Clinical Utility of Amino Acid PET-MRI in Children with CNS Neoplasms: A Territory-Wide Study from Hong Kong
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Imaging Methods and Analysis
2.3. Statistical Analysis
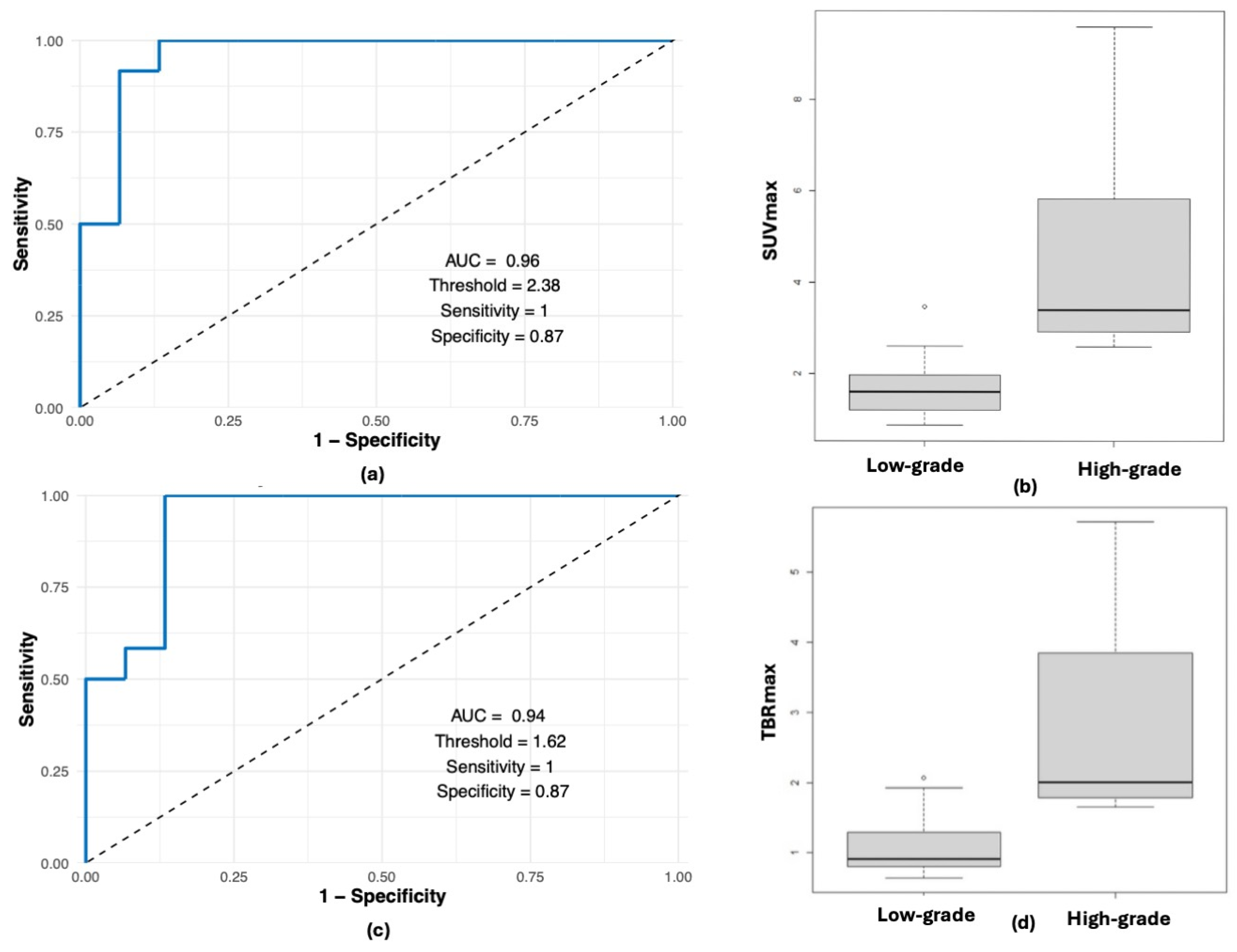
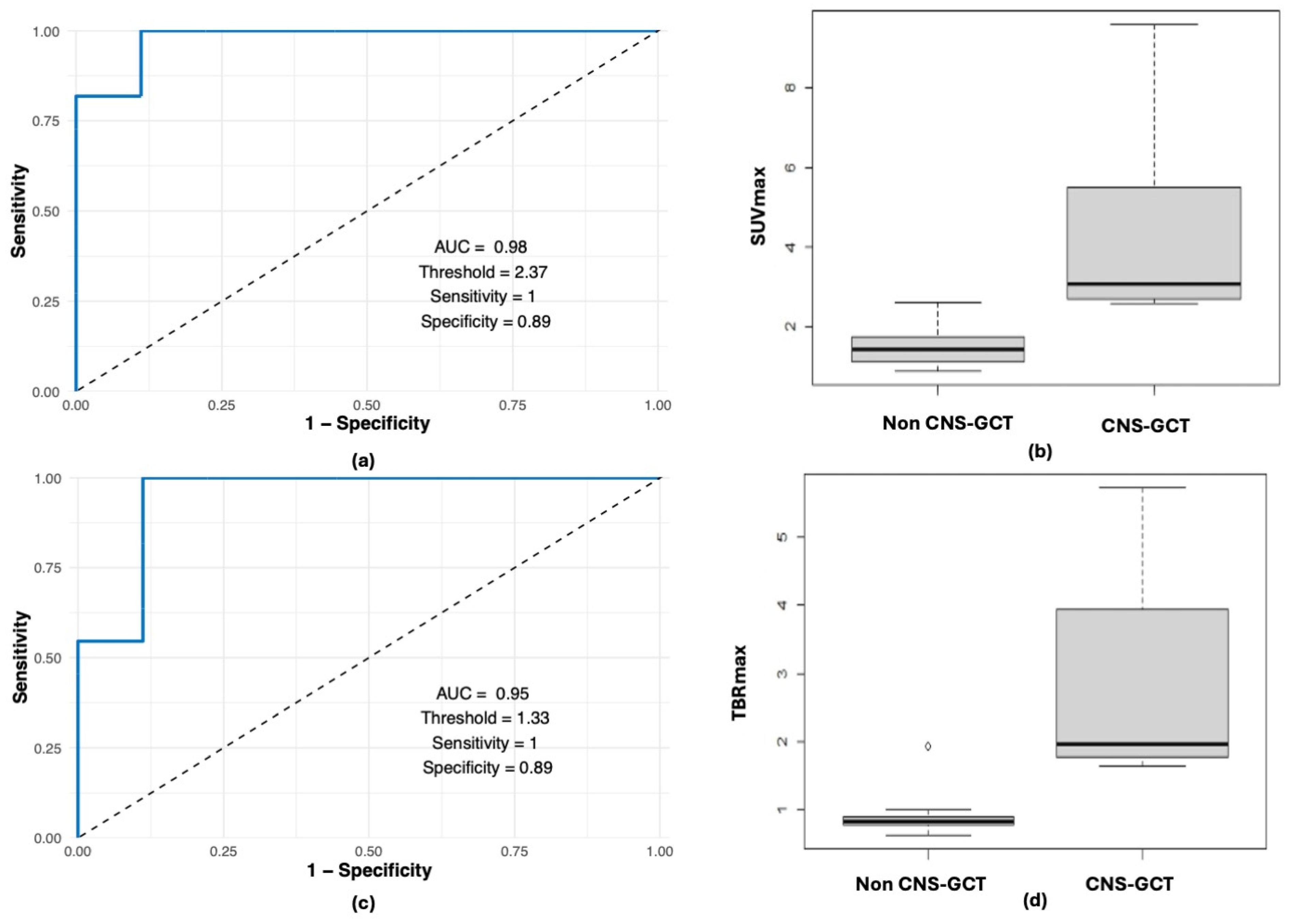
3. Results
3.1. Patient Demographics and Clinical Characteristics
3.2. PET-MRI Findings
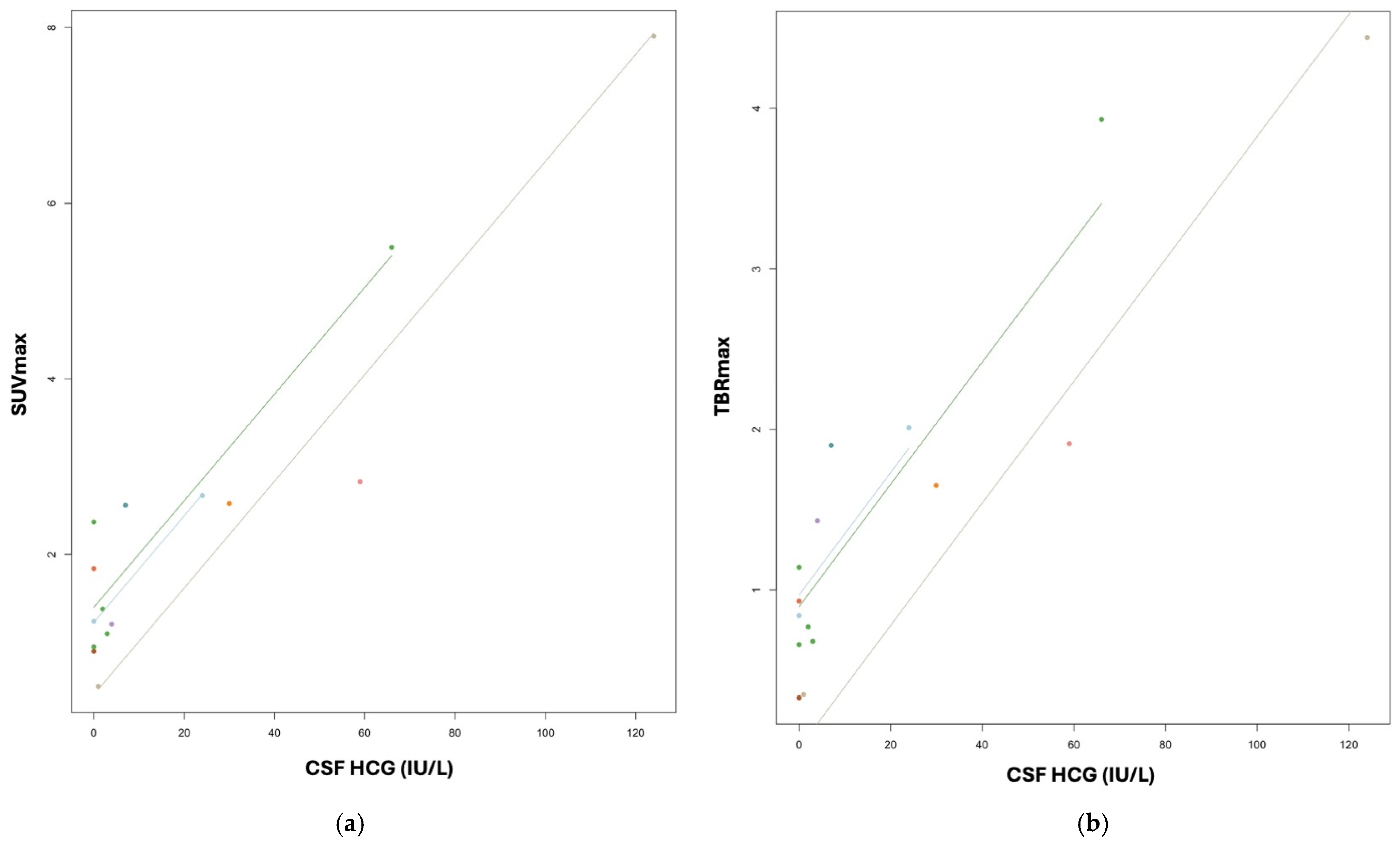
3.3. CNS-GCT
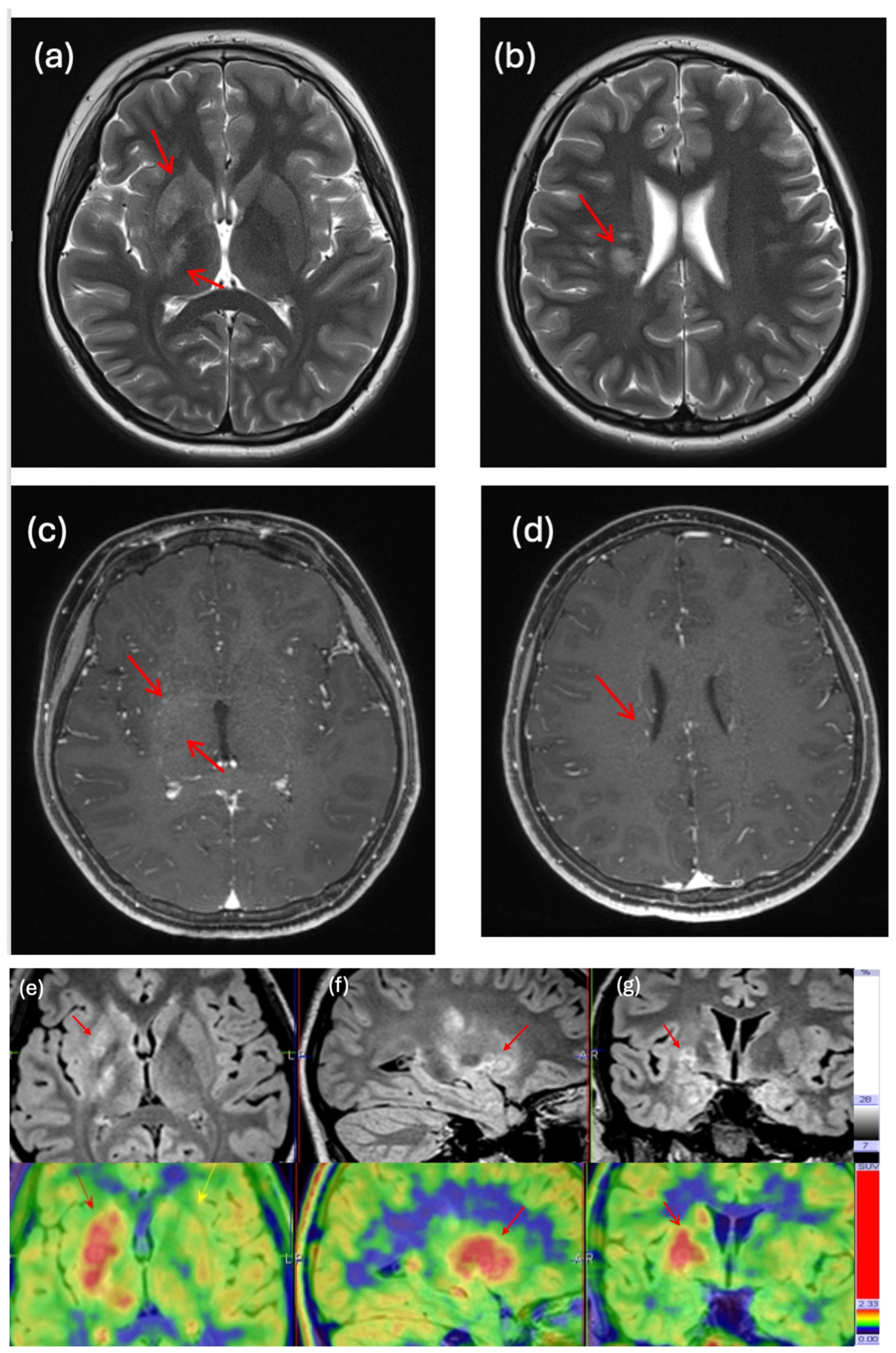
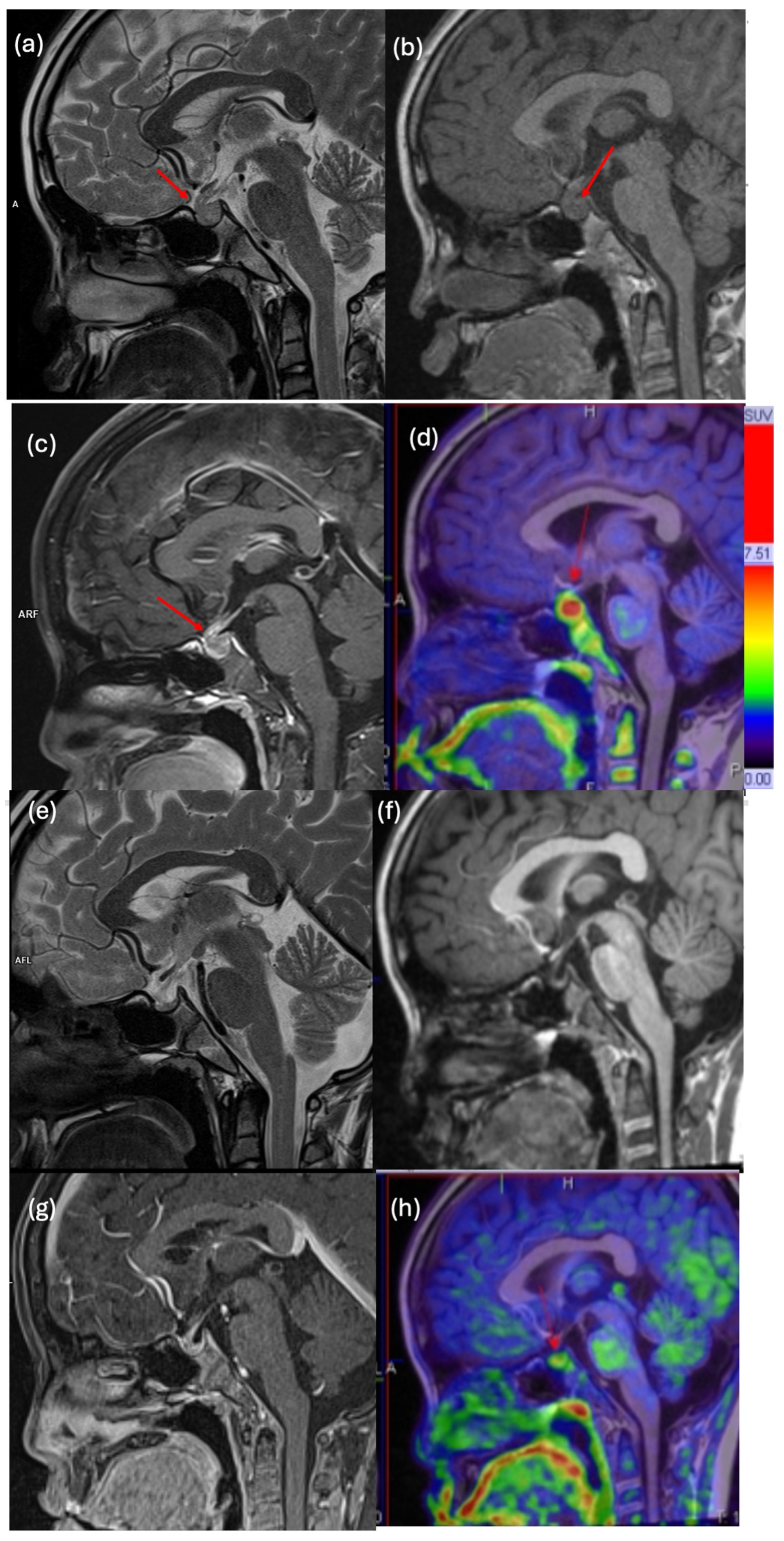
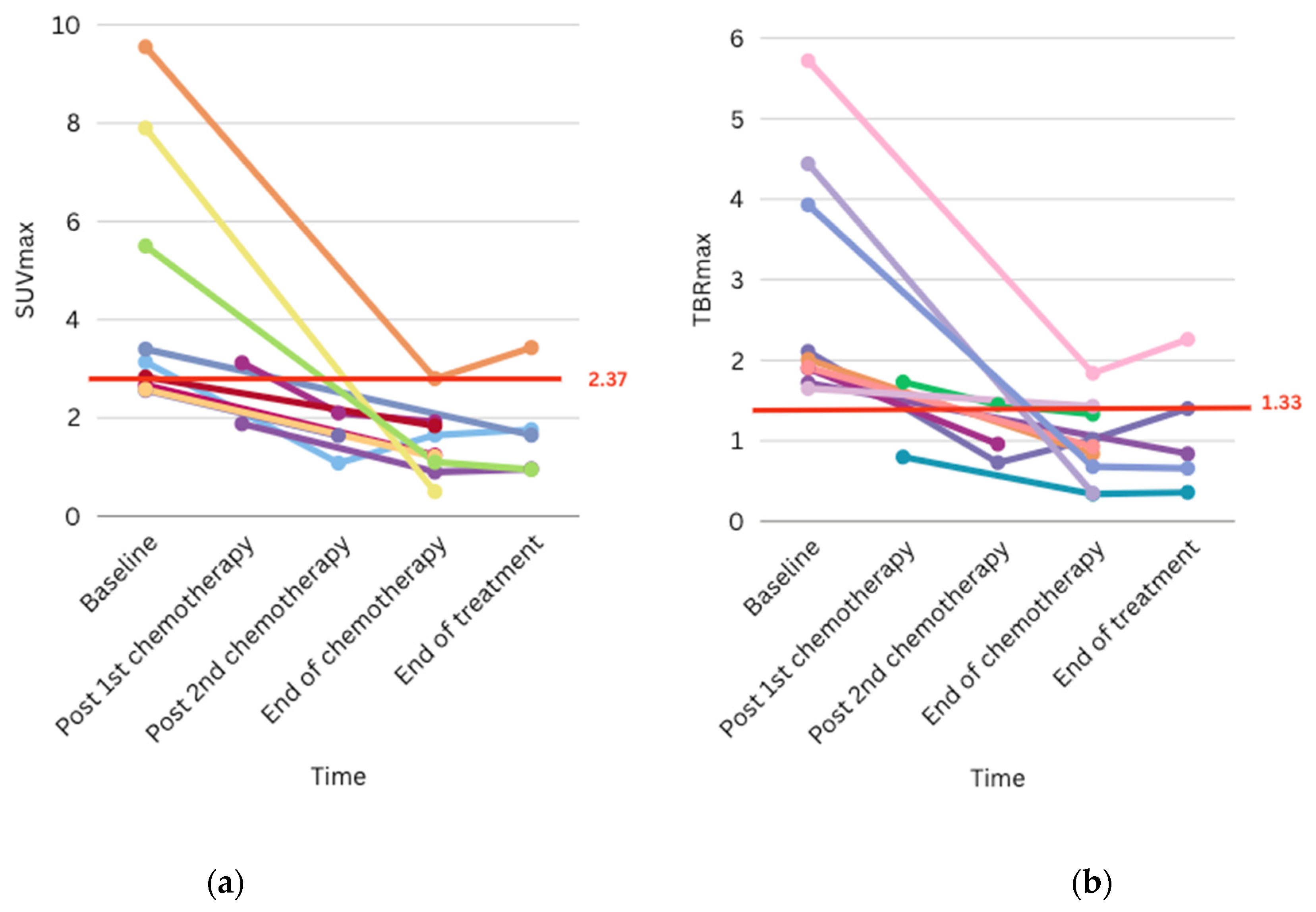
3.4. Clinical Impact and Illustrative Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFP | Alpha fetoprotein |
| ASCT2 | Alanine-serine-cysteine transporters |
| AUC | Area under the curve |
| AutoHSCT | Autologous haematopoietic stem cell transplantation |
| C | Carboplatin |
| CI | Confidence interval |
| CMR | Complete metabolic response |
| CNS | Central nervous system |
| CNS-GCT | Central nervous system germ cell tumour |
| CP angle | Cerebellopontine angle |
| CR | Complete remission |
| CSI | Craniospinal irradiation |
| CSF | Cerebrospinal fluid |
| DOPA | Fluorodopa |
| DTI | Diffusion tensor imaging |
| DWI | Diffusion-weighted imaging |
| Dx | Diagnosis |
| EOC | End of chemotherapy |
| EOT | End-of-treatment evaluation |
| FDG | Fluorodeoxyglucose |
| FET | 18-fluoroethyl-L-tyrosine |
| FLAIR | Fluid-attenuated inversion recovery |
| GBM | Glioblastoma |
| HCG | Beta human chorionic gonadotrophin |
| Ifo | Ifosfamide |
| LAT1 | L-type amino acid transporters |
| LCH | Langerhans cell histiocytosis |
| M | Metastatic disease |
| M0 | No metastatic disease |
| MET | C-11 methionine |
| MRI | Magnetic resonance imaging |
| MRS | MR spectroscopy |
| n | Number of patients |
| N | Number of scans |
| NA | Not available |
| NGGCT | Non-germinomatous germ cell tumour |
| PET-CT | Positron emission tomography–computer tomography |
| PET-MRI | Positron emission tomography–magnetic resonance imaging |
| PB | Involved field boost |
| ROC | Receiver operating characteristic curve |
| ROI | Region of interest |
| SWI | Susceptibility-weighted imaging |
| SUVmax | Maximal standardized uptake values |
| TBRmax | Tumour-to-background SUVmax ratios |
| VOI | Volume of interest |
| VP | Etoposide |
| WVI | Whole-ventricular irradiation |
References
- Erker, C.; Tamrazi, B.; Poussaint, T.Y.; Mueller, S.; Mata-Mbemba, D.; Franceschi, E.; Brandes, A.A.; Rao, A.; Haworth, K.B.; Wen, P.Y.; et al. Response assessment in paediatric high-grade glioma: Recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 2020, 21, e317–e329. [Google Scholar] [CrossRef]
- Albert, N.L.; Galldiks, N.; Ellingson, B.M.; Bent, M.J.v.D.; Chang, S.M.; Cicone, F.; de Groot, J.; Koh, E.-S.; Law, I.; Le Rhun, E.; et al. PET-based response assessment criteria for diffuse gliomas (PET RANO 1.0): A report of the RANO group. Lancet Oncol. 2024, 25, e29–e41. [Google Scholar] [CrossRef] [PubMed]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; la Fougère, C.; Langen, K.-J.; Lopci, E.; Lowe, V.; et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: Version 1.0. Eur. J. Nucl. Med. 2018, 46, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Verburg, N.; Koopman, T.; Yaqub, M.M.; Hoekstra, O.S.; Lammertsma, A.A.; Barkhof, F.; Pouwels, P.J.W.; Reijneveld, J.C.; Heimans, J.J.; Rozemuller, A.J.M.; et al. Improved detection of diffuse glioma infiltration with imaging combinations: A diagnostic accuracy study. Neuro-Oncology 2019, 22, 412–422. [Google Scholar] [CrossRef]
