Macrophages—Target and Tool in Tumor Treatment: Insights from Ovarian Cancer
Abstract
Simple Summary
Abstract
1. Introduction—Clinical Significance of Macrophages in Ovarian Cancer
2. The Biology of Ovarian Cancer
2.1. Epidemiology and Pathogenesis of Ovarian Cancer
2.2. Molecular Characterization and Development
2.3. Prognostic Factors
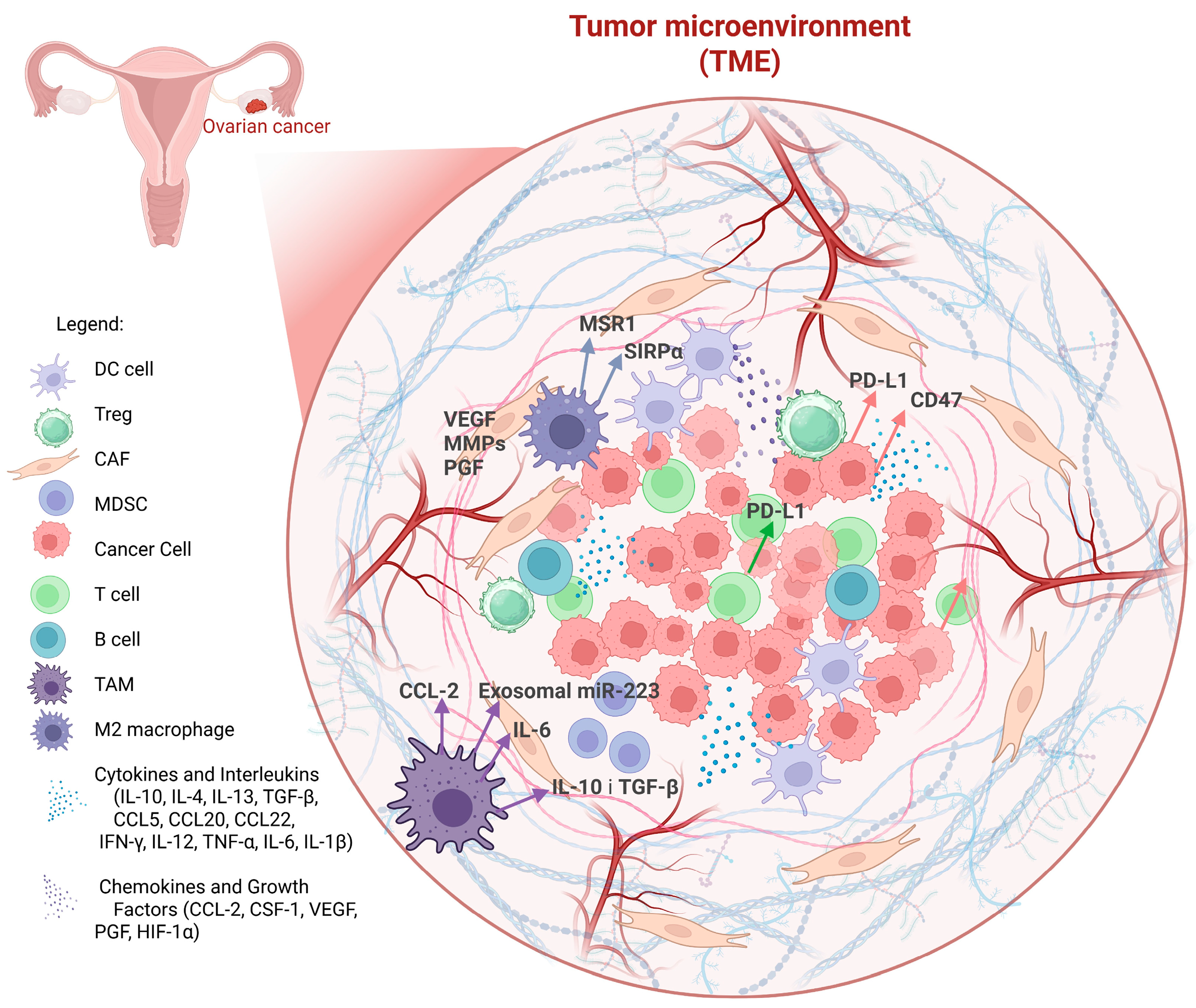
2.4. Composition of the Tumor Microenvironment (TME) and the Immune Cells Compartment in Ovarian Cancer
2.5. Characteristics and Biological Functions of Macrophages
2.6. Origin and Pathophysiological Role of TAMs
Interaction Between TAMs and Ovarian Tumor Cells—Recruitment and Characteristics
- Colony-stimulating factor-1 (CSF-1)—drives the differentiation of monocytes into macrophages [61].
- C-C motif chemokine ligand 2 (CCL2)—enhances monocyte recruitment to the TME [62].
- Vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1α (HIF-1α)—create a hypoxic and angiogenic environment that attracts and polarizes macrophages towards a pro-tumor phenotype [63].
