Microengineered Breast Cancer Models: Shaping the Future of Personalized Oncology
Simple Summary
Abstract
1. Introduction
1.1. Brief Overview of Breast Cancer Epidemiology
1.2. From Epidemiology to the Lab: Where Are We and What Should Be Improved?
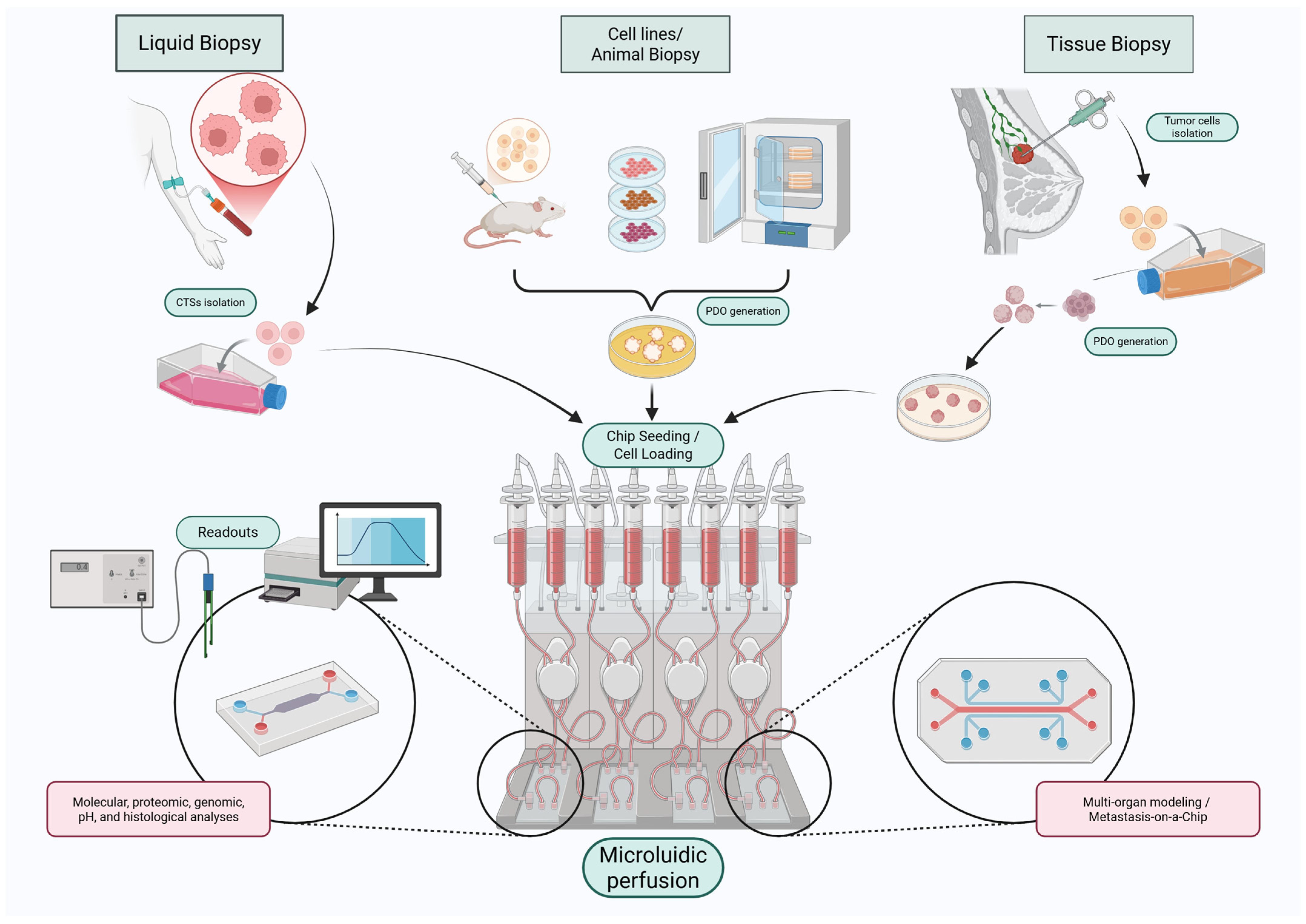
2. Breast Cancer-on-a-Chip Technologies: Addressing the Limitations
3. Breast Cancer Pathology
3.1. Genetic Alterations and Tumor Evolution
3.2. Immune System Interactions and Immune Evasion Impact on Breast Cancer Immunotherapy
3.3. Hormonal Signaling and Tumor–Immune Crosstalk
3.4. Implications for Therapeutic Targeting
4. Fabrication of Microfluidic Platforms
4.1. Fabrication Materials
4.2. Fabrication Techniques
5. The Role of the Tumor Microenvironment in Breast Cancer Progression
6. Microfluidic Modeling of Particular Subtypes
- Luminal A: The most common subtype; ER+/PR+, HER2−, low proliferation; generally, has a favorable prognosis and responds well to endocrine therapies [129].
- Luminal B: ER+, variable PR and HER2 expression; more aggressive and less responsive to hormone therapy.
- HER2-enriched: Defined by HER2 overexpression; typically, high-grade and associated with poorer outcomes [130].
- Triple-negative breast cancer (TNBC): Lacks ER, PR, and HER2 expression; often linked to BRCA1/2 mutations; associated with high proliferation, aggressiveness, and poor prognosis.
6.1. Breast Cancer-on-a-Chip Models of Ductal Carcinoma in Situ
6.2. Breast Cancer-on-a-Chip Models of Luminal a Subtype
6.3. Breast Cancer-on-a-Chip Models of Triple-Negative Breast Cancer (TNBC) Subtype
7. Modeling the Metastatic Process
7.1. Lymphatic Metastasis-on-a-Chip Models
7.2. Bone Metastasis-on-a-Chip Models
7.3. Brain Metastasis-on-a-Chip Models
7.4. Lung Metastasis-on-a-Chip Models
7.5. Liver Metastasis-on-a-Chip Models
7.6. Circulating Tumor Cells—Liquid Biopsy
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| ABCC5 | Multidrug resistance-associated protein 5 |
| AI | Artificial Intelligence |
| APOH | Apolipoprotein H |
| ASC | Adipose-derived Stem Cell |
| ASCs | Adipose-derived Stem Cells |
| BBB | Blood–Brain Barrier |
| BC | Breast Cancer |
| BCOC | Breast Cancer-on-a-Chip |
| BCSC | Breast Cancer Stem Cell |
| BM | Basement Membrane |
| BoC | Bone-on-a-Chip |
| BRCA1 | Breast Cancer gene 1 |
| BRCA2 | Breast Cancer gene 2 |
| CAFs | Cancer-Associated Fibroblasts |
| CAR | Chimeric Antigen Receptor |
| CD44 | Cluster of Differentiation 44 |
| CEA | Carcinoembryonic Antigen |
| CHECK2 | Checkpoint Kinase 2 |
| CK | Cytokeratin |
| COC | Cyclic Olefin Copolymer |
| CSF | Cerebrospinal Fluid |
| CTBP1 | C-terminal Binding Protein 1 |
| CTC | Circulating Tumor Cell |
| CTCs | Circulating Tumor Cells |
| DCIS | Ductal Carcinoma In Situ |
| DDR | DNA Damage Repair |
| DNA | Deoxyribonucleic Acid |
| DNMT | DNA Methyltransferase |
| E2F | E2F family of transcription factors |
| ECM | Extracellular Matrix |
| EGFR | Epidermal Growth Factor Receptor |
| EMA | European Medicines Agency |
| EMT | Epithelial-to-Mesenchymal Transition |
| ER | Estrogen Receptor |
| ER+ | Estrogen Receptor-Positive |
| ERBB2 | Erb-B2 Receptor Tyrosine Kinase 2 |
| EV | Extracellular Vesicle |
| EVs | Extracellular Vesicles |
| EMT | Epithelial–Mesenchymal Transition |
| EpCAM | Epithelial Cell Adhesion Molecule |
| ETS | Estrogen Receptor 1 |
| ESR1 | Electron Transport System |
| FDA | Food and Drug Administration |
| FEMC | Filter-Electrochemical Microfluidic Chip |
| FSS | Fluid Shear Stress |
| GelMA | Gelatin Methacryloyl |
| HDAC | human bone marrow-derived mesenchymal stem cells |
| hBM-MSCs | Histone Deacetylase |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HER2+ | Human Epidermal Growth Factor Receptor 2-Positive |
| HIF | Hypoxia-Inducible Factor |
| HR+ | Hormone Receptor Positive |
| HUVECs | Human Umbilical Vein Endothelial Cells |
| IL | Interleukin |
| IL-6 | Interleukin 6 |
| LAR | Luminal Androgen Receptor |
| LNOC | Lymph Node-on-a-Chip |
| LOC | Liver-on-a-Chip |
| LVI | Lympho-Vascular Invasion |
| MAP3K1 | Mitogen-Activated Protein Kinase 1 |
| MCF7 | Michigan Cancer Foundation-7 |
| MCF10A | Michigan Cancer Foundation-10A |
| MDA-MB-231 | MD Anderson Cancer Center-Metastatic Breast-231 cell line |
| MDSCs | Myeloid-Derived Suppressor Cells |
| MHC-I | Major Histocompatibility Complex Class I |
| MMP | Matrix Metalloproteinase |
| MNPs | Magnetic Nanoparticles |
| MSCs | Mesenchymal Stem Cells |
| MSL | Mesenchymal Stem-Like |
| MX-1 | Myxovirus Resistance Protein 1 |
| NK | Natural Killer |
| NLFs | Normal Lung Fibroblasts |
| Na2CO3 | Sodium Carbonate |
| OoAC | Organ-on-a-Chip |
| OoC | Organ-on-a-Chip |
| PALB2 | Partner and Localizer of BRCA2 |
| PARP2 | Poly(ADP-ribose) Polymerase 2 |
| PD-L1 | Programmed Death-Ligand 1 |
| PDMS | Polydimethylsiloxane |
| PDSs | Patient-Derived Scaffolds |
| PDX | Patient-Derived Xenograft |
| PEG | Polyethylene Glycol |
| PET | Positron Emission Tomography |
| PGA | Polyglycolic Acid |
| PIK3CA | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha |
| PLA | Polylactic Acid |
| PLGA | Poly(lactic-co-glycolic acid) |
| PlGF | Placenta Growth Factor |
| PMMA | Polymethyl Methacrylate |
| PMNs | Polymorphonuclear Neutrophils |
| PNI | Perineural Invasion |
| POMaC | Poly(octamethylene maleate (anhydride) citrate) |
| POSTN | Periostin |
| PR | Progesterone Receptor |
| PR+ | Progesterone Receptor Positive |
| RAD51 | RAD51 recombinase |
| RNA | Ribonucleic Acid |
| SFTA2 | Surfactant Associated 2 |
| SFTPB | Surfactant Protein B |
| SMA | Smooth Muscle Actin |
| SNS | Sympathetic Nervous System |
| SOX | Sulfur Oxides |
| TAMs | Tumor-Associated Macrophages |
| TAP | Transporter Associated with Antigen Processing |
| TGF | Transforming Growth Factor |
| TIL | Tumor-Infiltrating Lymphocytes |
| TIME | Tumor Immune Microenvironment |
| TLS | Tertiary Lymphoid Structures |
| TME | Tumor Microenvironment |
| TNBC | Triple-Negative Breast Cancer |
| TNM | Tumor Node Metastasis |
| ToC | Tumor-on-a-Chip |
| TP53 | Tumor Protein p53 |
| TPZ | Tirapazamine |
| VEGF | Vascular Endothelial Growth Factor |
| ZEB1 | Zinc Finger E-Box Binding Homeobox 1 |
| cfDNA | Cell-Free DNA |
| iPSC | Induced Pluripotent Stem Cell |
| miRNA | MicroRNA |
| pH | Potential of Hydrogen (Acidity Level) |
References
- Sedeta, E.T.; Jobre, B.; Avezbakiyev, B. Breast cancer: Global patterns of incidence, mortality, and trends. J. Clin. Oncol. 2023, 41, 10528. [Google Scholar] [CrossRef]
- Allahqoli, L.; Mazidimoradi, A.; Momenimovahed, Z.; Rahmani, A.; Hakimi, S.; Tiznobaik, A.; Gharacheh, M.; Salehiniya, H.; Babaey, F.; Alkatout, I. The Global Incidence, Mortality, and Burden of Breast Cancer in 2019: Correlation With Smoking, Drinking, and Drug Use. Front. Oncol. 2022, 12, 921015. [Google Scholar] [CrossRef]
- Sha, R.; Kong, X.M.; Li, X.Y.; Wang, Y.B. Global burden of breast cancer and attributable risk factors in 204 countries and territories, from 1990 to 2021: Results from the Global Burden of Disease Study 2021. Biomark. Res. 2024, 12, 87. [Google Scholar] [CrossRef]
- Ahmad, S.; Shaukat, M.T.; Rehman, W.U.; Mohsin, A.; Rehman, A.U.; Graff, S.L. The global and regional disease burden of breast cancer from 1980 to 2021: An analysis of GBD study 2021. JCO Oncol. Pract. 2024, 20, 146. [Google Scholar] [CrossRef]
