Real-Life Use Patterns of Androgen Receptor Pathway Inhibitors (ARPIs): A Nationwide Register-Based Study in Finland During 2012–2023
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Description of the Used Register Data and Definitions of the Key Studied Patterns
2.2. Ethics Statement
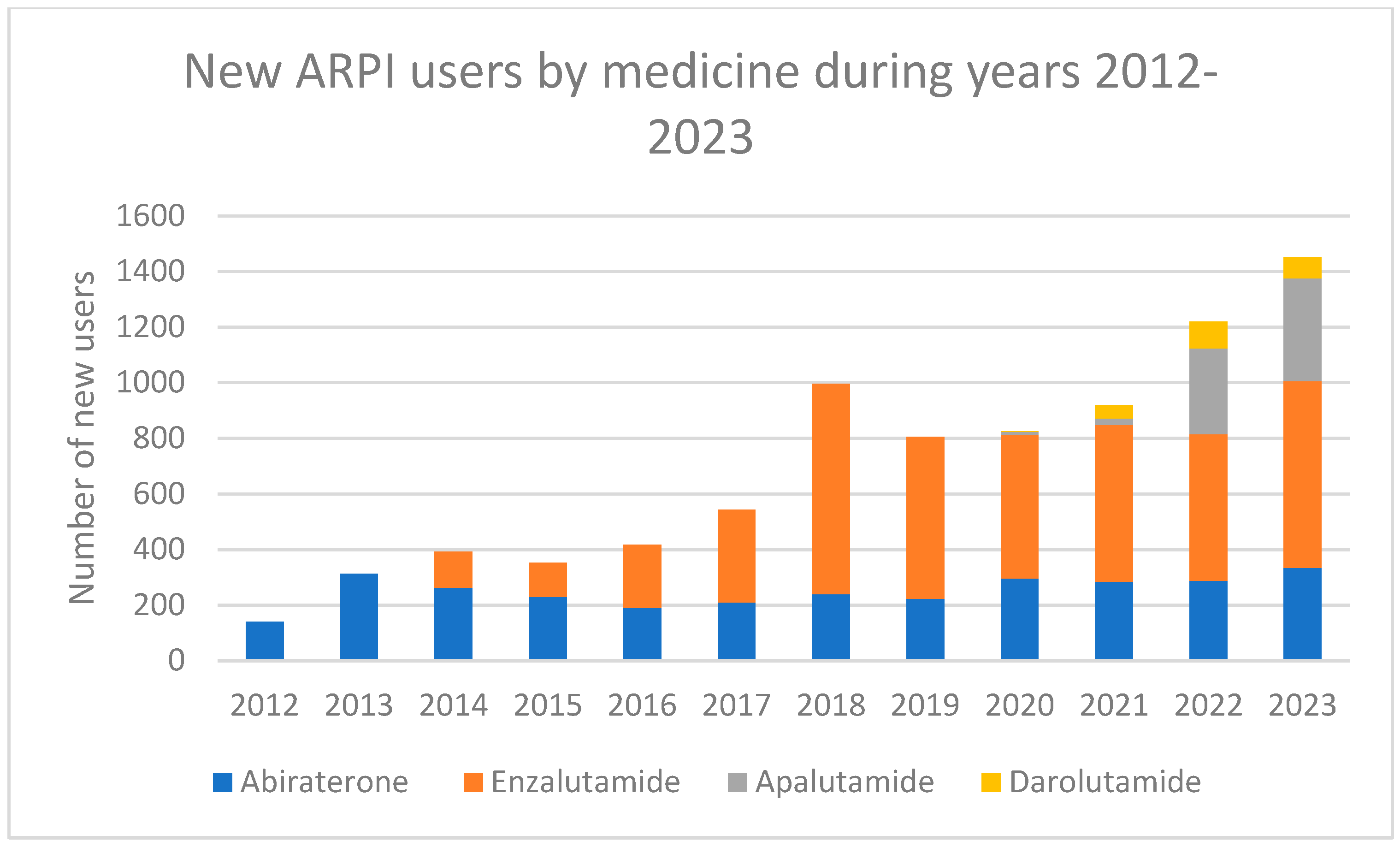
3. Results
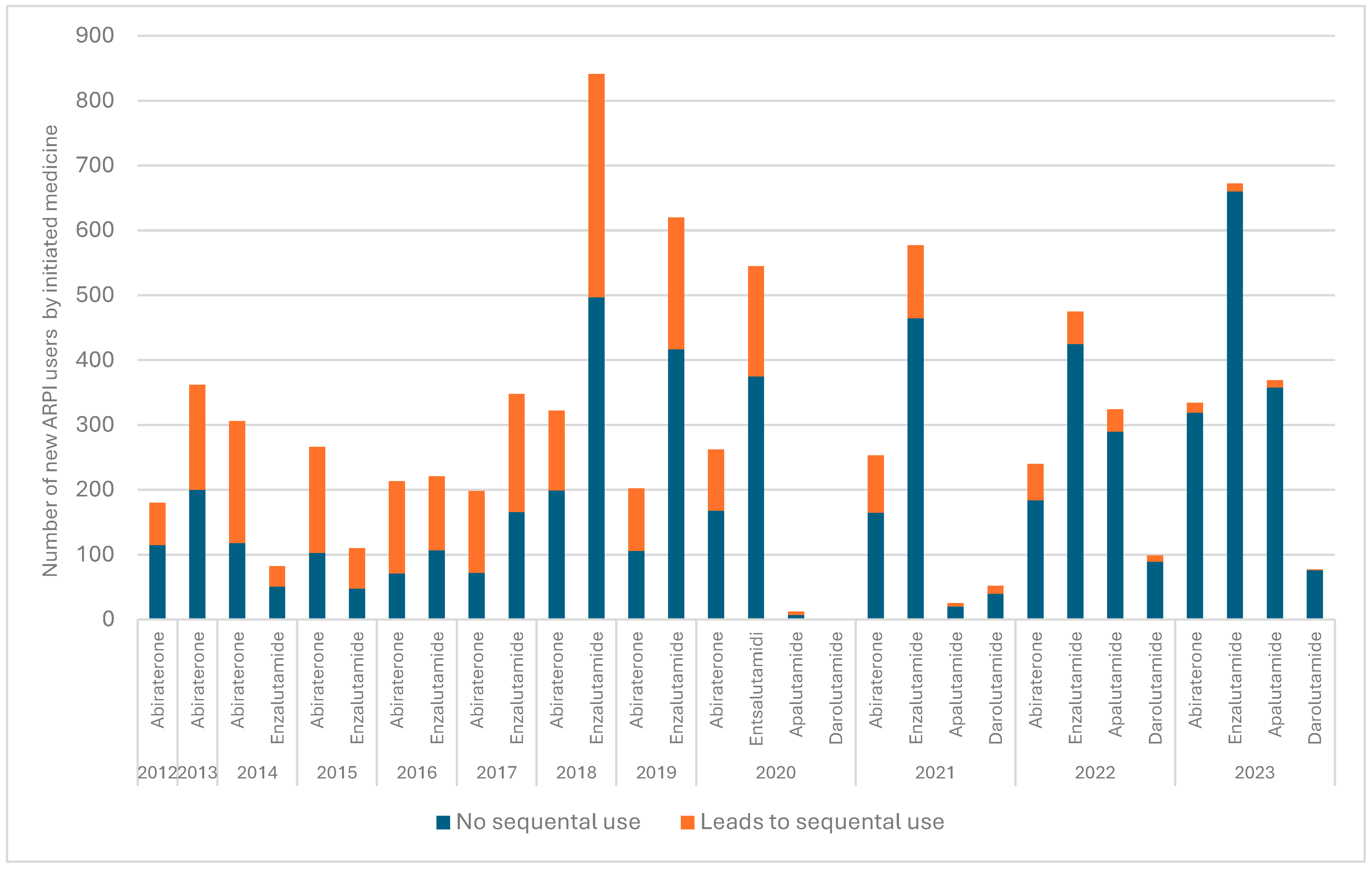
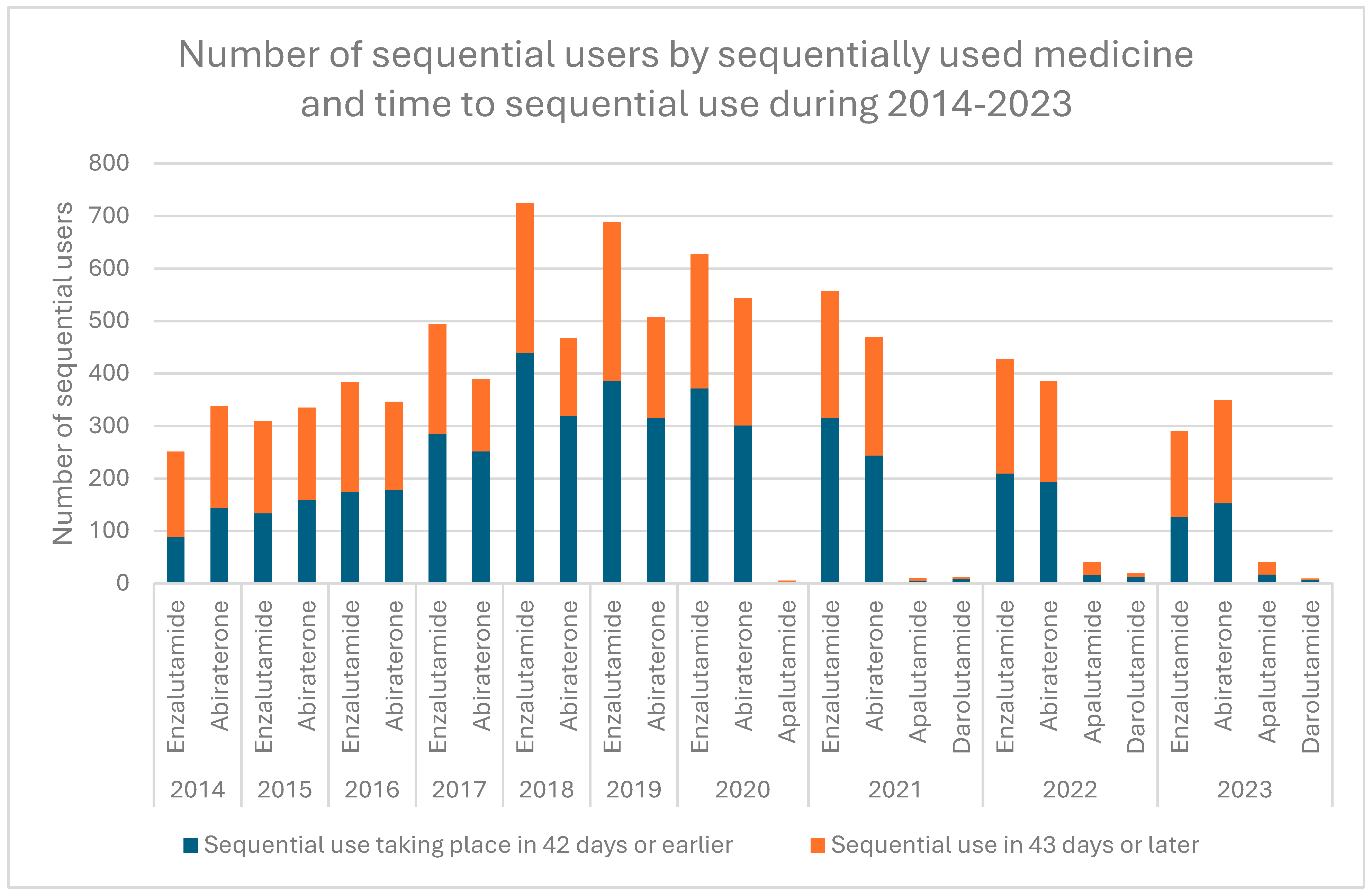
