Pre-Treatment PET Radiomics for Prediction of Disease-Free Survival in Cervical Cancer
Abstract
Simple Summary
Abstract
1. Introduction
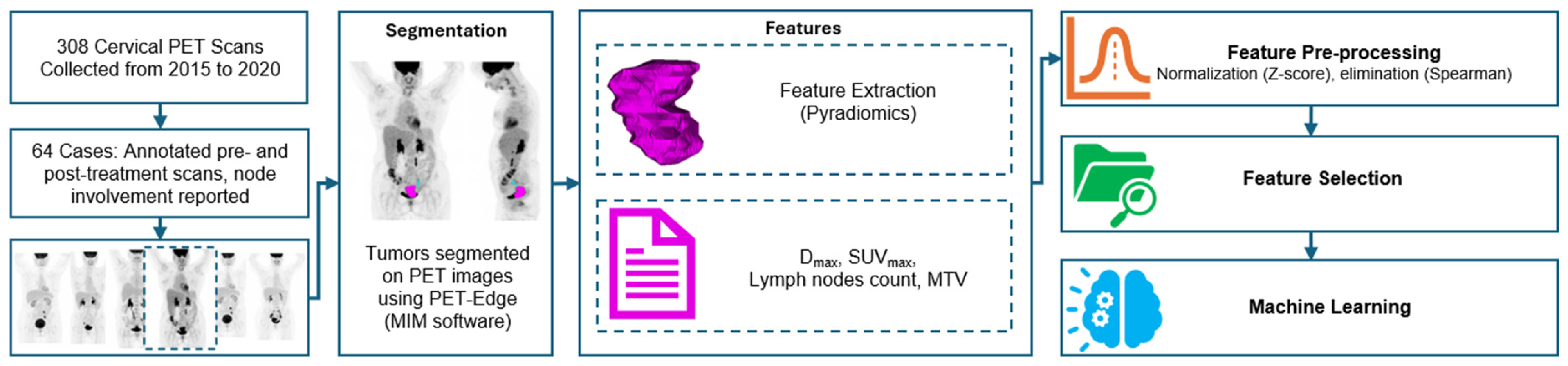
2. Materials and Methods
2.1. Patient Information
2.2. Segmentation
2.3. Radiomics Analysis
2.3.1. Feature Selection
2.3.2. Machine Learning Techniques
2.3.3. Nested Cross-Validation Process
2.3.4. External Validation
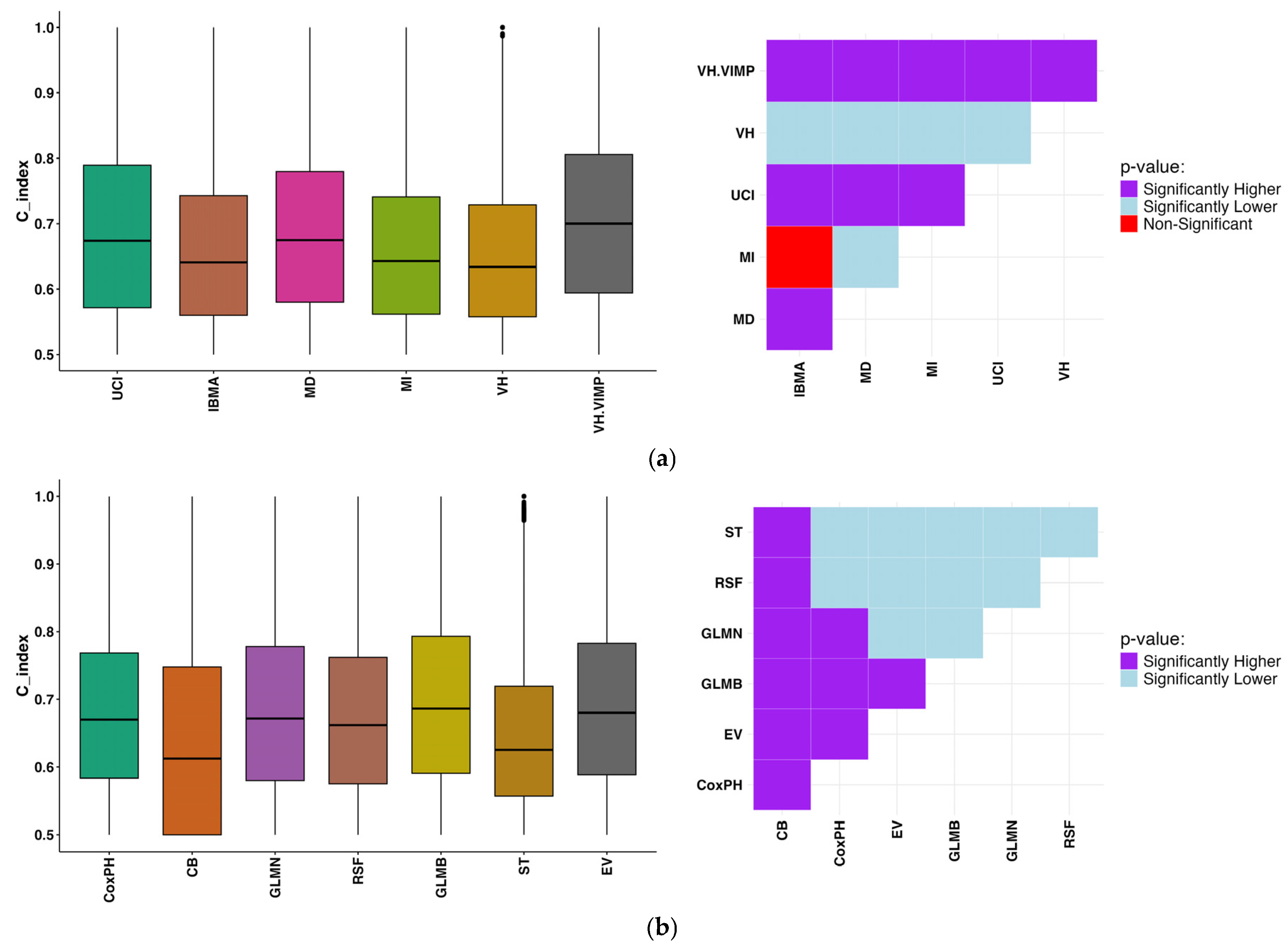
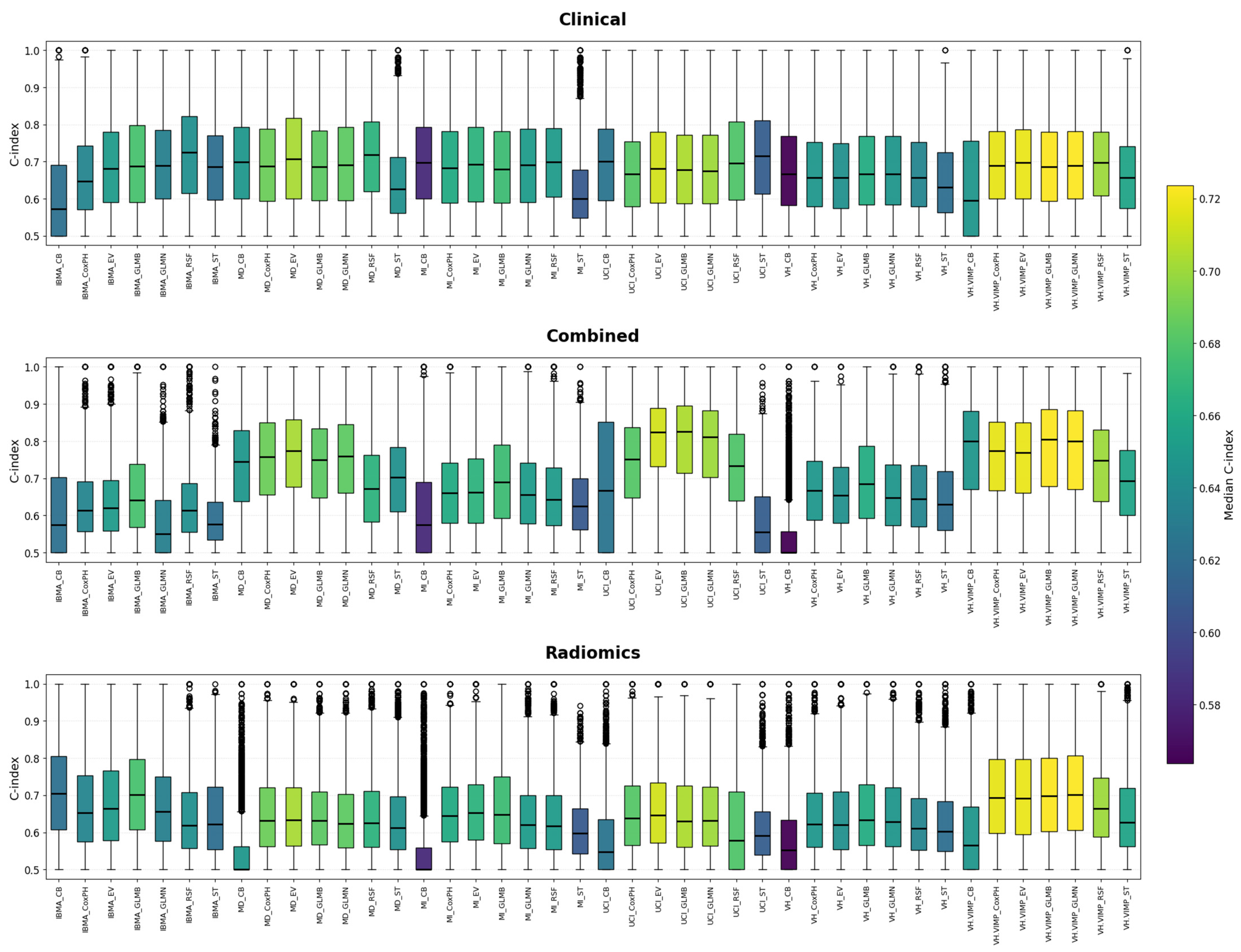
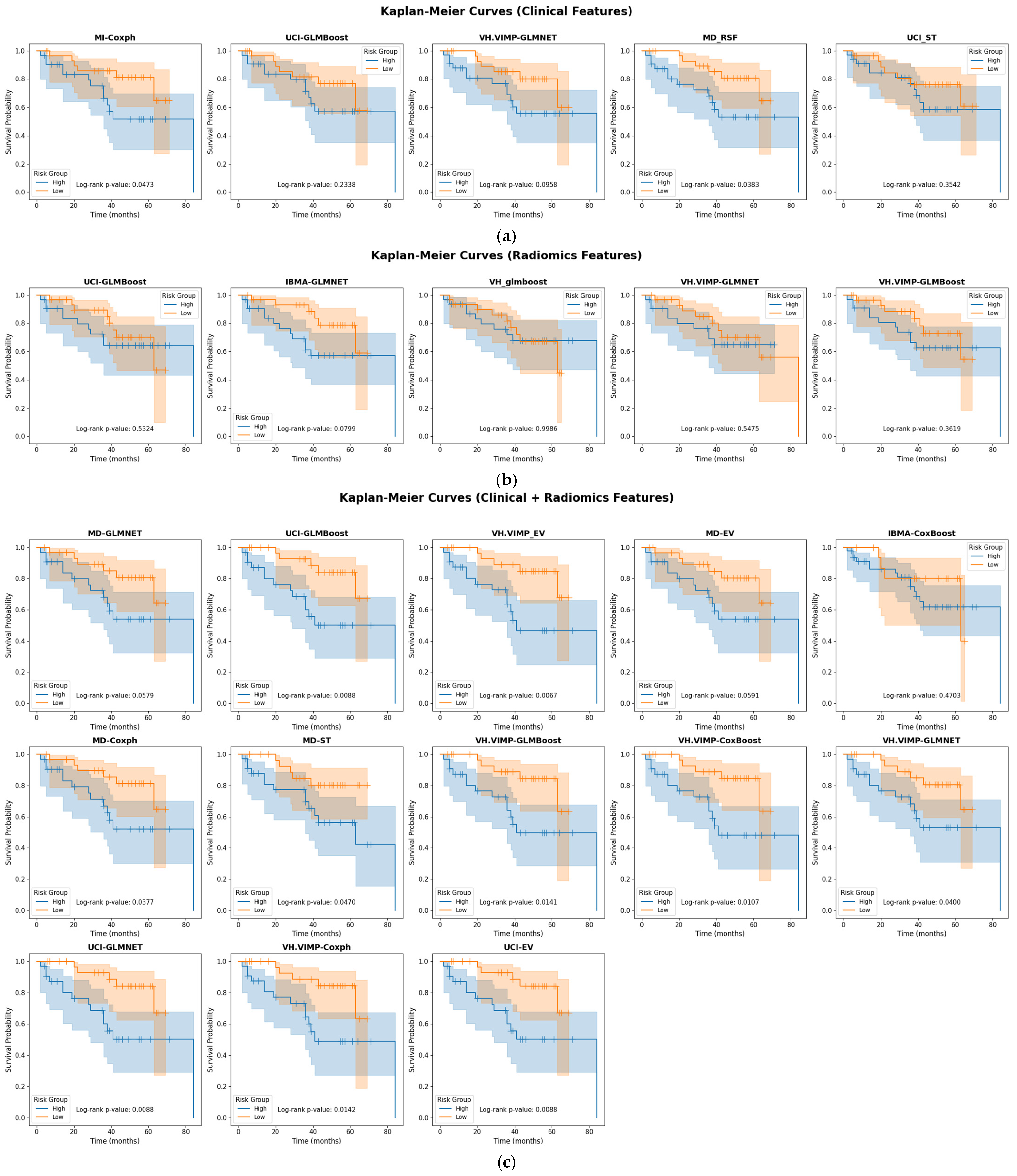
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Estimating the World Cancer Burden: Globocan 2000. Int. J. Cancer 2001, 94, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie-Meder, C.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients with Cervical Cancer. Virchows Arch. 2018, 472, 919–936. [Google Scholar] [CrossRef] [PubMed]
- Frille, A.; Arends, J.; Abenavoli, E.M.; Duke, S.A.; Ferrara, D.; Gruenert, S.; Hacker, M.; Hesse, S.; Hofmann, L.; Holm, S.H.; et al. “Metabolic fingerprints” of cachexia in lung cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2067–2069. [Google Scholar] [CrossRef] [PubMed]
- Sundar, L.K.; Beyer, T. Is automatic tumor segmentation on whole-body 18F-FDG PET images a clinical reality? J. Nucl. Med. 2024, 65, 995–997. [Google Scholar] [CrossRef]
- Yousefirizi, F.; Gowdy, C.; Klyuzhin, I.S.; Sabouri, M.; Tonseth, P.; Hayden, A.R.; Wilson, D.; Sehn, L.H.; Scott, D.W.; Steidl, C.; et al. Evaluating Outcome Prediction via Baseline, End-of-Treatment, and Delta Radiomics on PET-CT Images of Primary Mediastinal Large B-Cell Lymphoma. Cancers 2024, 16, 1090. [Google Scholar] [CrossRef]
