Gynecological Cancer Oncobiome Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
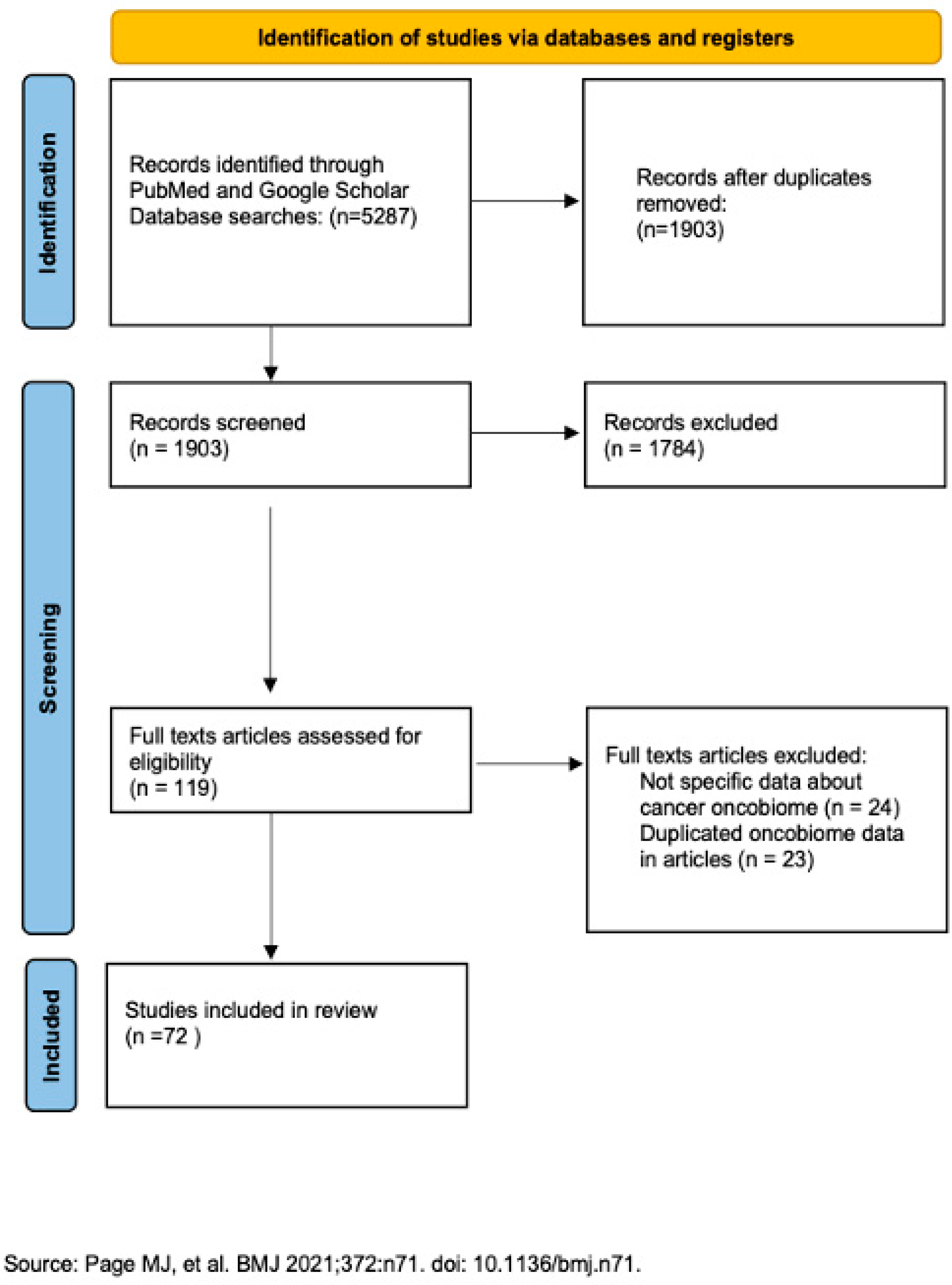
2. Materials and Methods
3. Results
3.1. Ovarian Cancer
3.2. Cervical Cancer
3.3. Uterine Corpus Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FIGO | The International Federation of Gynecology and Obstetrics |
| HPV | Human Papilloma Virus |
| HHV | Human Herpesvirus |
| TME | Tumor Microenvironment |
| Il | Inflammatory interleukin |
| TGFβ1 | Transforming growth factor β1 |
| TLR | Toll-like receptors |
| PAMPs | pathogen-associated molecular patterns |
| OS | Overall Survival |
| RME | Resident Microbiome of Endometrium |
| ROS | reactive oxygen species |
| TNF | Tumor necrosis factor |
| LCFA | long chain fatty acids |
| TCGA | The Cancer Genome Atlas |
| FRT | Female reproductive tract |
| GT | gastrointestinal tract |
| STD | Sexually Transmitted Diseases |
| ECM | extracellular matrix |
References
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 2021, 372, n71. [Google Scholar] [CrossRef]
- Available online: https://www.wcrf.org/cancer-trends/ovarian-cancer-statistics/ (accessed on 1 February 2023).