- Kadali, K.R.; Nierobisch, N.; Maibach, F.; Heesen, P.; Alcaide-Leon, P.; Hüllner, M.; Weller, M.; Kulcsar, Z.; Hainc, N. An effective MRI perfusion threshold based workflow to triage additional 18F-FET PET in posttreatment high grade glioma. Sci. Rep. 2025, 15, 7749. [Google Scholar] [CrossRef]
- Galldiks, N.; Niyazi, M.; Grosu, A.L.; Kocher, M.; Langen, K.-J.; Law, I.; Minniti, G.; Kim, M.M.; Tsien, C.; Dhermain, F.; et al. Contribution of PET imaging to radiotherapy planning and monitoring in glioma patients—A report of the PET/RANO group. Neuro-Oncology 2021, 23, 881–893. [Google Scholar] [CrossRef]
- Rosenschold, P.M.A.; Costa, J.; Engelholm, S.A.; Lundemann, M.J.; Law, I.; Ohlhues, L. Impact of [18F]-fluoro-ethyl-tyrosine PET imaging on target definition for radiation therapy of high-grade glioma. Neuro-Oncology 2014, 17, 757–763. [Google Scholar] [CrossRef]
- Albert, N.L.; Weller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.M.; Fougère, C.l.; Pope, W.; Law, I.; Arbizu, J.; et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-Oncology 2016, 18, 1199–1208. [Google Scholar] [CrossRef]
- Lam, J.; Lo, S.; Siu, D.; Cheng, P. Clinical Applications of Amino Acid Positron Emission Tomography–Magnetic Resonance Imaging in Neuro-Oncology: A Pictorial Essay. Hong Kong J. Radiol. 2025, 28, e128–e140. [Google Scholar] [CrossRef]
- Juhász, C.; Dwivedi, S.; Kamson, D.O.; Michelhaugh, S.K.; Mittal, S. Comparison of Amino Acid Positron Emission Tomographic Radiotracers for Molecular Imaging of Primary and Metastatic Brain Tumors. Mol. Imaging 2014, 13, 7290-2014. [Google Scholar] [CrossRef] [PubMed]
- Stöber, B.; Tanase, U.; Herz, M.; Seidl, C.; Schwaiger, M.; Senekowitsch-Schmidtke, R. Differentiation of tumour and inflammation: Characterisation of [methyl-3H]methionine (MET) and O-(2-[18F]fluoroethyl)-L-tyrosine (FET) uptake in human tumour and inflammatory cells. Eur. J. Nucl. Med. 2006, 33, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Pirotte, B.J.M.; Lubansu, A.; Massager, N.; Wikler, D.; Van Bogaert, P.; Levivier, M.; Brotchi, J.; Goldman, S. Clinical impact of integrating positron emission tomography during surgery in 85 children with brain tumors. J. Neurosurg. Pediatr. 2010, 5, 486–499. [Google Scholar] [CrossRef]
- Kertels, O.; Krauß, J.; Monoranu, C.M.; Samnick, S.; Dierks, A.; Kircher, M.; Mihovilovic, M.I.; Pham, M.; Buck, A.K.; Eyrich, M.; et al. [18F]FET-PET in children and adolescents with central nervous system tumors: Does it support difficult clinical decision-making? Eur. J. Nucl. Med. 2023, 50, 1699–1708. [Google Scholar] [CrossRef]
- Piccardo, A.; Tortora, D.; Mascelli, S.; Severino, M.; Piatelli, G.; Consales, A.; Pescetto, M.; Biassoni, V.; Schiavello, E.; Massollo, M.; et al. Advanced MR imaging and 18F-DOPA PET characteristics of H3K27M-mutant and wild-type pediatric diffuse midline gliomas. Eur. J. Nucl. Med. 2019, 46, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Morana, G.; Piccardo, A.; Puntoni, M.; Nozza, P.; Cama, A.; Raso, A.; Mascelli, S.; Massollo, M.; Milanaccio, C.; Garrè, M.L.; et al. Diagnostic and prognostic value of18F-DOPA PET and1H-MR spectroscopy in pediatric supratentorial infiltrative gliomas: A comparative study. Neuro-Oncology 2015, 17, 1637–1647. [Google Scholar] [CrossRef]
- Galldiks, N.; Kracht, L.W.; Berthold, F.; Miletic, H.; Klein, J.C.; Herholz, K.; Jacobs, A.H.; Heiss, W.D. [11C]-L-Methionine positron emission tomography in the management of children and young adults with brain tumors. J. Neuro-Oncol. 2010, 961, 231–239. [Google Scholar] [CrossRef]