- Yousefirizi, F.; Decazes, P.; Amyar, A.; Ruan, S.; Saboury, B.; Rahmim, A. AI-based detection, classification and prediction/prognosis in medical imaging: Towards radiophenomics. PET Clin. 2022, 17, 183–212. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Mukherjee, U.; Ghosh, S.; Ghosh, S.; Sarkar, S.K. Pattern of Failure with Locally Advanced Cervical Cancer– A Retrospective Audit and Analysis of Contributory Factors. Asian Pac. J. Cancer Prev. 2018, 19, 73–79. [Google Scholar]
- Chen, R.; Gong, Y.; Zou, D.; Wang, L.; Yuan, L.; Zhou, Q. Correlation between Subsets of Tumor-Infiltrating Immune Cells and Risk Stratification in Patients with Cervical Cancer. PeerJ 2019, 7, e7804. [Google Scholar] [CrossRef]
- Gandy, N.; Arshad, M.A.; Park, W.-H.E.; Rockall, A.G.; Barwick, T.D. FDG-PET Imaging in Cervical Cancer. Semin. Nucl. Med. 2019, 49, 461–470. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-Based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Rogers, W.; Thulasi Seetha, S.; Refaee, T.A.G.; Lieverse, R.I.Y.; Granzier, R.W.Y.; Ibrahim, A.; Keek, S.A.; Sanduleanu, S.; Primakov, S.P.; Beuque, M.P.L.; et al. Radiomics: From Qualitative to Quantitative Imaging. Br. J. Radiol. 2020, 93, 20190948. [Google Scholar] [CrossRef]
- Chicklore, S.; Goh, V.; Siddique, M.; Roy, A.; Marsden, P.K.; Cook, G.J.R. Quantifying Tumour Heterogeneity in 18F-FDG PET/CT Imaging by Texture Analysis. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 133–140. [Google Scholar] [CrossRef]
- Hatt, M.; Majdoub, M.; Vallières, M.; Tixier, F.; Le Rest, C.C.; Groheux, D.; Hindié, E.; Martineau, A.; Pradier, O.; Hustinx, R.; et al. 18F-FDG PET Uptake Characterization through Texture Analysis: Investigating the Complementary Nature of Heterogeneity and Functional Tumor Volume in a Multi-Cancer Site Patient Cohort. J. Nucl. Med. 2015, 56, 38–44. [Google Scholar] [CrossRef]
