Seven-Year PSA ≤ 0.2 ng/mL After High-Dose-Rate Brachytherapy Indicates Eligibility for Discontinuing PSA Surveillance in Prostate Cancer
Simple Summary
Abstract
1. Introduction
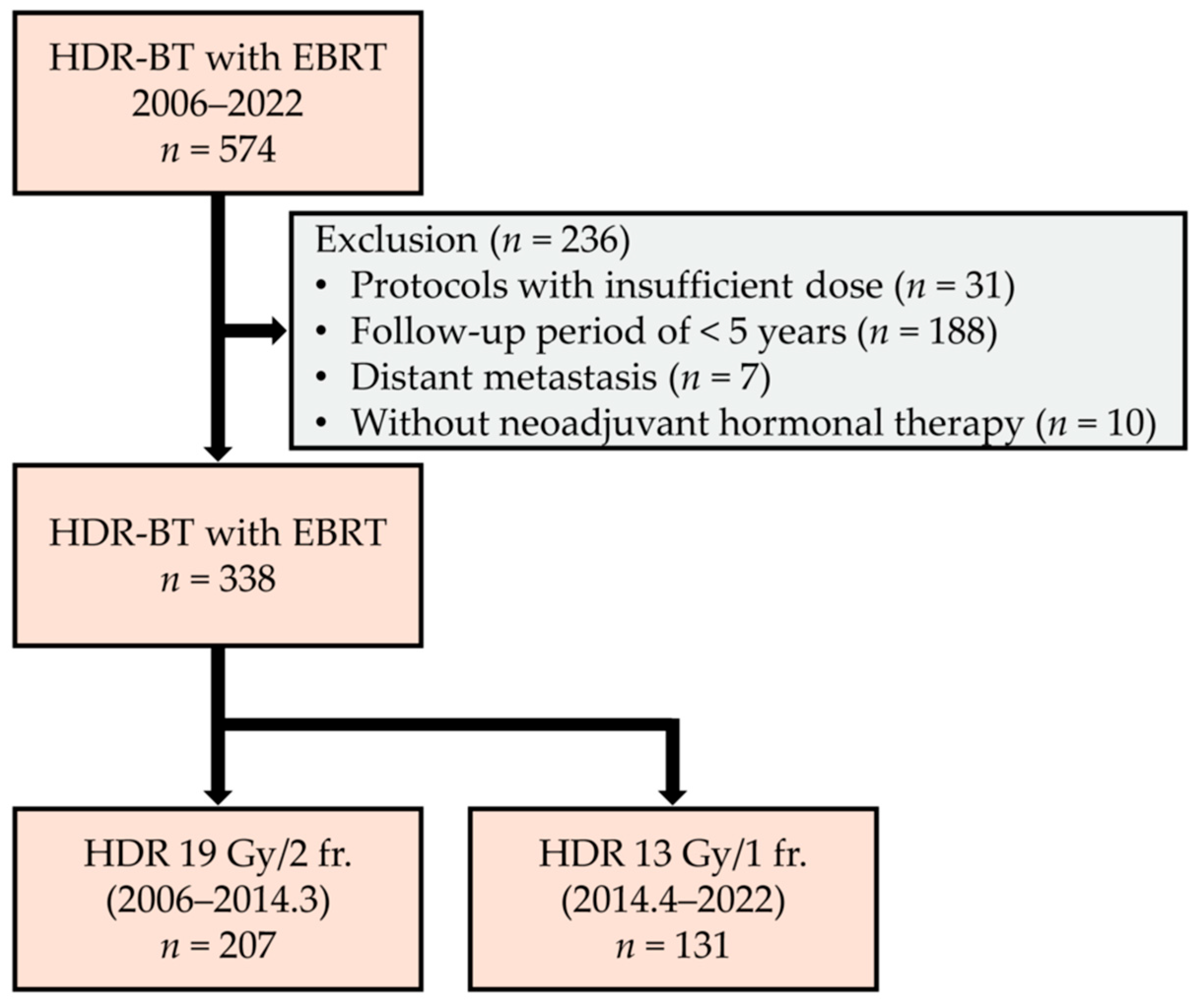
2. Materials and Methods
2.1. Patient Demographics
2.2. RT Protocol
2.3. Statistical Analyses
3. Results
3.1. Patient Characteristics
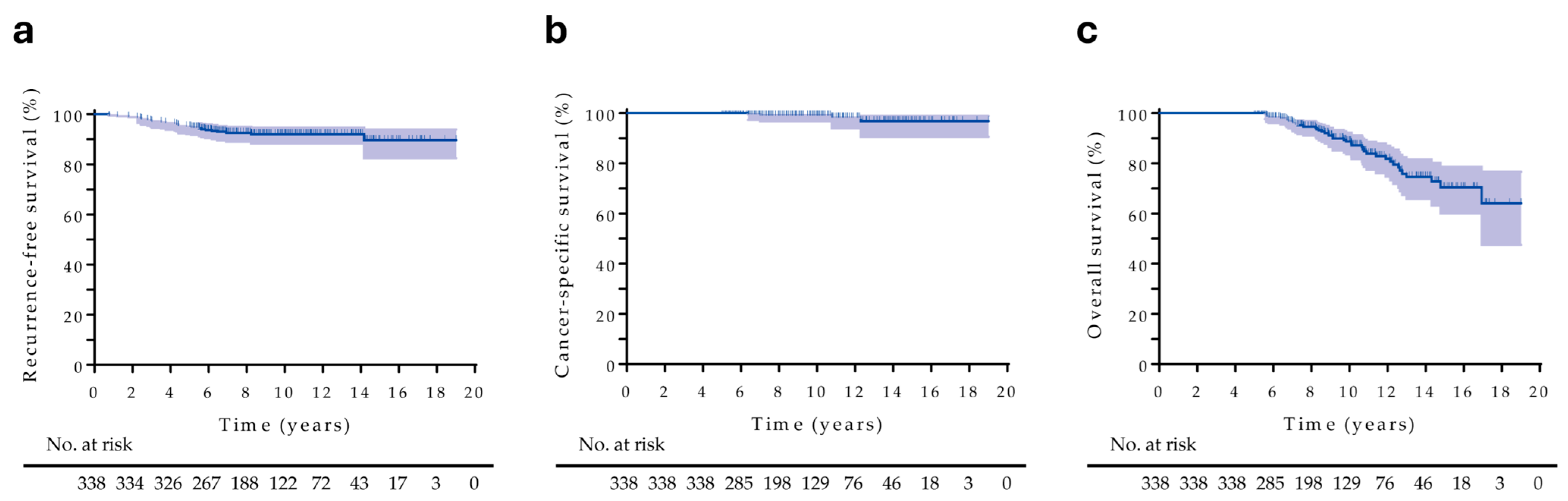
3.2. Treatment Outcomes