- Dunkl, V.; Cleff, C.; Stoffels, G.; Judov, N.; Sarikaya-Seiwert, S.; Law, I.; Bøgeskov, L.; Nysom, K.; Andersen, S.B.; Steiger, H.-J.; et al. The Usefulness of Dynamic O-(2-18F-Fluoroethyl)-l-Tyrosine PET in the Clinical Evaluation of Brain Tumors in Children and Adolescents. J. Nucl. Med. 2014, 56, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Pruis, I.J.; Verburg, F.A.; Balvers, R.K.; Harteveld, A.A.; Feelders, R.A.; Vernooij, M.W.; Smits, M.; Neggers, S.J.; van Zanten, S.E.V. [18F]FET PET/MRI: An Accurate Technique for Detection of Small Functional Pituitary Tumors. J. Nucl. Med. 2024, 65, 688–692. [Google Scholar] [CrossRef]
- Pirotte, B.; Acerbi, F.; Lubansu, A.; Goldman, S.; Brotchi, J.; Levivier, M. PET imaging in the surgical management of pediatric brain tumors. Child’s Nerv. Syst. 2007, 23, 739–751. [Google Scholar] [CrossRef]
- de Zwart, P.L.; van Dijken, B.R.; Holtman, G.A.; Stormezand, G.N.; Dierckx, R.A.; van Laar, P.J.; van der Hoorn, A. Diagnostic Accuracy of PET Tracers for the Differentiation of Tumor Progression from Treatment-Related Changes in High-Grade Glioma: A Systematic Review and Metaanalysis. J. Nucl. Med. 2020, 61, 498–504. [Google Scholar] [CrossRef]
- Mair, M.J.; Werner, J.-M.; Weller, J.; Barci, E.; Katzendobler, S.; Isakaj, J.; Berchtold, L.; Aras, T.; Stürzl, R.; Hennenberg, J.; et al. Prognostic stratification of newly diagnosed IDH-mutant gliomas by [18F]fluoroethyltyrosine and [11C]methionine PET—A retrospective, bicentric cohort study. Neuro-Oncology 2025, noaf196. [Google Scholar] [CrossRef]
- Marner, L.; Lundemann, M.; Sehested, A.; Nysom, K.; Borgwardt, L.; Mathiasen, R.; Wehner, P.S.; Henriksen, O.M.; Thomsen, C.; Skjøth-Rasmussen, J.; et al. Diagnostic accuracy and clinical impact of [18F]FET PET in childhood CNS tumors. Neuro-Oncology 2021, 23, 2107–2116. [Google Scholar] [CrossRef] [PubMed]
- Gauvain, K.; Ponisio, M.R.; Barone, A.; Grimaldi, M.; Parent, E.; Leeds, H.; Goyal, M.; Rubin, J.; McConathy, J. 18F-FDOPA PET/MRI for monitoring early response to bevacizumab in children with recurrent brain tumors. Neuro-Oncol. Pract. 2017, 5, 28–36. [Google Scholar] [CrossRef]
- Marner, L.; Nysom, K.; Sehested, A.; Borgwardt, L.; Mathiasen, R.; Henriksen, O.M.; Lundemann, M.; Rosenschöld, P.M.A.; Thomsen, C.; Bøgeskov, L.; et al. Early Postoperative18F-FET PET/MRI for Pediatric Brain and Spinal Cord Tumors. J. Nucl. Med. 2019, 60, 1053–1058. [Google Scholar] [CrossRef]
- Lohmann, P.; Stavrinou, P.; Lipke, K.; Bauer, E.K.; Ceccon, G.; Werner, J.-M.; Neumaier, B.; Fink, G.R.; Shah, N.J.; Langen, K.-J.; et al. FET PET reveals considerable spatial differences in tumour burden compared to conventional MRI in newly diagnosed glioblastoma. Eur. J. Nucl. Med. 2018, 46, 591–602. [Google Scholar] [CrossRef]
- Lucas, J.T.; Serrano, N.; Kim, H.; Li, X.; Snyder, S.E.; Hwang, S.; Li, Y.; Hua, C.-H.; Broniscer, A.; Merchant, T.E.; et al. 11C-Methionine positron emission tomography delineates non-contrast enhancing tumor regions at high risk for recurrence in pediatric high-grade glioma. J. Neuro-Oncology 2017, 132, 163–170. [Google Scholar] [CrossRef]
- Morana, G.; Piccardo, A.; Milanaccio, C.; Puntoni, M.; Nozza, P.; Cama, A.; Zefiro, D.; Cabria, M.; Rossi, A.; Garrè, M.L. Value of 18F-3,4-Dihydroxyphenylalanine PET/MR Image Fusion in Pediatric Supratentorial Infiltrative Astrocytomas: A Prospective Pilot Study. J. Nucl. Med. 2014, 55, 718–723. [Google Scholar] [CrossRef]
- Park, Y.-J.; Lee, J.W.; Cho, H.W.; Choe, Y.S.; Lee, K.-H.; Choi, J.Y.; Sung, K.W.; Moon, S.H. Value of C-11 methionine PET/CT in patients with intracranial germinoma. PLoS ONE 2022, 17, e0263690. [Google Scholar] [CrossRef]
