Immunotherapy-Induced Complete Response in dMMR Rectal Cancer—A Surgical Dilemma?
Abstract
Simple Summary
Abstract
1. Introduction
2. Clinical Evidence for Immunotherapy in dMMR Colorectal Cancer
2.1. Definitions of Complete Response
2.2. Landmark Clinical Trials
2.2.1. KEYNOTE-177: Foundation for Immunotherapy
2.2.2. CheckMate-142 & -8HW: Combination Immunotherapy
2.2.3. NICHE Studies: Neoadjuvant Immunotherapy
2.2.4. Dostarlimab: The 100% Complete Response Study
2.3. Additional Phase II Clinical Trials
2.3.1. PICC Study
2.3.2. Pembrolizumab Neoadjuvant Study
2.3.3. Sintilimab Neoadjuvant Study
2.4. Retrospective Studies and Case Series
2.4.1. A Single-Centre Real-World Study
2.4.2. A Multiple-Centre Cohort Study
2.4.3. Long Term Outcomes Study
2.4.4. Chinese Multicentre Experience
2.4.5. Sun Yat-Sen University Series
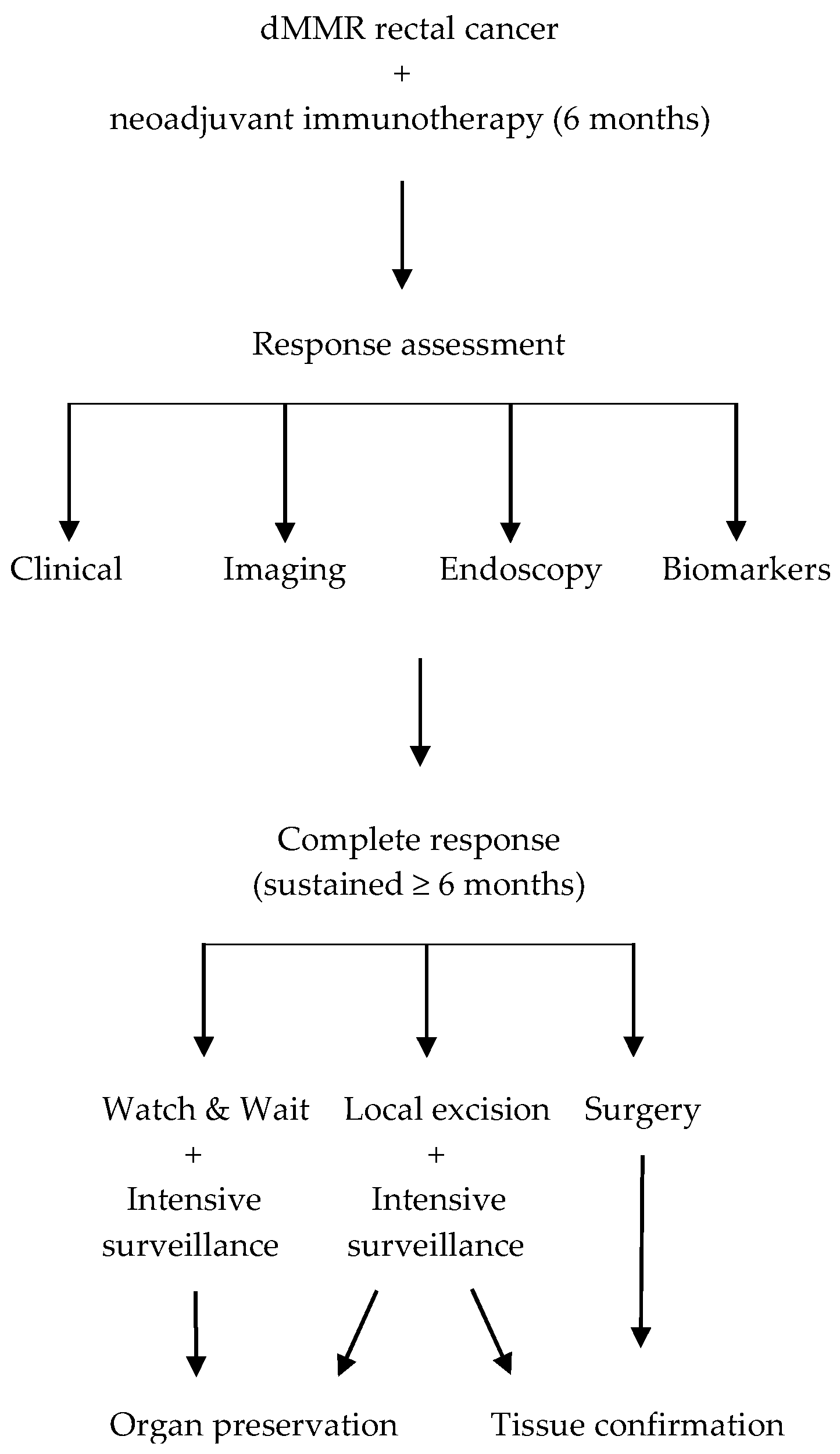
3. The Surgical Dilemma: Response Assessment and Decision-Making
3.1. Challenges in Response Evaluation
3.1.1. Pseudoprogression and Pseudoresidue
3.1.2. Limitations of Conventional Imaging
3.2. Response Assessment Strategies
3.2.1. Endoscopic Evaluation
3.2.2. Biomarker Integration
3.2.3. Imaging Techniques
3.3. Decision-Making Framework
3.3.1. cCR Criteria–Clinical Decision Factors
3.3.2. Treatment Selection Paradigms
- Local excision offers tissue confirmation while preserving organ function, providing pathological verification of complete response while minimising surgical morbidity (Figure 1). Favourable candidates for local excision are those with tumours located within 8–10 cm of the anal verge, measuring <4 cm in size, and readily accessible for full-thickness endoscopic excision. Additional selection criteria include a marked response to therapy and the absence of high-risk imaging features such as threatened mesorectal fascia, extramural vascular invasion, or bulky nodal involvement [24,37,38];
4. The Watch-and-Wait Approach
4.1. Immunotherapy-Specific Considerations for Organ Preservation
4.1.1. Enhanced Complete Response
4.1.2. Different Response Kinetics and Immune Memory
4.2. Patient Selection Criteria
- Confirmed dMMR/MSI-H status through validated testing methods;
- Local disease without distant metastases;
- Accessibility for surveillance procedures (Table 2).
- Clinical complete response by multimodal assessment;
- Sustained response for at least 6 months;
- Negative ctDNA when available (Table 2).
- Thorough understanding of the immunotherapy approach;
- Ability to comply with intensive follow-up requirements;
- Strong preference for organ preservation (Table 2).
| Watch-and-Wait | Surgery |
|---|---|
| Sustained complete response ≥6 months | Incomplete or partial response |
| Young age with quality of life priorities | Patient preference for definitive treatment |
| Strong patient preference for organ preservation | Compliance concerns with intensive surveillance |