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Banerjee, S.; Wei, Z.; Tan, F.; Peck, K.N.; Shih, N.; Feldman, M.; Rebbeck, T.R.; Alwine, J.C.; Robertson, E.S. Distinct microbiological signatures associated with triple negative breast cancer. Sci. Rep. 2015, 5, 15162. [Google Scholar] [CrossRef]
- Rosa, M.I.; Silva, G.D.; Waleska, T.d.A.S.E.P.; Souza, M.V.; Panatto, A.P.R.; Simon, C.S.; Madeira, K.; Medeiros, L.R. The Prevalence of Human Papillomavirus in Ovarian Cancer. Int. J. Gynecol. Cancer 2013, 23, 437–441. [Google Scholar] [CrossRef] [PubMed]
- DeCaprio, J.A. Merkel cell polyomavirus and Merkel cell carcinoma. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160276. [Google Scholar] [CrossRef] [PubMed]
- Dockrell, D.H.; Smith, T.F.; Paya, C.V. Human Herpesvirus 6. Mayo Clin. Proc. 1999, 74, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Huang, G.; Gao, T.; Huang, T.; Zou, M.; Zou, Y.; Duan, S. Epigenetic Changes Associated with Interleukin-10. Front. Immunol. 2020, 11, 1105. [Google Scholar] [CrossRef]
- A Al-Shabanah, O.; Hafez, M.M.; Hassan, Z.K.; Sayed-Ahmed, M.M.; Abozeed, W.N.; Al-Rejaie, S.S.; A Alsheikh, A. Human papillomavirus genotyping and integration in ovarian cancer Saudi patients. Virol. J. 2013, 10, 343. [Google Scholar] [CrossRef]
- Werner, J.; A DeCarlo, C.; Escott, N.; Zehbe, I.; Ulanova, M. Expression of integrins and Toll-like receptors in cervical cancer: Effect of infectious agents. J. Endotoxin Res. 2011, 18, 55–69. [Google Scholar] [CrossRef]
- Shanmughapriya, S.; SenthilKumar, G.; Vinodhini, K.; Das, B.C.; Vasanthi, N.; Natarajaseenivasan, K. Viral and bacterial aetiologies of epithelial ovarian cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2311–2317. [Google Scholar] [CrossRef]
- Zhou, B.; Sun, C.; Huang, J.; Xia, M.; Guo, E.; Li, N.; Lu, H.; Shan, W.; Wu, Y.; Li, Y.; et al. The biodiversity Composition of Microbiome in Ovarian Carcinoma Patients. Sci. Rep. 2019, 9, 1691. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Nené, N.R.; Reisel, D.; Leimbach, A.; Franchi, D.; Jones, A.; Evans, I.; Knapp, S.; Ryan, A.; Ghazali, S.; Timms, J.F.; et al. Association between the cervicovaginal microbiome, BRCA1 mutation status, and risk of ovarian cancer: A case-control study. Lancet Oncol. 2019, 20, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, A.; Kawabata, A.; Shirahige, K.; Akiyama, T.; Okamoto, A.; Sutani, T. Altered cervicovaginal microbiota in premenopausal ovarian cancer patients. Gene 2022, 811, 146083. [Google Scholar] [CrossRef]
- Narunsky-Haziza, L.; Sepich-Poore, G.D.; Livyatan, I.; Asraf, O.; Martino, C.; Nejman, D.; Gavert, N.; Stajich, J.E.; Amit, G.; González, A.; et al. Pan-cancer analyses reveal cancer-type-specific fungal ecologies and bacteriome interactions. Cell 2022, 185, 3789–3806.e17. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wang, S.-Y.; Shen, H.; Yao, X.-F.; Zhang, F.-L.; Lai, D. The marine-derived fungal metabolite, terrein, inhibits cell proliferation and induces cell cycle arrest in human ovarian cancer cells. Int. J. Mol. Med. 2014, 34, 1591–1598. [Google Scholar] [CrossRef]
- Yang, Y.; Meng, Y.; Xu, Z.; Zhang, Q.; Li, M.; Kong, F.; Zhang, S.; Li, X.; Zhu, Y. Leveraging microbiome signatures to predict tumor immune microenvironment and prognosis of patients with endometrial carcinoma. Discov. Oncol. 2025, 16, 299. [Google Scholar] [CrossRef]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef]
- Available online: https://www.wcrf.org/cancer-trends/cervical-cancer-statistics/ (accessed on 1 February 2023).