- Fujii, Y.; Saito, Y.; Ogawa, T.; Fujii, S.; Kamitani, H.; Kondo, S.; Horie, Y.; Togawa, M.; Senda, M.; Maegaki, Y.; et al. Basal ganglia germinoma: Diagnostic value of MR spectroscopy and 11C-methionine positron emission tomography. J. Neurol. Sci. 2008, 270, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Okochi, Y.; Nihashi, T.; Fujii, M.; Kato, K.; Okada, Y.; Ando, Y.; Maesawa, S.; Takebayashi, S.; Wakabayashi, T.; Naganawa, S. Clinical use of 11C-methionine and 18F-FDG-PET for germinoma in central nervous system. Ann. Nucl. Med. 2013, 28, 94–102. [Google Scholar] [CrossRef]
- Lee, J.; Lee, B.L.; Yoo, K.H.; Sung, K.W.; Koo, H.H.; Lee, S.J.; Choi, J.Y.; Lee, K.-H.; Lee, J.I.; Shin, H.-J.; et al. Atypical basal ganglia germinoma presenting as cerebral hemiatrophy: Diagnosis and follow-up with 11C-methionine positron emission tomography. Child’s Nerv. Syst. 2008, 25, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kawai, N.; Miyake, K.; Yamamoto, Y.; Nishiyama, Y.; Maeda, Y.; Kageji, T.; Tamiya, T. Use of 11C-methionine positron emission tomography in basal germinoma: Assessment of treatment response and residual tumor. Child’s Nerv. Syst. 2009, 25, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Misch, M.; Guggemos, A.; Driever, P.H.; Koch, A.; Grosse, F.; Steffen, I.G.; Plotkin, M.; Thomale, U.-W. 18F-FET-PET guided surgical biopsy and resection in children and adolescence with brain tumors. Child’s Nerv. Syst. 2014, 31, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Grosse, F.; Wedel, F.; Thomale, U.-W.; Steffen, I.; Koch, A.; Brenner, W.; Plotkin, M.; Driever, P.H. Benefit of Static FET PET in Pretreated Pediatric Brain Tumor Patients with Equivocal Conventional MRI Results. Klin. Padiatr. 2021, 233, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.; Werner, J.-M.; Ceccon, G.S.; Rosen, E.K.; Wollring, M.M.; Stetter, I.; Lohmann, P.; Mottaghy, F.M.; Marner, L.; Law, I.; et al. Diagnosis of treatment-related changes in children and adolescents with brain and spinal tumors: A cost-effectiveness analysis using MRI and [18 F]FET PET. Eur. J. Nucl. Med. 2025, 1–11. [Google Scholar] [CrossRef]
- Kawai, N.; Miyake, K.; Nishiyama, Y.; Yamamoto, Y.; Miki, A.; Haba, R.; Imai, T.; Tamiya, T.; Nagao, S. Targeting optimal biopsy location in basal ganglia germinoma using 11C-methionine positron emission tomography. Surg. Neurol. 2008, 70, 408–413. [Google Scholar] [CrossRef]
- Chung, J.-K.; Kim, Y.; Kim, S.-K.; Lee, Y.; Paek, S.; Yeo, J.; Jeong, J.; Lee, D.; Jung, H.; Lee, M. Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo- or isometabolic on 18F-FDG PET. Eur. J. Nucl. Med. 2001, 29, 176–182. [Google Scholar] [CrossRef]
- Sato, N.; Suzuki, M.; Kuwata, N.; Kuroda, K.; Wada, T.; Beppu, T.; Sera, K.; Sasaki, T.; Ogawa, A. Evaluation of the malignancy of glioma using 11 C-methionine positron emission tomography and proliferating cell nuclear antigen staining. Neurosurg. Rev. 1999, 22, 210–214. [Google Scholar] [CrossRef]
| Paper | Modality of PET | Tracer | Patient Number (Age) | Diagnosis | Indication of Scan | Conclusion |
|---|---|---|---|---|---|---|
| Pirotte et al., 2007 [19] | PET-CT | FDG, MET | 126 patients | Brain tumour | Provide additional information as MRI was inconclusive; select biopsy target; delineate tissue for resection | PET images helped with surgical management at diagnostic, surgical, and postoperative steps |
| Lee et al., 2008 [31] | PET-CT | MET | 3 (9, 10, and 14 years) | Germinoma (basal ganglia) | Facilitate diagnosis and monitor treatment response | MET PET images helped with diagnosis and disease evaluation |
| Kawai et al., 2009 [32] | PET-CT | MET | 3 (9, 17, and 18 years) | Germinoma (basal ganglia, thalamus) | Monitor for treatment response | MET PET helped with treatment response monitoring and identified lesions not visualized on MRI scan |
