Donor-Derived Vγ9Vδ2 T Cells for Acute Myeloid Leukemia: A Promising “Off-the-Shelf” Immunotherapy Approach
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Peripheral Blood PBMCs and Primary AML Cells
2.3. GMP-Grade Donor-Derived Vγ9Vδ2 T Cell Generation
2.4. Flow Cytometric Profiling of γδ T Cells
2.5. Flow Cytometric Apoptosis Assays
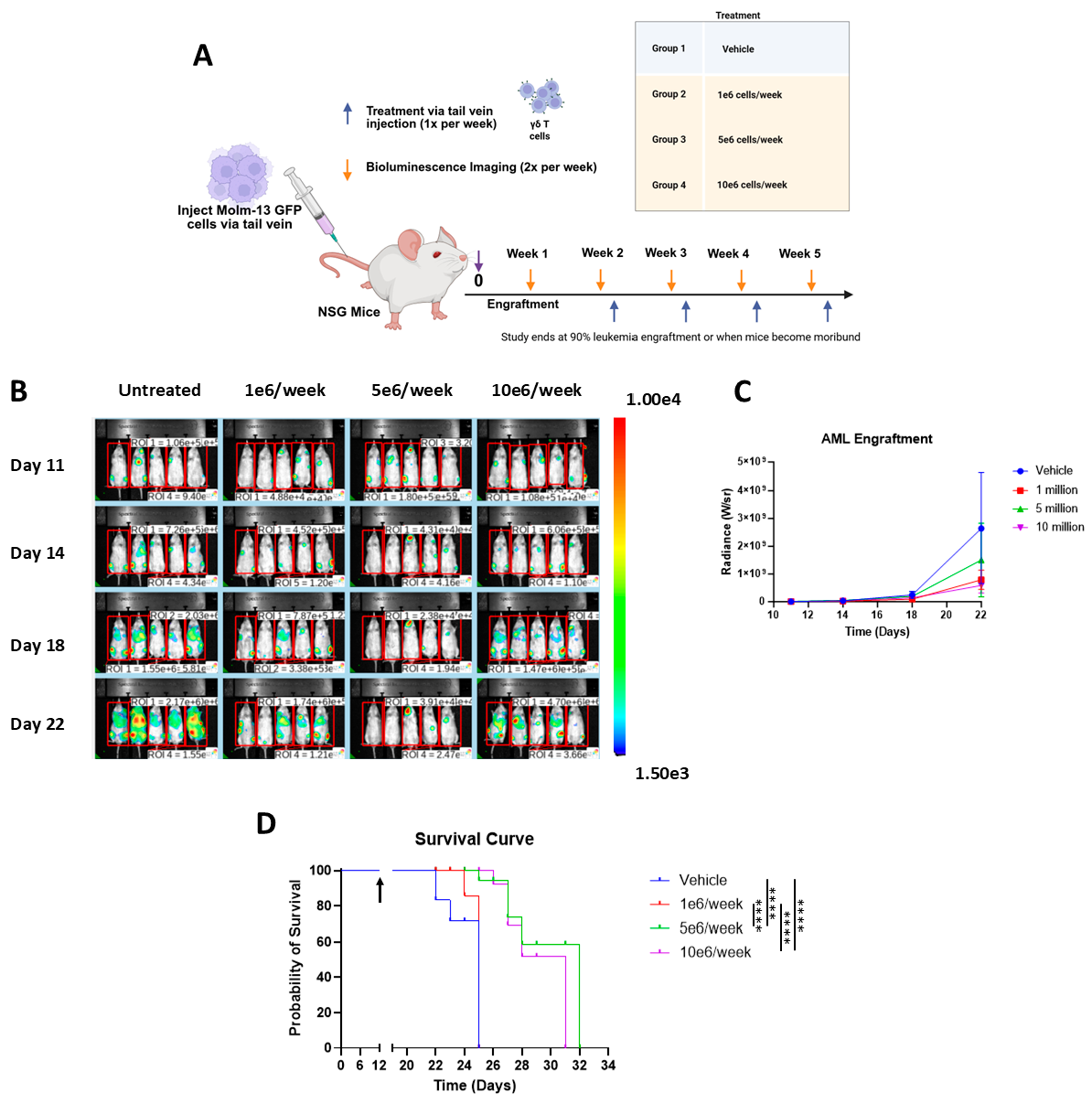
2.6. Vγ9Vδ2 T Cell Therapy in Molm-13 GFP Luc AML Xenograft Model
2.7. Vγ9Vδ2 T Cells and Venetoclax Combination Therapy in OCI-AML3 GFP Luc AML Xenograft Model
2.8. Statistical Analysis
3. Results
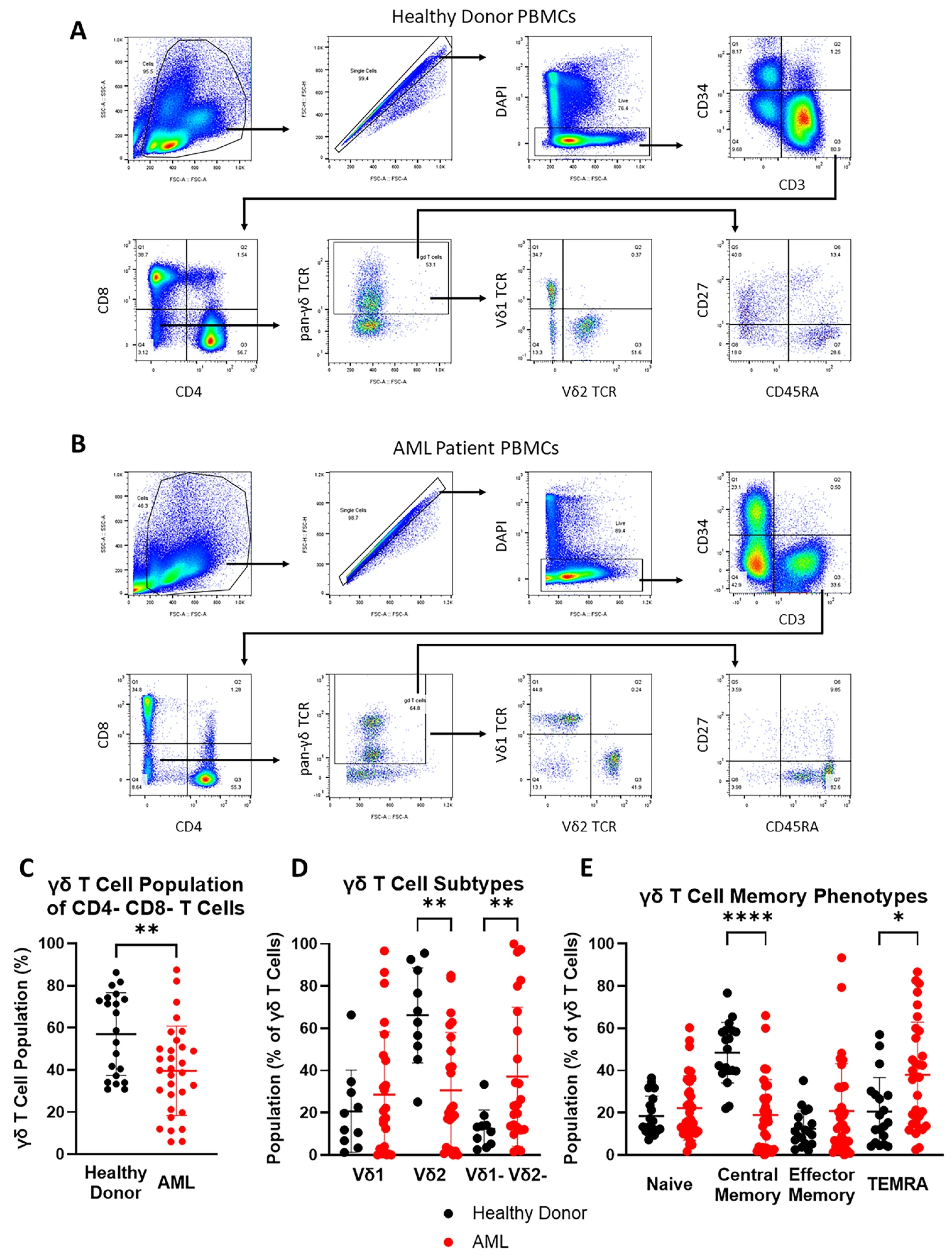
3.1. Vδ2 T Cells Are Less Abundant and TEMRA Phenotype Is More Prevalent in Peripheral Blood γδ T Cell Population of AML Patients
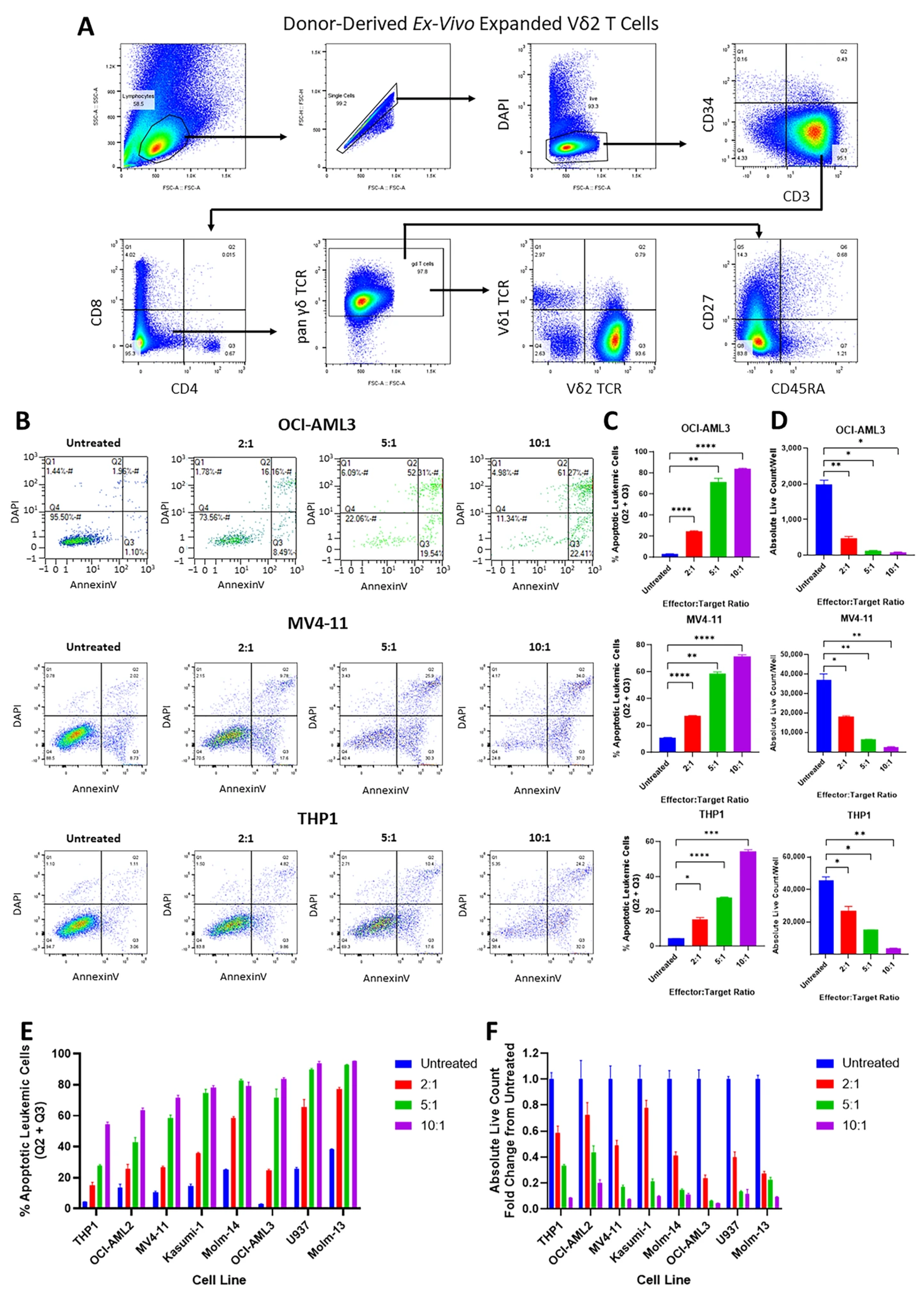
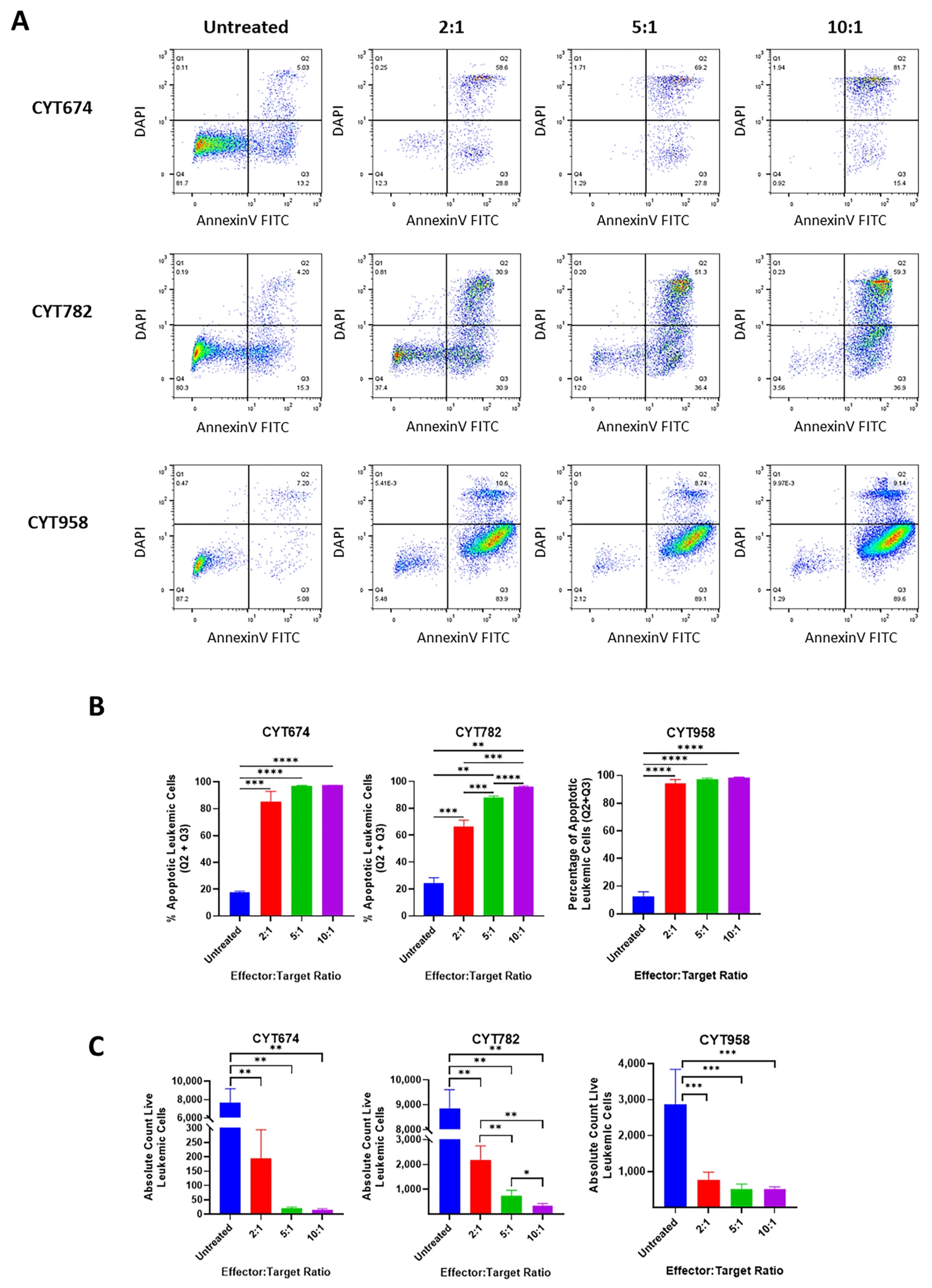
3.2. Vγ9Vδ2 T Cells Are Cytotoxic Against AML Cell Lines and Primary AML Cells
3.3. Vγ9Vδ2 T Cells Alone Extend Survival in Aggressive AML Xenograft Model
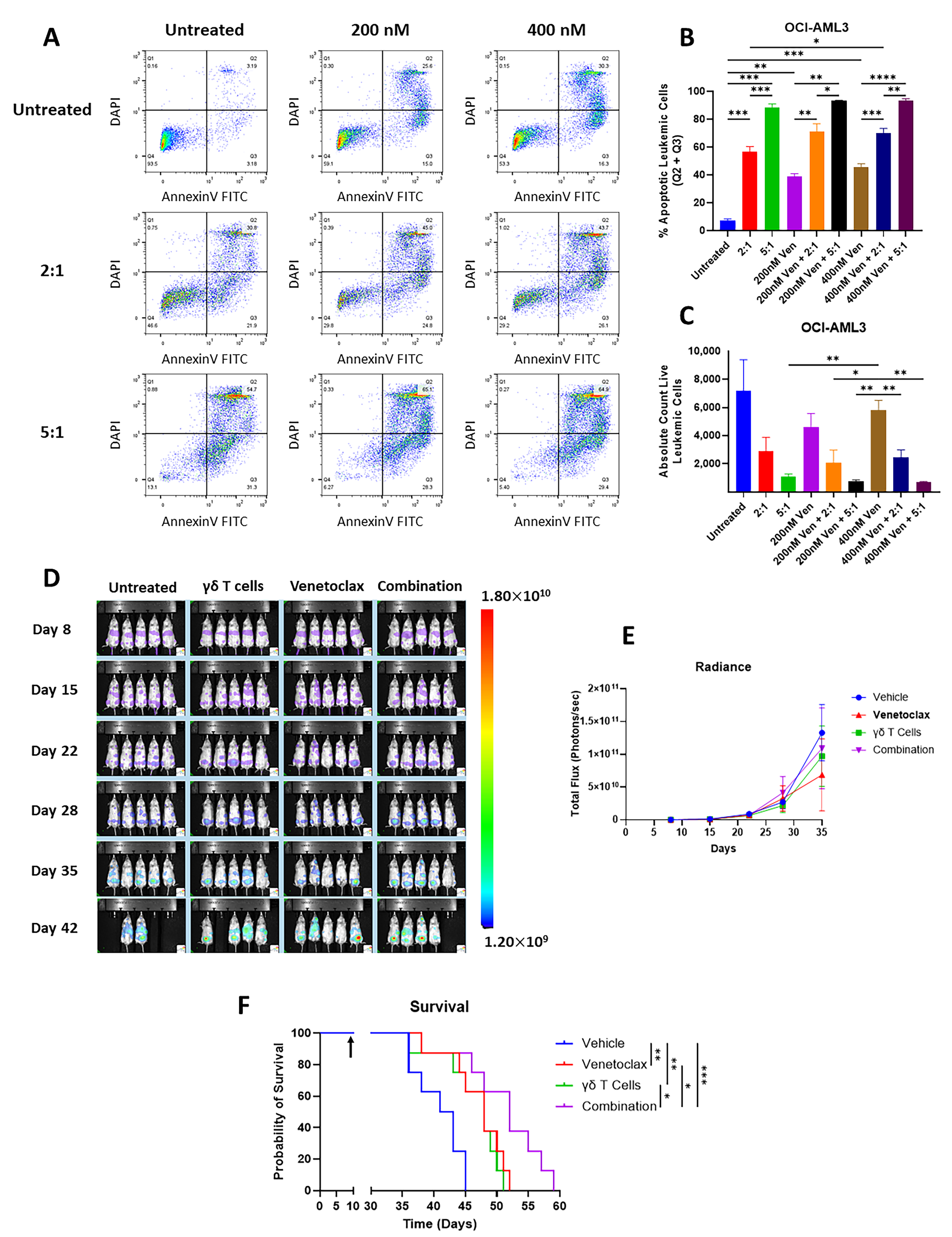
3.4. Vγ9Vδ2 T Cells Are Cytotoxic to Venetoclax-Resistant AML Cells and Vγ9Vδ2 T Cells Alone or in Combination with Venetoclax Extends Survival in Venetoclax-Resistant AML Xenograft Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vakiti, A.; Reynolds, S.B.; Mewawalla, P. Acute Myeloid Leukemia; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Short, N.J.; Rytting, M.E.; Cortes, J.E. Acute myeloid leukaemia. Lancet 2018, 392, 593–606. [Google Scholar] [CrossRef]
- Kolitz, J.E. Acute Myeloid Leukemia in Adults: Overveiw; Larson, R.A., Rosmarin, A.G., Eds.; UpToDate [Internet]: Waltham, MA, USA, 2022. [Google Scholar]
- Ohmoto, A.; Fuji, S. Clinical status of induction therapy incorporating a hypomethylating agent for newly diagnosed adult acute myeloid leukemia compared to the standard 7 + 3 regimen. Expert Rev. Hematol. 2023, 16, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Fernandez, C.; Hauch, O.; Klibanov, O.M.; Chaudhary, T.; Rives, V. Therapies for acute myeloid leukemia in patients ineligible for standard induction chemotherapy: A systematic review. Future Oncology 2023, 19, 789–810. [Google Scholar] [CrossRef]