- Altazi, B.A.; Fernandez, D.C.; Zhang, G.G.; Hawkins, S.; Naqvi, S.M.; Kim, Y.; Hunt, D.; Latifi, K.; Biagioli, M.; Venkat, P.; et al. Investigating Multi-Radiomic Models for Enhancing Prediction Power of Cervical Cancer Treatment Outcomes. Phys. Med. 2018, 46, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-C.; Chen, S.-W.; Wu, K.-C.; Hsieh, T.-C.; Liang, J.-A.; Hung, Y.-C.; Yeh, L.-S.; Chang, W.-C.; Lin, W.-C.; Yen, K.-Y.; et al. Prediction of Local Relapse and Distant Metastasis in Patients with Definitive Chemoradiotherapy-Treated Cervical Cancer by Deep Learning from [18F]-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography. Eur. Radiol. 2019, 29, 6741–6749. [Google Scholar] [CrossRef] [PubMed]
- Rahmim, A.; Toosi, A.; Salmanpour, M.R.; Dubljevic, N.; Janzen, I.; Shiri, I.; Yuan, R.; Ho, C.; Zaidi, H.; MacAulay, C.; et al. Tensor Radiomics: Paradigm for Systematic Incorporation of Multi-Flavoured Radiomics Features. Quant. Imaging Med. Surg. 2023, 13, 7680–7694. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, Z.; Salimi, Y.; Hajianfar, G.; Wolf, N.B.; Knappe, L.; Xhepa, G.; Gleyzolle, A.; Ricoeur, A.; Garibotto, V.; Mainta, I.; et al. The Role of Biomarkers and Dosimetry Parameters in Overall and Progression Free Survival Prediction for Patients Treated with Personalized 90Y Glass Microspheres SIRT: A Preliminary Machine Learning Study. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 4111–4126. [Google Scholar] [CrossRef]