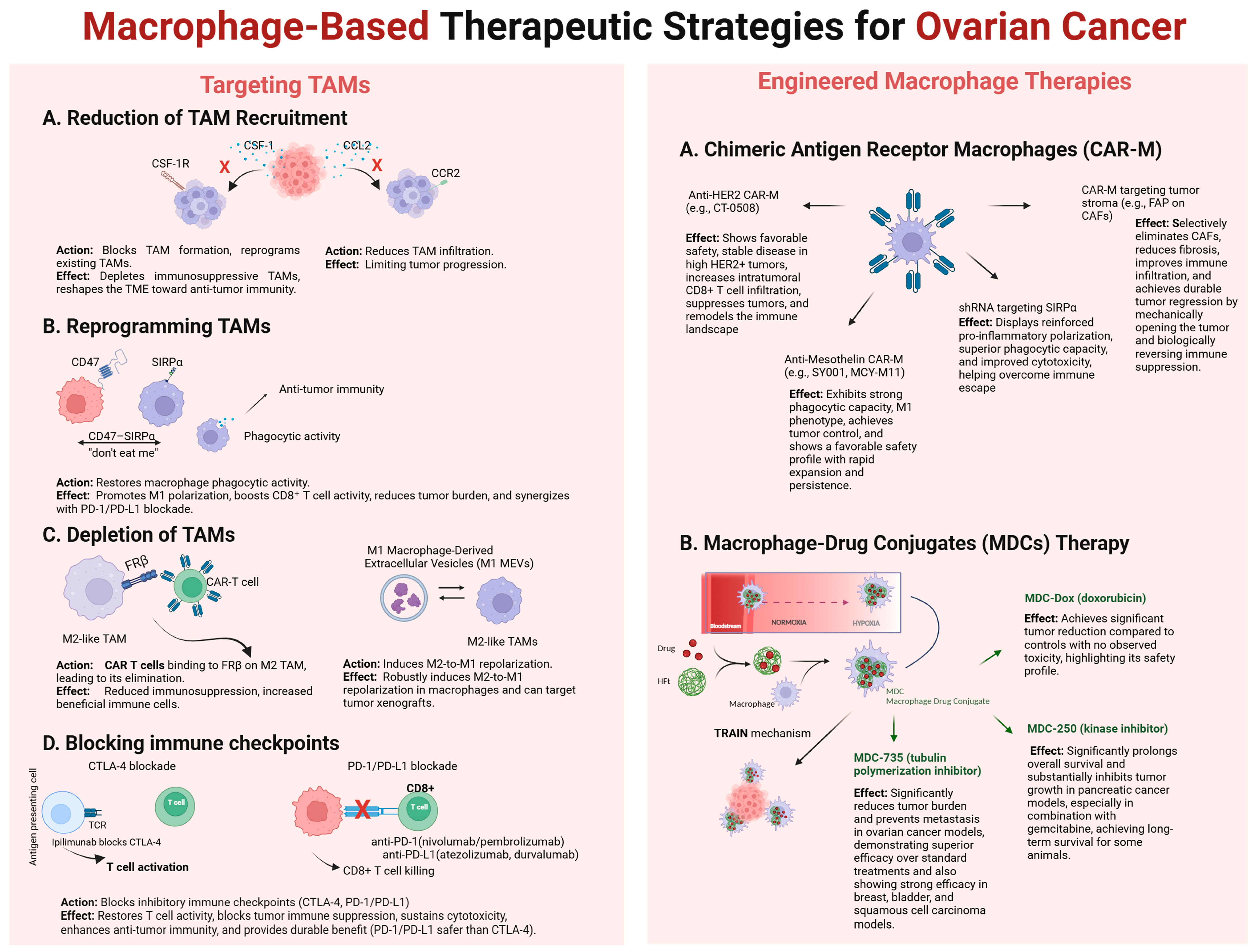
3. Macrophages—Target in Ovarian Cancer Therapy
3.1. Blocking the Migration and Recruitment of Macrophages into the Tumor Microenvironment
3.2. Reprogramming TAMs
3.3. Selective Depletion of Macrophages
3.4. Blocking Immune Checkpoints
3.5. Effectiveness of TAM-Targeted Therapy—Summary
4. Macrophages—A Tool in Ovarian Cancer Therapy
4.1. Chimeric Antigen Receptor (CAR)
4.2. CAR-M
4.3. Macrophages as a Delivery Vehicle
4.4. CAR-M and MDC Therapies: Divergent Challenges and Emerging Opportunities
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACT | Adoptive Cell Transfer |
| ADU-S100 | (MIW815)—A STING agonist |
| BCMA | B-cell Maturation Antigen |
| BRCA1 | Breast cancer type 1 susceptibility protein gene |
| BRCA2 | Breast cancer type 2 susceptibility protein gene |
| CAFs | Cancer-Associated Fibroblasts |
| CAR | Chimeric Antigen Receptor |
| CAR-M | Chimeric Antigen Receptor Macrophages |
| CAR-NK | Chimeric Antigen Receptor Natural Killer cells |
| CAR-T | Chimeric Antigen Receptor T cells |
| CCL2 | C-C Motif Chemokine Ligand 2 |
| CCR2 | C-C Chemokine Receptor Type 2 |
| CSF-1 | Colony-Stimulating Factor 1 |
| CSF-1R | Colony-Stimulating Factor 1 Receptor |
| CTL | Cytotoxic T Lymphocytes |
| CTLA-4 | Cytotoxic T-Lymphocyte-Associated Protein 4 |
| DNAM-1 | DNAX Accessory Molecule-1 |
| ECM | Extracellular Matrix |
| EMT | Epithelial-to-Mesenchymal Transition |
| EOC | Epithelial Ovarian Cancer |
| FAP | Fibroblast Activation Protein (a CAR-M target) |
| FDA | Food and Drug Administration |
| FRβ | Folate Receptor Beta |
| GvHD | Graft-versus-Host Disease |
| HFt | Human Heavy-Chain Ferritin |
| HER2 | Human Epidermal growth factor Receptor 2 |
| HGSC | High-Grade Serous Carcinoma |
| HIF-1α | Hypoxia-Inducible Factor 1-alpha |
| HRR | Homologous Recombination Repair |
| ICIs | Immune Checkpoint Inhibitors |
| IFN-γ | Interferon Gamma |
| IL-10 | Interleukin 10 |
| iPSC | Induced Pluripotent Stem Cell |
| LGSC | Low-Grade Serous Carcinoma |
| M1 | Classically activated macrophages (pro-inflammatory) |
| M1 MEVs | M1 macrophage-derived extracellular vesicles |
| M2 | Alternatively activated macrophages (immunosuppressive) |
| MDCs | Macrophage–Drug Conjugates |
| MDSCs | Myeloid-Derived Suppressor Cells |
| MHC | Major Histocompatibility Complex |
| MICA/MICB | MHC Class I Chain-Related Protein A/B |
| MMPs | Matrix Metalloproteinases |
| NF-kB | Nuclear factor kB |
| NK | Natural Killer |
| PARP | Poly(ADP-ribose) Polymerase |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| PGF | Placental Growth Factor |
| RMI | Risk of Malignancy Index |
| ROS | Reactive Oxygen Species |
| TAMs | Tumor-Associated Macrophages |
| TCR | T Cell Receptor |
| TGF-β | Transforming Growth Factor Beta |
| TME | Tumor Microenvironment |
| TNF-α | Tumor Necrosis Factor Alpha |
| TRAIN | TRAnsfer of Iron-binding proteiN |
| TRUCKs | T cells redirected for universal cytokine-mediated killing |
| Tregs | Regulatory T Cells |
| ULBP | UL16-Binding Proteins |
| VEGF | Vascular Endothelial Growth Factor |
| ctDNA | Circulating Tumor DNA |
| scFv | Single-Chain Variable Fragment |
| scRNA-seq | Single-Cell RNA Sequencing |
References
- Mantovani, A.; Sica, A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr. Opin. Immunol. 2010, 22, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Carey, P.; Low, E.; Harper, E.; Stack, M.S. Metalloproteinases in Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 3403. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, H.; Mao, Y.; Wang, X.; Zhang, X.; Yu, X.; Tian, J.; Lei, Z.; Li, C.; Han, Q.; et al. Apoptotic SKOV3 cells stimulate M0 macrophages to differentiate into M2 macrophages and promote the proliferation and migration of ovarian cancer cells by activating the ERK signaling pathway. Int. J. Mol. Med. 2019, 45, 10–22. [Google Scholar] [CrossRef]
- Bertout, J.A.; Patel, S.A.; Simon, M.C. The impact of O2 availability on human cancer. Nat. Rev. Cancer 2008, 8, 967–975. [Google Scholar] [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Yang, J.; Zhao, X.; Wei, X. Tumor Microenvironment in Ovarian Cancer: Function and Therapeutic Strategy. Front. Cell Dev. Biol. 2020, 8, 758. [Google Scholar] [CrossRef]
- Xu, C.; Chen, J.; Tan, M.; Tan, Q. The role of macrophage polarization in ovarian cancer: From molecular mechanism to therapeutic potentials. Front. Immunol. 2025, 16, 1543096. [Google Scholar] [CrossRef]
- Cao, W.; Chen, H.-D.; Yu, Y.-W.; Li, N.; Chen, W.-Q. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin. Med, J. 2021, 134, 783–791. [Google Scholar] [CrossRef]