| Galldiks et al., 2010 [16] | PET-CT | MET | 39 (mean 15 ± 5 years) | Pilocytic astrocytoma, astrocytoma, glioblastoma, medulloblastoma, ependymoma, atypical teratoid rhabdoid tumour, desmoplastic infantile ganglioglioma, non-tumour lesions, etc. | Differentiate tumours and non-tumourous brain lesions | MET PET images can differentiate tumourous and non-tumourous brain lesions at a threshold of 1.48 but are unable to differentiate between high-grade and low-grade tumours |
| Pirotte et al., 2010 [12] | PET-CT | FDG, MET | 85 patients | Glioblastoma, anaplastic astrocytoma, ganglioglioma, oligodendroglioma, pilocytic astrocytoma, primitive neuroectodermal tumour, ependymoma, CNS-GCT, etc. | Plan for biopsy target when MRI unable to yield accurate information | PET-CT data influenced surgical decisions and procedures by differentiating indolent and active components, improving target selection and diagnostic yield of biopsies, providing prognostic information, reducing tissue amount needed for biopsy, delineating lesions better, guiding resection, improving detection of residual tumour, and avoiding unnecessary reoperation |
| Okochi et al., 2013 [30] | PET-CT | FDG, MET | 10 patients (mean 13.4 ± 7 years) | CNS-GCT | Diagnosis | MET is a good tracer for diagnosing CNS germinoma and is useful for planning biopsies; FDG on the other hand is unable to produce sufficient image contrast |
| Dunkl et al., 2014 [17] | PET-CT | FET | 48 patients (median 13 years) | Brain tumour | Diagnosis, treatment response, residual evaluation | FET PET helped with diagnosis, monitoring treatment response, and detecting residuals |
| Misch et al., 2015 [33] | PET-CT | FET | 26 patients (median 12 ± 6.6 years) | Astrocytoma, ependymoma, pilocytic astrocytoma, glioblastoma, ganglioglioma, medulloblastoma, dysembryoplastic neuroepithelial tumour, etc. | Surgical planning | FET PET helped with target selection with decent specificity and high sensitivity |
| Lucas Jr. et al., 2017 [26] | PET-CT | MET | 31 patients (median 10.5 years) | High-grade glioma | Prognosis | MET PET delineates regions at increased risk of recurrence and improves prognostic assessment and target definition for radiotherapy |
| Marner et al., 2019 [24] | PET-MRI | FET | 22 patients (mean 9.5 years) | Diffuse midline glioma, atypical teratoid rhabdoid tumour, CNS-GCT, ganglioglioma, neuroepithelial tumour, ependymoma, pilocytic astrocytoma, etc. | Postoperative assessment | FET PET-MRI, when supplemented with MRI, improves specificity for detecting residual tumours |
| Grosse et al., 2021 [34] | PET-CT | FET | 17 patients (median 12 years) | Medulloblastoma, low-grade glioma, high-grade glioma, CNS-GCT, choroid plexus tumour | Treatment response assessment | FET PET discriminated between residual/recurrent tumours and post-therapeutic changes |
| Park et al., 2022 [28] | PET-CT | MET | 21 patients (median 16 years) | CNS-GCT | Diagnosis and monitoring | MET PET is useful as a diagnostic tool for CNS-GCT; PET avidity also strongly correlates with pre-treatment serum HCG |
| Kertels et al., 2023 [13] | PET-CT | FET | 21 patients (mean 8.6 ± 5.2 years) | Glioblastoma, low-grade glioma, diffuse intrinsic pontine glioma, CNS-GCT, optic pathway glioma, primitive neuroectodermal tumour, pilocytic astrocytoma, ependymoma, etc. | Diagnosis confirmation, treatment planning | FET PET impacted patient management by avoidance of invasive surgery or biopsy, biopsy guidance, change in treatment, and confirmation of diagnosis |
| Rosen et al., 2025 [35] | PET-CT and PET-MRI | FET | 80 patients from studies by Marner et al. and Dunkl et al. | CNS neoplasms | Diagnosis of treatment-related changes (cost-effective analysis) | FET PET is cost-effective for identification of treatment-related changes in pre-treated CNS tumours in children |