- Hajianfar, G.; Haddadi Avval, A.; Hosseini, S.A.; Nazari, M.; Oveisi, M.; Shiri, I.; Zaidi, H. Time-to-Event Overall Survival Prediction in Glioblastoma Multiforme Patients Using Magnetic Resonance Imaging Radiomics. Radiol. Med. 2023, 128, 1521–1534. [Google Scholar] [CrossRef]
- Yang, F.; Young, L.; Grigsby, P. Predictive Value of Standardized Intratumoral Metabolic Heterogeneity in Locally Advanced Cervical Cancer Treated with Chemoradiation. Int. J. Gynecol. Cancer 2016, 26, 777–784. [Google Scholar] [CrossRef]
- Ho, K.-C.; Fang, Y.-H.D.; Chung, H.-W.; Yen, T.-C.; Ho, T.-Y.; Chou, H.-H.; Hong, J.-H.; Huang, Y.-T.; Wang, C.-C.; Lai, C.-H. A Preliminary Investigation into Textural Features of Intratumoral Metabolic Heterogeneity in (18)F-FDG PET for Overall Survival Prognosis in Patients with Bulky Cervical Cancer Treated with Definitive Concurrent Chemoradiotherapy. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 166–175. [Google Scholar]
- Mu, W.; Liang, Y.; Hall, L.O.; Tan, Y.; Balagurunathan, Y.; Wenham, R.; Wu, N.; Tian, J.; Gillies, R.J. 18F-FDG PET/CT Habitat Radiomics Predicts Outcome of Patients with Cervical Cancer Treated with Chemoradiotherapy. Radiol. Artif. Intell. 2020, 2, e190218. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Lovinfosse, P.; Hermesse, J.; Decuypere, M.; Rousseau, C.; Lucia, F.; Schick, U.; Reinhold, C.; Robin, P.; Hatt, M.; et al. [18F]FDG PET Radiomics to Predict Disease-Free Survival in Cervical Cancer: A Multi-Scanner/Center Study with External Validation. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3432–3443. [Google Scholar] [CrossRef] [PubMed]
- de Alencar, N.R.G.; Machado, M.A.D.; Mourato, F.A.; de Oliveira, M.L.; Moraes, T.F.; Mattos Junior, L.A.R.; Chang, T.-M.C.; de Azevedo, C.R.A.S.; Brandão, S.C.S. Exploratory Analysis of Radiomic as Prognostic Biomarkers in 18F-FDG PET/CT Scan in Uterine Cervical Cancer. Front. Med. 2022, 9, 1046551. [Google Scholar] [CrossRef] [PubMed]