| Excellent compliance potential | Limited access to specialised monitoring |
| Access to specialised surveillance | High-risk tumour features |
4.3. Surveillance Protocols
4.4. Salvage Surgery Considerations
4.5. Clinical Outcomes
4.5.1. Exceptional Efficacy Results
4.5.2. Functional Results
5. The Surgical Approach
5.1. Factors Favouring Surgery
5.1.1. Patient Preference and Psychological Considerations
5.1.2. Compliance and Access Considerations
5.2. Surgical Outcomes
5.2.1. Pathological Complete Response Confirmation
5.2.2. Perioperative Safety and Complications
5.3. Future Directions in Surgical Management
6. Comparative Outcomes: Watch-and-Wait Versus Surgery
6.1. Oncological Outcomes
6.2. Quality of Life Considerations
7. Current Clinical Trials and Future Directions
7.1. Ongoing Clinical Trials
7.2. Future Research Priorities
7.2.1. Treatment Optimisation
7.2.2. Novel Therapeutic Development
7.2.3. Biomarker Development and Validation
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TME | Total mesorectal excision |
| dMMR | Deficient mismatch repair |
| MSI-H | High microsatellite instability |
| ICI | Immune checkpoint inhibitors |
| CR | Complete response |
| pCR | Pathological complete response |
| cCR | Clinical complete response |
| rCT | Radiological complete response |
| AE | Adverse events |
| Retro | Retrospective |
| NR | Not reported |
| MCRC | Metastatic colorectal cancer |
| LACRC | Locally advanced colorectal cancer |
| LACC | Locally advanced colon cancer |
| LARC | Locally advanced rectal cancer |
| OR | Objective response |
| PFS | Progression free survival |
| DFS | Disease free survival |
| DRE | Digital rectal examination |
| WW | Watch-and-wait |
| NCCN | National comprehensive cancer network |
| ESMO | European society of medical oncology |
| ASCO | American society of clinical oncology |
| ctDNA | Circulating tumour DNA |
| nIT | Neoadjuvant immunotherapy |
| MSK | Memorial Sloan Kettering |
| FS | Flexible sigmoidoscopy |
| CT CAP | Computed tomography chest-abdomen-pelvis |
| MRI | Magnetic resonance imaging |
| PET | Positron emission tomography |
| FDG | Fluorodeoxyglucose |
| mrTRG | Magnetic resonance tumour response grade |
| RECIST | Response evaluation criteria in solid tumours |
| PD | Progressive disease |
| iUPD | Unconfirmed progressive disease |
| iCPD | Confirmed progressive disease |
| AJCC | American Joint Committee on Cancer |
References
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D.; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. S4), iv22–iv40. [Google Scholar] [CrossRef]
- Cercek, A.; Dos Santos Fernandes, G.; Roxburgh, C.S.; Ganesh, K.; Ng, S.; Sanchez-Vega, F.; Yaeger, R.; Segal, N.H.; Reidy-Lagunes, D.L.; Varghese, A.M.; et al. Mismatch repair-deficient rectal cancer and resistance to neoadjuvant chemotherapy. Clin. Cancer Res. 2020, 26, 3271–3279. [Google Scholar] [CrossRef]