- Reddy, J.P.; Atkinson, R.L.; Larson, R.; Burks, J.K.; Smith, D.; Debeb, B.G.; Ruffell, B.; Creighton, C.J.; Bambhroliya, A.; Reuben, J.M.; et al. Mammary stem cell and macrophage markers are enriched in normal tissue adjacent to inflammatory breast cancer. Breast Cancer Res. Treat. 2018, 171, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Xu, J.; Aysola, K.; Qin, Y.; Okoli, C.; Hariprasad, R.; Chinemerem, U.; Gates, C.; Reddy, A.; Danner, O.; et al. Epithelial ovarian cancer: An overview. World J. Transl. Med. 2014, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wilczyński, J.; Paradowska, E.; Wilczyński, M. High-Grade Serous Ovarian Cancer—A Risk Factor Puzzle and Screening Fugitive. Biomedicines 2024, 12, 229. [Google Scholar] [CrossRef]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Women’s Health 2019, 11, 287–299. [Google Scholar] [CrossRef]
- Gaona-Luviano, P.; Medina-Gaona, L.A.; Magaña-Pérez, K. Epidemiology of ovarian cancer. Chin. Clin. Oncol. 2020, 9, 47. [Google Scholar] [CrossRef]
- Petrucelli, N.; Daly, M.B.; Pal, T. BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer; University of Washington: Seattle, DC, USA, 1998. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1247/ (accessed on 25 September 2025).
- Huang, J.; Chen, J.; Huang, Q. Diagnostic value of HE4 in ovarian cancer: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 231, 35–42. [Google Scholar] [CrossRef]
- Antoniou, A.; Pharoah, P.D.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg, A.; et al. Average Risks of Breast and Ovarian Cancer Associated with BRCA1 or BRCA2 Mutations Detected in Case Series Unselected for Family History: A Combined Analysis of 22 Studies. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef]
- Iqbal, J.; Ragone, A.; Lubinski, J.; Lynch, H.T.; Moller, P.; Ghadirian, P.; Foulkes, W.D.; Armel, S.; Eisen, A.; Neuhausen, S.L.; et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br. J. Cancer 2012, 107, 2005–2009. [Google Scholar] [CrossRef]
- Jeong, K.-Y.; Park, M.H. The Significance of Targeting Poly (ADP-Ribose) Polymerase-1 in Pancreatic Cancer for Providing a New Therapeutic Paradigm. Int. J. Mol. Sci. 2021, 22, 3509. [Google Scholar] [CrossRef] [PubMed]
- Lisio, M.-A.; Fu, L.; Goyeneche, A.; Gao, Z.-H.; Telleria, C. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef]
- Song, H.; Dicks, E.; Ramus, S.J.; Tyrer, J.P.; Intermaggio, M.P.; Hayward, J.; Edlund, C.K.; Conti, D.; Harrington, P.; Fraser, L.; et al. Contribution of Germline Mutations in the RAD51B, RAD51C, and RAD51D Genes to Ovarian Cancer in the Population. J. Clin. Oncol. 2015, 33, 2901–2907. [Google Scholar] [CrossRef] [PubMed]
- Graffeo, R.; Rana, H.; Conforti, F.; Bonanni, B.; Cardoso, M.; Paluch-Shimon, S.; Pagani, O.; Goldhirsch, A.; Partridge, A.; Lambertini, M.; et al. Moderate penetrance genes complicate genetic testing for breast cancer diagnosis: ATM, CHEK2, BARD1 and RAD51D. Breast 2022, 65, 32–40. [Google Scholar] [CrossRef]
- Davar, R.; Yalamanchili, M. Identification of a Panel of Biomarkers for the Early Detection of Ovarian Cancer. J. Stud. Res. 2022, 11, 2. [Google Scholar] [CrossRef]
- Ngu, S.F.; Chai, Y.K.; Choi, K.M.; Leung, T.W.; Li, J.; Kwok, G.S.T.; Chu, M.M.Y.; Tse, K.Y.; Cheung, V.Y.T.; Ngan, H.Y.S.; et al. Diagnostic Performance of Risk of Malignancy Algorithm (ROMA), Risk of Malignancy Index (RMI) and Expert Ultrasound Assessment in a Pelvic Mass Classified as Inconclusive by International Ovarian Tumour Analysis (IOTA) Simple Rules. Cancers 2022, 14, 810. [Google Scholar] [CrossRef]
- Ali, A.T.; Al-Ani, O.; Al-Ani, F. Epidemiology and risk factors for ovarian cancer. Menopausal Rev. 2023, 22, 93–104. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, C.; Huang, Z.; Li, Z.; Zhang, Q.; He, Y. In Silico screening of circulating tumor DNA, circulating microRNAs, and long non-coding RNAs as diagnostic molecular biomarkers in ovarian cancer: A comprehensive meta-analysis. PLoS ONE 2021, 16, e0250717. [Google Scholar] [CrossRef]
- Haunschild, C.E.; Tewari, K.S. The current landscape of molecular profiling in the treatment of epithelial ovarian cancer. Gynecol. Oncol. 2021, 160, 333–345. [Google Scholar] [CrossRef]
- Jaliffa, C.; Rogel, U.; Sen, I.; Singer, G. Comprehensive Genomic Characterization in Ovarian Low-Grade and Chemosensitive and Chemoresistant High-Grade Serous Carcinomas. Oncology 2024, 102, 979–987. [Google Scholar] [CrossRef]
- Hollis, R.L.; Gourley, C. Genetic and molecular changes in ovarian cancer. Cancer Biol. Med. 2016, 13, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, G.; Li, C.; Sparovec, T.G.; Dekanski, A.; Torstensson, S.; Risal, S.; Ohlsson, C.; Hirschberg, A.L.; Petropoulos, S.; Deng, Q.; et al. Single-cell profiling of the human endometrium in polycystic ovary syndrome. Nat. Med. 2025, 31, 1925–1938. [Google Scholar] [CrossRef]
- Xiang, X.; Tao, X.; Hua, K.; Jiang, H.; Ding, J. Single-cell RNA sequencing reveals tumor heterogeneity in small cell neuroendocrine cervical carcinoma. Commun. Biol. 2025, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Avci, C.B.; Bagca, B.G.; Nikanfar, M.; Takanlou, L.S.; Takanlou, M.S.; Nourazarian, A. Tumor microenvironment and cancer metastasis: Molecular mechanisms and therapeutic implications. Front. Pharmacol. 2024, 15, 1442888. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Padzińska-Pruszyńska, I.B.; Taciak, B.; Kiraga, Ł.; Smolarska, A.; Górczak, M.; Kucharzewska, P.; Kubiak, M.; Szeliga, J.; Matejuk, A.; Król, M. Targeting Cancer: Microenvironment and Immunotherapy Innovations. Int. J. Mol. Sci. 2024, 25, 13569. [Google Scholar] [CrossRef] [PubMed]