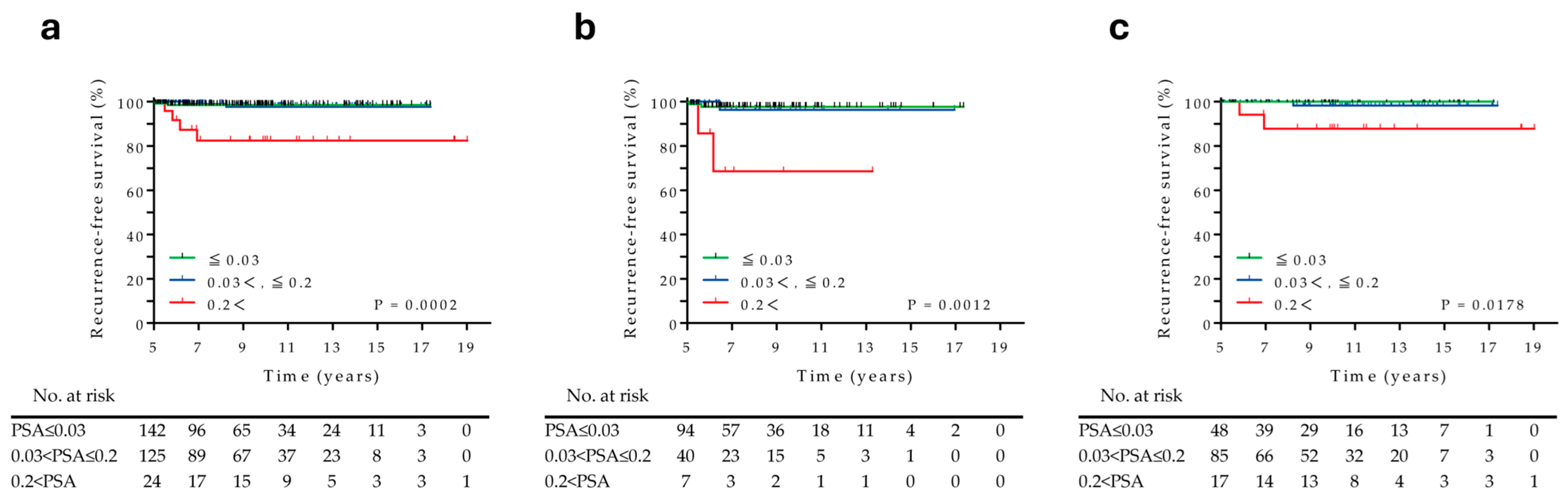
3.3. Prognostic Factors Associated with RT Recurrences over 5 Years
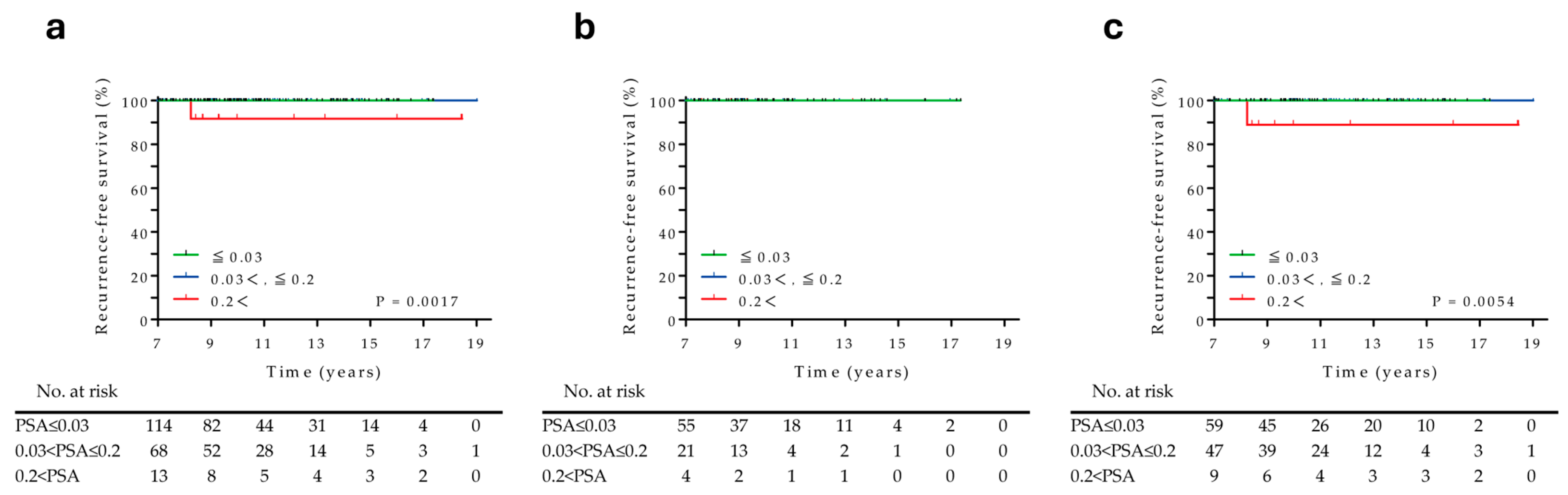
3.4. Prognostic Factors Associated with RT Recurrences over 7 Years
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADT | Androgen deprivation therapy |
| CSS | Cancer-specific survival |
| EBRT | External beam radiotherapy |
| HDR-BT | High-dose-rate brachytherapy |
| HT | Hormonal therapy |
| NCCN | National Comprehensive Cancer Network |
| OS | Overall survival |
| PC | Prostate cancer |
| PSA | Prostate-specific antigen |
| RFS | Recurrence-free survival |
| RT | Radiotherapy |
References
- Spratt, D.; Srinivas, S.; Schaeffer, E.; Adra, N.; Ahmed, B.; An, Y.; Bitting, R.; Chapin, B.; Cheng, H.; Cho, S.; et al. Prostate Cancer, Version 2.2025, NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 17 September 2025).