- Schrock, A.B.; Ouyang, C.; Sandhu, J.; Batra, P.; Bernards, S.S.; Chou, J.; Chaudhary, K.R.; Iacobuzio-Donahue, C.A.; Loree, J.M.; Meropol, N.J.; et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann. Oncol. 2019, 30, 1096–1103. [Google Scholar] [CrossRef]
- Zhao, P.; Li, L.; Jiang, X.; Li, Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J. Hematol. Oncol. 2019, 12, 54. [Google Scholar] [CrossRef]
- Fan, W.X.; Su, F.; Zhang, Y.; Zhang, X.-L.; Du, Y.-Y.; Gao, Y.-J.; Li, W.-L.; Hu, W.-Q.; Zhao, J. Oncological characteristics, treatments and prognostic outcomes in MMR-deficient colorectal cancer. Biomark. Res. 2024, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.A.; et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.J.; Van Cutsem, E.; Limon, M.L.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: The phase II CheckMate 142 study. J. Clin. Oncol. 2022, 40, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Diaz LAJr Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; de la Fourchardiere, C.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef]
- Chalabi, M.; Verschoor, Y.L.; van den Berg, J.; Sikorska, K.; Beets, G.; van Lent, A.U.; Grootscholten, C.; Maas, M.; Mertz, M.; Veninga, V.; et al. Neoadjuvant immune checkpoint inhibition in locally advanced MMR-deficient colon cancer: The NICHE-2 study. Ann. Oncol. 2022, 33, S1389. [Google Scholar] [CrossRef]
- de Gooyer, P.G.M.; Verschoor, Y.L.; van den Dungen, L.D.W.; Balduzzi, S.; Marsman, H.A.; Geukes Foppen, M.H.G.; Grootscholten, C.; Dokter, S.; den Hartog, A.G.; Verbeek, W.H.M.; et al. Neoadjuvant nivolumab and relatlimab in locally advanced MMR-deficient colon cancer: A phase 2 trial. Nat. Med. 2024, 30, 3284–3290. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Pretta, A.; Ziranu, P.; Giampieri, R.; Pinna, G.; Randon, G.; Donisi, C.; Farris, A.; Pusceddu, S.; Manca, A.; Manca, A.; et al. Mismatch repair system protein deficiency as a resistance factor for locally advanced rectal adenocarcinoma patients receiving neoadjuvant chemo-radiotherapy. Br. J. Cancer. 2023, 129, 1619–1624. [Google Scholar] [CrossRef]
- Roudko, V.; Cimen Bozkus, C.; Greenbaum, B.; Lucas, A.; Samstein, R.; Bhardwaj, N. Lynch syndrome and MSI-H cancers: From mechanisms to “off-the-shelf” cancer vaccines. Front. Immunol. 2021, 12, 757804. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. Durable complete responses to PD-1 blockade alone in mismatch repair-deficient locally advanced rectal cancer. J. Clin. Oncol. 2024, 42 (Suppl. S17), LBA3512. [Google Scholar] [CrossRef]