3.1. Patterns Related to Sequential ARPI Use
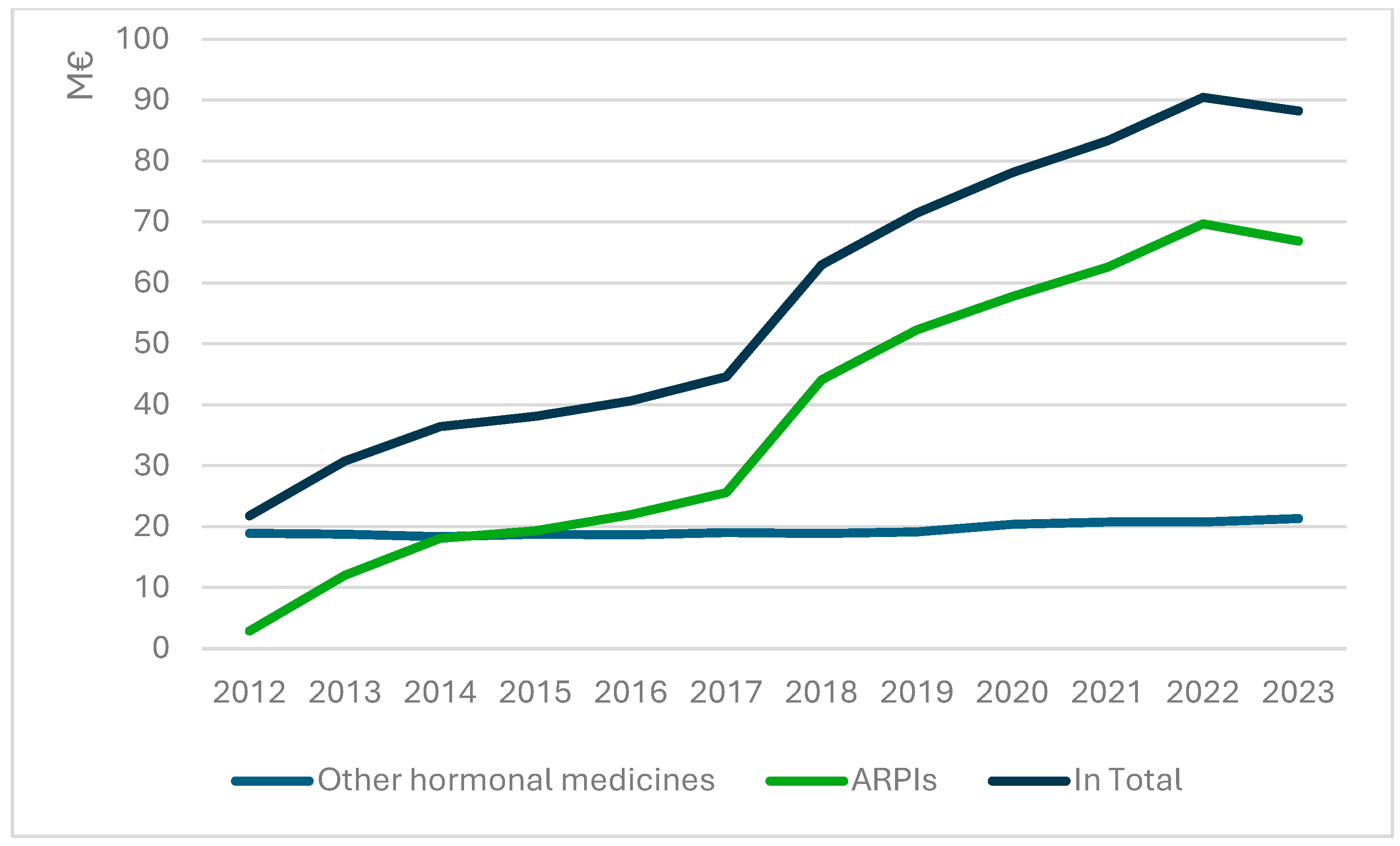
3.2. Costs of Hormonal Prostate Cancer Medicines and Sequential ARPI Use
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Pitkäniemi, J.M.; Malila, N.K.; Heikkinen, S.; Seppä, K. Cancer in Finland in 2022. Finnish Cancer Registry. Available online: https://syoparekisteri.fi/assets/themes/ssy3/factsheets/syopa_2022_tilastoraportti.html (accessed on 15 September 2025).