- Ishiyama, H.; Kamitani, N.; Kawamura, H.; Kato, S.; Aoki, M.; Kariya, S.; Matsumura, T.; Kaidu, M.; Yoshida, K.; Hashimoto, Y.; et al. Nationwide multi-institutional retrospective analysis of high-dose-rate brachytherapy combined with external beam radiotherapy for localized prostate cancer: An Asian Prostate HDR-BT Consortium. Brachytherapy 2017, 16, 503–510. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Kotsuma, T.; Komiya, A.; Kariya, S.; Konishi, K.; Nonomura, N.; Ogawa, K.; Tanaka, E.; Nishimura, K.; Fujiuchi, Y.; et al. Nationwide, Multicenter, Retrospective Study on High-Dose-Rate Brachytherapy as Monotherapy for Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 952–961. [Google Scholar] [CrossRef]
- Hsu, I.C.; Rodgers, J.P.; Shinohara, K.; Purdy, J.; Michalski, J.; Roach, M., 3rd; Vigneault, E.; Ivker, R.A.; Pryzant, R.M.; Kuettel, M.; et al. Long-Term Results of NRG Oncology/RTOG 0321: A Phase II Trial of Combined High Dose Rate Brachytherapy and External Beam Radiation Therapy for Adenocarcinoma of the Prostate. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Sakurai, T.; Takamatsu, S.; Iwamoto, H.; Yaegashi, H.; Iijima, M.; Kawaguchi, S.; Nohara, T.; Shigehara, K.; Izumi, K.; et al. The effectiveness of high-dose-rate brachytherapy with external beam radiotherapy for clinically locally advanced and node-positive prostate cancer: Long-term results of a retrospective study. Int. J. Clin. Oncol. 2021, 26, 2310–2317. [Google Scholar] [CrossRef]
- Slevin, F.; Zattoni, F.; Checcucci, E.; Cumberbatch, M.G.K.; Nacchia, A.; Cornford, P.; Briers, E.; De Meerleer, G.; De Santis, M.; Eberli, D.; et al. A Systematic Review of the Efficacy and Toxicity of Brachytherapy Boost Combined with External Beam Radiotherapy for Nonmetastatic Prostate Cancer. Eur. Urol. Oncol. 2023, 7, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Astrom, L.; Grusell, E.; Sandin, F.; Turesson, I.; Holmberg, L. Two decades of high dose rate brachytherapy with external beam radiotherapy for prostate cancer. Radiother. Oncol. 2018, 127, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Wedde, T.B.; Smastuen, M.C.; Brabrand, S.; Fossa, S.D.; Kaasa, S.; Tafjord, G.; Russnes, K.M.; Hellebust, T.P.; Lilleby, W. Ten-year survival after High-Dose-Rate Brachytherapy combined with External Beam Radiation Therapy in high-risk prostate cancer: A comparison with the Norwegian SPCG-7 cohort. Radiother. Oncol. 2019, 132, 211–217. [Google Scholar] [CrossRef]
- Hjalm-Eriksson, M.; Nilsson, S.; Brandberg, Y.; Johansson, H.; Lennernas, B.; Lundell, G.; Castellanos, E.; Ullen, A. High rate of local control and cure at 10 years after treatment of prostate cancer with external beam radiotherapy and high-dose-rate brachytherapy: A single centre experience. Acta Oncol. 2021, 60, 1301–1307. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Rojas, A.M.; Ostler, P.J.; Bryant, L.; Lowe, G.J. Randomised trial of external-beam radiotherapy alone or with high-dose-rate brachytherapy for prostate cancer: Mature 12-year results. Radiother. Oncol. 2021, 154, 214–219. [Google Scholar] [CrossRef]