| n = 37 | |
|---|---|
| Age (median, range, years) | 11.2 (0.6–17) |
| Sex (n, percentage) | |
| F | 15 (41%) |
| M | 22 (59%) |
| Grading (n, percentage) | |
| High-grade | 21 (56%) |
| Low-grade | 8 (22%) |
| Non-oncological | 8 (22%) |
| Diagnosis (n, percentage) | |
| CNS-GCT | 15 (40%) |
| Thickened pituitary stalk (non-oncological) | 7 (19%) |
| Low-grade glioma | 6 (16%) |
| High-grade glioma | 4 (11%) |
| Medulloblastoma | 2 (5.5%) |
| Langerhans cell histiocytosis | 2 (5.5%) |
| Demyelination | 1 (3%) |
| Site | |
| Pituitary/suprasellar | 18 (49%) |
| Pineal gland | 6 (16%) |
| Cerebellum | 5 (14%) |
| Basal ganglia | 4 (11%) |
| Brainstem | 3 (8%) |
| Ventricles | 3 (8%) |
| Frontal lobe | 2 (5%) |
| Cerebellopontine angle | 1 (3%) |
| Spine | 1 (3%) |
| Tracer (N = no of scans, percentage) | N = 63 |
| FET | 48 (76%) |
| MET | 15 (24%) |
| Indication of scan (N = no of scans, percentage) | N = 63 |
| Diagnosis | 26 (41%) |
| Monitor/response assessment | 30 (48%) |
| Evaluation of residual lesion or suspected relapse | 7 (11%) |
| No | Sex | Age | Diagnosis | Stage | CSF AFP | CSF HCG | Serum AFP | Serum HCG | Treatment | Outcome | Purpose | SUVmax | TBRmax | Follow Up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 17 | Bifocal germinoma (Pineal, suprasellar, multiventricular) | M | <3 | 66 | <3 | 1 | 4 cycles VP/C + WVI + PB | Relapse | Dx | 5.5 | 3.93 | 26 |
| 2 | M | 14 | Suprasellar germinoma | M0 | <3 | <1 | <3 | <2 | 4 cycles VP/C + WVI + PB | CR | Dx | 9.55 | 5.72 | 29 |
| 3 | M | 11 | Right basal ganglia germinoma | M0 | <2 | <1 | <3 | <1 | 4 cycles VP/C + WVI + PB | CR | Dx | 3 | 1.78 | 63 |
| 4 | M | 13 | Basal ganglia germinoma | M0 | 3 | 30 | 3 | 12 | 4 cycles VP/C + WVI + PB | CR | Dx | 2.58 | 1.65 | 34 |
| 5 | F | 9 | Bifocal germinoma (suprasellar, 4th ventricle) | M | 3 | 124 | 3 | 377 | 6 cycles C/VP/Ifo + CSI + WVI + PB | CR | Dx | 7.9 | 4.44 | 23 |
| 6 | M | 16 | Suprasellar germinoma | M0 | <3 | 1 | <3 | <1 | 4 cycles VP/C + WVI + PB | CR | Dx | 3.14 | 2.11 | 8 |
| 7 | F | 17 | Suprasellar germinoma | M0 | <1 | 33 | 1 | 3 | 4 cycles VP/C + WVI + PB | CR | Assess response (post 1st chemo) | 1.88 | 0.8 | 7 |
| 8 | F | 10 | Suprasellar germinoma | M0 | 3 | 148 | 3 | 14 | 6 cycles of C/VP/Ifo + WVI + PB | CR | Dx | 3.4 | 1.72 | 21 |
| 9 | M | 14 | Suprasellar germinoma | M0 | 3 | 7 | 3 | 2.1 | 4 cycles VP/C+ WVI + PB | CR | Rule out relapse | 2.3 | 1.53 | 22 |
| 10 | M | 8 | NGGCT (left basal ganglia, ventricle) | M | 26 | 35 | 18 | 24 | 6 cycles of C/VP/Ifo + WVI + PB | CR | EOC | 1.05 | 1.18 | 19 |
| 11 | M | 16 | Germinoma (pineal) | M0 | <3 | 8 | <3 | 1 | 4 cycles VP/C | NA | Assess response (post 1st chemo) | 3.12 | 1.73 | 3 |
| 12 | M | 12 | Bifocal germinoma (suprasellar, pineal) | M0 | <3 | 24 | <2.5 | 13 | 4 cycles VP/C | NA | Dx | 2.67 | 2.01 | 3 |
| 13 | M | 15 | Bifocal germinoma (suprasellar, pineal) | M0 | <3 | 59 | 3 | 12 | 4 cycles VP/C | NA | Dx | 2.83 | 1.91 | 3 |
| 14 | M | 11 | Bifocal germinoma (suprasellar, pineal) | M0 | <3 | 7 | 1 | <1 | 4 cycles VP/C | NA | Dx | 2.56 | 1.9 | 2 |
| 15 | M | 14 | Germinoma (pineal) | M0 | <3 | 4 | <3 | 2 | 4 cycles VP/C | NA | Dx | 5 | 2.12 | 1 |
| Avoidance of Biopsy/Surgery | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient No | Age (Years) | Sex | Diagnosis | Site | Indication of Scan | Tracer | SUVmax | TBRmax | Conclusion |
| 15 | 9 | F | Thickened pituitary stalk | Pituitary | Diagnosis | FET | 1.09 | 0.91 | Suggestive of non-neoplastic lesion, biopsy not recommended. Followed-up for 14 months. |
| 16 | 14 | M | Thickened pituitary stalk | Pituitary | Diagnosis | FET | 1.74 | 0.83 | Suggestive of non-neoplastic lesion, biopsy not recommended. Followed-up for 24 months. |