- Medeiros, B.C.; Satram-Hoang, S.; Hurst, D.; Hoang, K.Q.; Momin, F.; Reyes, C. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann. Hematol. 2015, 94, 1127–1138. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Maiti, A.; Rausch, C.R.; Cortes, J.E.; Pemmaraju, N.; Daver, N.G.; Ravandi, F.; Garcia-Manero, G.; Borthakur, G.; Naqvi, K.; Ohanian, M.; et al. Outcomes of relapsed or refractory acute myeloid leukemia after frontline hypomethylating agent and venetoclax regimens. Haematologica 2021, 106, 894–898. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, X. Cellular immunotherapy for hematological malignancy: Recent progress and future perspectives. Cancer Biol. Med. 2021, 18, 966–980. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Goldenson, B.H.; Kaufman, D.S. Into the multiverse of gene edited NK cell-based therapeutic strategies. Cell Stem Cell 2021, 28, 2041–2043. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lal, G. Regulatory and effector functions of gamma-delta (gammadelta) T cells and their therapeutic potential in adoptive cellular therapy for cancer. Int. J. Cancer 2016, 139, 976–985. [Google Scholar] [CrossRef]
- Ou, L.; Wang, H.; Liu, Q.; Zhang, J.; Lu, H.; Luo, L.; Shi, C.; Lin, S.; Dong, L.; Guo, Y.; et al. Dichotomous and stable gamma delta T-cell number and function in healthy individuals. J. Immunother. Cancer 2021, 9, e002274. [Google Scholar] [CrossRef] [PubMed]
- Deseke, M.; Prinz, I. Ligand recognition by the γδ TCR and discrimination between homeostasis and stress conditions. Cell. Mol. Immunol. 2020, 17, 914–924. [Google Scholar] [CrossRef]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Klyuchnikov, E.; Badbaran, A.; Massoud, R.; Fritsche-Friedland, U.; Janson, D.; Ayuk, F.; Wolschke, C.; Bacher, U.; Kröger, N. Enhanced Immune Reconstitution of γδ T Cells after Allogeneic Stem Cell Transplantation Overcomes the Negative Impact of Pretransplantation Minimal Residual Disease-Positive Status in Patients with Acute Myelogenous Leukemia. Transplant. Cell. Ther. 2021, 27, 841–850. [Google Scholar]