- Kloosterman, D.J.; Akkari, L. Macrophages at the interface of the co-evolving cancer ecosystem. Cell 2023, 186, 1627–1651. [Google Scholar] [CrossRef]
- Tai, Y.-T.; Lin, W.-C.; Ye, J.; Chen, D.T.-H.; Chen, K.-C.; Wang, D.Y.-T.; Tan, T.Z.; Wei, L.-H.; Huang, R.Y.-J. Spatial Profiling of Ovarian Clear Cell Carcinoma Reveals Immune-Hot Features. Mod. Pathol. 2024, 38, 100630. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and hot tumors: From molecular mechanisms to targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 274. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and Tissue Specialization. Annu. Rev. Immunol. 2015, 33, 643–675. [Google Scholar] [CrossRef]
- Vogel, D.Y.; Heijnen, P.D.; Breur, M.; de Vries, H.E.; Tool, A.T.; Amor, S.; Dijkstra, C.D. Macrophages migrate in an activation-dependent manner to chemokines involved in neuroinflammation. J. Neuroinflamm. 2014, 11, 23. [Google Scholar] [CrossRef]
- Curi, R.; de Siqueira Mendes, R.; de Campos Crispin, L.A.; Norata, G.D.; Sampaio, S.C.; Newsholme, P. A past and present overview of macrophage metabolism and functional outcomes. Clin. Sci. 2017, 131, 1329–1342. [Google Scholar] [CrossRef]
- Tugal, D.; Liao, X.; Jain, M.K. Transcriptional Control of Macrophage Polarization. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1135–1144. [Google Scholar] [CrossRef]
- Rongvaux, A.; Willinger, T.; Martinek, J.; Strowig, T.; Gearty, S.V.; Teichmann, L.L.; Saito, Y.; Marches, F.; Halene, S.; Palucka, A.K.; et al. Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol. 2014, 32, 364–372. [Google Scholar] [CrossRef]
- Tong, N.; He, Z.; Ma, Y.; Wang, Z.; Huang, Z.; Cao, H.; Xu, L.; Zou, Y.; Wang, W.; Yi, C.; et al. Tumor Associated Macrophages, as the Dominant Immune Cells, Are an Indispensable Target for Immunologically Cold Tumor-Glioma Therapy? Front. Cell Dev. Biol. 2021, 9, 706286. [Google Scholar] [CrossRef]
- Saeed, A.F. Tumor-Associated Macrophages: Polarization, Immunoregulation, and Immunotherapy. Cells 2025, 14, 741. [Google Scholar] [CrossRef]
- Ghamangiz, S.; Jafari, A.; Maleki-Kakelar, H.; Azimi, H.; Mazloomi, E. Reprogram to heal: Macrophage phenotypes as living therapeutics. Life Sci. 2025, 371, 123601. [Google Scholar] [CrossRef]
- Xu, J.; Ding, L.; Mei, J.; Hu, Y.; Kong, X.; Dai, S.; Bu, T.; Xiao, Q.; Ding, K. Dual roles and therapeutic targeting of tumor-associated macrophages in tumor microenvironments. Signal Transduct. Target. Ther. 2025, 10, 268. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, A.; Lynn, R.C.; Poussin, M.; Eiva, M.A.; Shaw, L.C.; O’cOnnor, R.S.; Minutolo, N.G.; Casado-Medrano, V.; Lopez, G.; Matsuyama, T.; et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat. Commun. 2021, 12, 877. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.G.; Robinson, J.M.; Sharma, P.; Rodriguez-Garcia, A.; Poussin, M.A.; Nickerson-Nutter, C.; Powell, D.J. Folate Receptor Beta as a Direct and Indirect Target for Antibody-Based Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 5572. [Google Scholar] [CrossRef] [PubMed]
- Dizman, N.; Buchbinder, E.I. Cancer Therapy Targeting CD47/SIRPα. Cancers 2021, 13, 6229. [Google Scholar] [CrossRef] [PubMed]
- Sikic, B.I.; Lakhani, N.; Patnaik, A.; Shah, S.A.; Chandana, S.R.; Rasco, D.; Colevas, A.D.; O’rOurke, T.; Narayanan, S.; Papadopoulos, K.; et al. First-in-Human, First-in-Class Phase I Trial of the Anti-CD47 Antibody Hu5F9-G4 in Patients with Advanced Cancers. J. Clin. Oncol. 2019, 37, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, N.J.; Stewart, D.; Richardson, D.L.; Dockery, L.E.; Van Le, L.; Call, J.; Rangwala, F.; Wang, G.; Ma, B.; Metenou, S.; et al. First-in-human phase I trial of the bispecific CD47 inhibitor and CD40 agonist Fc-fusion protein, SL-172154 in patients with platinum-resistant ovarian cancer. J. Immunother. Cancer 2025, 13, e010565. [Google Scholar] [CrossRef]
- Yu, M.; Wu, Y.; Li, Q.; Hong, W.; Hu, X.; Yang, Y.; Lu, T.; Zhao, X.; Wei, X. Colony-stimulating factor-1 receptor inhibition combined with paclitaxel exerts effective antitumor effects in the treatment of ovarian cancer. Genes Dis. 2024, 11, 100989. [Google Scholar] [CrossRef] [PubMed]
- Gaudreau, P.-O.; Allard, B.; Turcotte, M.; Stagg, J. CD73-adenosine reduces immune responses and survival in ovarian cancer patients. OncoImmunology 2016, 5, e1127496. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz-Sanabria, A.; Baliu-Piqué, M.; Saiz-Ladera, C.; Rojas, K.; Manzano, A.; Marquina, G.; Casado, A.; Cimas, F.J.; Pérez-Segura, P.; Pandiella, A.; et al. Genomic Signatures of Immune Activation Predict Outcome in Advanced Stages of Ovarian Cancer and Basal-Like Breast Tumors. Front. Oncol. 2020, 9, 1486. [Google Scholar] [CrossRef]