- Kovachev, S.M. Cervical cancer and vaginal microbiota changes. Arch. Microbiol. 2019, 202, 323–327. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, M.; Qin, L.; Wan, B.; Wang, H. A meta-analysis of the relationship between vaginal microecology, human papillomavirus infection and cervical intraepithelial neoplasia. Infect. Agents Cancer 2019, 14, 29. [Google Scholar] [CrossRef]
- Łaniewski, P.; Barnes, D.; Goulder, A.; Cui, H.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Linking cervicovaginal immune signatures, HPV and microbiota composition in cervical carcinogenesis in non-Hispanic and Hispanic women. Sci. Rep. 2018, 8, 7593. [Google Scholar] [CrossRef] [PubMed]
- Audirac-Chalifour, A.; Torres-Poveda, K.; Bahena-Román, M.; Téllez-Sosa, J.; Martínez-Barnetche, J.; Cortina-Ceballos, B.; López-Estrada, G.; Delgado-Romero, K.; I Burguete-García, A.; Cantú, D.; et al. Cervical Microbiome and Cytokine Profile at Various Stages of Cervical Cancer: A Pilot Study. PLoS ONE 2016, 11, e0153274. [Google Scholar] [CrossRef] [PubMed]
- Sims, T.T.; Colbert, L.E.; Zheng, J.; Medrano, A.Y.D.; Hoffman, K.L.; Ramondetta, L.; Jazaeri, A.; Jhingran, A.; Schmeler, K.M.; Daniel, C.R.; et al. Gut microbial diversity and genus-level differences identified in cervical cancer patients versus healthy controls. Gynecol. Oncol. 2019, 155, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Lakins, M.A.; Ghorani, E.; Munir, H.; Martins, C.P.; Shields, J.D. Cancer-associated fibroblasts induce antigen-specific deletion of CD8+ T Cells to protect tumour cells. Nat. Commun. 2018, 9, 948. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Lehne, B.; Bhatia, R.; Lyons, D.; Paraskevaidis, E.; Li, J.V.; et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci. Rep. 2015, 5, 16865. [Google Scholar] [CrossRef]
- Jiang, L.; Duan, B.; Jia, P.; Zhang, Y.; Yan, X. The Role of Intratumor Microbiomes in Cervical Cancer Metastasis. Cancers 2023, 15, 509. [Google Scholar] [CrossRef]
- Routy, B.; le Chatelier, E.; DeRosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Fan, Q.; Wu, Y.; Li, M.; An, F.; Yao, L.; Wang, M.; Wang, X.; Yuan, J.; Jiang, K.; Li, W.; et al. Lactobacillus spp. create a protective micro-ecological environment through regulating the core fucosylation of vaginal epithelial cells against cervical cancer. Cell Death Dis. 2021, 12, 1094. [Google Scholar] [CrossRef]
- Zhong, K.; Tong, L.; Liu, L.; Zhou, X.; Liu, X.; Zhang, Q.; Zhou, S. Immunoregulatory and antitumor activity of schizophyllan under ultrasonic treatment. Int. J. Biol. Macromol. 2015, 80, 302–308. [Google Scholar] [CrossRef]
- Lichty, B.D.; Breitbach, C.J.; Stojdl, D.F.; Bell, J.C. Going viral with cancer immunotherapy. Nat. Rev. Cancer 2014, 14, 559–567. [Google Scholar] [CrossRef]
- Available online: https://www.wcrf.org/cancer-trends/endometrial-cancer-statistics/ (accessed on 1 February 2023).