| 9 | 14 | M | Germinoma | Pituitary | Rule out relapse | FET | 2.3 | 1.53 | Favours post-treatment residual, not indicated for biopsy/resection. |
| 17 | 7 | F | Thickened pituitary stalk | Pituitary | Diagnosis | FET | 0.87 | 0.64 | Suggestive of non-neoplastic lesion, biopsy not recommended. Followed-up for 21 months. |
| 18 | 1 | M | Congenital pontine tumour | Brainstem | End-of-treatment evaluation for residual tumour | MET | 1.05 | 0.76 | MRI after chemotherapy showed no reduction in tumour size. PET-MRI showed low metabolic activity, suggestive of post-treatment changes. Biopsy not recommended. CSF molecular study suggestive of low-grade lesion. Maintenance weekly vinblastine given till 1 year old. Patient followed-up for 2 years without progression. |
| 19 | 7 | M | LCH of frontal bone | Pituitary | Diagnosis/staging | FET | 0.81 | 0.82 | MRI showed suspected pituitary stalk thickening; PET-MRI showed no increase in metabolic uptake, not suggestive of LCH involvement of pituitary. Biopsy avoided. Spontaneous resolution and followed-up for 2 years. |
| 20 | 4 | M | Low-grade glioma | Basal Ganglia | Diagnosis | FET | 2.18 | 1.01 | Low metabolic uptake, suggestive of low-grade lesion; biopsy not recommended. Later developed seizure with biopsy confirming low-grade lesion. |
| 21 | 10 | F | Thickened pituitary stalk | Pituitary | Diagnosis | FET | 1.6 | 0.75 | Suggestive of non-neoplastic lesion, biopsy not recommended. Followed-up for 17 months. |
| 22 | 8 | F | Thickened pituitary stalk | Pituitary | Diagnosis | MET | 2.6 | 1.93 | Suggestive of non-neoplastic lesion, biopsy not recommended. Followed-up for 28 months. |
| 23 | 17 | F | Thickened pituitary stalk | Pituitary | Diagnosis | FET | 1.42 | 0.85 | Suggestive of non-neoplastic lesion, biopsy not recommended. |
| 19 | Monitor | FET | 1.76 | 0.89 | Follow-up MRI showed interval increase in size of thickened stalk; FET PET showed no increased metabolic uptake; plan for continued surveillance. Followed-up for 23 months. | ||||
| 24 | 15 | F | GBM | Frontal lobe | Evaluate residual | FET | 3.4 | 2.01 | MRI unable to differentiate pseudoprogression versus true progression; PET-MRI showed increased FET uptake suggestive of possible residual active neoplasm; surveillance suggested. Decision-making facilitated with the additional information from PET-MRI without the need for biopsy. |
| 25 | 7 | F | Thickened pituitary stalk | Pituitary | Diagnosis | FET | 1.31 | 0.79 | Suggestive of non-neoplastic lesion, biopsy not recommended. Followed-up for 18 months. |
| 26 | 6 | M | Medulloblastoma | Cerebellum | Rule out relapse | FET | 0 | 0 | MRI showed new enhancing focus. No FET uptake suggestive of active high-grade lesion. Surgery or biopsy avoided. In remission for 21 months. |
| 10 | 8 | M | NGGCT | Basal ganglia | Evaluate residual | FET | 0.73 | 0.82 | MRI showed residual lesion; PET-MRI showed no increase in metabolic uptake, suggestive of post-treatment changes. In remission for 18 months. |
| Refine tumour grading/diagnosis | |||||||||
| 27 | 4 | M | Diffuse midline glioma | Brainstem | Diagnosis | FET | 6.16 | 3.76 | Biopsy result unable to differentiate low-grade or high-grade glioma due to low cellularity on tissue slides. PET-MRI showed intra-lesional metabolic heterogeneity and high metabolic uptake in the unbiopsied region. Next-generation sequencing later revealed H3F3A mutation. |
| 28 | 16 | M | GBM | Frontal | Diagnosis | MET | 4 | 1.8 | Surgical resection with pathology showed low-grade glioma but high MET uptake suggestive of high-grade lesion. Eventually methylation revealed diagnosis of glioblastoma. |