- Lan, T.; Lu, Y.; He, J.; Zhan, C.; Wang, X.; Shao, X.; Hu, Z. Global, reginal, national burden and risk factors in female breast cancer from 1990 to 2021. iScience 2024, 27, 111045. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Zhang, T.; Zheng, J.; Lin, W.; Cai, J.; Zou, J.; Chen, Y.; Xie, Y.; Chen, Y.; et al. The Global, Regional, and National Burden and Trends of Breast Cancer From 1990 to 2019: Results From the Global Burden of Disease Study 2019. Front. Oncol. 2021, 11, 689562. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, Z.; Bao, L.; Shu, P. The global burden of breast cancer in women from 1990 to 2030: Assessment and projection based on the global burden of disease study 2019. Front. Oncol. 2024, 14, 1364397. [Google Scholar] [CrossRef]
- Jaiswal, C.; Mandal, B.B. A 3D In Vitro Triculture Hybrid Model Recapitulating Tumor Stromal Interaction of Triple-Negative Breast Cancer as a High Throughput Anticancer Drug Screening Platform. Adv. Ther. 2024, 7, 2300450. [Google Scholar] [CrossRef]
- Moccia, C.; Haase, K. Engineering Breast Cancer On-chip—Moving Toward Subtype Specific Models. Front. Bioeng. Biotechnol. 2021, 9, 694218. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-M.; Mu, H.-Y.; Chu, L.-A.; Lin, Y.-H.; Li, J.; Liu, C.-Y.; Huang, H.-C.; Cheng, S.-L.; Lee, T.-Y.; Lee, H.M.; et al. Abstract 6771: Tumor-microenvironment-on-chip: An ex vivo drug screening platform enabling real-time observation of regional tumor responses during drug development and clinical treatments. Cancer Res. 2024, 84, 6771. [Google Scholar] [CrossRef]
- Karlin, N.J.; Wong, D.A. Mesenchymal Neoplasms and Primary Lymphomas of the Breast. In The Breast: Comprehensive Management of Benign and Malignant Diseases; Elsevier: Amsterdam, The Netherlands, 2018; pp. 156–168. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, L.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef]
- Kalot, R.; Mhanna, R.; Talhouk, R. Organ-on-a-chip platforms as novel advancements for studying heterogeneity, metastasis, and drug efficacy in breast cancer. Pharmacol. Ther. 2022, 237, 108156. [Google Scholar] [CrossRef]
- Jin, L.; Qu, Y.; Gomez, L.J.; Chung, S.; Han, B.; Gao, B.; Yue, Y.; Gong, Y.; Liu, X.; Amersi, F.; et al. Characterization of primary human mammary epithelial cells isolated and propagated by conditional reprogrammed cell culture. Oncotarget 2017, 9, 11503–11514. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Kenny, P.A.; Radisky, D.C. Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: The role of extracellular matrix and its degrading enzymes. Cold Spring Harb. Symp. Quant. Biol. 2005, 70, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Furuta, S.; Bissell, M.J. Pathways involved in formation of mammary organoid architecture have keys to understanding drug resistance and to discovery of druggable targets. Cold Spring Harb. Symp. Quant. Biol. 2016, 81, 207–217. [Google Scholar] [CrossRef][Green Version]
- Menshykau, D.; Michos, O.; Lang, C.; Conrad, L.; McMahon, A.P.; Iber, D. Image-based modeling of kidney branching morphogenesis reveals GDNF-RET based Turing-type mechanism and pattern-modulating WNT11 feedback. Nat. Commun. 2019, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Lara, R.; Annabi, N. Microengineered cancer-on-a-chip platforms to study the metastatic microenvironment. Lab. Chip 2016, 16, 4063–4081. [Google Scholar] [CrossRef]
- Gioiella, F.; Urciuolo, F.; Imparato, G.; Brancato, V.; Netti, P.A. An Engineered Breast Cancer Model on a Chip to Replicate ECM-Activation In Vitro during Tumor Progression. Adv. Healthc. Mater. 2016, 5, 3074–3084. [Google Scholar] [CrossRef]
- Holen, I.; Speirs, V.; Morrissey, B.; Blyth, K. In vivo models in breast cancer research: Progress, challenges and future directions. Dis. Model. Mech. 2017, 10, 359–371. [Google Scholar] [CrossRef]
- Komen, J.; van Neerven, S.M.; Berg, A.v.D.; Vermeulen, L.; van der Meer, A.D. Mimicking and surpassing the xenograft model with cancer-on-chip technology. eBioMedicine 2021, 66, 103303. [Google Scholar] [CrossRef]
- Wan, L.; Neumann, C.A.; Leduc, P.R. Tumor-on-a-chip for integrating a 3D tumor microenvironment: Chemical and mechanical factors. Lab. Chip 2020, 20, 873–888. [Google Scholar] [CrossRef]
- Ran, R.; Wang, H.; Hou, F.; Liu, Y.; Hui, Y.; Petrovsky, N.; Zhang, F.; Zhao, C. A Microfluidic Tumor-on-a-Chip for Assessing Multifunctional Liposomes’ Tumor Targeting and Anticancer Efficacy. Adv. Healthc. Mater. 2019, 8, e1900015. [Google Scholar] [CrossRef]
- Ai, X.; Zhao, L.; Lu, Y.-Y.; Hou, Y.; Lv, T.; Jiang, Y.; Tu, P.; Guo, X. Integrated Array Chip for High-Throughput Screening of Species Differences in Metabolism. Anal. Chem. 2020, 92, 11696–11704. [Google Scholar] [CrossRef]
- Duncan, B.B.; Dunbar, C.E.; Ishii, K. Applying a clinical lens to animal models of CAR-T cell therapies. Mol. Ther. Methods Clin. Dev. 2022, 27, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Testa, M.; Gaggianesi, M.; D’accardo, C.; Porcelli, G.; Turdo, A.; Di Marco, C.; Patella, B.; Di Franco, S.; Modica, C.; Di Bella, S.; et al. A Novel Tumor on Chip Mimicking the Breast Cancer Microenvironment for Dynamic Drug Screening. Int. J. Mol. Sci. 2025, 26, 1028. [Google Scholar] [CrossRef] [PubMed]
- Neagu, A.N.; Whitham, D.; Bruno, P.; Versaci, N.; Biggers, P.; Darie, C.C. Tumor-on-chip platforms for breast cancer continuum concept modeling. Front. Bioeng. Biotechnol. 2024, 12, 1436393. [Google Scholar] [CrossRef] [PubMed]
- Noe-Kim, V.; Ozcelikkale, A.; Han, B. Multifaceted Transport Characteristics of Nanomedicine: Needs for Characterization in Dynamic Environment. Mol. Pharm. 2014, 10, 2111–2126. [Google Scholar]
- Zhang, Z.; Hao, R.; Guo, Q.; Zhang, S.; Wang, X. TP53 Mutation Infers a Poor Prognosis and Is Correlated to Immunocytes Infiltration in Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 759154. [Google Scholar] [CrossRef]
- Dailey, G.P.; Rabiola, C.A.; Lei, G.; Wei, J.; Yang, X.-Y.; Wang, T.; Liu, C.-X.; Gajda, M.; Hobeika, A.C.; Summers, A.; et al. Vaccines targeting ESR1 activating mutations elicit anti-tumor immune responses and suppress estrogen signaling in therapy resistant ER+ breast cancer. Hum. Vaccines Immunother. 2024, 20, 2309693. [Google Scholar] [CrossRef]
- Goldberg, J.; Qiao, N.; Guerriero, J.L.; Gross, B.; Meneksedag, Y.; Lu, Y.F.; Philips, A.V.; Rahman, T.; Meric-Bernstam, F.; Roszik, J.; et al. Estrogen Receptor Mutations as Novel Targets for Immunotherapy in Metastatic Estrogen Receptor-positive Breast Cancer. Cancer Res. Commun. 2024, 4, 496–504. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, Y.; Zhang, M.; Yu, W.; Kang, L. Mechanisms of endocrine resistance in hormone receptor-positive breast cancer. Front. Oncol. 2024, 14, 1448687. [Google Scholar] [CrossRef]
- Chen, C.; Lin, C.-J.; Pei, Y.-C.; Ma, D.; Liao, L.; Li, S.-Y.; Fan, L.; Di, G.-H.; Wu, S.-Y.; Liu, X.-Y.; et al. Comprehensive genomic profiling of breast cancers characterizes germline-somatic mutation interactions mediating therapeutic vulnerabilities. Cell Discov. 2023, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Houlahan, K.E.; Khan, A.; Greenwald, N.F.; Vivas, C.S.; West, R.B.; Angelo, M.; Curtis, C. Germline-mediated immunoediting sculpts breast cancer subtypes and metastatic proclivity. Science 2024, 384, eadh8697. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Heredia, J.; Souza, C.A.; Trincado, J.L.; Gonzalez-Cao, M.; Gonçalves-Ribeiro, S.; Gil, S.R.; Pravdyvets, D.; Cedeño, S.; Callari, M.; Marra, A.; et al. Converging and evolving immuno-genomic routes toward immune escape in breast cancer. Nat. Commun. 2024, 15, 1302. [Google Scholar] [CrossRef]