- Kuźmycz, O.; Kowalczyk, A.; Bolanowska, A.; Drozdzowska, A.; Lach, J.; Wierzbińska, W.; Kluz, T.; Stączek, P. A comprehensive analysis of the uterine microbiome in endometrial cancer patients—Identification of Anaerococcus as a potential biomarker and carcinogenic cofactor. Front. Cell. Infect. Microbiol. 2025, 15, 1511625. [Google Scholar] [CrossRef]
- Stabile, G.; Doria, A.; Bruno, M.; D’indinosante, M.; Gallotta, V.; Fanfani, F.; Scambia, G.; Restaino, S.; Vizzielli, G.; Carlucci, S.; et al. The Role of the Endometrial Microbiota in Endometrial Cancer: A Systematic Review of the Literature. J. Clin. Med. 2024, 13, 7135. [Google Scholar] [CrossRef] [PubMed]
- Marconi, C.; Cruciani, F.; Vitali, B.; Donders, G.G. Correlation of Atopobium vaginae Amount with Bacterial Vaginosis Markers. J. Low. Genit. Tract Dis. 2012, 16, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Walther-António, M.R.S.; Chen, J.; Multinu, F.; Hokenstad, A.; Distad, T.J.; Cheek, E.H.; Keeney, G.L.; Creedon, D.J.; Nelson, H.; Mariani, A.; et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 2016, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Caselli, E.; Soffritti, I.; D’Accolti, M.; Piva, I.; Greco, P.; Bonaccorsi, G. Atopobium vaginae And Porphyromonas somerae Induce Proinflammatory Cytokines Expression In Endometrial Cells: A Possible Implication for Endometrial Cancer? Cancer Manag. Res. 2019, 11, 8571–8575. [Google Scholar] [CrossRef]
- Hawkins, G.; Clark, L.; Sullivan, S.; Keku, T.; Brewster, W.; Bae-Jump, V. Impact of ethnicity and obesity on the uterine microbiome in women and mice with endometrial cancer. Gynecol. Oncol. 2019, 154, 77. [Google Scholar] [CrossRef]
- Walsh, D.M.; Hokenstad, A.N.; Chen, J.; Sung, J.; Jenkins, G.D.; Chia, N.; Nelson, H.; Mariani, A.; Walther-Antonio, M.R.S. Postmenopause as a key factor in the composition of the Endometrial Cancer Microbiome (ECbiome). Sci. Rep. 2019, 9, 19213. [Google Scholar] [CrossRef]
- Takai, N.; Narahara, H. Human Endometrial and Ovarian Cancer Cells: Histone Deacetylase Inhibitors Exhibit Antiproliferative Activity, Potently Induce Cell Cycle Arrest, and Stimulate Apoptosis. Curr. Med. Chem. 2007, 14, 2548–2553. [Google Scholar] [CrossRef]
- Zhao, S.-S.; Chen, L.; Yang, J.; Wu, Z.-H.; Wang, X.-Y.; Zhang, Q.; Liu, W.-J.; Liu, H.-X. Altered Gut Microbial Profile Accompanied by Abnormal Fatty Acid Metabolism Activity Exacerbates Endometrial Cancer Progression. Microbiol. Spectr. 2022, 10, e0261222. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2020, 19, 77–94. [Google Scholar] [CrossRef]
- Thomas, R.M.; Jobin, C. The Microbiome and Cancer: Is the ‘Oncobiome’ Mirage Real? Trends Cancer 2015, 1, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Ragonnaud, E.; Biragyn, A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun. Ageing 2021, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Picardo, S.L.; Coburn, B.; Hansen, A.R. The microbiome and cancer for clinicians. Crit. Rev. Oncol. 2019, 141, 1–12. [Google Scholar] [CrossRef]
- Andriamihaja, M.; Lan, A.; Beaumont, M.; Audebert, M.; Wong, X.; Yamada, K.; Yin, Y.; Tomé, D.; Carrasco-Pozo, C.; Gotteland, M.; et al. The deleterious metabolic and genotoxic effects of the bacterial metabolite p-cresol on colonic epithelial cells. Free Radic. Biol. Med. 2015, 85, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Thomas-White, K.; Forster, S.C.; Kumar, N.; Van Kuiken, M.; Putonti, C.; Stares, M.D.; Hilt, E.E.; Price, T.K.; Wolfe, A.J.; Lawley, T.D. Culturing of female bladder bacteria reveals an interconnected urogenital microbiota. Nat. Commun. 2018, 9, 1557. [Google Scholar] [CrossRef]
- Plottel, C.S.; Blaser, M.J. Microbiome and Malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef]