- Hu, H.; Kang, L.; Zhang, J.; Liu, Y.; Li, Y.; Wang, Z.; Zhang, X.; Li, Z.; Zhang, Y.; Li, J.; et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): A single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 2022, 7, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Gögenur, I.; Justesen, T.F.; Tarpgaard, L.S.; Bulut, M.; Hansen, T.F.; Jensen, L.H.; Rahr, H.B.; Kirkegaard, T.; Balsevicius, L.; Raskov, H.; et al. Neoadjuvant pembrolizumab in stage I–III deficient mismatch repair colon cancer: A clinical trial. Ann. Surg. 2024. Epub ahead of print. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, J.; Li, Y.; Wang, Z.; Liu, Y.; Zhang, X.; Li, Z.; Zhang, Y.; Li, J.; Wang, L.; et al. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: An open-label, single-centre phase 2 study. Lancet Gastroenterol. Hepatol. 2023, 8, 422–431. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Li, Y.; Wang, Z.; Zhang, J.; Li, Z.; Zhang, Y.; Li, J.; Wang, L.; Chen, G.; et al. Efficacy and safety of neoadjuvant monoimmunotherapy with PD-1 inhibitor for dMMR/MSI-H locally advanced colorectal cancer: A single-centre real-world study. Front. Immunol. 2022, 13, 913483. [Google Scholar] [CrossRef]
- Yang, R.; Wu, T.; Yu, J.; Cai, X.; Li, G.; Li, X.; Zhang, Y.; Chen, L.; Wang, H.; Liu, Q.; et al. Locally advanced rectal cancer with dMMR/MSI-H may be excused from surgery after neoadjuvant anti-PD-1 monotherapy: A multiple-center, cohort study. Front. Immunol. 2023, 14, 1182299. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liao, L.; Xiao, B.; Zhang, Y.; Wang, Z.; Liu, Y.; Li, Y.; Chen, G.; Zhang, X.; Li, J.; et al. Long-term outcomes of dMMR/MSI-H rectal cancer treated with anti-PD-1-based immunotherapy as curative-intent treatment. J. Natl. Compr. Cancer Netw. 2024, 22, e237096. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.X.; Xiao, B.Y.; Cheng, Y.; Zhang, Y.; Li, Y.; Li, X.; Zhang, J.; Li, Z.; Wang, Z.; Liu, Y.; et al. Anti-PD-1-based immunotherapy as curative-intent treatment in dMMR/MSI-H rectal cancer: A multicentre cohort study. Eur. J. Cancer. 2022, 174, 176–184. [Google Scholar] [CrossRef]
- Xie, Y.; Lin, J.; Zhang, N.; Wang, Z.; Liu, Y.; Li, Y.; Zhang, J.; Li, Z.; Chen, G.; Zhang, X.; et al. Prevalent pseudoprogression and pseudoresidue in patients with rectal cancer treated with neoadjuvant immune checkpoint inhibitors. J. Natl. Compr. Canc Netw. 2023, 21, 133–142. [Google Scholar] [CrossRef]
- André, T.; Le, D.T.; Lenz, H.J.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; García-Carbonero, R.; et al. Nivolumab plus ipilimumab in microsatellite-instability-high metastatic colorectal cancer. N. Engl. J. Med. 2024, 391, 2014–2026. [Google Scholar] [CrossRef]
- Chalabi, M. Defying all odds in MMR-deficient rectal cancers. Cancer Cell. 2022, 40, 914–916. [Google Scholar] [CrossRef]
- Tokmak, H.; Demir, N.; Çulpan, H.C. Total lesion glycolysis (TLG) on 18F-FDG PET/CT as a potential predictor of pathological complete response in locally advanced rectal cancer after total neoadjuvant therapy: A retrospective study. Diagnostics 2025, 15, 1800. [Google Scholar] [CrossRef]