- Moura, T.; Laranjeira, P.; Caramelo, O.; Gil, A.M.; Paiva, A. Breast Cancer and Tumor Microenvironment: The Crucial Role of Immune Cells. Curr. Oncol. 2025, 32, 143. [Google Scholar] [CrossRef]
- Grant, G.; Ferrer, C.M. The role of the immune tumor microenvironment in shaping metastatic dissemination, dormancy, and outgrowth. Trends Cell Biol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.-W.; Liu, C.-C.; Zhang, Y.-W.; Liu, Y.-M.; Chen, L.; Xiong, X.; Shao, Z.-M.; Yu, K.-D. MAP3K1 mutations confer tumor immune heterogeneity in hormone receptor–positive HER2-negative breast cancer. J. Clin. Investig. 2025, 135. [Google Scholar] [CrossRef]
- Barb, A.C.; Fenesan, M.P.; Pirtea, M.; Margan, M.M.; Tomescu, L.; Melnic, E.; Cimpean, A.M. Tertiary Lymphoid Structures (TLSs) and Stromal Blood Vessels Have Significant and Heterogeneous Impact on Recurrence, Lymphovascular and Perineural Invasion amongst Breast Cancer Molecular Subtypes. Cells 2023, 12, 1176. [Google Scholar] [CrossRef]
- Salemme, V.; Centonze, G.; Cavallo, F.; Defilippi, P.; Conti, L. The Crosstalk Between Tumor Cells and the Immune Microenvironment in Breast Cancer: Implications for Immunotherapy. Front. Oncol. 2021, 11, 610303. [Google Scholar] [CrossRef]
- E Yates, M.; Waltermire, H.; Mori, K.; Li, Z.; Li, Y.; Guzolik, H.; Wang, X.; Liu, T.; Atkinson, J.M.; Hooda, J.; et al. ESR1 Fusions Invoke Breast Cancer Subtype-Dependent Enrichment of Ligand-Independent Oncogenic Signatures and Phenotypes. Endocrinology 2024, 165, bqae111. [Google Scholar] [CrossRef]
- Saha, T.; Lukong, K.E. Decoding estrogen receptor and GPER biology: Structural insights and therapeutic advances in ERα−positive breast cancer. Front. Oncol. 2025, 15, 1513225. [Google Scholar] [CrossRef]
- Williams, A.E. Obesity Enhances Breast Cancer Risk, Metastasis, and Response to Immunotherapy. 2024. Available online: https://search.library.wisc.edu/digital/AYORWH24YVA73O86 (accessed on 1 July 2025).
- Kundu, M.; Butti, R.; Panda, V.K.; Malhotra, D.; Das, S.; Mitra, T.; Kapse, P.; Gosavi, S.W.; Kundu, G.C. Modulation of the tumor microenvironment and mechanism of immunotherapy-based drug resistance in breast cancer. Mol. Cancer 2024, 23, 1–27. [Google Scholar] [CrossRef]
- Banik, S.; Uchil, A.; Kalsang, T.; Chakrabarty, S.; Ali, A.; Srisungsitthisunti, P.; Mahato, K.K.; Surdo, S.; Mazumder, N. The revolution of PDMS microfluidics in cellular biology. Crit. Rev. Biotechnol. 2022, 43, 465–483. [Google Scholar] [CrossRef]
- Chuchuy, J.; Rogal, J.; Ngo, T.; Stadelmann, K.; Antkowiak, L.; Achberger, K.; Liebau, S.; Schenke-Layland, K.; Loskill, P. Integration of Electrospun Membranes into Low-Absorption Thermoplastic Organ-on-Chip. ACS Biomater. Sci. Eng. 2021, 7, 3006–3017. [Google Scholar] [CrossRef]
- Urbaczek, A.C.; Leão, P.A.G.C.; de Souza, F.Z.R.; Afonso, A.; Alberice, J.V.; Cappelini, L.T.D.; Carlos, I.Z.; Carrilho, E. Endothelial Cell Culture Under Perfusion On A Polyester-Toner Microfluidic Device. Sci. Rep. 2017, 7, 10466. [Google Scholar] [CrossRef]
- Thompson, B.L.; Ouyang, Y.; Duarte, G.R.M.; Carrilho, E.; Krauss, S.T.; Landers, J.P. Inexpensive, rapid prototyping of microfluidic devices using overhead transparencies and a laser print, cut and laminate fabrication method. Nat. Protoc. 2015, 10, 875–886. [Google Scholar] [CrossRef]
- Yang, X.; Li, K.; Zhang, X.; Liu, C.; Guo, B.; Wen, W.; Gao, X. Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing. Lab. A Chip 2018, 18, 486–495. [Google Scholar] [CrossRef]
- Tran, R.T.; Thevenot, P.; Gyawali, D.; Chiao, J.C.; Tang, L.; Yang, J. Synthesis and characterization of a biodegradable elastomer featuring a dual crosslinking mechanism. Soft Matter 2010, 6, 2449. [Google Scholar] [CrossRef]
- Nguyen, T.; Jung, S.H.; Lee, M.S.; Park, T.E.; Ahn, S.K.; Kang, J.H. Robust chemical bonding of PMMA microfluidic devices to porous PETE membranes for reliable cytotoxicity testing of drugs. Lab. Chip 2019, 19, 3706–3713. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.A.; Ataya, S.; Silberschmidt, V.V. Temperature-dependent mechanical behaviour of PMMA: Experimental analysis and modelling. Polym. Test. 2017, 58, 86–95. [Google Scholar] [CrossRef]
- Shuai, C.; Wu, P.; Zhong, Y.; Feng, P.; Gao, C.; Huang, W.; Zhou, Z.; Chen, L.; Shuai, C. Polyetheretherketone/poly (glycolic acid) blend scaffolds with biodegradable properties. J. Biomater. Sci. Polym. Ed. 2016, 27, 1434–1446. [Google Scholar] [CrossRef] [PubMed]
- Nahak, B.K.; Mishra, A.; Preetam, S.; Tiwari, A. Advances in Organ-on-a-Chip Materials and Devices. ACS Appl. Bio Mater. 2022, 5, 3576–3607. [Google Scholar] [CrossRef]
- Hassan, S.; Heinrich, M.; Cecen, B.; Prakash, J.; Zhang, Y.S. Biomaterials for on-chip organ systems. In Biomaterials for Organ and Tissue Regeneration: New Technologies and Future Prospects; Woodhead Publishing: Sawston, UK, 2020; pp. 669–707. [Google Scholar] [CrossRef]
- Zamprogno, P.; Wüthrich, S.; Achenbach, S.; Thoma, G.; Stucki, J.D.; Hobi, N.; Schneider-Daum, N.; Lehr, C.-M.; Huwer, H.; Geiser, T.; et al. Second-generation lung-on-a-chip with an array of stretchable alveoli made with a biological membrane. Commun. Biol. 2021, 4, 168. [Google Scholar] [CrossRef]
- Nashimoto, Y.; Hayashi, T.; Kunita, I.; Nakamasu, A.; Torisawa, Y.-S.; Nakayama, M.; Takigawa-Imamura, H.; Kotera, H.; Nishiyama, K.; Miura, T.; et al. Integrating perfusable vascular networks with a three-dimensional tissue in a microfluidic device. Integr. Biol. 2017, 9, 506–518. [Google Scholar] [CrossRef]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef]
- Cao, U.M.N.; Zhang, Y.; Chen, J.; Sayson, D.; Pillai, S.; Tran, S.D. Microfluidic Organ-on-A-chip: A Guide to Biomaterial Choice and Fabrication. Int. J. Mol. Sci. 2023, 24, 3232. [Google Scholar] [CrossRef]
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A guide to the organ-on-a-chip. Nat. Rev. Methods Prim. 2022, 2, 33. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Ribeiro, N.; Soares, G.C.; Santos-Rosales, V.; Concheiro, A.; Alvarez-Lorenzo, C.; García-González, C.A.; Oliveira, A.L. A new era for sterilization based on supercritical CO2 technology. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2019, 108, 399–428. [Google Scholar] [CrossRef] [PubMed]
- Jeibouei, S.; Monfared, A.K.; Hojat, A.; Aref, A.R.; Shams, F.; Dolati, M.; Moradi, A.; Hosseini, M.; Javadi, S.M.; Ajoudanian, M.; et al. Human-derived Tumor-On-Chip model to study the heterogeneity of breast cancer tissue. Mater. Sci. Eng. C 2024, 162, 213915. [Google Scholar] [CrossRef]
- Alamán-Díez, P.; García-Gareta, E.; Arruebo, M.; Pérez, M.Á. A bone-on-a-chip collagen hydrogel-based model using pre-differentiated adipose-derived stem cells for personalized bone tissue engineering. J. Biomed. Mater. Res. A 2023, 111, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Grasman, J.M.; O’Brien, M.P.; Ackerman, K.; Gagnon, K.A.; Wong, G.M.; Pins, G.D. The Effect of Sterilization Methods on the Structural and Chemical Properties of Fibrin Microthread Scaffolds. Macromol. Biosci. 2016, 16, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, L.E.; Lütgebaucks, C.; Lewis, D.M.; Gerecht, S. Patterning microscale extracellular matrices to study endothelial and cancer cell interactions in vitro. Lab. Chip 2012, 12, 4244–4248. [Google Scholar] [CrossRef]