- Godder, K.T.; Henslee-Downey, P.J.; Mehta, J.; Park, B.S.; Chiang, K.-Y.; Abhyankar, S.; Lamb, L.S. Long term disease-free survival in acute leukemia patients recovering with increased γδ T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant. 2007, 39, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Minculescu, L.; Marquart, H.V.; Ryder, L.P.; Andersen, N.S.; Schjoedt, I.; Friis, L.S.; Kornblit, B.T.; Petersen, S.L.; Haastrup, E.; Fischer-Nielsen, A.; et al. Improved Overall Survival, Relapse-Free-Survival, and Less Graft-vs.-Host-Disease in Patients with High Immune Reconstitution of TCR Gamma Delta Cells 2 Months After Allogeneic Stem Cell Transplantation. Front. Immunol. 2019, 10, 1997. [Google Scholar] [CrossRef] [PubMed]
- Perko, R.; Kang, G.; Sunkara, A.; Leung, W.; Thomas, P.G.; Dallas, M.H. Gamma Delta T Cell Reconstitution is Associated with Fewer Infections and Improved Event-Free Survival after Hematopoietic Stem Cell Transplantation for Pediatric Leukemia. Biol. Blood Marrow Transplant. 2015, 21, 130–136. [Google Scholar] [CrossRef]
- Zhao, Y.; Niu, C.; Cui, J. Gamma-delta (gammadelta) T cells: Friend or foe in cancer development? J. Transl. Med. 2018, 16, 3. [Google Scholar] [CrossRef]
- Almeida, A.R.; Correia, D.V.; Fernandes-Platzgummer, A.; da Silva, C.L.; da Silva, M.G.; Anjos, D.R.; Silva-Santos, B. Delta One T Cells for Immunotherapy of Chronic Lymphocytic Leukemia: Clinical-Grade Expansion/Differentiation and Preclinical Proof of Concept. Clin. Cancer Res. 2016, 22, 5795–5804. [Google Scholar] [CrossRef]