- Fox, D.A.; Bhamidipati, D.; Konishi, T.; Kaur, H.; You, N.; Raghav, K.P.S.; Ge, P.S.; Messick, C.; Johnson, B.; Morris, V.K.; et al. Endoscopic and imaging outcomes of PD-1 therapy in localised dMMR colorectal cancer. Eur. J. Cancer. 2023, 194, 113356. [Google Scholar] [CrossRef]
- Tie, J.; Wang, Y.; Tomasetti, C.; Li, L.; Springer, S.; Kinde, I.; Silliman, N.; Tacey, M.; Wong, H.L.; Christie, M.; et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci. Transl. Med. 2016, 8, 346ra92. [Google Scholar] [CrossRef]
- Gong, J.; Aguirre, F.; Hazelett, D.; Alvarez, R.; Zhou, L.; Hendifar, A.; Osipov, A.; Zaghiyan, K.; Cho, M.; Gangi, A.; et al. Circulating tumor DNA dynamics and response to immunotherapy in colorectal cancer. Mol. Clin. Oncol. 2022, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Yu, J.; Ding, P.R. Nonoperative management of dMMR/MSI-H colorectal cancer following neoadjuvant immunotherapy: A narrative review. Clin. Colon. Rectal Surg. 2023, 36, 378–384. [Google Scholar] [CrossRef]
- Jiménez de Los Santos, M.E.; Reyes-Pérez, J.A.; Sandoval-Nava, R.M.; Villalobos-Juárez, J.L.; Villaseñor-Navarro, Y.; Vela-Sarmiento, I.; Sollozo-Dupont, I. The apparent diffusion coefficient is a useful biomarker in predicting treatment response in patients with locally advanced rectal cancer. Acta Radiol. Open. 2020, 9, 2058460120957295. [Google Scholar] [CrossRef]
- Eijkelenkamp, H.; Grimbergen, G.; McDonald, B.; Rutgers, R.; Schakel, T.; Beijst, C.; Philippens, M.; Meijer, G.; Intven, M. Repeatability of rectal cancer apparent diffusion coefficient measurements on a 1.5 T MR-linac. Phys. Imaging Radiat. Oncol. 2025, 33, 100720. [Google Scholar] [CrossRef]
- Nilbert, M.; Eberhard, J.; Engdahl Severin, J.; Edelhamre, M.; Torle, F.; Korkocic, D.; Jörgren, F. Surgical decision-making following neoadjuvant immunotherapy for dMMR rectal cancer; case reports and review of the literature. Acta Oncol. 2023, 62, 1945–1951. [Google Scholar] [CrossRef]
- Hofheinz, R.D.; Fokas, E.; Benhaim, L.; Price, T.J.; Arnold, D.; Beets-Tan, R.; Guren, M.G.; Hospers, G.A.P.; Lonardi, S.; Nagtegaal, I.D.; et al. Localised rectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2025, 36, 1007–1024. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.J.; Kennedy, E.B.; Berlin, J.; Brown, G.; Chalabi, M.; Cho, M.T.; Cusnir, M.; Dorth, J.; George, M.; Kachnic, L.A.; et al. Management of locally advanced rectal cancer: ASCO guideline. J. Clin. Oncol. 2024, 42, 3355–3375. [Google Scholar] [CrossRef] [PubMed]
- Smits, L.J.H.; van Lieshout, A.S.; Grüter, A.A.J.; Horsthuis, K.; Tuynman, J.B. Multidisciplinary management of early rectal cancer—The role of surgical local excision in current and future clinical practice. Surg. Oncol. 2022, 40, 101687. [Google Scholar] [CrossRef] [PubMed]