- Rothbauer, M.; Zirath, H.; Ertl, P. Recent advances in microfluidic technologies for cell-to-cell interaction studies. Lab. Chip 2018, 18, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Cintrón, K.M.; Gong, M.M.; Ayuso, J.M.; Tomko, L.A.; Beebe, D.J.; Virumbrales-Muñoz, M.; Ponik, S.M. Breast Fibroblasts and ECM Components Modulate Breast Cancer Cell Migration through the Secretion of MMPs in a 3D Microfluidic Co-Culture Model. Cancers 2020, 12, 1173. [Google Scholar] [CrossRef] [PubMed]
- Otterlei, M.; Østgaard, K.; Skjåk-Bræk, G.; Smidsr, O.; Soon-Shiong, P.; Espevik, T. Induction of Cytokine Production from Human Monocytes Stimulated with Alginate. J. Immunother. 1991, 10, 286–291. [Google Scholar] [CrossRef]
- Osório, L.A.; Silva, E.; Mackay, R.E. A Review of Biomaterials and Scaffold Fabrication for Organ-on-a-Chip (OOAC) Systems. Bioengineering 2021, 8, 113. [Google Scholar] [CrossRef]
- Kajtez, J.; Buchmann, S.; Vasudevan, S.; Birtele, M.; Rocchetti, S.; Pless, C.J.; Heiskanen, A.; Barker, R.A.; Martínez-Serrano, A.; Parmar, M.; et al. 3D-Printed Soft Lithography for Complex Compartmentalized Microfluidic Neural Devices. Adv. Sci. 2020, 7, 2001150. [Google Scholar] [CrossRef]
- Ferrari, E.; Nebuloni, F.; Rasponi, M.; Occhetta, P. Photo and Soft Lithography for Organ-on-Chip Applications. Methods Mol. Biol. 2022, 2373, 1–19. [Google Scholar] [CrossRef]
- Ding, Y.; Hassan, M.H.; Bakker, O.; Hinduja, S.; Bártolo, P. A Review on Microcellular Injection Moulding. Materials 2021, 14, 4209. [Google Scholar] [CrossRef]
- Cameron, N.S.; Roberge, H.; Veres, T.; Jakeway, S.C.; Crabtree, H.J. High fidelity, high yield production of microfluidic devices by hot embossing lithography: Rheology and stiction. Lab. Chip 2006, 6, 936–941. [Google Scholar] [CrossRef]
- Yi, D.; Marcelot, C.; Romana, I.; Tassé, M.; Fazzini, P.-F.; Peres, L.; Ratel-Ramond, N.; Decorse, P.; Warot-Fonrose, B.; Viau, G.; et al. Etching suppression as a means to Pt dendritic ultrathin nanosheets by seeded growth. Nanoscale 2022, 15, 1739–1753. [Google Scholar] [CrossRef]
- Schmid, J.; Schwarz, S.; Fischer, M.; Sudhop, S.; Clausen-Schaumann, H.; Schieker, M.; Huber, R. A laser-cutting-based manufacturing process for the generation of three-dimensional scaffolds for tissue engineering using Polycaprolactone/Hydroxyapatite composite polymer. J. Tissue Eng. 2019, 10, 2041731419859157. [Google Scholar] [CrossRef] [PubMed]
- Kafle, A.; Luis, E.; Silwal, R.; Pan, H.M.; Shrestha, P.L.; Bastola, A.K. 3D/4D Printing of Polymers: Fused Deposition Modelling (FDM), Selective Laser Sintering (SLS), and Stereolithography (SLA). Polymers 2021, 13, 3101. [Google Scholar] [CrossRef]
- Ebrahimbaygi, P.; Khazaei, M.R.; Valadbeigi, P.; Rostaminasab, G.; Mikaeili, A.; Jouybari, T.A.; Rezakhani, L. Recent advances in scaffold based electrospun for breast cancer research. Polym. Adv. Technol. 2024, 35, e6499. [Google Scholar] [CrossRef]
- Nazarnezhad, S.; Gorgani, S.; Kargozar, S. Cancer diagnosis and therapy by electrospun nanofibers: Opportunities and challenges. In Biomaterials for Precision Cancer Medicine; Woodhead Publishing: Sawston, UK, 2025; pp. 457–484. [Google Scholar] [CrossRef]
- Maffini, M.V.; Soto, A.M.; Calabro, J.M.; Ucci, A.A.; Sonnenschein, C. The stroma as a crucial target in rat mammary gland carcinogenesis. J. Cell Sci. 2004, 117, 1495–1502. [Google Scholar] [CrossRef]
- Guo, Q.; Betts, C.; Pennock, N.; Mitchell, E.; Schedin, P. Mammary Gland Involution Provides a Unique Model to Study the TGF-β Cancer Paradox. J. Clin. Med. 2017, 6, 10. [Google Scholar] [CrossRef]
- Cohen, N.; Shani, O.; Raz, Y.; Sharon, Y.; Hoffman, D.; Abramovitz, L.; Erez, N. Fibroblasts drive an immunosuppressive and growth-promoting microenvironment in breast cancer via secretion of Chitinase 3-like 1. Oncogene 2017, 36, 4457–4468. [Google Scholar] [CrossRef]
- Asano, K.; Nelson, C.M.; Nandadasa, S.; Aramaki-Hattori, N.; Lindner, D.J.; Alban, T.; Inagaki, J.; Ohtsuki, T.; Oohashi, T.; Apte, S.S.; et al. Stromal Versican Regulates Tumor Growth by Promoting Angiogenesis. Sci. Rep. 2017, 7, 17225. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, B.; Li, Z.; Li, J.; Sun, S.; Sun, S. Cancer-associated adipocytes: Key players in breast cancer progression. J. Hematol. Oncol. 2019, 12, 95. [Google Scholar] [CrossRef]
- Hillers, L.E.; D’Amato, J.V.; Chamberlin, T.; Paderta, G.; Arendt, L.M. Obesity-Activated Adipose-Derived Stromal Cells Promote Breast Cancer Growth and Invasion. Neoplasia 2018, 20, 1161. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Bunnell, B.A.; Frazier, T.; Rowan, B.; Shah, F.; Thomas-Porch, C.; Wu, X. Adipose-derived stromal/stem cells: A primer. Organogenesis 2013, 9, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.; Campbell, J.M.; Coates, R.N.; Render, K.M.; Byrne, C.E.; Martin, E.C.; Melvin, A.T. Evaluation of intercellular communication between breast cancer cells and adipose-derived stem cells via passive diffusion in a two-layer microfluidic device. Lab. A Chip 2020, 20, 2009–2019. [Google Scholar] [CrossRef]
- Hamel, K.M.; Frazier, T.P.; Williams, C.; Duplessis, T.; Rowan, B.G.; Gimble, J.M.; Sanchez, C.G. Adipose Tissue in Breast Cancer Microphysiological Models to Capture Human Diversity in Preclinical Models. Int. J. Mol. Sci. 2024, 25, 2728. [Google Scholar] [CrossRef] [PubMed]
- Adriance, M.C.; Inman, J.L.; Petersen, O.W.; Bissell, M.J. Myoepithelial cells: Good fences make good neighbors. Breast Cancer Res. 2005, 7, 190–197. [Google Scholar] [CrossRef]
- Pandey, P.R.; Saidou, J.; Watabe, K. Role of myoepithelial cells in breast tumor progression. Front. Biosci. 2010, 15, 226–236. [Google Scholar] [CrossRef]
- Man, Y.-g. Focal degeneration of aged or injured myoepithelial cells and the resultant auto-immunoreactions are trigger factors for breast tumor invasion. Med. Hypotheses 2007, 69, 1340–1357. [Google Scholar] [CrossRef]
- Man, Y.-G.; Stojadinovic, A.; Mason, J.; Avital, I.; Bilchik, A.; Bruecher, B.; Protic, M.; Nissan, A.; Izadjoo, M.; Zhang, X.; et al. Tumor-Infiltrating Immune Cells Promoting Tumor Invasion and Metastasis: Existing Theories. J. Cancer 2013, 4, 84–95. [Google Scholar] [CrossRef]
- Duffy, M.J.; Maguire, T.M.; Hill, A.; McDermott, E.; O’Higgins, N. Metalloproteinases: Role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000, 2, 252–257. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Segovia-Mendoza, M.; Morales-Montor, J. Immune tumor microenvironment in breast cancer and the participation of estrogens and its receptors into cancer physiopathology. Front. Immunol. 2019, 10, 348. [Google Scholar] [CrossRef]
- Belli, C.; Trapani, D.; Viale, G.; D’Amico, P.; Duso, B.A.; Della Vigna, P.; Orsi, F.; Curigliano, G. Targeting the microenvironment in solid tumors. Cancer Treat. Rev. 2018, 65, 22–32. [Google Scholar] [CrossRef]
- Soysal, S.D.; Tzankov, A.; Muenst, S.E. Role of the Tumor Microenvironment in Breast Cancer. Pathobiology 2015, 82, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Naylor, M.J. Tumour Microenvironment-Immune Cell Interactions Influencing Breast Cancer Heterogeneity and Disease Progression. Front. Oncol. 2022, 12, 876451. [Google Scholar] [CrossRef] [PubMed]
- Akinsipe, T.; Mohamedelhassan, R.; Akinpelu, A.; Pondugula, S.R.; Mistriotis, P.; Avila, L.A.; Suryawanshi, A. Cellular interactions in tumor microenvironment during breast cancer progression: New frontiers and implications for novel therapeutics. Front. Immunol. 2024, 15, 1302587. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, T.J.M.; Ravi, K.; Suresh, A.P.; Acharya, A.P.; Nikkhah, M. Engineered Tumor-Immune Microenvironment On-a-Chip to Study T Cell—Macrophage Interaction in Breast Cancer Progression. Adv. Healthc. Mater. 2024, 13, e2303658. [Google Scholar] [CrossRef]