- Brierley, J.; Gospodarowicz, M.; Wittekind, C. The TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar]
- Sakurai, T.; Takamatsu, S.; Shibata, S.; Iwata, K.; Taka, M.; Gabata, T.; Kumano, T.; Makino, T.; Mizokami, A. Toxicity and clinical outcomes of single-fraction high-dose-rate brachytherapy combined with external beam radiotherapy for high-/very high-risk prostate cancer: A dosimetric analysis of toxicity. Jpn. J. Radiol. 2020, 38, 1197–1208. [Google Scholar] [CrossRef]
- Roach, M., 3rd; Hanks, G.; Thames, H., Jr.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef]
- National Cancer Center Japan. Cancer Information Service. Prostate Cancer. Available online: https://ganjoho.jp/public/cancer/prostate/index.html (accessed on 17 September 2025).
- Kohjimoto, Y.; Uemura, H.; Yoshida, M.; Hinotsu, S.; Takahashi, S.; Takeuchi, T.; Suzuki, K.; Shinmoto, H.; Tamada, T.; Inoue, T.; et al. Japanese clinical practice guidelines for prostate cancer 2023. Int. J. Urol. 2024, 31, 1180–1222. [Google Scholar] [CrossRef] [PubMed]
- Slevin, F.; Rodda, S.L.; Bownes, P.; Murray, L.; Bottomley, D.; Wilkinson, C.; Adiotomre, E.; Al-Qaisieh, B.; Dugdale, E.; Hulson, O.; et al. A comparison of outcomes for patients with intermediate and high risk prostate cancer treated with low dose rate and high dose rate brachytherapy in combination with external beam radiotherapy. Clin. Transl. Radiat. Oncol. 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Demanes, D.J.; Rodriguez, R.R.; Schour, L.; Brandt, D.; Altieri, G. High-dose-rate intensity-modulated brachytherapy with external beam radiotherapy for prostate cancer: California endocurietherapy’s 10-year results. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.J.; Rojas, A.M.; Bownes, P.J.; Lowe, G.J.; Ostler, P.J.; Bryant, L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother. Oncol. 2012, 103, 217–222. [Google Scholar] [CrossRef]
- Khor, R.; Duchesne, G.; Tai, K.H.; Foroudi, F.; Chander, S.; Van Dyk, S.; Garth, M.; Williams, S. Direct 2-arm comparison shows benefit of high-dose-rate brachytherapy boost vs external beam radiation therapy alone for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 679–685. [Google Scholar] [CrossRef]
- Miszczyk, M.; Magrowski, L.; Krzysztofiak, T.; Stando, R.; Majewski, W.; Stawiski, K.; Masri, O.; Ciepal, J.; Depowska, G.; Chimiak, K.; et al. Brachytherapy boost improves survival and decreases risk of developing distant metastases compared to external beam radiotherapy alone in intermediate and high risk group prostate cancer patients. Radiother. Oncol. 2023, 183, 109632. [Google Scholar] [CrossRef]