- Wo, J.Y.; Ashman, J.B.; Bhadkamkar, N.A.; Bradfield, L.; Chang, D.T.; Hanna, N.; Hawkins, M.; Holtz, M.; Kim, E.; Kelly, P.; et al. Radiation therapy for rectal cancer: An ASTRO clinical practice guideline focused update. Pract. Radiat. Oncol. 2025, 15, 124–143. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.C.; Guerra, G.R.; Warrier, S.K.; Ramsay, R.G.; Heriot, A.G. Outcome and salvage surgery following “watch and wait” for rectal cancer after neoadjuvant therapy: A systematic review. Dis. Colon. Rectum. 2017, 60, 335–345. [Google Scholar] [CrossRef]
- Stupart, D.; Win, A.K.; Winship, I.M.; Jenkins, M. Fertility after young-onset colorectal cancer: A study of subjects with Lynch syndrome. Color. Dis. 2015, 17, 787–793. [Google Scholar] [CrossRef]
- Yao, S.; Lan, H.; Han, Y.; Mao, C.; Yang, M.; Zhang, X.; Jin, K. From organ preservation to selective surgery: How immunotherapy changes colorectal surgery? Surg. Open Sci. 2023, 15, 44–53. [Google Scholar] [CrossRef]
- Baraka, B.; Shandakova, M.; Abosheaisha, H.; Nassar, M. Immunotherapy-induced thyroid dysfunction: An updated review. Egypt. J. Intern. Med. 2023, 35, 21. [Google Scholar] [CrossRef]
- Jacques, J.P.; Valadares, M.; Moura, L.; Oliveira, G.; Naves, L. Frequency and clinical characteristics of hypophysitis induced by immune checkpoint inhibitors: A systematic review. Front. Endocrinol. 2023, 14, 997464. [Google Scholar] [CrossRef]
- Pennings, A.J.; Kimman, M.L.; Gielen, A.H.C.; Beets, G.L.; Melenhorst, J.; Breukink, S.O.; Van De Velde, C.J.H.; Bos, A.C.; Dekker, E.; Tanis, P.J.; et al. Burden of disease experienced by patients following a watch-and-wait policy for locally advanced rectal cancer: A qualitative study. Color. Dis. 2021, 23, 2870–2878. [Google Scholar] [CrossRef]
- Cui, H.X.; Yang, X.Q.; Zhao, G.Y.; Wang, F.J.; Liu, X.; Li, Y.; Zhang, W.; Chen, J.; Wu, L.; Zhou, Q.; et al. The neoadjuvant immunotherapy for non-metastatic mismatch repair-deficient colorectal cancer: A systematic review. Front. Immunol. 2025, 16, 1540751. [Google Scholar] [CrossRef]
- Chalabi, M.; Verschoor, Y.L.; Batista Tan, P.; Balduzzi, S.; Van Lent, A.U.; Grootscholten, C.; Dokter, S.; Büller, N.V.; Grotenhuis, B.A.; Kuhlmann, K.; et al. Neoadjuvant immunotherapy in locally advanced mismatch repair–deficient colon cancer. N. Engl. J. Med. 2024, 390, 1949–1958. [Google Scholar] [CrossRef]
- Cercek, A.; Bachet, J.B.; Capdevila, J.; Starling, N.; Chen, E.X.; Di Bartolomeo, M.; Yoshino, T.; Vlahovic, G.; Zografos, E.; O’Donnell, S.; et al. Phase II, single-arm, open-label study of dostarlimab monotherapy in previously untreated patients with stage II/III dMMR/MSI-H locally advanced rectal cancer. J. Clin. Oncol. 2023, 41, TPS3639. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Study of Induction PD-1 Blockade in Subjects with Locally Advanced Mismatch Repair Deficient Rectal Adenocarcinoma. Identifier NCT04165772. 2021. Available online: https://clinicaltrials.gov/study/NCT04165772 (accessed on 13 July 2025).