- Nerger, B.A.; Brun, P.T.; Nelson, C.M. Microextrusion printing cell-laden networks of type I collagen with patterned fiber alignment and geometry. Soft Matter 2019, 15, 5728–5738. [Google Scholar] [CrossRef]
- Gong, X.; Kulwatno, J.; Mills, K.L. Rapid fabrication of collagen bundles mimicking tumor-associated collagen architectures. Acta Biomater. 2020, 108, 128–141. [Google Scholar] [CrossRef]
- Dittmer, J.; Leyh, B. The impact of tumor stroma on drug response in breast cancer. Semin. Cancer Biol. 2015, 31, 3–15. [Google Scholar] [CrossRef]
- Garre, E.; Gustafsson, A.; Leiva, M.C.; Håkansson, J.; Ståhlberg, A.; Kovács, A.; Landberg, G. Breast Cancer Patient-Derived Scaffolds Can Expose Unique Individual Cancer Progressing Properties of the Cancer Microenvironment Associated with Clinical Characteristics. Cancers 2022, 14, 2172. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, N.M.; Kevrekidis, P.G.; Shahriyari, L. Oxygen, angiogenesis, cancer and immune interplay in breast tumour microenvironment: A computational investigation. R. Soc. Open Sci. 2024, 11, 240718. [Google Scholar] [CrossRef]
- Seager, R.; Pabla, S.; Gandhi, S.; Senosain, M.-F.; Parikh, H.; Van Roey, E.; Gao, S.; Pulivendula, Y.; DePietro, P.; Nesline, M.K.; et al. Interaction between VEGF-A and immune checkpoint targets in triple-negative breast cancer and mechanisms of immune evasion and tumor progression. J. Clin. Oncol. 2024, 42, 1096. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Liu, C.; Li, Y.; Sun, C.; Wu, J.; Wu, Q. Crosstalk and plasticity driving between cancer-associated fibroblasts and tumor microenvironment: Significance of breast cancer metastasis. J. Transl. Med. 2023, 21, 827. [Google Scholar] [CrossRef] [PubMed]
- Offeddu, G.S.; Cambria, E.; Shelton, S.E.; Haase, K.; Wan, Z.; Possenti, L.; Nguyen, H.T.; Gillrie, M.R.; Hickman, D.; Knutson, C.G.; et al. Personalized models of breast cancer desmoplasia reveal biomechanical determinants of drug penetration. Adv. Sci. 2024, 11, 2402757. [Google Scholar] [CrossRef]
- Bohn, T.; Rapp, S.; Luther, N.; Klein, M.; Bruehl, T.-J.; Kojima, N.; Lopez, P.A.; Hahlbrock, J.; Muth, S.; Endo, S.; et al. Tumor immunoevasion via acidosis-dependent induction of regulatory tumor-associated macrophages. Nat. Immunol. 2018, 19, 1319–1329. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Du, J.Z.; Mao, C.Q.; Yuan, Y.Y.; Yang, X.Z.; Wang, J. Tumor extracellular acidity-activated nanoparticles as drug delivery systems for enhanced cancer therapy. Biotechnol. Adv. 2014, 32, 789–803. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Q.; Zhang, H.; Han, S.; Liu, N.; Ren, H.; Guo, H.; Xu, F. Construction of cancer-on-a-chip for drug screening. Drug Discov. Today 2021, 26, 1875–1890. [Google Scholar] [CrossRef]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, Q.; Chen, B.; Liang, G.-T.; Li, W.-X.; Zhou, X.-M.; Liu, D.-Y. Study on Microenvironment Acidification by Microfluidic Chip with Multilayer-paper Supported Breast Cancer Tissue. Chin. J. Anal. Chem. 2013, 41, 822–827. [Google Scholar] [CrossRef]
- Xu, H.; Rahimpour, S.; Nesvick, C.L.; Zhang, X.; Ma, J.; Zhang, M.; Zhang, G.; Wang, L.; Yang, C.; Hong, C.S.; et al. Activation of hypoxia signaling induces phenotypic transformation of glioma cells: Implications for bevacizumab antiangiogenic therapy. Oncotarget 2015, 6, 11882–11893. [Google Scholar] [CrossRef]
- Lee, I.; Woo, J.H.; Lee, M.; Jeon, T.J.; Kim, S.M. Hypoxic physiological environments in a gas-regulated microfluidic device. Micromachines 2019, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, W.; Wang, Y.; Wang, J.-C.; Tu, Q.; Liu, R.; Wang, J. Construction of oxygen and chemical concentration gradients in a single microfluidic device for studying tumor cell–drug interactions in a dynamic hypoxia microenvironment. Lab. A Chip 2012, 13, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Palacio-Castañeda, V.; Kooijman, L.; Venzac, B.; Verdurmen, W.P.R.; Le Gac, S. Metabolic Switching of Tumor Cells under Hypoxic Conditions in a Tumor-on-a-chip Model. Micromachines 2020, 11, 382. [Google Scholar] [CrossRef]
- Lin, X.; Chen, Q.; Liu, W.; Zhang, J.; Wang, S.; Lin, Z.; Lin, J.-M. Oxygen-induced cell migration and on-line monitoring biomarkers modulation of cervical cancers on a microfluidic system. Sci. Rep. 2015, 5, 9643. [Google Scholar] [CrossRef]
- Robey, I.F.; Baggett, B.K.; Kirkpatrick, N.D.; Roe, D.J.; Dosescu, J.; Sloane, B.F.; Hashim, A.I.; Morse, D.L.; Raghunand, N.; Gatenby, R.A.; et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009, 69, 2260–2268. [Google Scholar] [CrossRef]
- Lam, S.F.; Bishop, K.W.; Mintz, R.; Fang, L.; Achilefu, S. Calcium carbonate nanoparticles stimulate cancer cell reprogramming to suppress tumor growth and invasion in an organ-on-a-chip system. Sci. Rep. 2021, 11, 9246. [Google Scholar] [CrossRef]
- Avci, C.B.; Bagca, B.G.; Nikanfar, M.; Takanlou, L.S.; Takanlou, M.S.; Nourazarian, A. Tumor microenvironment and cancer metastasis: Molecular mechanisms and therapeutic implications. Front. Pharmacol. 2024, 15, 1442888. [Google Scholar] [CrossRef] [PubMed]
- Erez, N. Abstract IA013: Stromal and immune plasticity shape the metastatic microenvironment. Cancer Res. 2024, 84, IA013. [Google Scholar] [CrossRef]
- Nguyen, M.; De Ninno, A.; Mencattini, A.; Mermet-Meillon, F.; Fornabaio, G.; Evans, S.S.; Cossutta, M.; Khira, Y.; Han, W.; Sirven, P.; et al. Dissecting Effects of Anti-cancer Drugs and Cancer-Associated Fibroblasts by On-Chip Reconstitution of Immunocompetent Tumor Microenvironments. Cell Rep. 2018, 25, 3884–3893.e3. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Smith, A.M.; Garson, C.J.; Hassani, I.; Seeto, W.J.; Pant, K.; Arnold, R.D.; Prabhakarpandian, B.; Lipke, E.A. A Microvascularized Tumor-mimetic Platform for Assessing Anti-cancer Drug Efficacy. Sci. Rep. 2018, 8, 3171. [Google Scholar] [CrossRef] [PubMed]
- Eliyatkin, N.; Yalcin, E.; Zengel, B.; Aktaş, S.; Vardar, E. Molecular Classification of Breast Carcinoma: From Traditional, Old-Fashioned Way to A New Age, and A New Way. J. Breast Health 2015, 11, 59–66. [Google Scholar] [CrossRef]
- Xiong, X.; Zheng, L.W.; Ding, Y.; Chen, Y.F.; Cai, Y.W.; Wang, L.P.; Huang, L.; Liu, C.C.; Shao, Z.M.; Yu, K.D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef]
- Fragomeni, S.M.; Sciallis, A.; Jeruss, J.S. Molecular Subtypes and Local-Regional Control of Breast Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 95–120. [Google Scholar] [CrossRef]
- Gomes Do Nascimento, R.; Otoni, K.M. Histological and molecular classification of breast cancer: What do we know? Mastology 2020, 30, 20200024. [Google Scholar] [CrossRef]
- Burstein, M.D.; Tsimelzon, A.; Poage, G.M.; Covington, K.R.; Contreras, A.; Fuqua, S.A.; Savage, M.I.; Osborne, C.K.; Hilsenbeck, S.G.; Chang, J.C.; et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin. Cancer Res. 2015, 21, 1688–1698. [Google Scholar] [CrossRef] [PubMed]
- Bischel, L.L.; Beebe, D.J.; Sung, K.E. Microfluidic model of ductal carcinoma in situ with 3D, organotypic structure. BMC Cancer 2015, 15, 12. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Gillette, A.; Lugo-Cintrón, K.; Acevedo-Acevedo, S.; Gomez, I.; Morgan, M.; Heaster, T.; Wisinski, K.B.; Palecek, S.P.; Skala, M.C.; et al. Organotypic microfluidic breast cancer model reveals starvation-induced spatial-temporal metabolic adaptations. EBioMedicine 2018, 37, 144–157. [Google Scholar] [CrossRef]