- Hatt, M.; Tixier, F.; Pierce, L.; Kinahan, P.E.; Le Rest, C.C.; Visvikis, D. Characterization of PET/CT Images Using Texture Analysis: The Past, the Present… Any Future? Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 151–165. [Google Scholar] [CrossRef]
- Reuzé, S.; Orlhac, F.; Chargari, C.; Nioche, C.; Limkin, E.; Riet, F.; Escande, A.; Haie-Meder, C.; Dercle, L.; Gouy, S.; et al. Prediction of Cervical Cancer Recurrence Using Textural Features Extracted from 18F-FDG PET Images Acquired with Different Scanners. Oncotarget 2017, 8, 43169–43179. [Google Scholar] [CrossRef]
- Collarino, A.; Feudo, V.; Pasciuto, T.; Florit, A.; Pfaehler, E.; de Summa, M.; Bizzarri, N.; Annunziata, S.; Zannoni, G.F.; de Geus-Oei, L.-F.; et al. Is PET Radiomics Useful to Predict Pathologic Tumor Response and Prognosis in Locally Advanced Cervical Cancer? J. Nucl. Med. 2024, 65, 962–970. [Google Scholar] [CrossRef]
- Grigsby, P.W. The Prognostic Value of PET and PET/CT in Cervical Cancer. Cancer Imaging 2008, 8, 146–155. [Google Scholar] [CrossRef]
- Yousefirizi, F.; Bloise, I.; Martineau, P.; Wilson, D.; Benard, F.; Bradshaw, T.B.; Arman, R.; Uribe, C. Reproducibility of a Semi-Automatic Gradient-Based Segmentation Approach for Lymphoma PET. In Proceedings of the EANM Abstract Book, a Supplement of the European Journal of Nuclear Medicine and Molecular Imaging (EJNMMI); Springer Science & Business Media: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Crandall, J.P.; Fraum, T.J.; Lee, M.; Jiang, L.; Grigsby, P.; Wahl, R.L. Repeatability of 18F-FDG PET Radiomic Features in Cervical Cancer. J. Nucl. Med. 2021, 62, 707–715. [Google Scholar] [CrossRef]