- Sun, G.; Liu, Y. Tertiary lymphoid structures in ovarian cancer. Front. Immunol. 2024, 15, 1465516. [Google Scholar] [CrossRef]
- Zeng, X.-Y.; Xie, H.; Yuan, J.; Jiang, X.-Y.; Yong, J.-H.; Zeng, D.; Dou, Y.-Y.; Xiao, S.-S. M2-like tumor-associated macrophages-secreted EGF promotes epithelial ovarian cancer metastasis via activating EGFR-ERK signaling and suppressing lncRNA LIMT expression. Cancer Biol. Ther. 2019, 20, 956–966. [Google Scholar] [CrossRef]
- Bialasek, M.; Kubiak, M.; Gorczak, M.; Braniewska, A.; Kucharzewska-Siembieda, P.; Krol, M.; Taciak, B. Exploiting Iron-Binding Proteins for Drug Delivery. Ournal Physiol. Pharmacol. 2019, 70, 675–685. [Google Scholar] [CrossRef]
- Kirkham, P. Oxidative stress and macrophage function: A failure to resolve the inflammatory response. Biochem. Soc. Trans. 2007, 35, 284–287. [Google Scholar] [CrossRef]
- Liu, D.; Liu, L.; Zhao, X.; Zhang, X.; Chen, X.; Che, X.; Wu, G. A comprehensive review on targeting diverse immune cells for anticancer therapy: Beyond immune checkpoint inhibitors. Crit. Rev. Oncol. 2025, 210, 104702. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef]
- Truxova, I.; Cibula, D.; Spisek, R.; Fucikova, J. Targeting tumor-associated macrophages for successful immunotherapy of ovarian carcinoma. J. Immunother. Cancer 2023, 11, e005968. [Google Scholar] [CrossRef]
- Steitz, A.M.; Steffes, A.; Finkernagel, F.; Unger, A.; Sommerfeld, L.; Jansen, J.M.; Wagner, U.; Graumann, J.; Müller, R.; Reinartz, S. Tumor-associated macrophages promote ovarian cancer cell migration by secreting transforming growth factor beta induced (TGFBI) and tenascin C. Cell Death Dis. 2020, 11, 249. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, J.; Zhang, B.; Liang, G.; Chen, X.; Yao, F.; Xu, X.; Wu, H.; He, Q.; Ding, L.; et al. Imatinib prevents lung cancer metastasis by inhibiting M2-like polarization of macrophages. Pharmacol. Res. 2018, 133, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Fang, Y.; Ma, Y.; Wang, F.; Wang, Y.; Jia, J.; Yang, Y.; Sun, W.; Zhou, Q.; Li, Z. Angiogenesis and targeted therapy in the tumour microenvironment: From basic to clinical practice. Clin. Transl. Med. 2025, 15, e70313. [Google Scholar] [CrossRef] [PubMed]
- Garlisi, B.; Lauks, S.; Aitken, C.; Ogilvie, L.M.; Lockington, C.; Petrik, D.; Eichhorn, J.S.; Petrik, J. The Complex Tumor Microenvironment in Ovarian Cancer: Therapeutic Challenges and Opportunities. Curr. Oncol. 2024, 31, 3826–3844. [Google Scholar] [CrossRef]
- Tariq, M.; Zhang, J.; Liang, G.; Ding, L.; He, Q.; Yang, B. Macrophage Polarization: Anti-Cancer Strategies to Target Tumor-Associated Macrophage in Breast Cancer. J. Cell. Biochem. 2017, 118, 2484–2501. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Xie, S.; Wang, Y.; Wu, F.; Chen, Y.; Bian, J.; Gao, X. Development of anti-cancer drugs for tumor-associated macrophages: A comprehensive review and mechanistic insights. Front. Mol. Biosci. 2024, 11, 1463061. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. npj Precis. Oncol. 2024, 8, 31. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, C.; Li, J.; Xiao, J.; Duan, X. CD47-SIRPα axis in cancer therapy: Precise delivery of CD47-targeted therapeutics and design of anti-phagocytic drug delivery systems. Med. Drug Discov. 2022, 15, 100139. [Google Scholar] [CrossRef]
- Huo, X.; Tian, T.; Zhang, X.; Zhou, N. Comparative effectiveness and safety of treatment regimens for recurrent advanced ovarian cancer: A systematic review and network meta-analysis. World, J. Surg. Oncol. 2025, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Zhang, P.; Lei, X.; Du, L.; Qu, B. Insights into CSF-1/CSF-1R signaling: The role of macrophage in radiotherapy. Front. Immunol. 2025, 16, 1530890. [Google Scholar] [CrossRef]
- Hume, D.A.; MacDonald, K.P.A. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 2012, 119, 1810–1820. [Google Scholar] [CrossRef]
- Anfray, C.; Ummarino, A.; Andón, F.T.; Allavena, P. Current Strategies to Target Tumor-Associated-Macrophages to Improve Anti-Tumor Immune Responses. Cells 2019, 9, 46. [Google Scholar] [CrossRef]
- Yang, Y.-I.; Wang, Y.-Y.; Ahn, J.-H.; Kim, B.-H.; Choi, J.-H. CCL2 overexpression is associated with paclitaxel resistance in ovarian cancer cells via autocrine signaling and macrophage recruitment. Biomed. Pharmacother. 2022, 153, 113474. [Google Scholar] [CrossRef]
- Su, P.; Li, O.; Ke, K.; Jiang, Z.; Wu, J.; Wang, Y.; Mou, Y.; Jin, W. Targeting tumor-associated macrophages: Critical players in tumor progression and therapeutic strategies (Review). Int. J. Oncol. 2024, 64, 60. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Sweis, R.F.; Kasper, S.; Hamid, O.; Bhatia, S.; Dummer, R.; Stradella, A.; Long, G.V.; Spreafico, A.; Shimizu, T.; et al. Combination of the STING Agonist MIW815 (ADU-S100) and PD-1 Inhibitor Spartalizumab in Advanced/Metastatic Solid Tumors or Lymphomas: An Open-Label, Multicenter, Phase Ib Study. Clin. Cancer Res. 2022, 29, 110–121. [Google Scholar] [CrossRef]