- Choi, Y.; Hyun, E.; Seo, J.; Blundell, C.; Kim, H.C.; Lee, E.; Lee, S.H.; Moon, A.; Moon, W.K.; Huh, D. A microengineered pathophysiological model of early-stage breast cancer. Lab. A Chip 2015, 15, 3350–3357. [Google Scholar] [CrossRef]
- Nashimoto, Y.; Okada, R.; Hanada, S.; Arima, Y.; Nishiyama, K.; Miura, T.; Yokokawa, R. Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid. Biomaterials 2020, 229, 119547. [Google Scholar] [CrossRef]
- Montanez-Sauri, S.I.; Sung, K.E.; Berthier, E.; Beebe, D.J. Enabling screening in 3D microenvironments: Probing matrix and stromal effects on the morphology and proliferation of T47D breast carcinoma cells. Integr. Biol. 2013, 5, 631–640. [Google Scholar] [CrossRef]
- Aung, A.; Kumar, V.; Theprungsirikul, J.; Davey, S.K.; Varghese, S. An Engineered Tumor-on-a-Chip Device with Breast Cancer–Immune Cell Interactions for Assessing T-cell Recruitment. Cancer Res. 2020, 80, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Dereli-Korkut, Z.; Akaydin, H.D.; Ahmed, A.H.R.; Jiang, X.; Wang, S. Three dimensional microfluidic cell arrays for ex vivo drug screening with mimicked vascular flow. Anal. Chem. 2014, 86, 2997–3004. [Google Scholar] [CrossRef] [PubMed]
- Khoo, B.L.; Grenci, G.; Lim, J.S.Y.; Lim, Y.P.; Fong, J.; Yeap, W.H.; Bin Lim, S.; Chua, S.L.; Wong, S.C.; Yap, Y.-S.; et al. Low-dose anti-inflammatory combinatorial therapy reduced cancer stem cell formation in patient-derived preclinical models for tumour relapse prevention. Br. J. Cancer 2019, 120, 407–423. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, F.; Reis-Filho, J.S. Pathogenesis of Triple-Negative Breast Cancer. Annu. Rev. Pathol. Mech. Dis. 2021, 17, 181–204. [Google Scholar] [CrossRef]
- Turdo, A.; Gaggianesi, M.; Di Franco, S.; Veschi, V.; D’aCcardo, C.; Porcelli, G.; Iacono, M.L.; Pillitteri, I.; Verona, F.; Militello, G.; et al. Effective targeting of breast cancer stem cells by combined inhibition of Sam68 and Rad51. Oncogene 2022, 41, 2196–2209. [Google Scholar] [CrossRef]
- Liu, Y.; Burness, M.L.; Martin-Trevino, R.; Guy, J.; Bai, S.; Harouaka, R.; Brooks, M.D.; Shang, L.; Fox, A.; Luther, T.K.; et al. RAD51 mediates resistance of cancer stem cells to PARP inhibition in triple-negative breast cancer. Clin. Cancer Res. 2017, 23, 514–522. [Google Scholar] [CrossRef]
- Chi, C.W.; Lao, Y.H.; Ahmed, A.H.R.; Benoy, E.C.; Li, C.; Dereli-Korkut, Z.; Fu, B.M.; Leong, K.W.; Wang, S. High-Throughput Tumor-on-a-Chip Platform to Study Tumor–Stroma Interactions and Drug Pharmacokinetics. Adv. Healthc. Mater. 2020, 9, 2000880. [Google Scholar] [CrossRef]
- Shirure, V.S.; Bi, Y.; Curtis, M.B.; Lezia, A.; Goedegebuure, M.M.; Goedegebuure, S.P.; Aft, R.; Fields, R.C.; George, S.C. Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids. Lab. A Chip 2018, 18, 3687–3702. [Google Scholar] [CrossRef]
- Devadas, D.; Moore, T.A.; Walji, N.; Young, E.W.K. A microfluidic mammary gland coculture model using parallel 3D lumens for studying epithelial-endothelial migration in breast cancer. Biomicrofluidics 2019, 13, 064122. [Google Scholar] [CrossRef]
- Kulwatno, J.; Gong, X.; Devaux, R.; Herschkowitz, J.I.; Mills, K.L. An Organotypic Mammary Duct Model Capturing Matrix Mechanics-Dependent Ductal Carcinoma in Situ Progression. Tissue Eng. Part. A 2021, 27, 454–466. [Google Scholar] [CrossRef]
- Shields, J.D.; Fleury, M.E.; Yong, C.; Tomei, A.A.; Randolph, G.J.; Swartz, M.A. Autologous Chemotaxis as a Mechanism of Tumor Cell Homing to Lymphatics via Interstitial Flow and Autocrine CCR7 Signaling. Cancer Cell 2007, 11, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Ma, Y.; Wu, C.; Shiau, C.; Segall, J.E.; Wu, M. Tumor spheroids under perfusion within a 3D microfluidic platform reveal critical roles of cell-cell adhesion in tumor invasion. Sci. Rep. 2020, 10, 9648. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-R.; Ospina-Muñoz, N.; Noe-Kim, V.; Yang, Y.; Elzey, B.D.; Konieczny, S.F.; Han, B.; Choi, J. Subtype-specific characterization of breast cancer invasion using a microfluidic tumor platform. PLoS ONE 2020, 15, e0234012. [Google Scholar] [CrossRef] [PubMed]
- Imparato, G.; Urciuolo, F.; Netti, P.A. Organ on Chip Technology to Model Cancer Growth and Metastasis. Bioengineering 2022, 9, 28. [Google Scholar] [CrossRef]
- Jouybar, M.; de Winde, C.M.; Wolf, K.; Friedl, P.; Mebius, R.E.; den Toonder, J.M.J. Cancer-on-chip models for metastasis: Importance of the tumor microenvironment. Trends Biotechnol. 2024, 42, 431–448. [Google Scholar] [CrossRef]
- Tang, T.; Zhang, L.; Li, C.; Zhou, T. Gastric and adrenal metastasis from breast cancer: Case report and review of literature. Medicine 2020, 99, e18812. [Google Scholar] [CrossRef]
- Moradi, A.A.; Moradi, M.; Hosseini, S.; Garmsiri, A.; Bahari, E.; Bahrami, F.; Sheikhesmaeili, F.; Ghaderi, B.; Yousefinejad, V.; Bahrami, T. Organotropism of breast cancer metastasis: A comprehensive approach to the shared gene network. Gene Rep. 2023, 30, 101749. [Google Scholar] [CrossRef]
- Sigdel, I.; Gupta, N.; Faizee, F.; Khare, V.M.; Tiwari, A.K.; Tang, Y. Biomimetic Microfluidic Platforms for the Assessment of Breast Cancer Metastasis. Front. Bioeng. Biotechnol. 2021, 9, 633671. [Google Scholar] [CrossRef]
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Marada, S.; Madu, C.; Lu, Y. Role of transcription factors in metastasis of breast cancer. Open Explor. 2024, 5, 936–949. [Google Scholar] [CrossRef]
- Jiang, W.-J.; Zhou, T.-H.; Huang, H.-J.; Li, L.-S.; Tan, H.; Zhang, R.; Wang, Q.-S.; Feng, Y.-M. Breast Cancer Subtype–Specific Organotropism Is Dictated by FOXF2-Regulated Metastatic Dormancy and Recovery. Cancer Res. 2025, 85, 644–659. [Google Scholar] [CrossRef]
- Wu, Q.; Tian, P.; He, D.; Jia, Z.; He, Y.; Luo, W.; Lv, X.; Wang, Y.; Zhang, P.; Liang, Y.; et al. SCUBE2 mediates bone metastasis of luminal breast cancer by modulating immune-suppressive osteoblastic niches. Cell Res. 2023, 33, 464–478. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, R.; Jin, X.; Bai, X.; Tang, W.; Wang, L.; Karako, K.; Zhu, W. Advances in research on receptor heterogeneity in breast cancer liver metastasis. Biosci. Trends 2025, 19, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Rashid, N.S.; Grible, J.M.; Clevenger, C.V.; Harrell, J.C. Breast cancer liver metastasis: Current and future treatment approaches. Clin. Exp. Metastasis 2021, 38, 263–277. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, K.; Lim, S.H.; Kim, H.S.; Yoo, K.H.; Jung, K.S.; Song, H.-N.; Hong, M.; Do, I.-G.; Ahn, T.; et al. Mutational profiling of brain metastasis from breast cancer: Matched pair analysis of targeted sequencing between brain metastasis and primary breast cancer. Oncotarget 2015, 6, 43731–43742. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Fu, Z.; Li, Y.; Zhang, M.; Zhang, D.; Liu, L.; Jin, Q.; Chen, X.; Xie, H. Mechanisms of organotropism in breast cancer and predicting metastasis to distant organs using deep learning. Discov. Oncol. 2025, 16, 1056. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, X.; He, W.; Zhao, Y.; Hao, S.; Wu, Q.; Li, S.; Zhang, S.; Shi, M. Fluid shear stress and tumor metastasis. Am. J. Cancer Res. 2018, 8, 763–777. [Google Scholar]
- Fan, R.; Emery, T.; Zhang, Y.; Xia, Y.; Sun, J.; Wan, J. Circulatory shear flow alters the viability and proliferation of circulating colon cancer cells. Sci. Rep. 2016, 6, 27073. [Google Scholar] [CrossRef]
- Labelle, M.; Hynes, R.O. The initial hours of metastasis: The importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012, 2, 1091–1099. [Google Scholar] [CrossRef]