- Prada Gomez, P.J.; Rivero Perez, A.L.; Carballido Rodriguez, J.; Anchuelo Latorre, J.; Fabregat Borras, R.; Gutierrez Ruiz, M.; Rodriguez-Acosta Caballero, C.; Carrascal Gordillo, C.F.; Galdos Barroso, M.P.; Navarrete Solano, P.A. Long-Term Outcomes After High-Dose-Rate Brachytherapy and Hypofractionated External Beam Radiotherapy in Very High-Risk Prostate Cancer: A 24-Year Follow-Up. Biomedicines 2025, 13, 1310. [Google Scholar] [CrossRef]
- Makino, T.; Sakurai, T.; Takamatsu, S.; Kano, H.; Naito, R.; Iwamoto, H.; Yaegashi, H.; Kawaguchi, S.; Shigehara, K.; Nohara, T.; et al. Biochemical response to neoadjuvant hormonal therapy predicts long-term prostate cancer survival outcomes after high-dose-rate brachytherapy with external beam radiotherapy. Sci. Rep. 2025, 15, 5118. [Google Scholar] [CrossRef] [PubMed]
- Tsumura, H.; Satoh, T.; Ishiyama, H.; Tabata, K.; Komori, S.; Sekiguchi, A.; Ikeda, M.; Kurosaka, S.; Fujita, T.; Kitano, M.; et al. Prostate-specific antigen nadir after high-dose-rate brachytherapy predicts long-term survival outcomes in high-risk prostate cancer. J. Contemp. Brachytherapy 2016, 8, 95–103. [Google Scholar] [CrossRef]
- Nakazono, M.; Urabe, F.; Iwatani, K.; Imai, Y.; Tashiro, K.; Honda, M.; Koike, Y.; Shimomura, T.; Sato, S.; Takahashi, H.; et al. Patients with PSA below 0.2 ng/mL at 8 years post high-dose-rate brachytherapy have an extremely low risk of subsequent recurrence. Int. J. Urol. 2023, 30, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Noble, D.J.; Doyle, E.; Tramonti, G.; Law, A.B.; Sundaramurthy, A.; Brush, J.P.; Keanie, J.; Wood, C.; Drewell, P.; Keough, W.; et al. Defining Biochemical Cure After Low Dose Rate Prostate Brachytherapy: External Validation of 4-year Prostate-specific Antigen Nadir as a Predictor of 10- and 15-year Disease-free Survival. Clin. Oncol. 2022, 34, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Nagore, G.; Moreno-Olmedo, E.; Suarez-Gironzini, V.; Aakki, L.; Li, R.G.; Gomez, E.; Garcia, A.; Beltran, L.; Gomez-Iturriaga, A. Long-term outcomes of ultra-hypofractionated 2 fractions single day HDR brachytherapy in localized prostate cancer. Radiother. Oncol. 2023, 186, 109807. [Google Scholar] [CrossRef]
- Geara, F.B.; Bulbul, M.; Khauli, R.B.; Andraos, T.Y.; Abboud, M.; Al Mousa, A.; Sarhan, N.; Salem, A.; Ghatasheh, H.; Alnsour, A.; et al. Nadir PSA is a strong predictor of treatment outcome in intermediate and high risk localized prostate cancer patients treated by definitive external beam radiotherapy and androgen deprivation. Radiat. Oncol. 2017, 12, 149. [Google Scholar] [CrossRef]
- Coelho, M.O.; Dal Col, L.S.; Capibaribe, D.M.; Salgado, C.M.; Travassos, T.C.; Junior, V.J.; Monti, C.R.; Reis, L.O. PSA nadir predicts biochemical recurrence after external beam radiation therapy combined to high dose rate brachytherapy in the treatment of prostate cancer. Am. J. Clin. Exp. Urol. 2022, 10, 52–62. [Google Scholar] [PubMed]