| 29 | 2 | M | Pilocytic astrocytoma | Cerebellum | Diagnosis | MET | 1.99 | 1.34 | MRI unable to differentiate high-grade or low-grade lesion; PET MRI showed mild increase in uptake suggestive of low-grade neoplasm. Eventually had surgical resection due to increased tumour size; diagnosis of pilocytic astrocytoma made. |
| 3 | 11 | M | Germinoma | Basal ganglia | Diagnosis | MET | 3 | 1.78 | First MRI suggestive of infarction; high MET uptake on PET-MRI suggestive of high-grade neoplasm. |
| 14 | 11 | M | Germinoma | Suprasellar and pineal | Diagnosis | FET | 2.56 | 1.9 | First MRI identified only the pineal lesion; PET-MRI showed high metabolic uptake over suprasellar region and pineal region; diagnosis of bifocal germinoma made after histological confirmation of suprasellar lesion. |
| Treatment guidance or modification | |||||||||
| 30 | 17 | F | LCH | CP angle | Evaluate residual | FET | 1.39 | 0.55 | Post-treatment MRI showed similar residual lesion compared to pre-treatment scan; unsure whether active lesion or not. PET-MRI showed mild increased FET uptake, possible remaining active lesions. Surgery performed for residual lesion. |
| 31 | 1 | F | Ganglioglioma | Cerebellum | Diagnosis | MET | 1.53 | 1.25 | MRI showed incidental enhancing lesion; PET-MRI showed mild MET uptake, suggestive of low-grade neoplasm; hence decision was made for surgical resection. |
| 32 | 16 | M | Pilocytic astrocytoma | Cerebellum | Diagnosis | FET | 1.94 | 1.59 | Initial MRI showed 2 cm cerebellar lesion; observation planned; repeated scan with PET-MRI showed high uptake suggestive of neoplasm; hence decision was made for surgical resection. |
| 33 | 8 | M | Medulloblastoma | Spine | Evaluate relapse | FET | 1.82 | NA | MRI spine inconclusive for isolated spinal relapse; high uptake on PET-MRI supported diagnosis of spinal metastasis; treatment for relapse medulloblastoma initiated with response. |
| 34 | 0.75 | F | High-grade glioma (ROS1 fusion positive) | Temporal-parietal | Response assessment | FET | 2.73 | 1.16 | MRI inconclusive for residual tumour; PET-MRI showed high uptake, suggestive of disease progression, hence switched to targeted therapy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, E.R.; Cheng, P.W.; Lo, S.S.M.; Siu, C.W.Y.; Fu, E.C.H.; Yau, J.P.W.; Lee, A.C.W.; Wong, K.C.; Kan, E.Y.L.; Lau, S.S.N.; et al. Clinical Utility of Amino Acid PET-MRI in Children with CNS Neoplasms: A Territory-Wide Study from Hong Kong. Cancers 2025, 17, 3233. https://doi.org/10.3390/cancers17193233
Lu ER, Cheng PW, Lo SSM, Siu CWY, Fu ECH, Yau JPW, Lee ACW, Wong KC, Kan EYL, Lau SSN, et al. Clinical Utility of Amino Acid PET-MRI in Children with CNS Neoplasms: A Territory-Wide Study from Hong Kong. Cancers. 2025; 17(19):3233. https://doi.org/10.3390/cancers17193233
Chicago/Turabian StyleLu, Evelyn R., Pui Wai Cheng, Sherman S. M. Lo, Chloe W. Y. Siu, Eric C. H. Fu, Jeffrey P. W. Yau, Anselm C. W. Lee, Kwok Chun Wong, Elaine Y. L. Kan, Sarah S. N. Lau, and et al. 2025. "Clinical Utility of Amino Acid PET-MRI in Children with CNS Neoplasms: A Territory-Wide Study from Hong Kong" Cancers 17, no. 19: 3233. https://doi.org/10.3390/cancers17193233
APA StyleLu, E. R., Cheng, P. W., Lo, S. S. M., Siu, C. W. Y., Fu, E. C. H., Yau, J. P. W., Lee, A. C. W., Wong, K. C., Kan, E. Y. L., Lau, S. S. N., Ho, W. W. S., Cheng, K. K. F., Chan, E. K. Y., Ng, H. K., Kan, A. N. C., Chan, G. C. F., Ku, D. T. L., Shing, M. M. K., Liu, A. P. Y., & Siu, D. Y. W. (2025). Clinical Utility of Amino Acid PET-MRI in Children with CNS Neoplasms: A Territory-Wide Study from Hong Kong. Cancers, 17(19), 3233. https://doi.org/10.3390/cancers17193233