- World Health Organization. Cancer, Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 15 September 2025).
- James, N.D.; Tannock, I.; N’Dow, J.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. The Lancet Commission on Prostate Cancer: Planning for the Surge in Cases. Lancet 2024, 403, 1683–1722. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Yu, X.Q.; Smith, D.P.; O’Connell, D.L. A Population-Based Study of Progression to Metastatic Prostate Cancer in Australia. Cancer Epidemiol. 2015, 39, 617–622. [Google Scholar] [CrossRef]
- Kirby, M.; Hirst, C.; Crawford, E.D. Characterising the Castration-Resistant Prostate Cancer Population: A Systematic Review. Int. J. Clin. Pract. 2011, 65, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Beltran, H. Clinical and Biological Features of Neuroendocrine Prostate Cancer. Curr. Oncol. Rep. 2021, 23, 15. [Google Scholar] [CrossRef]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel Plus Prednisone or Mitoxantrone Plus Prednisone for Advanced Prostate Cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef]
- Kellokumpu-Lehtinen, P.L.; Härmenberg, U.; Joensuu, T.; McDermott, R.; Hervonen, P.; Ginman, C.; Luukkaa, M.; Nyandoto, P.; Hemminki, A.; Nilsson, S.; et al. 2-Weekly Versus 3-Weekly Docetaxel to Treat Castration-Resistant Advanced Prostate Cancer: A Randomised, Phase 3 Trial. Lancet Oncol. 2013, 14, 117–124. [Google Scholar] [CrossRef]
- Desai, K.; McManus, J.M.; Sharifi, N. Hormonal Therapy for Prostate Cancer. Endocr. Rev. 2021, 42, 354–373. [Google Scholar]
- Sandhu, S.; Moore, C.M.; Chiong, E.; Beltran, H.; Bristow, R.G.; Williams, S.G. Prostate Cancer. Lancet 2021, 398, 1075–1090. [Google Scholar] [CrossRef]
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.-P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone Plus Cabazitaxel or Mitoxantrone for Metastatic Castration-Resistant Prostate Cancer Progressing after Docetaxel Treatment: A Randomised Open-Label Trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Kellokumpu-Lehtinen, P.-L.; Marttila, T.; Jekunen, A.; Hervonen, P.; Klintrup, K.; Kataja, V.; Utriainen, T.; Luukkaa, M.; Leskinen, M.; Pulkkanen, K.; et al. Biweekly Cabazitaxel Is a Safe Treatment Option for Metastatic Castration-Resistant Prostate Cancer (mCRPC) Patients after Docetaxel—A Final Analysis of the Prosty II. Anticancer Res. 2020, 40, 6915–6921. [Google Scholar] [CrossRef]
- de Wit, R.; de Bono, J.; Sternberg, C.N.; Fizazi, K.; Tombal, B.; Wülfing, C.; Kramer, G.; Eymard, J.-C.; Bamias, A.; Carles, J.; et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 2506–2518. [Google Scholar] [CrossRef]
- Khalaf, D.J.; Annala, M.; Taavitsainen, S.; Finch, D.L.; Oja, C.; Vergidis, J.; Zulfiqar, M.; Sunderland, K.; A Azad, A.; Kollmannsberger, C.K.; et al. Optimal Sequencing of Enzalutamide and Abiraterone Acetate Plus Prednisone in Metastatic Castration-Resistant Prostate Cancer: A Multicentre, Randomised, Open-Label, Phase 2, Crossover Trial. Lancet Oncol. 2019, 20, 1730–1739. [Google Scholar] [CrossRef]
- Mori, K.; Miura, N.; Mostafaei, H.; Quhal, F.; Motlagh, R.S.; Pradere, B.; Kimura, S.; Kimura, T.; Egawa, S.; Briganti, A.; et al. Sequential Therapy of Abiraterone and Enzalutamide in Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Prostate Cancer Prostatic Dis. 2020, 23, 539–548. [Google Scholar] [CrossRef]
