Zamay TN, Glazyrin YE, Minic Z, Sidorov SA, Komissarova VA, et al. Targeting CTC Heterogeneity: Aptamer-Based Liquid Biopsy Predicts Outcome in Lung Cancer. Cancers. 2025; 17(19):3244. https://doi.org/10.3390/cancers17193244
Chicago/Turabian StyleKrat, Alexey V., Galina S. Zamay, Dmitry V. Veprintsev, Daria A. Kirichenko, Olga S. Kolovskaya, Tatiana N. Zamay, Yury E. Glazyrin, Zoran Minic, Semen A. Sidorov, Valeria A. Komissarova, and et al. 2025. "Targeting CTC Heterogeneity: Aptamer-Based Liquid Biopsy Predicts Outcome in Lung Cancer" Cancers 17, no. 19: 3244. https://doi.org/10.3390/cancers17193244
APA StyleKrat, A. V., Zamay, G. S., Veprintsev, D. V., Kirichenko, D. A., Kolovskaya, O. S., Zamay, T. N., Glazyrin, Y. E., Minic, Z., Sidorov, S. A., Komissarova, V. A., Zukov, R. A., Berezovski, M. V., & Kichkailo, A. S. (2025). Targeting CTC Heterogeneity: Aptamer-Based Liquid Biopsy Predicts Outcome in Lung Cancer. Cancers, 17(19), 3244. https://doi.org/10.3390/cancers17193244