- Siegers, G.M.; Dhamko, H.; Wang, X.-H.; Mathieson, A.M.; Kosaka, Y.; Felizardo, T.C.; Medin, J.A.; Tohda, S.; Schueler, J.; Fisch, P.; et al. Human Vδ1 γδ T cells expanded from peripheral blood exhibit specific cytotoxicity against B-cell chronic lymphocytic leukemia-derived cells. Cytotherapy 2011, 13, 753–764. [Google Scholar] [CrossRef]
- Kimura, Y.; Nagai, N.; Tsunekawa, N.; Sato-Matsushita, M.; Yoshimoto, T.; Cua, D.J.; Iwakura, Y.; Yagita, H.; Okada, F.; Tahara, H.; et al. IL-17A-producing CD30+ Vδ1 T cells drive inflammation-induced cancer progression. Cancer Sci. 2016, 107, 1206–1214. [Google Scholar] [CrossRef]
- Wistuba-Hamprecht, K.; Martens, A.; Haehnel, K.; Foppen, M.G.; Yuan, J.; Postow, M.A.; Wong, P.; Romano, E.; Khammari, A.; Dreno, B.; et al. Proportions of blood-borne Vδ1+ and Vδ2+ T-cells are associated with overall survival of melanoma patients treated with ipilimumab. Eur. J. Cancer 2016, 64, 116–126. [Google Scholar] [CrossRef]
- Wistuba-Hamprecht, K.; Di Benedetto, S.; Schilling, B.; Sucker, A.; Schadendorf, D.; Garbe, C.; Weide, B.; Pawelec, G. Phenotypic characterization and prognostic impact of circulating γδ and αβ T-cells in metastatic malignant melanoma. Int. J. Cancer 2016, 138, 698–704. [Google Scholar] [CrossRef]
- Rong, L.; Li, K.; Li, R.; Liu, H.M.; Sun, R.; Liu, X.Y. Analysis of tumor-infiltrating gamma delta T cells in rectal cancer. World J. Gastroenterol. 2016, 22, 3573–3580. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Dunn, Z.S.; Guo, W.; Rosenthal, C.J.; Penn, N.E.; Yu, Y.; Zhou, K.; Li, Z.; Ma, F.; Li, M.; et al. Unlocking the potential of allogeneic Vδ2 T cells for ovarian cancer therapy through CD16 biomarker selection and CAR/IL-15 engineering. Nat. Commun. 2023, 14, 6942. [Google Scholar] [CrossRef]
- Karunakaran, M.M.; Willcox, C.R.; Salim, M.; Paletta, D.; Fichtner, A.S.; Noll, A.; Starick, L.; Nöhren, A.; Begley, C.R.; Berwick, K.A.; et al. Butyrophilin-2A1 Directly Binds Germline-Encoded Regions of the Vγ9Vδ2 TCR and Is Essential for Phosphoantigen Sensing. Immunity 2020, 52, 487–498.e486. [Google Scholar] [CrossRef]