- Graziani, G.; Tentori, L.; Navarra, P. Ipilimumab: A novel immunostimulatory monoclonal antibody for the treatment of cancer. Pharmacol. Res. 2012, 65, 9–22. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.-A.; Reed, K.; et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Lipson, E.J.; Drake, C.G. Ipilimumab: An Anti-CTLA-4 Antibody for Metastatic Melanoma. Clin. Cancer Res. 2011, 17, 6958–6962. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Pu, Y.; Ji, Q. Tumor-Associated Macrophages Regulate PD-1/PD-L1 Immunosuppression. Front. Immunol. 2022, 13, 874589. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, B.; Zhou, H.; Yang, S.; Yang, L.; Hong, Y. Development of a prognostic immune cell-based model for ovarian cancer using multiplex immunofluorescence. J. Transl. Med. 2025, 23, 688. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Han, R.; Ogurek, S.; Xue, C.; Wu, L.M.; Zhang, L.; Zhang, L.; Hu, J.; Phoenix, T.N.; Waggoner, S.N.; et al. Glioblastoma genetic drivers dictate the function of tumor-associated macrophages/microglia and responses to CSF1R inhibition. Neuro-Oncology 2021, 24, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, T.; Cheng, Y.; Li, F.; Qi, S.; Mao, M.; Wu, J.; Liu, Q.; Zhang, X.; Li, X.; et al. Identification of hypoxic macrophages in glioblastoma with therapeutic potential for vasculature normalization. Cancer Cell 2024, 42, 815–832.e12. [Google Scholar] [CrossRef]
- Pennisi, G.; Valeri, F.; Burattini, B.; Bruzzaniti, P.; Sturiale, C.L.; Talacchi, A.; Papacci, F.; Olivi, A.; Della Pepa, G.M. Targeting Macrophages in Glioblastoma: Current Therapies and Future Directions. Cancers 2025, 17, 2687. [Google Scholar] [CrossRef]
- Farhangnia, P.; Khorramdelazad, H.; Nickho, H.; Delbandi, A.-A. Current and future immunotherapeutic approaches in pancreatic cancer treatment. J. Hematol. Oncol. 2024, 17, 40. [Google Scholar] [CrossRef]
- Pan, D.; Li, X.; Qiao, X.; Wang, Q. Immunosuppressive tumor microenvironment in pancreatic cancer: Mechanisms and therapeutic targets. Front. Immunol. 2025, 16, 1582305. [Google Scholar] [CrossRef]
- Minaei, E.; Ranson, M.; Aghmesheh, M.; Sluyter, R.; Vine, K.L. Enhancing Pancreatic Cancer Immunotherapy: Leveraging Localized Delivery Strategies through the Use of Implantable Devices and Scaffolds. J. Control. Release 2024, 373, 145–160. [Google Scholar] [CrossRef]
- Yu, Z.; Zou, J.; Xu, F. Tumor-associated macrophages affect the treatment of lung cancer. Heliyon 2024, 10, e29332. [Google Scholar] [CrossRef]
- Rannikko, J.H.; Hollmén, M. Clinical landscape of macrophage-reprogramming cancer immunotherapies. Br. J. Cancer 2024, 131, 627–640. [Google Scholar] [CrossRef]
- Ding, J.; Guo, C.; Hu, P.; Chen, J.; Liu, Q.; Wu, X.; Cao, Y.; Wu, J. CSF1 is involved in breast cancer progression through inducing monocyte differentiation and homing. Int. J. Oncol. 2016, 49, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Melaiu, O.; Vanni, G.; Portarena, I.; Pistolese, C.A.; Anemona, L.; Pomella, S.; Bei, R.; Buonomo, O.C.; Roselli, M.; Mauriello, A.; et al. The Combination of Immune Checkpoint Blockade with Tumor Vessel Normalization as a Promising Therapeutic Strategy for Breast Cancer: An Overview of Preclinical and Clinical Studies. Int. J. Mol. Sci. 2023, 24, 3226. [Google Scholar] [CrossRef]
- Schweer, D.; McAtee, A.; Neupane, K.; Richards, C.; Ueland, F.; Kolesar, J. Tumor-Associated Macrophages and Ovarian Cancer: Implications for Therapy. Cancers 2022, 14, 2220. [Google Scholar] [CrossRef]
- McDermott, M.S.; O’BRien, N.A.; Hoffstrom, B.; Gong, K.; Lu, M.; Zhang, J.; Luo, T.; Liang, M.; Jia, W.; Hong, J.J.; et al. Preclinical Efficacy of the Antibody–Drug Conjugate CLDN6–23-ADC for the Treatment of CLDN6-Positive Solid Tumors. Clin. Cancer Res. 2023, 29, 2131–2143. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, C.; Li, X.; Yang, F.; Wang, N.; Ji, G.; Liu, Q.; Zhu, H.; Xu, S.; Li, H. Unraveling the role of M2 TAMs in ovarian cancer dynamics: A systematic review. J. Transl. Med. 2025, 23, 623. [Google Scholar] [CrossRef]
- Liu, M.; Liu, L.; Song, Y.; Li, W.; Xu, L. Targeting macrophages: A novel treatment strategy in solid tumors. J. Transl. Med. 2022, 20, 586. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, Z.; Ren, M.; Li, S.; Zhang, L.; Zhang, X.; Liu, F. Stromal Infiltration of Tumor-Associated Macrophages Conferring Poor Prognosis of Patients with Basal-Like Breast Carcinoma. J. Cancer 2018, 9, 2308–2316. [Google Scholar] [CrossRef]
- Karwicka, K.; Wawer, J.; Czabak, O.; Kocki, J.; Hus, M. Innowacyjna terapia CAR-T w leczeniu nowotworów hematologicznych—Wybrane aspekty genetyczne i immunologiczne. Hematologia 2020, 11, 166–182. [Google Scholar] [CrossRef]