- D’antonio, D.L.; Marchetti, S.; Pignatelli, P.; Piattelli, A.; Curia, M.C. The Oncobiome in Gastroenteric and Genitourinary Cancers. Int. J. Mol. Sci. 2022, 23, 9664. [Google Scholar] [CrossRef]
- Gong, Z.; Luna, Y.; Yu, P.; Fan, H. Lactobacilli Inactivate Chlamydia trachomatis through Lactic Acid but Not H2O2. PLoS ONE 2014, 9, e107758. [Google Scholar] [CrossRef] [PubMed]
- Conti, C.; Malacrino, C.; Mastromarino, P. Inhibition of herpes simplex virus type 2 by vaginal lactobacilli. J. Physiol. Pharmacol. 2009, 60, 19–26. [Google Scholar] [PubMed]
- A Cadieux, P.; Burton, J.; Devillard, E.; Reid, G. Lactobacillus by-products inhibit the growth and virulence of uropathogenic Escherichia coli. J. Physiol. Pharmacol. 2009, 60, 13–18. [Google Scholar] [PubMed]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef]
- Borgdorff, H.; Van Der Veer, C.; Van Houdt, R.; Alberts, C.J.; De Vries, H.J.; Bruisten, S.M.; Snijder, M.B.; Prins, M.; E Geerlings, S.; Van Der Loeff, M.F.S.; et al. The association between ethnicity and vaginal microbiota composition in Amsterdam, the Netherlands. PLoS ONE 2017, 12, e0181135. [Google Scholar] [CrossRef]
- Martin, D.H.; Marrazzo, J.M. The Vaginal Microbiome: Current Understanding and Future Directions. J. Infect. Dis. 2016, 214, S36–S41. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef]
- Muhleisen, A.L.; Herbst-Kralovetz, M.M. Menopause and the vaginal microbiome. Maturitas 2016, 91, 42–50. [Google Scholar] [CrossRef]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Bieda, J.; Chaemsaithong, P.; Miranda, J.; Chaiworapongsa, T.; Ravel, J. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome 2014, 2, 18. [Google Scholar] [CrossRef]
- Ingerslev, K.; Hogdall, E.; Schnack, T.H.; Skovrider-Ruminski, W.; Hogdall, C.; Blaakaer, J. The potential role of infectious agents and pelvic inflammatory disease in ovarian carcinogenesis. Infect. Agents Cancer 2017, 12, 25. [Google Scholar] [CrossRef]
- Livyatan, I.; Nejman, D.; Shental, N.; Straussman, R. Characterization of the human tumor microbiome reveals tumor-type specific intra-cellular bacteria. OncoImmunology 2020, 9, 1800957. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef]
- Mao, Y.; Keller, E.T.; Garfield, D.H.; Shen, K.; Wang, J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2012, 32, 303–315. [Google Scholar] [CrossRef]
| Bacteria | Viruses | Fungi and Parasites | |
|---|---|---|---|
| Ovarian cancer | Proteobacteria Firmicutes | Retroviridae, Hepadnaviridae, Papillomaviridae, Flaviviridae, Polyomaviridae and Herpesviridae | Cladosporia Dipylidium, Trichuris, Leishmania and Babesia |
| Cervical cancer | Gardnerella vaginalis Prevotellabivia Sneathia | HPV | Schizophyllum |
| Uterine corpus cancer | Shigella | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łatkiewicz, T.; Rasoul-Pelińska, K.; Kułak, K.; Tarkowski, R.; Kułak, A.; Puzio, I. Gynecological Cancer Oncobiome Systematic Review. Cancers 2025, 17, 3227. https://doi.org/10.3390/cancers17193227
Łatkiewicz T, Rasoul-Pelińska K, Kułak K, Tarkowski R, Kułak A, Puzio I. Gynecological Cancer Oncobiome Systematic Review. Cancers. 2025; 17(19):3227. https://doi.org/10.3390/cancers17193227
Chicago/Turabian StyleŁatkiewicz, Tomasz, Karolina Rasoul-Pelińska, Krzysztof Kułak, Rafał Tarkowski, Anna Kułak, and Iwona Puzio. 2025. "Gynecological Cancer Oncobiome Systematic Review" Cancers 17, no. 19: 3227. https://doi.org/10.3390/cancers17193227
APA StyleŁatkiewicz, T., Rasoul-Pelińska, K., Kułak, K., Tarkowski, R., Kułak, A., & Puzio, I. (2025). Gynecological Cancer Oncobiome Systematic Review. Cancers, 17(19), 3227. https://doi.org/10.3390/cancers17193227