- Herrmann, T.; Fichtner, A.S.; Karunakaran, M.M. An Update on the Molecular Basis of Phosphoantigen Recognition by Vγ9Vδ2 T Cells. Cells 2020, 9, 1433. [Google Scholar] [CrossRef]
- Rao, A.; Agrawal, A.; Borthakur, G.; Battula, V.L.; Maiti, A. Gamma delta T cells in acute myeloid leukemia: Biology and emerging therapeutic strategies. J. Immunother. Cancer 2024, 12, e007981. [Google Scholar] [CrossRef]
- Vydra, J.; Cosimo, E.; Lesný, P.; Wanless, R.S.; Anderson, J.; Clark, A.G.; Scott, A.; Nicholson, E.K.; Leek, M. A Phase I Trial of Allogeneic γδ T Lymphocytes From Haploidentical Donors in Patients with Refractory or Relapsed Acute Myeloid Leukemia. Clin. Lymphoma Myeloma Leuk. 2023, 23, e232–e239. [Google Scholar] [CrossRef] [PubMed]
- Anane, L.H.; Edwards, K.M.; Burns, V.E.; van Zanten, J.J.V.; Drayson, M.T.; Bosch, J.A. Phenotypic characterization of γδ T cells mobilized in response to acute psychological stress. Brain Behav. Immun. 2010, 24, 608–614. [Google Scholar] [CrossRef]
- Caccamo, N.; Meraviglia, S.; Ferlazzo, V.; Angelini, D.; Borsellino, G.; Poccia, F.; Battistini, L.; Dieli, F.; Salerno, A. Differential requirements for antigen or homeostatic cytokines for proliferation and differentiation of human Vγ9Vδ2 naive, memory and effector T cell subsets. Eur. J. Immunol. 2005, 35, 1764–1772. [Google Scholar] [CrossRef]
- Angelini, D.F.; Borsellino, G.; Poupot, M.; Diamantini, A.; Poupot, R.; Bernardi, G.; Poccia, F.; Fournié, J.J.; Battistini, L. FcγRIII discriminates between 2 subsets of Vγ9Vδ2 effector cells with different responses and activation pathways. Blood 2004, 104, 1801–1807. [Google Scholar] [CrossRef]
- Coscia, M.; Vitale, C.; Peola, S.; Foglietta, M.; Rigoni, M.; Griggio, V.; Castella, B.; Angelini, D.; Chiaretti, S.; Riganti, C.; et al. Dysfunctional Vγ9Vδ2 T cells are negative prognosticators and markers of dysregulated mevalonate pathway activity in chronic lymphocytic leukemia cells. Blood 2012, 120, 3271–3279. [Google Scholar] [CrossRef][Green Version]