- Orlhac, F.; Nioche, C.; Klyuzhin, I.; Rahmim, A.; Buvat, I. Radiomics in PET Imaging: A Practical Guide for Newcomers. PET Clin. 2021, 16, 597–612. [Google Scholar] [CrossRef]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.-C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- De Jay, N.; Papillon-Cavanagh, S.; Olsen, C.; El-Hachem, N.; Bontempi, G.; Haibe-Kains, B. MRMRe: An R Package for Parallelized MRMR Ensemble Feature Selection. Bioinformatics 2013, 29, 2365–2368. [Google Scholar] [CrossRef] [PubMed]
- Ishwaran, H.; Kogalur, U.B.; Chen, X.; Minn, A.J. Random Survival Forests for High-Dimensional Data. Stat. Anal. Data Min. 2011, 4, 115–132. [Google Scholar] [CrossRef]
- Annest, A.; Bumgarner, R.E.; Raftery, A.E.; Yeung, K.Y. Iterative Bayesian Model Averaging: A Method for the Application of Survival Analysis to High-Dimensional Microarray Data. BMC Bioinform. 2009, 10, 72. [Google Scholar] [CrossRef]
- Ishwaran, H.; Kogalur, U.B.; Gorodeski, E.Z.; Minn, A.J.; Lauer, M.S. High-Dimensional Variable Selection for Survival Data. J. Am. Stat. Assoc. 2010, 105, 205–217. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. Cox Proportional-Hazards Regression for Survival Data. In An R and S-PLUS Companion to Applied Regression; Sage: Thousand Oaks, CA, USA, 2002; Volume 2002. [Google Scholar]
- Binder, H.; Allignol, A.; Schumacher, M.; Beyersmann, J. Boosting for High-Dimensional Time-to-Event Data with Competing Risks. Bioinformatics 2009, 25, 890–896. [Google Scholar] [CrossRef]
- Hastie, T.; Qian, J. Glmnet Vignette. Available online: https://hastie.su.domains/Papers/Glmnet_Vignette.pdf (accessed on 25 July 2016).