- Loria, R.; Vici, P.; Di Lisa, F.S.; Soddu, S.; Maugeri-Saccà, M.; Bon, G. Cross-Resistance Among Sequential Cancer Therapeutics: An Emerging Issue. Front. Oncol. 2022, 12, 877380. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
- Fizazi, K.; Gillessen, S. Updated Treatment Recommendations for Prostate Cancer from the ESMO Clinical Practice Guideline Considering Treatment Intensification and Use of Novel Systemic Agents. Ann. Oncol. 2023, 34, 557–563. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer. Part II–2024 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2024, 86, 148–163. [Google Scholar]
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Bärnighausen, T.; et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories from 2020 to 2050. JAMA Oncol. 2023, 9, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Leighl, N.B.; Nirmalakumar, S.; Ezeife, D.A.; Gyawali, B. An Arm and a Leg: The Rising Cost of Cancer Medicines and Impact on Access. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e1–e12. [Google Scholar] [CrossRef] [PubMed]
- Rannikko, A.; Hölsä, O.; Ågesen, T.; Ekman, M.; Mattila, R. Real-World Treatment Patterns and Survival Outcomes in Men with Metastatic Castration-Resistant Prostate Cancer in Finland: A National, Population-Based Cohort Study. Acta Oncol. 2025, 64, 173–178. [Google Scholar] [CrossRef]
- Data Resources Catalogue. Available online: https://aineistokatalogi.fi/catalog (accessed on 18 June 2024).
- Paulamäki, J.; Jyrkkä, J.; Hyttinen, V. Prevalence of Potentially Inappropriate Medication Use in Older Population: Comparison of the Finnish Meds75+ Database with Eight Published Criteria. BMC Geriatr. 2023, 23, 139. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.Y.; Anton, A.; Xie, O.; Tan, N.; O’Haire, S.; Maleki, S.; Inderjeeth, A.; Parente, P.; Spain, L.; Gibbs, P.; et al. Impact of Comorbidities and Drug Interactions in Patients with Metastatic Castration-Resistant Prostate Cancer Receiving Androgen Receptor Pathway Inhibitors. JCO Oncol. Pract. 2024, 20, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Hoeh, B.; Siech, C.; Humke, C.; Welte, M.; Ahrens, M.; Würnschimmel, C.; Tilki, D.; Steuber, T.; Graefen, M.; et al. Association Between Frailty and Specific Comorbidities on Oncological Outcomes in Metastatic Hormone-Sensitive and Castration-Resistant Prostate Cancer. Urol. Oncol. 2025, 43, 397.e9–397.e16. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, A.; Yanagisawa, T.; Parizi, M.K.; Laukhtina, E.; Klemm, J.; Fazekas, T.; Mori, K.; Kimura, S.; Briganti, A.; Ploussard, G.; et al. Cardiovascular Events Among Men with Prostate Cancer Treated with Androgen Receptor Signaling Inhibitors: A Systematic Review, Meta-Analysis, and Network Meta-Analysis. Prostate Cancer Prostatic Dis. 2025, 28, 298–308. [Google Scholar] [CrossRef]
- Gillessen, S.; Bossi, A.; Davis, I.D.; de Bono, J.; Fizazi, K.; James, N.D.; Mottet, N.; Shore, N.; Small, E.; Smith, M.; et al. Management of Patients with Advanced Prostate Cancer. Part I: Intermediate-/High-Risk and Locally Advanced Disease, Biochemical Relapse, and Side Effects of Hormonal Treatment: Report of the Advanced Prostate Cancer Consensus Conference 2022. Eur. Urol. 2023, 83, 267–293. [Google Scholar] [CrossRef]
- Stucki, M.; Dosch, S.; Gnädinger, M. Real-World Treatment Patterns and Medical Costs of Prostate Cancer Patients in Switzerland—A Claims Data Analysis. Eur. J. Cancer 2024, 204, 114072. [Google Scholar] [CrossRef]