- Maiti, A.; Peterlin, P.; Morillo, D.; Torregrosa-Diaz, J.-M.; Ulrickson, M.; Penedo, A.; De Gassart, A.; Wieduwild, E.; Valentin, E.; Mairesse, M.; et al. Abstract CT024: γ9δ2 T-cell (γδTC) activation and azacitidine-venetoclax (AV) for older/unfit adults with newly diagnosed (ND) acute myeloid leukemia (AML) induces high rates of complete remission (CR): Preliminary efficacy, safety, pharmacodynamics (PD) and dose selection of ICT01 in the phase 1 study EVICTION. Cancer Res. 2025, 85 (Suppl. S2), CT024. [Google Scholar][Green Version]
- Du, S.H.; Li, Z.; Chen, C.; Tan, W.K.; Chi, Z.; Kwang, T.W.; Xu, X.H.; Wang, S. Co-Expansion of Cytokine-Induced Killer Cells and Vγ9Vδ2 T Cells for CAR T-Cell Therapy. PLoS ONE 2016, 11, e0161820. [Google Scholar] [CrossRef]
- Vera, J.; Savoldo, B.; Vigouroux, S.; Biagi, E.; Pule, M.; Rossig, C.; Wu, J.; Heslop, H.E.; Rooney, C.M.; Brenner, M.K.; et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood 2006, 108, 3890–3897. [Google Scholar] [CrossRef]
- Ong, F.; Kim, K.; Konopleva, M.Y. Venetoclax resistance: Mechanistic insights and future strategies. Cancer Drug Resist. 2022, 5, 380–400. [Google Scholar] [CrossRef]
- Reis-Silva, C.S.M.; Branco, P.C.; Lima, K.; Silva, F.L.; Moreno, P.R.H.; Guallar, V.; Costa-Lotufo, L.V.; Machado-Neto, J.A. Embelin potentiates venetoclax-induced apoptosis in acute myeloid leukemia cells. Toxicol. Vitro 2021, 76, 105207. [Google Scholar] [CrossRef]
- Knaus, H.A.; Berglund, S.; Hackl, H.; Blackford, A.L.; Zeidner, J.F.; Montiel-Esparza, R.; Mukhopadhyay, R.; Vanura, K.; Blazar, B.R.; Karp, J.E.; et al. Signatures of CD8+ T cell dysfunction in AML patients and their reversibility with response to chemotherapy. JCI Insight 2018, 3, e120974. [Google Scholar] [CrossRef]
- Zhou, H.; Jia, B.; Annageldiyev, C.; Minagawa, K.; Zhao, C.; Mineishi, S.; Ehmann, W.C.; Naik, S.G.; Cioccio, J.; Wirk, B.; et al. CD26lowPD-1+ CD8 T cells are terminally exhausted and associated with leukemia progression in acute myeloid leukemia. Front. Immunol. 2023, 14, 1169144. [Google Scholar] [CrossRef]