- Jackson, H.J.; Rafiq, S.; Brentjens, R.J. Driving CAR T-cells forward. Nat. Rev. Clin. Oncol. 2016, 13, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.; Abken, H. TRUCKs: The fourth generation of CARs. Expert Opin. Biol. Ther. 2015, 15, 1145–1154. [Google Scholar] [CrossRef]
- Koneru, M.; Purdon, T.J.; Spriggs, D.; Koneru, S.; Brentjens, R.J. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. OncoImmunology 2015, 4, e994446. [Google Scholar] [CrossRef] [PubMed]
- Andreou, T.; Neophytou, C.; Mpekris, F.; Stylianopoulos, T. Expanding Immunotherapy Beyond CAR T Cells: Engineering Diverse Immune Cells to Target Solid Tumors. Cancers 2025, 17, 2917. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Wang, H.; Zuo, D.; Xu, J.; Feng, Y.; Xue, D.; Zhang, L.; Lin, L.; Zhang, J. A clinical study of autologous chimeric antigen receptor macrophage targeting mesothelin shows safety in ovarian cancer therapy. J. Hematol. Oncol. 2024, 17, 116. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, K.; Saio, M.; Suwa, T.; Frey, A.B.; Umemura, N.; Imai, H.; Ouyang, G.-F.; Osada, S.; Balazs, M.; Adany, R.; et al. Skewing the Th cell phenotype toward Th1 alters the maturation of tumor-infiltrating mononuclear phagocytes. J. Leukoc. Biol. 2008, 84, 679–688. [Google Scholar] [CrossRef]
- Henze, A.T.; Mazzone, M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest. 2016, 126, 3672–3679. [Google Scholar] [CrossRef]
- Klichinsky, M.; Ruella, M.; Shestova, O.; Lu, X.M.; Best, A.; Zeeman, M.; Schmierer, M.; Gabrusiewicz, K.; Anderson, N.R.; Petty, N.E.; et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 2020, 38, 947–953. [Google Scholar] [CrossRef]
- Reiss, K.A.; Angelos, M.G.; Dees, E.C.; Yuan, Y.; Ueno, N.T.; Pohlmann, P.R.; Johnson, M.L.; Chao, J.; Shestova, O.; Serody, J.S.; et al. CAR-macrophage therapy for HER2-overexpressing advanced solid tumors: A phase 1 trial. Nat. Med. 2025, 31, 1171–1182. [Google Scholar] [CrossRef]
- Sloas, C.; Gill, S.; Klichinsky, M. Engineered CAR-Macrophages as Adoptive Immunotherapies for Solid Tumors. Front. Immunol. 2021, 12, 783305. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Ma, P.; Zha, Y.; Zhang, J.; Lei, A.; Li, N. CAR-macrophage: An extensive immune enhancer to fight cancer. EBioMedicine 2022, 76, 103873. [Google Scholar] [CrossRef]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Kelly, P.; Davison, R.; Bliss, E.; McGee, J. Macrophages in human breast disease: A quantitative immunohistochemical study. Br. J. Cancer 1988, 57, 174–177. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, X.; Liu, X.; Shen, Y.; Wang, K. Macrophages as an active tumour-targeting carrier of SN38-nanoparticles for cancer therapy. J. Drug Target. 2017, 26, 458–465. [Google Scholar] [CrossRef]
- Muthana, M.; Giannoudis, A.; Scott, S.D.; Fang, H.-Y.; Coffelt, S.B.; Morrow, F.J.; Murdoch, C.; Burton, J.; Cross, N.; Burke, B.; et al. Use of Macrophages to Target Therapeutic Adenovirus to Human Prostate Tumors. Cancer Res. 2011, 71, 1805–1815. [Google Scholar] [CrossRef]
- Muthana, M.; Kennerley, A.J.; Hughes, R.; Fagnano, E.; Richardson, J.; Paul, M.; Murdoch, C.; Wright, F.; Payne, C.; Lythgoe, M.F.; et al. Directing cell therapy to anatomic target sites in vivo with magnetic resonance targeting. Nat. Commun. 2015, 6, 8009. [Google Scholar] [CrossRef] [PubMed]
- Chernajovsky, Y.; Layward, L.; Lemoine, N. Fighting cancer with oncolytic viruses. BMJ 2006, 332, 170–172. [Google Scholar] [CrossRef]
- Choi, J.; Kim, H.-Y.; Ju, E.J.; Jung, J.; Park, J.; Chung, H.-K.; Lee, J.S.; Lee, J.S.; Park, H.J.; Song, S.Y.; et al. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials 2012, 33, 4195–4203. [Google Scholar] [CrossRef]
- Taciak, B.; Bialasek, M.; Kubiak, M.; Marszalek, I.; Gorczak, M.; Osadchuk, O.; Kurpiel, D.; Strzemecki, D.; Barwik, K.; Skorzynski, M.; et al. Harnessing macrophage-drug conjugates for allogeneic cell-based therapy of solid tumors via the TRAIN mechanism. Nat. Commun. 2025, 16, 1327. [Google Scholar] [CrossRef] [PubMed]
- Magdalena, K.; Irene, B.; Paola, B.; Tomasz, R.; Alberto, B. Cellular Targeted Active Ingredient Delivery System. U.S. Patent US20240060045A1, 22 September 2022. [Google Scholar]
- Sun, M.; Bialasek, M.; Mayoux, M.; Lin, M.-S.; Buck, A.; Marszałek, I.; Taciak, B.; Bühler, M.; Górczak, M.; Kucharzewska, P.; et al. Adoptive cell therapy with macrophage-drug conjugates facilitates cytotoxic drug transfer and immune activation in glioblastoma models. Sci. Transl. Med. 2025, 17, eadr4058. [Google Scholar] [CrossRef]
- Meyron-Holtz, E.G.; Fibach, E.; Gelvan, D.; Konijn, A.M. Binding and uptake of exogenous isoferritins by cultured human erythroid precursor cells. Br. J. Haematol. 1994, 86, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Morva, A.; Arroyo, A.B.; Andreeva, L.; Tapia-Abellán, A.; Luengo-Gil, G. Unleashing the power of CAR-M therapy in solid tumors: A comprehensive review. Front. Immunol. 2025, 16, 1615760. [Google Scholar] [CrossRef] [PubMed]
| Region | Incidence | Mortality Rate (Per 100,000) |