- Ishwaran, H.; Kogalur, U.B.; Blackstone, E.H.; Lauer, M.S. Random Survival Forests. Aoas 2008, 2, 841–860. [Google Scholar] [CrossRef]
- Hothorn, T.; Bühlmann, P.; Kneib, T.; Schmid, M.; Hofner, B. Model-Based Boosting 2.0. J. Mach. Learn. Res. 2010, 11, 2109–2113. [Google Scholar]
- Loh, W.-Y. Classification and Regression Trees: Classification and Regression Trees. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2011, 1, 14–23. [Google Scholar] [CrossRef]
- Yusufaly, T.I.; Zou, J.; Nelson, T.J.; Williamson, C.W.; Simon, A.; Singhal, M.; Liu, H.; Wong, H.; Saenz, C.C.; Mayadev, J.; et al. Improved Prognosis of Treatment Failure in Cervical Cancer with Nontumor PET/CT Radiomics. J. Nucl. Med. 2022, 63, 1087–1093. [Google Scholar] [CrossRef]
- Li, H.; Zhu, M.; Jian, L.; Bi, F.; Zhang, X.; Fang, C.; Wang, Y.; Wang, J.; Wu, N.; Yu, X. Radiomic Score as a Potential Imaging Biomarker for Predicting Survival in Patients with Cervical Cancer. Front. Oncol. 2021, 11, 706043. [Google Scholar] [CrossRef]
- Tian, X.; Sun, C.; Liu, Z.; Li, W.; Duan, H.; Wang, L.; Fan, H.; Li, M.; Li, P.; Wang, L.; et al. Prediction of Response to Preoperative Neoadjuvant Chemotherapy in Locally Advanced Cervical Cancer Using Multicenter CT-Based Radiomic Analysis. Front. Oncol. 2020, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cui, Y.; Chang, C.; Zhou, Z.; Zhang, Y.; Ma, C.; Yin, Y.; Wang, R. Development and Validation of a 18F-FDG PET/CT Radiomics Nomogram for Predicting Progression Free Survival in Locally Advanced Cervical Cancer: A Retrospective Multicenter Study. BMC Cancer 2024, 24, 150. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, R.; Liu, Q.; Sun, D.; Yang, H.; Pan, H. Song Radiomics Model of 18F-FDG PET/CT Imaging for Predicting Disease-Free Survival of Early-Stage Uterine Cervical Squamous Cancer. Cancer Biomark. 2022, 33, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, Q.; Zhao, L.; Feng, X.; Wang, Y.; Zou, Y.; Li, Q. A Systematic Review and Meta-Analysis of the Prognostic Impact of Pretreatment Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Parameters. Diagnostics 2021, 11, 1258. [Google Scholar] [CrossRef]
- Calles-Sastre, L.; Mucientes-Rasilla, J.; M, S.-F.L.L.; Royuela, A.; Garcia-Espantaleón, N.M.; Herrero, G.S.; Perez-Medina, T. Prognostic Significance of Metabolic Tumor Volume and Total Lesion Glycolysis in Patients with Advanced Cervical Carcinoma. Rev. Esp. Med. Nucl. Imagen Mol. 2019, 38, 17–21. [Google Scholar] [CrossRef]
- Chen, S.-W.; Shen, W.-C.; Hsieh, T.-C.; Liang, J.-A.; Hung, Y.-C.; Yeh, L.-S.; Chang, W.-C.; Lin, W.-C.; Yen, K.-Y.; Kao, C.-H. Textural Features of Cervical Cancers on FDG-PET/CT Associate with Survival and Local Relapse in Patients Treated with Definitive Chemoradiotherapy. Sci. Rep. 2018, 8, 11859. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, H.-L.; Zhang, X.-L.; Tian, Z.-F.; Xu, X.-Q.; Tang, W.-W. Multiparametric Magnetic Resonance Imaging-Derived Radiomics for the Prediction of Disease-Free Survival in Early-Stage Squamous Cervical Cancer. Eur. Radiol. 2022, 32, 2540–2551. [Google Scholar] [CrossRef]
- Moore, K.N.; Java, J.J.; Slaughter, K.N.; Rose, P.G.; Lanciano, R.; DiSilvestro, P.A.; Thigpen, J.T.; Lee, Y.-C.; Tewari, K.S.; Chino, J.; et al. Is Age a Prognostic Biomarker for Survival among Women with Locally Advanced Cervical Cancer Treated with Chemoradiation? An NRG Oncology/Gynecologic Oncology Group Ancillary Data Analysis. Gynecol. Oncol. 2016, 143, 294–301. [Google Scholar] [CrossRef]
- Kocak, B.; Akinci D’Antonoli, T.; Mercaldo, N.; Alberich-Bayarri, A.; Baessler, B.; Ambrosini, I.; Andreychenko, A.E.; Bakas, S.; Beets-Tan, R.G.H.; Bressem, K.; et al. METhodological RadiomICs Score (METRICS): A Quality Scoring Tool for Radiomics Research Endorsed by EuSoMII. Insights Imaging 2024, 15, 8. [Google Scholar] [CrossRef]