- Barros, M.S.; de Araújo, N.D.; Magalhães-Gama, F.; Pereira Ribeiro, T.L.; Alves Hanna, F.S.; Tarragô, A.M.; Malheiro, A.; Costa, A.G. γδ T Cells for Leukemia Immunotherapy: New and Expanding Trends. Front. Immunol. 2021, 12, 729085. [Google Scholar] [CrossRef]
- Wu, K.; Wang, L.M.; Liu, M.; Xiu, Y.; Hu, Y.; Fu, S.; Huang, H.; Xu, B.; Xiao, H. The CD226-ERK1/2-LAMP1 pathway is an important mechanism for Vγ9Vδ2 T cell cytotoxicity against chemotherapy-resistant acute myeloid leukemia blasts and leukemia stem cells. Cancer Sci. 2021, 112, 3233–3242. [Google Scholar] [CrossRef]
- Jiang, S.; Zheng, S.; Yao, C.; Ning, D.; Zou, S.; Zhan, J.; Lan, T.; Yi, T.; Jin, Z.; Wu, X. Heterogeneity of γδ T-cell subsets and their clinical correlation in patients with AML. Front. Immunol. 2025, 16, 1552235. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, H.; Wu, M.; Peng, G.; He, Y.; Wan, N.; Zeng, Y. Targeting the NKG2D/NKG2D-L axis in acute myeloid leukemia. Biomed. Pharmacother. 2021, 137, 111299. [Google Scholar] [CrossRef] [PubMed]
- Xingchi, C.; Sun, G.; Sui, Y.; Ma, W.; Zhang, Y.; Zhu, X. Venetoclax Enhances Human Γδt Cells Anti-Leukemia Immunity through Metabolic Reprogramming. Blood 2024, 144 (Suppl. S1), 2059. [Google Scholar] [CrossRef]
- Han, J.; Goldstein, L.A.; Gastman, B.R.; Froelich, C.J.; Yin, X.M.; Rabinowich, H. Degradation of Mcl-1 by Granzyme B: Implications for bim-mediated mitochondrial apoptotic events. J. Biol. Chemistry 2004, 279, 22020–22029. [Google Scholar] [CrossRef]
- Catalán, E.; Jaime-Sánchez, P.; Aguiló, N.; Simon, M.M.; Froelich, C.J.; Pardo, J. Mouse cytotoxic T cell-derived granzyme B activates the mitochondrial cell death pathway in a Bim-dependent fashion. J. Biol. Chem. 2015, 290, 6868–6877. [Google Scholar] [CrossRef]
| Population | Healthy Donor | AML | Difference | |||
|---|---|---|---|---|---|---|
| Mean % | SD % | Mean % | SD % | Mean % | p-Value | |