- European Medicines Agency. Study of Induction PD-1 Blockade in Subjects with Locally Advanced Mismatch Repair Deficient Rectal Adenocarcinoma. EudraCT Number 2022-500646-14-00. 2022. Available online: https://euclinicaltrials.eu/ctis-public/view/2022-500646-14-00 (accessed on 13 July 2025).
- ClinicalTrials.gov. Response to Immunotherapy in MMR-deficient Localized Colorectal Cancer. Identifier NCT05662527. 2023. Available online: https://clinicaltrials.gov/study/NCT05662527 (accessed on 10 July 2025).
- Huang, C.Y.; Bai, M.H.; Shen, J.W.; Sun, Q.Q.; Feng, Y.R.; Chen, Q.P.; Mao, W.; Ju, H.X.; Zhu, J. Anus preservation in low rectal adenocarcinoma based on MMR/MSI status (APRAM): A study protocol for a randomised, controlled, open-label, multicentre phase III trial. BMC Cancer 2024, 24, 57. [Google Scholar] [CrossRef]
- Gong, C.; Liang, J.; Tang, J.; Zou, S.; Pei, W.; Zhou, H.; Liu, Z.; Zhang, W.; Jiang, Z.; Qu, W.; et al. Neoadjuvant treatment with a bispecific antibody cadonilimab in dMMR/MSI-H locally advanced colorectal cancer: Preliminary results from a phase II trial. Immuno-Oncol. Technol. 2024, 24, 100759. [Google Scholar] [CrossRef]
- Kamrani, A.; Nasiri, H.; Hassanzadeh, A.; Ahmadian Heris, J.; Mohammadinasab, R.; Sadeghvand, S.; Sadeghi, M.; Valedkarimi, Z.; Hosseinzadeh, R.; Shomali, N.; et al. New immunotherapy approaches for colorectal cancer: Focusing on CAR-T cell, BiTE, and oncolytic viruses. Cell Commun. Signal. 2024, 22, 56. [Google Scholar] [CrossRef]
- Jia, W.; Shen, X.; Guo, Z.; Cheng, X.; Zhao, R. The future of cancer vaccines against colorectal cancer. Expert. Opin. Biol. Ther. 2024, 24, 269–284. [Google Scholar] [CrossRef] [PubMed]
- González-Montero, J.; Rojas, C.I.; Burotto, M. Predictors of response to immunotherapy in colorectal cancer. Oncologist 2024, 29, 824–832. [Google Scholar] [CrossRef] [PubMed]
| Phase | Treatment | N | Setting | CR Rate (%) | Grade 3–4 AE (%) | Median Follow-Up (mo) | Recurrence (%) | |
|---|---|---|---|---|---|---|---|---|
| KEYNOTE-177 [8] | III | Pembrolizumab | 153 | MCRC | 13.1 rCR | 22 | 44.5 | NR |
| CheckMate-142 [7] | II | Nivolumab Nivolumab + Ipilimumab | 74 45 | MCRC | 2.7 rCR 13 rCR | 20 6 | 12 29 | NR |
| NICHE-1 [10] NICHE-2 [11] NICHE-3 [12] | II | Nivolumab + Ipilimumab Nivolumab + relatlimab | 32 111 59 | LACC | 69 pCR 68 pCR 68 pCR | 12 4 10 | 25 26 8 | 0 0 2 |
| Cercek A., et al., 2022 [13] 2024 [16] | II II | Dostarlimab + WW | 12 41 | LARC | NR 100 cCR | 0 | 12 28.9 | 0 |