- Bradshaw, T.J.; Huemann, Z.; Hu, J.; Rahmim, A. A Guide to Cross-Validation for Artificial Intelligence in Medical Imaging. Radiol. Artif. Intell. 2023, 5, e220232. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, B.; Wang, S.; Jin, Y.; Wang, F.; Ding, Y.; Chen, Q.; Chen, L.; Li, Y.; Li, M.; et al. Association of MRI-Derived Radiomic Biomarker with Disease-Free Survival in Patients with Early-Stage Cervical Cancer. Theranostics 2020, 10, 2284–2292. [Google Scholar] [CrossRef]
- Lucia, F.; Bourbonne, V.; Pleyers, C.; Dupré, P.-F.; Miranda, O.; Visvikis, D.; Pradier, O.; Abgral, R.; Mervoyer, A.; Classe, J.-M.; et al. Multicentric Development and Evaluation of 18F-FDG PET/CT and MRI Radiomics Models to Predict Para-Aortic Lymph Node Involvement in Locally Advanced Cervical Cancer. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2514–2528. [Google Scholar] [CrossRef]
- Lucia, F.; Visvikis, D.; Desseroit, M.-C.; Miranda, O.; Malhaire, J.-P.; Robin, P.; Pradier, O.; Hatt, M.; Schick, U. Prediction of Outcome Using Pretreatment 18F-FDG PET/CT and MRI Radiomics in Locally Advanced Cervical Cancer Treated with Chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 768–786. [Google Scholar] [CrossRef]
- Lucia, F.; Visvikis, D.; Vallières, M.; Desseroit, M.-C.; Miranda, O.; Robin, P.; Bonaffini, P.A.; Alfieri, J.; Masson, I.; Mervoyer, A.; et al. External Validation of a Combined PET and MRI Radiomics Model for Prediction of Recurrence in Cervical Cancer Patients Treated with Chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 864–877. [Google Scholar] [CrossRef]
| Patient Characteristic | |
|---|---|
| Age (year) | 47.9 ± 14.5 |
| Node involvement in Pre-treatment PET (%) | 61.9 |
| Average nodes per patient (with node involvement) | ~2 |
| T-stage- Cervical Staging (%) | |
| IB1 | 9.5 |
| IB2 | 17.5 |
| IIA | 14.3 |
| IIB | 47.6 |
| IIIA | 1.6 |
| IIIB | 9.5 |
| Recurrence (%) | 31.7 |
| Recurrence location (%) | |
| Cervix/Uterus (%) | 15 |
| Pelvic (%) | 35 |
| Paraaortic nodes (%) | 15 |
| Distant (%) | 35 |
| Median follow-up (month) | 3.53 |
| Treatment | |
| Radiotherapy (%) | 4.7 (3/63) * |
| Concurrent Chemoradiotherapy (chemoRT) (%) | 95.2 (60/63) * |
| Neoadjuvant Chemotherapy before PET (%) | 7.9 (5/63) * |
| Negative Post-treatment Scan (%) | 73.0 |
| Recurrence in patients with Negative post-treatment scan (%) | 21.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousefirizi, F.; Hajianfar, G.; Sabouri, M.; Holloway, C.; Tonseth, P.; Alexander, A.; Yusufaly, T.I.; Mell, L.K.; Harsini, S.; Bénard, F.; et al. Pre-Treatment PET Radiomics for Prediction of Disease-Free Survival in Cervical Cancer. Cancers 2025, 17, 3218. https://doi.org/10.3390/cancers17193218
Yousefirizi F, Hajianfar G, Sabouri M, Holloway C, Tonseth P, Alexander A, Yusufaly TI, Mell LK, Harsini S, Bénard F, et al. Pre-Treatment PET Radiomics for Prediction of Disease-Free Survival in Cervical Cancer. Cancers. 2025; 17(19):3218. https://doi.org/10.3390/cancers17193218
Chicago/Turabian StyleYousefirizi, Fereshteh, Ghasem Hajianfar, Maziar Sabouri, Caroline Holloway, Pete Tonseth, Abraham Alexander, Tahir I. Yusufaly, Loren K. Mell, Sara Harsini, François Bénard, and et al. 2025. "Pre-Treatment PET Radiomics for Prediction of Disease-Free Survival in Cervical Cancer" Cancers 17, no. 19: 3218. https://doi.org/10.3390/cancers17193218
APA StyleYousefirizi, F., Hajianfar, G., Sabouri, M., Holloway, C., Tonseth, P., Alexander, A., Yusufaly, T. I., Mell, L. K., Harsini, S., Bénard, F., Zaidi, H., Uribe, C., & Rahmim, A. (2025). Pre-Treatment PET Radiomics for Prediction of Disease-Free Survival in Cervical Cancer. Cancers, 17(19), 3218. https://doi.org/10.3390/cancers17193218