| CD8+ T cells | 41.32 | 9.29 | 27.97 | 13.74 | −13.35 | 0.0004 |
| CD8+ CD4+ T cells | 3.13 | 4.85 | 1.13 | 1.25 | −2.00 | ns |
| CD4+ T cells | 50.55 | 10.88 | 56.06 | 20.63 | 5.51 | ns |
| CD4- CD8- T cells | 5.00 | 2.90 | 14.84 | 19.37 | 9.84 | 0.031 |
| γδ T cells | 57.03 | 19.60 | 39.62 | 21.14 | −17.41 | 0.0039 |
| Vδ1 T cells | 20.70 | 19.50 | 28.63 | 29.67 | 7.93 | ns |
| Vδ2 T cells | 66.21 | 22.54 | 30.59 | 27.46 | −35.62 | 0.0023 |
| Vδ1- Vδ2- T cells | 12.37 | 9.04 | 37.09 | 32.96 | 24.72 | 0.006 |
| Naïve γδ T cells | 18.54 | 9.42 | 22.28 | 15.22 | 3.74 | ns |
| CM γδ T cells | 48.45 | 14.36 | 18.95 | 16.78 | −29.50 | <0.0001 |
| EM γδ T cells | 12.40 | 8.49 | 20.83 | 22.43 | 8.43 | ns |
| TEMRA γδ T cells | 20.63 | 16.10 | 37.94 | 24.94 | 17.31 | 0.0163 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckstrom, A.; Tyagi, A.; Siddiqui, M.; Borgman, J.; Zeng, J.; Rao, A.; Maiti, A.; Battula, V.L. Donor-Derived Vγ9Vδ2 T Cells for Acute Myeloid Leukemia: A Promising “Off-the-Shelf” Immunotherapy Approach. Cancers 2025, 17, 3166. https://doi.org/10.3390/cancers17193166
Eckstrom A, Tyagi A, Siddiqui M, Borgman J, Zeng J, Rao A, Maiti A, Battula VL. Donor-Derived Vγ9Vδ2 T Cells for Acute Myeloid Leukemia: A Promising “Off-the-Shelf” Immunotherapy Approach. Cancers. 2025; 17(19):3166. https://doi.org/10.3390/cancers17193166
Chicago/Turabian StyleEckstrom, Amanda, Anudishi Tyagi, Maryam Siddiqui, Jenny Borgman, Jieming Zeng, Adishwar Rao, Abhishek Maiti, and Venkata Lokesh Battula. 2025. "Donor-Derived Vγ9Vδ2 T Cells for Acute Myeloid Leukemia: A Promising “Off-the-Shelf” Immunotherapy Approach" Cancers 17, no. 19: 3166. https://doi.org/10.3390/cancers17193166
APA StyleEckstrom, A., Tyagi, A., Siddiqui, M., Borgman, J., Zeng, J., Rao, A., Maiti, A., & Battula, V. L. (2025). Donor-Derived Vγ9Vδ2 T Cells for Acute Myeloid Leukemia: A Promising “Off-the-Shelf” Immunotherapy Approach. Cancers, 17(19), 3166. https://doi.org/10.3390/cancers17193166