- Olsen, T.A.; Filson, C.P.; Richards, T.B.; Ekwueme, D.U.; Howard, D.H. The Cost of Metastatic Prostate Cancer in the United States. Urol. Pract. 2023, 10, 41–47. [Google Scholar] [CrossRef]
- Cao, B.; Kim, M.; Reizine, N.M.; Moreira, D.M. Adverse Events and Androgen Receptor Signaling Inhibitors in the Treatment of Prostate Cancer: A Systematic Review and Multivariate Network Meta-Analysis. Eur. Urol. Oncol. 2023, 6, 237–250. [Google Scholar] [CrossRef]
| Number of Patients Initiating Treatment | 8369 | |||
| Median age of the users, years at the time of treatment initiation (range) | 75.1 (41.0–98.1) | |||
| Number of ARPI users who used | n | % | ||
| One ARPI | 5684 | 67.9% | ||
| Two ARPIs | 2537 | 30.3% | ||
| Three ARPIs | 134 | 1.6% | ||
| Four ARPIs | 14 | 0.2% | ||
| Patterns related to the first line of treatment | ||||
| The medicine chosen in the first line | n | % | ||
| Abiraterone | 2977 | 35.6 | ||
| Enzalutamide | 4438 | 53.0 | ||
| Apalutamide | 727 | 8.7 | ||
| Darolutamide | 227 | 2.7 | ||
| Treatment duration characteristics | ||||
| Median duration of first-line use | Months | |||
| Overall | 8.7 | |||
| Leads to sequential use | 8.3 | |||
| Not leading to sequential use | 9.6 | |||
| Median duration of first-line use by medicine | Months | |||
| Abiraterone | 6.6 | |||
| Enzalutamide | 10.2 | |||
| Apalutamide | 9.3 | |||
| Darolutamide | 12.1 | |||
| Use patterns related to sequential use | ||||
| Time to sequential use * | Months | |||
| Median | 1.4 | |||
| Minimum | 0 | |||
| Maximum | 45.1 | |||
| Median duration of sequential use by medicine used sequentially (users) | Months | |||
| Abiraterone (n = 1354) | 6.4 | |||
| Enzalutamide (n = 1321) | 6.9 | |||
| Apalutamide (n = 5) | 3.5 | |||
| Darolutamide (n = 5) | 11.4 | |||
| Observed combinations in sequential use | users, n | % of all users | % of all sequential users | |
| Abiraterone- enzalutamide | 1249 | 14.9 | 47% | |
| Enzalutamide-abiraterone | 1214 | 14.5 | 45% | |
| Enzalutamide- abiraterone-enzalutamide | 63 | 0.8 | 2% | |
| Abiraterone- enzalutamide-abiraterone | 61 | 0.7 | 2% | |
| Other combinations (altogether 14 different ones) ** | 98 | 1.2 | 4% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurko, T.; Heino, P.; Kellokumpu-Lehtinen, P.-L.; Sarnola, K.; Koskinen, H.; Bärlund, M. Real-Life Use Patterns of Androgen Receptor Pathway Inhibitors (ARPIs): A Nationwide Register-Based Study in Finland During 2012–2023. Cancers 2025, 17, 3162. https://doi.org/10.3390/cancers17193162
Kurko T, Heino P, Kellokumpu-Lehtinen P-L, Sarnola K, Koskinen H, Bärlund M. Real-Life Use Patterns of Androgen Receptor Pathway Inhibitors (ARPIs): A Nationwide Register-Based Study in Finland During 2012–2023. Cancers. 2025; 17(19):3162. https://doi.org/10.3390/cancers17193162
Chicago/Turabian StyleKurko, Terhi, Pekka Heino, Pirkko-Liisa Kellokumpu-Lehtinen, Kati Sarnola, Hanna Koskinen, and Maarit Bärlund. 2025. "Real-Life Use Patterns of Androgen Receptor Pathway Inhibitors (ARPIs): A Nationwide Register-Based Study in Finland During 2012–2023" Cancers 17, no. 19: 3162. https://doi.org/10.3390/cancers17193162
APA StyleKurko, T., Heino, P., Kellokumpu-Lehtinen, P.-L., Sarnola, K., Koskinen, H., & Bärlund, M. (2025). Real-Life Use Patterns of Androgen Receptor Pathway Inhibitors (ARPIs): A Nationwide Register-Based Study in Finland During 2012–2023. Cancers, 17(19), 3162. https://doi.org/10.3390/cancers17193162