| PICC [17] | II | Toralimab + celecoxib Toralimab | 17 17 | LACRC | 88 65 | 3 | 14.9 | 0 |
| Gögenur I., et al. [18] | II | Pembrolizumab | 42 | LACC | 46 pCR | 7.1 | NR | NR |
| Chen G., et al. [19] | II | nIT + surgery nIT + WW | 6 9 | LARC | 75 pCR + cCR | 6 | 17.2 | 0 |
| Zhang X., et al. [20] | Retro | nIT + surgery nIT + WW | 29 3 | LACRC | 75.9 pCR | 0 | 14 | 0 0 |
| Yang R., et al. [21] | Retro | nIT + surgery nIT + WW | 13 7 | LARC | 84.6 pCR | 0 | 25 | 0 0 |
| Yu J., et al. [22] | Retro | nIT + WW | 24 | LARC | 100 cCR | 8.4 | 29.1 | 0 |
| Wang Q.X., et al. [23] | Retro | nIT + surgery nIT + WW | 10 19 | LARC | pCR NR | 15 | 17.1 | 0 |
| Xie Y., et al. [24] | Retro | nIT + surgery nIT + WW | 12 1 | LARC | 92 pCR | NR | NR | NR |
| Time Period | History and Physical Examination | CEA | DRE and Proctoscopy or FS | MRI Pelvis | CT CAP |
|---|---|---|---|---|---|
| 0–24 months | Every 3–6 months | Every 3–6 months | Every 3–4 months | Every 6 months | Every 6–12 months |
| 24–60 months | Every 6 months | Every 6 months | Every 6 months | Every 6 months (until the 36th month) | Every 6–12 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loufopoulos, P.; Perivoliotis, K.; Chatziathanasiou, D.; Frountzas, M.; Sukha, A.; Alrebdi, A.; Eddama, M.M.R.; Kontovounisios, C.; Qiu, S.; Tekkis, P.; et al. Immunotherapy-Induced Complete Response in dMMR Rectal Cancer—A Surgical Dilemma? Cancers 2025, 17, 3153. https://doi.org/10.3390/cancers17193153
Loufopoulos P, Perivoliotis K, Chatziathanasiou D, Frountzas M, Sukha A, Alrebdi A, Eddama MMR, Kontovounisios C, Qiu S, Tekkis P, et al. Immunotherapy-Induced Complete Response in dMMR Rectal Cancer—A Surgical Dilemma? Cancers. 2025; 17(19):3153. https://doi.org/10.3390/cancers17193153
Chicago/Turabian StyleLoufopoulos, Panagiotis, Konstantinos Perivoliotis, Danai Chatziathanasiou, Maximos Frountzas, Anisha Sukha, Abdullah Alrebdi, Mohammad Mahmoud Rajab Eddama, Christos Kontovounisios, Shengyang Qiu, Paris Tekkis, and et al. 2025. "Immunotherapy-Induced Complete Response in dMMR Rectal Cancer—A Surgical Dilemma?" Cancers 17, no. 19: 3153. https://doi.org/10.3390/cancers17193153
APA StyleLoufopoulos, P., Perivoliotis, K., Chatziathanasiou, D., Frountzas, M., Sukha, A., Alrebdi, A., Eddama, M. M. R., Kontovounisios, C., Qiu, S., Tekkis, P., & Rasheed, S. (2025). Immunotherapy-Induced Complete Response in dMMR Rectal Cancer—A Surgical Dilemma? Cancers, 17(19), 3153. https://doi.org/10.3390/cancers17193153