- Lee, H.J.; Diaz, M.F.; Price, K.M.; Ozuna, J.A.; Zhang, S.; Sevick-Muraca, E.M.; Hagan, J.P.; Wenzel, P.L. Fluid shear stress activates YAP1 to promote cancer cell motility. Nat. Commun. 2017, 8, 14122. [Google Scholar] [CrossRef]
- Toh, Y.C.; Raja, A.; Yu, H.; Van Noort, D. A 3D microfluidic model to recapitulate cancer cell migration and invasion. Bioengineering 2018, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Choi, J.H.; Kim, K.J.; Shin, M.; Choi, J.W. Microfluidic System to Analyze the Effects of Interleukin 6 on Lymphatic Breast Cancer Metastasis. Front. Bioeng. Biotechnol. 2021, 8, 611802. [Google Scholar] [CrossRef]
- German, S.V.; Abalymov, A.A.; Kurochkin, M.A.; Kan, Y.; Gorin, D.A.; Novoselova, M.V. Plug-and-Play Lymph Node-on-Chip: Secondary Tumor Modeling by the Combination of Cell Spheroid, Collagen Sponge and T-Cells. Int. J. Mol. Sci. 2023, 24, 3183. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Gong, M.M.; Skala, M.C.; Harari, P.M.; Beebe, D.J. Human Tumor-Lymphatic Microfluidic Model Reveals Differential Conditioning of Lymphatic Vessels by Breast Cancer Cells. Adv. Healthc. Mater. 2020, 9, 1900925. [Google Scholar] [CrossRef]
- Hao, S.; Ha, L.; Cheng, G.; Wan, Y.; Xia, Y.; Sosnoski, D.M.; Mastro, A.M.; Zheng, S.Y. A Spontaneous 3D Bone-On-a-Chip for Bone Metastasis Study of Breast Cancer Cells. Small 2018, 14, 1702787. [Google Scholar] [CrossRef]
- Marturano-Kruik, A.; Nava, M.M.; Yeager, K.; Chramiec, A.; Hao, L.; Robinson, S.; Guo, E.; Raimondi, M.T.; Vunjak-Novakovic, G. Human bone perivascular niche-on-a-chip for studying metastatic colonization. Proc. Natl. Acad. Sci. USA 2018, 115, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Crippa, M.; Talò, G.; Lamouline, A.; Bolis, S.; Arrigoni, C.; Bersini, S.; Moretti, M. A microfluidic model of human vascularized breast cancer metastasis to bone for the study of neutrophil-cancer cell interactions. Mater. Today Bio 2022, 17, 100460. [Google Scholar] [CrossRef]
- Bersini, S.; Jeon, J.S.; Dubini, G.; Arrigoni, C.; Chung, S.; Charest, J.L.; Moretti, M.; Kamm, R.D. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials 2014, 35, 2454–2461. [Google Scholar] [CrossRef] [PubMed]
- Conceição, F.; Sousa, D.M.; Loessberg-Zahl, J.; Vollertsen, A.R.; Neto, E.; Søe, K.; Paredes, J.; Leferink, A.; Lamghari, M. A metastasis-on-a-chip approach to explore the sympathetic modulation of breast cancer bone metastasis. Mater. Today Bio 2022, 13, 100219. [Google Scholar] [CrossRef]
- Terceiro, L.E.L.; Ikeogu, N.M.; Lima, M.F.; Edechi, C.A.; Nickel, B.E.; Fischer, G.; Leygue, E.; McManus, K.J.; Myal, Y. Navigating the Blood–Brain Barrier: Challenges and Therapeutic Strategies in Breast Cancer Brain Metastases. Int. J. Mol. Sci. 2023, 24, 12034. [Google Scholar] [CrossRef]
- Xu, H.; Li, Z.; Yu, Y.; Sizdahkhani, S.; Ho, W.S.; Yin, F.; Wang, L.; Zhu, G.; Zhang, M.; Jiang, L.; et al. A dynamic in vivo-like organotypic blood-brain barrier model to probe metastatic brain tumors. Sci. Rep. 2016, 6, 36670. [Google Scholar] [CrossRef]
- TWesterhof, T.M.; Yang, B.A.; Merrill, N.M.; Yates, J.A.; Altemus, M.; Russell, L.; Miller, A.J.; Bao, L.; Wu, Z.; Ulintz, P.J.; et al. Blood–Brain Barrier Remodeling in an Organ-on-a-Chip Device Showing Dkk1 to be a Regulator of Early Metastasis. Adv. NanoBiomed Res. 2023, 3, 2200036. [Google Scholar] [CrossRef]
- Cui, K.; Chen, W.; Cao, R.; Xie, Y.; Wang, P.; Wu, Y.; Wang, Y.; Qin, J. Brain organoid-on-chip system to study the effects of breast cancer derived exosomes on the neurodevelopment of brain. Cell Regen. 2022, 11, 7. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Y.; Liu, X.; Liao, X.; He, J.; Niu, L. The Clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer 2019, 19, 1091. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Mark, M.T.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Zarin, B.; Rafiee, L.; Abdollahi, S.; Vatani, M.; Hassani, M.; Sanati-Nezhad, A.; Javanmard, S.H. Studying breast cancer lung metastasis using a multi-compartment microfluidic device with a mimetic tumor-stroma interaction model. Transl. Oncol. 2025, 53, 102303. [Google Scholar] [CrossRef]
- Kong, J.; Luo, Y.; Jin, D.; An, F.; Zhang, W.; Liu, L.; Li, J.; Fang, S.; Li, X.; Yang, X.; et al. A novel microfluidic model can mimic organ-specific metastasis of circulating tumor cells. Oncotarget 2016, 7, 78421–78432. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.; Kim, I.; Ro, J.; Kim, J.; Min, Y.; Park, J.; Sunkara, V.; Park, Y.S.; Michael, I.; et al. Three-dimensional human liver-chip emulating premetastatic niche formation by breast cancer-derived extracellular vesicles. ACS Nano 2020, 14, 14971–14988. [Google Scholar] [CrossRef]
- Tian, H.; Pang, J.; Qin, K.; Yuan, W.; Kong, J.; Ma, H.; He, J.; Yang, X.; Luo, Y.; Lu, Y.; et al. A Novel Tissue-Based Liver–Kidney-on-a-Chip Can Mimic Liver Tropism of Extracellular Vesicles Derived from Breast Cancer Cells. Biotechnol. J. 2020, 15, 1900107. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Miao, X.; Song, Q.; Ding, H.; Rajan, S.A.P.; Skardal, A.; Votanopoulos, K.I.; Dai, K.; Zhao, W.; Lu, B.; et al. Detection of lineage-reprogramming efficiency of tumor cells in a 3D-printed liver-on-a-chip model. Theranostics 2023, 13, 4905–4918. [Google Scholar] [CrossRef]
- Cayrefourcq, L.; Alix-Panabières, C. Clinical relevance of liquid biopsy in breast cancer: Update in 2020. Expert. Rev. Mol. Diagn. 2020, 20, 913–919. [Google Scholar] [CrossRef]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid biopsy: Current technology and clinical applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef]
- Cai, S.; Deng, Y.; Wang, Z.; Zhu, J.; Huang, C.; Du, L.; Wang, C.; Yu, X.; Liu, W.; Yang, C.; et al. Development and clinical validation of a microfluidic-based platform for CTC enrichment and downstream molecular analysis. Front. Oncol. 2023, 13, 1238332. [Google Scholar] [CrossRef]
- Malik, S.; Zaheer, S. The impact of liquid biopsy in breast cancer: Redefining the landscape of non-invasive precision oncology. J. Liq. Biopsy 2025, 8, 100299. [Google Scholar] [CrossRef]
- Macaraniag, C.; Zhou, J.; Li, J.; Putzbach, W.; Hay, N.; Papautsky, I. Microfluidic isolation of breast cancer circulating tumor cells from microvolumes of mouse blood. Electrophoresis 2023, 44, 1859–1867. [Google Scholar] [CrossRef]
- Abdulla, A.; Zhang, Z.; Ahmad, K.Z.; Warden, A.R.; Li, H.; Ding, X. Rapid and efficient capturing of circulating tumor cells from breast cancer Patient’s whole blood via the antibody functionalized microfluidic (AFM) chip. Biosens. Bioelectron. 2022, 201, 113965. [Google Scholar] [CrossRef]
- Hyun, K.-A.; Koo, G.-B.; Han, H.; Sohn, J.; Choi, W.; Kim, S.-I.; Jung, H.-I.; Kim, Y.-S. Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget 2016, 7, 24677–24687. [Google Scholar] [CrossRef] [PubMed]
- Galletti, G.; Sung, M.S.; Vahdat, L.T.; Shah, M.A.; Santana, S.M.; Altavilla, G.; Kirby, B.J.; Giannakakou, P. Isolation of breast cancer and gastric cancer circulating tumor cells by use of an anti HER2-based microfluidic device. Lab. Chip 2014, 14, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Loeian, M.S.; Aghaei, S.M.; Farhadi, F.; Rai, V.; Yang, H.W.; Johnson, M.D.; Aqil, F.; Mandadi, M.; Rai, S.N.; Panchapakesan, B. Liquid biopsy using the nanotube-CTC-chip: Capture of invasive CTCs with high purity using preferential adherence in breast cancer patients. Lab. A Chip 2019, 19, 1899–1915. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, X.; Gao, W.; Wang, Y.; Jia, C.; Cong, H. A label-free microfluidic chip for the highly selective isolation of single and cluster CTCs from breast cancer patients. Transl. Oncol. 2021, 14, 100959. [Google Scholar] [CrossRef]