|---|---|---|
| North America | 8.4 | 4.9 |
| Europe | 7.8 | 5.1 |
| Asia | 6.2 | 4.0 |
| Africa | 3.5 | 2.7 |
| Latin America | 5.4 | 3.6 |
| Risk Factors | Description | Impact or Risk |
|---|---|---|
| Genetic Mutations | homologous recombination repair (HRR) pathway genes (BRCA1, BRCA2, TP53, RAD51C, PALB2) | High |
| Reproductive and Hormonal Factors | Early menarche, late menopause, nulliparity, hormone replacement therapy (HRT) | Moderate |
| Lifestyle and Environmental Factors | Smoking, obesity, high-fat diets | Low to moderate |
| Inflammation | Endometriosis, pelvic inflammatory disease | Moderate |
| Prognostic Factor | Impact on Survival |
|---|---|
| Tumor Microenvironment | High CD8+ T-cell infiltration correlates with better prognosis |
| BRCA Mutations | Improved response to PARP inhibitors and platinum-based chemotherapy |
| Circulating Biomarkers | ctDNA and exosomal RNA predict treatment response |
| Chemoresistance Genes | ABC transporters and drug efflux genes contribute to resistance |
| Inflammatory Markers | IL-6 and TNF-alpha associated with poor prognosis |
| Feature | Immune “Hot” Tumors | Immune “Cold” Tumors |
|---|---|---|
| Immune Cell Infiltration | High (especially CD8+ T cells) | Low |
| Gene Expression Profile | High expression of immune activation and IFN-γ pathways | Low immune gene expression; suppressive markers may dominate |
| Response to Immunotherapy | Often responsive | Generally unresponsive |
| Presence of TLS (Tertiary Lymphoid Structures) | Common | Rare or absent |
| Tumor Microenvironment (TME) | Inflamed, immunologically active | Immune-excluded or immunosuppressed |
| Common Immune Cell Types | CD8+ T cells, Th1 cells, dendritic cells | Tregs, M2 macrophages, few effector T cells |
| Spatial Pattern | Dense clusters of immune cells within tumor core and periphery | Sparse immune presence, often restricted to stromal edges |
| Therapeutic Strategy | Checkpoint inhibitors, adoptive T cell therapy | Combination therapies to induce immune infiltration |
| Function | Mechanism | Key Factors |
|---|---|---|
| Promotion of Tumor Invasion | TAMs secrete matrix metalloproteinases (MMPs), particularly MMP-2 and MMP-9, which degrade the extracellular matrix (ECM) and facilitate tumor cell invasion. TAMs also enhance epithelial-to-mesenchymal transition (EMT), supporting metastasis [64,65]. | MMP-2, MMP-9, EMT [64,65]. |
| Fostering Tumor Angiogenesis | TAMs promote neovascularization by releasing VEGF, PDGF, and angiopoietins. They also secrete pro-inflammatory cytokines like TNF-α and IL-6, which stimulate endothelial proliferation and increase vascular permeability [66]. | VEGF, PDGF, angiopoietins, TNF-α, IL-6 [66]. |
| Immune Suppression and Therapy Resistance | TAMs produce immunosuppressive cytokines (IL-10, TGF-β) that inhibit cytotoxic T cells and expand regulatory T cells. They upregulate immune checkpoint molecules (e.g., PD-L1), contributing to immune evasion. TAMs also support chemotherapy resistance via interactions with cancer stem cells, aiding tumor survival and dormancy [64,67]. | IL-10, TGF-β, PD-L1, cancer stem cell signaling [64,67]. |
| Tumor Type | TAM-Targeted Approach | Key Observations/Effects |
|---|---|---|
| Glioblastoma | CSF-1R inhibitors, TAM reprogramming [85] | Monotherapy often shows limited efficacy; improved outcomes observed in combination with chemotherapy or anti-angiogenic therapy [85,86,87]. |
| Pancreatic Cancer | TAM recruitment blockade (CCR2), CSF-1R inhibitors [88] | Moderate efficacy; outcome dependent on combination with chemotherapy [89,90]. |
| Lung Cancer | TAM polarization reprogramming, CSF-1R inhibitors [91]. | Variable results depending on histological subtype; some studies suggest synergy with immunotherapy [92]. |
| Breast Cancer | CSF-1R inhibitors, TAM depletion or repolarization [93] | Promising preclinical results; combination with chemotherapy or immune checkpoint blockade enhances response [94]. |
| Ovarian Cancer | CSF-1R inhibitors, TAM polarization modulation [95] | Preclinical and early clinical data suggest comparable or slightly higher efficacy relative to other solid tumors [96]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górczak, M.; Kiraga, Ł. Macrophages—Target and Tool in Tumor Treatment: Insights from Ovarian Cancer. Cancers 2025, 17, 3182. https://doi.org/10.3390/cancers17193182
Górczak M, Kiraga Ł. Macrophages—Target and Tool in Tumor Treatment: Insights from Ovarian Cancer. Cancers. 2025; 17(19):3182. https://doi.org/10.3390/cancers17193182
Chicago/Turabian StyleGórczak, Małgorzata, and Łukasz Kiraga. 2025. "Macrophages—Target and Tool in Tumor Treatment: Insights from Ovarian Cancer" Cancers 17, no. 19: 3182. https://doi.org/10.3390/cancers17193182
APA StyleGórczak, M., & Kiraga, Ł. (2025). Macrophages—Target and Tool in Tumor Treatment: Insights from Ovarian Cancer. Cancers, 17(19), 3182. https://doi.org/10.3390/cancers17193182