- Parvin, D.; Hashemi, Z.S.; Shokati, F.; Mohammadpour, Z.; Bazargan, V. Immunomagnetic Isolation of HER2-Positive Breast Cancer Cells Using a Microfluidic Device. ACS Omega 2023, 8, 21745–21754. [Google Scholar] [CrossRef]
- Gwak, H.; Park, S.; Kim, J.; Lee, J.D.; Kim, I.-S.; Kim, S.-I.; Hyun, K.-A.; Jung, H.-I. Microfluidic chip for rapid and selective isolation of tumor-derived extracellular vesicles for early diagnosis and metastatic risk evaluation of breast cancer. Biosens. Bioelectron. 2021, 192, 113495. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Sun, M.; Feng, B.; Shen, H.; Zhu, J.; Chen, X.; Yu, S. A filter-electrochemical microfluidic chip for multiple surface protein analysis of exosomes to detect and classify breast cancer. Biosens. Bioelectron. 2023, 239, 115590. [Google Scholar] [CrossRef]
- Lim, J.; Kang, B.; Son, H.Y.; Mun, B.; Huh, Y.-M.; Rho, H.W.; Kang, T.; Moon, J.; Lee, J.-J.; Seo, S.B.; et al. Microfluidic device for one-step detection of breast cancer-derived exosomal mRNA in blood using signal-amplifiable 3D nanostructure. Biosens. Bioelectron. 2022, 197, 113753. [Google Scholar] [CrossRef]
- Mun, B.; Jeong, H.; Kim, R.; Gu, B.; Kim, J.; Son, H.Y.; Rho, H.W.; Lim, E.-K.; Haam, S. 3D-Nanostructured microfluidic device arranged in a herringbone pattern for the highly effective capture of HER2-Positive cancer-derived exosomes in urine. Chem. Eng. J. 2024, 482. [Google Scholar] [CrossRef]
- Wnorowski, A.; Yang, H.; Wu, J.C. Progress, obstacles, and limitations in the use of stem cells in organ-on-a-chip models. Adv. Drug Deliv. Rev. 2019, 140, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Firatligil-Yildirir, B.; Bati-Ayaz, G.; Tahmaz, I.; Bilgen, M.; Pesen-Okvur, D.; Yalcin-Ozuysal, O. On-chip determination of tissue-specific metastatic potential of breast cancer cells. Biotechnol. Bioeng. 2021, 118, 3799–3810. [Google Scholar] [CrossRef]
- Maulana, T.I.; Teufel, C.; Cipriano, M.; Roosz, J.; Lazarevski, L.; Hil, F.E.v.D.; Scheller, L.; Orlova, V.; Koch, A.; Hudecek, M.; et al. Breast cancer-on-chip for patient-specific efficacy and safety testing of CAR-T cells. Cell Stem Cell 2024, 31, 989–1002.e9. [Google Scholar] [CrossRef] [PubMed]
- Tajeddin, A.; Mustafaoglu, N. Design and fabrication of organ-on-chips: Promises and challenges. Micromachines 2021, 12, 1443. [Google Scholar] [CrossRef] [PubMed]
- Poh, W.T.; Stanslas, J. The new paradigm in animal testing—‘3Rs alternatives’. Regul. Toxicol. Pharmacol. 2024, 153, 105705. [Google Scholar] [CrossRef]
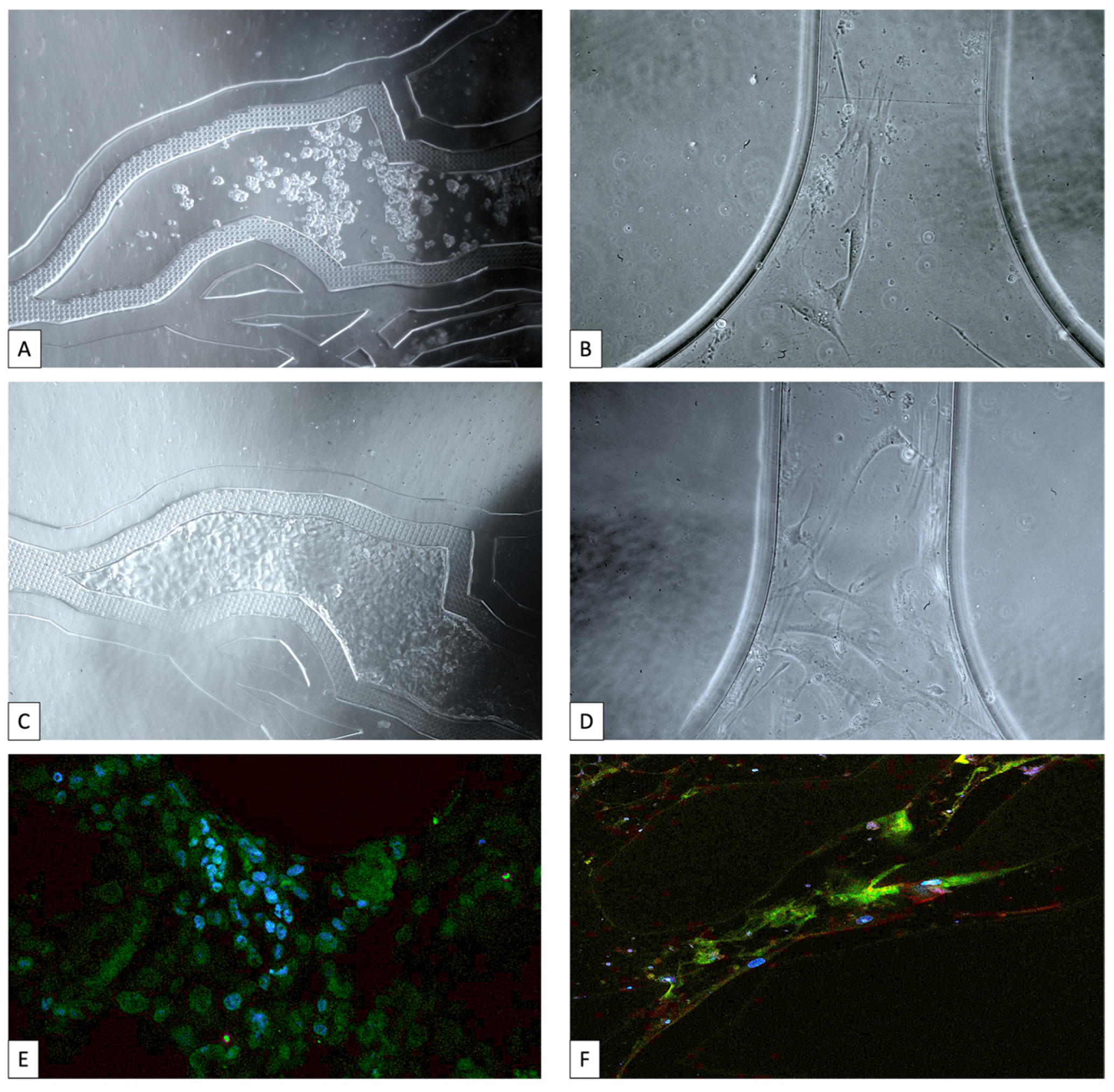
| Model Type | Key Limitations | Breast Cancer-on-a-Chip Solutions |
|---|---|---|
| 2D In Vitro Cultures |
|
|
|
| |
| Animal Models |
|
|
|
| |
| Static 3D Models |
|
|
|
|
| Technique | Advantages | Limitations | Typical Materials |
|---|---|---|---|
| Soft Lithography | High-resolution, biocompatible, cleanroom-free | Needs master mold | PDMS, polystyrene |
| Photolithography | High precision and batch fabrication | Cleanroom required, expensive | Glass, silicon |
| Injection Molding | Scalable, reproducible | High setup cost, inflexible design | PMMA, COC |
| Hot Embossing | Good replication fidelity | Heat-sensitive, slow cycles | PC, PLA |
| Etching | Sub-micron resolution | Toxic reagents, complex process | Glass, silk fibroin |
| Laser Cutting | Rapid prototyping, low-cost | Thermal damage, toxic residues | Paper, PET |
| 3D Printing | Customizable, broad material range | Size resolution, slow throughput | PEG, collagen, alginate |
| Electrospinning | Biomimetic ECM, tunable fibers | Requires integration with microfluidics | PCL, PLA, gelatin |
| Target Organ | Preferred Subtypes | Metastasis Drivers | OoC Features Modeled |
|---|---|---|---|
| Bone | ER+ luminal | CXCL12–CXCR4 axis, dormancy mechanisms | Triculture chips, shear stress, vascular mimicry |
| Brain | TNBC, HER2+ | BBB disruption, astrocyte signaling | BBB-on-a-chip, astrocyte/EC co-cultures |
| Liver | TNBC | EVs, fibronectin expression | LOC with induced hepatocytes, EV signaling |
| Lung | TNBC, luminal B | β4 integrin, CXCL12 gradient | Lung-on-chip, HUVEC layers, exosome tracking |
| Lymph Nodes | All | IL-6 signaling, VEGF-mediated remodeling | LN-on-chip, lymphatic EC co-culture |
| Research Gap. | Description | Future Need |
|---|---|---|
| Lack of Standardization and Reproducibility | No harmonized protocols for chip design, ECM, or readouts; hinders reproducibility. | Establish standardized fabrication and validation protocols. |
| Incomplete TME Representation | Key cell types (myoepithelial cells, adipocytes, lymphoid aggregates) often excluded. | Integrate full immune–stromal– adipose–myoepithelial complexity. |
| Limited Modeling of Tumor Heterogeneity | BCOC rarely captures polyclonality or spatial tumor heterogeneity. | Design chips with spatial/temporal heterogeneity and clonal tracking. |
| Short-Term Culture Limitations | Most platforms limited to short durations (<1 week), impeding chronic drug modeling. | Develop long-term perfused models with treatment simulation capabilities. |
| Single-Organ Metastasis Modeling | Few models recreate multi-organ metastatic cascades or pre-metastatic niche formation. | Connect multi-organ systems with real-time tracking of tumor migration. |
| Neglect of Biomechanical Forces | Models lack simulation of stiffness, compression, and tissue deformation. | Incorporate mechanical strain, pressure, and tension cues. |
| Low Scalability for Personalized Testing | Platforms are low-throughput, unsuitable for real-time therapeutic screening. | Miniaturize for multiplexed patient-on-a-chip drug screening. |
| Unclear Regulatory Integration | Despite regulatory interest, there is no defined validation path for BCOC in preclinical pipelines. | Define regulatory roadmaps and align with FDA/EMA guidelines. |
| Trial ID | Application | Technology | Target Analyte |
|---|---|---|---|
| NCT02948751 | Leptomeningeal metastasis detection | OncoCEE™ microfluidics | CSF-derived CTCs and cfDNA |
| NCT04239105 | CTC detection, therapy monitoring | Microfluidics + Raman spectroscopy | Peripheral blood CTCs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popoiu, T.-A.; Cimpean, A.M.; Bojin, F.; Cerbu, S.; Gug, M.-C.; Pirvu, C.-A.; Pantea, S.; Neagu, A. Microengineered Breast Cancer Models: Shaping the Future of Personalized Oncology. Cancers 2025, 17, 3160. https://doi.org/10.3390/cancers17193160
Popoiu T-A, Cimpean AM, Bojin F, Cerbu S, Gug M-C, Pirvu C-A, Pantea S, Neagu A. Microengineered Breast Cancer Models: Shaping the Future of Personalized Oncology. Cancers. 2025; 17(19):3160. https://doi.org/10.3390/cancers17193160
Chicago/Turabian StylePopoiu, Tudor-Alexandru, Anca Maria Cimpean, Florina Bojin, Simona Cerbu, Miruna-Cristiana Gug, Catalin-Alexandru Pirvu, Stelian Pantea, and Adrian Neagu. 2025. "Microengineered Breast Cancer Models: Shaping the Future of Personalized Oncology" Cancers 17, no. 19: 3160. https://doi.org/10.3390/cancers17193160
APA StylePopoiu, T.-A., Cimpean, A. M., Bojin, F., Cerbu, S., Gug, M.-C., Pirvu, C.-A., Pantea, S., & Neagu, A. (2025). Microengineered Breast Cancer Models: Shaping the Future of Personalized Oncology. Cancers, 17(19), 3160. https://doi.org/10.3390/cancers17193160