- Miszczyk, M.; Magrowski, L.; Masri, O.; Jablon’ska, I.; Nowicka, Z.; Krzysztofiak, T.; Wojcieszek, P.; Lipka-Rajwa, A.; Ciepal, J.; Depowska, G.; et al. Prostate-specific antigen kinetics and metastasis-free survival in patients treated with external beam radiotherapy combined with high-dose-rate brachytherapy boost and androgen deprivation therapy for localized prostate cancer. J. Contemp. Brachytherapy 2022, 14, 15–22. [Google Scholar] [CrossRef]
- Crook, J.M.; Tang, C.; Thames, H.; Blanchard, P.; Sanders, J.; Ciezki, J.; Keyes, M.; Morris, W.J.; Merrick, G.; Catton, C.; et al. A biochemical definition of cure after brachytherapy for prostate cancer. Radiother. Oncol. 2020, 149, 64–69. [Google Scholar] [CrossRef]
| All Patients | No Recurrence at 5 Years | No Recurrence at 7 Years | |
|---|---|---|---|
| Number of patients | 338 | 291 | 195 |
| Follow-up (year) | |||
| Median (range) | 8.9 (5.0–19.0) | 9.1 (5.0–19.0) | 10.1 (7.0–19.0) |
| Age (year), n (%) | |||
| Median (range) | 69 (50–83) | 69 (50–83) | 67 (51–81) |
| <70 | 190 (56.2) | 167 (57.4) | 126 (64.6) |
| ≥70 | 148 (43.8) | 124 (42.6) | 69 (35.4) |
| PSA at diagnosis (ng/mL), n (%) | |||
| Median (range) | 12.25 (2.26–557.64) | 12.20 (2.26–557.64) | 12.20 (2.59–557.64) |
| ≤20 | 228 (67.5) | 196 (67.4) | 136 (69.7) |
| >20, ≤40 | 70 (20.7) | 63 (21.6) | 40 (20.5) |
| >40 | 40 (11.8) | 32 (11.0) | 19 (9.7) |
| Grade Group, n (%) | |||
| ≤3 | 153 (45.3) | 131 (45.0) | 97 (49.7) |
| 4 | 102 (30.2) | 88 (30.2) | 54 (27.7) |
| 5 | 82 (24.3) | 71 (24.4) | 43 (22.1) |
| Unknown | 1 (0.3) | 1 (0.3) | 1 (0.5) |
| Clinical T stage, n (%) | |||
| cT1 | 27 (8.0) | 23 (7.9) | 15 (7.7) |
| cT2 | 159 (47.0) | 138 (47.4) | 105 (53.8) |
| cT3 | 131 (38.8) | 112 (38.5) | 68 (34.9) |
| cT4 | 21 (6.2) | 18 (6.2) | 7 (3.6) |
| Clinical N stage, n (%) | |||
| cN0 | 320 (94.7) | 278 (95.5) | 188 (96.4) |
| cN1 | 18 (5.3) | 13 (4.5) | 7 (3.6) |
| Adjuvant hormonal therapy, n (%) | |||
| Yes | 162 (47.9) | 141 (48.5) | 80 (41.0) |
| HDR-BT protocol, n (%) | |||
| 9.5Gy × 2 | 207 (61.2) | 180 (61.9) | 150 (76.9) |
| 13Gy × 1 | 131 (38.8) | 111 (38.1) | 45 (23.1) |
| Pre HDR-BT PSA (ng/mL) | |||
| Median (range) | 0.057 (0–25.442) | 0.053 (0–25.442) | 0.046 (0–10.066) |
| PSA at 5 years (ng/mL) | |||
| Median (range) | 0.033 (0–3.540) | 0.032 (0–1.322) | 0.035 (0–0.781) |
| Covariant | Recurrence-Free Survival | ||||
|---|---|---|---|---|---|
| Univariate | Multivariate | ||||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Age (year) | <70 | Reference | Reference | ||
| ≥70 | 1.62 (0.41–6.50) | 0.493 | 1.48 (0.30–7.38) | 0.630 | |
| PSA at diagnosis (ng/mL) | ≤20 | Reference | Reference | ||
| >20, ≤40 | 2.34 (0.52–10.47) | 0.266 | 1.54 (0.26–9.03) | 0.632 | |
| >40 | 1.59 (0.18–14.26) | 0.678 | 0.14 (0.00–5.38) | 0.288 | |
| Pre HDR-BT PSA (ng/mL) | ≤0.05 | Reference | Reference | ||
| >0.05 | 2.25 (0.45–11.17) | 0.320 | 4.51 (0.46–44.13) | 0.195 | |
| Clinical T stage | <T3 | Reference | Reference | ||
| ≥T3 | 4.21 (0.85–20.89) | 0.079 | 11.66 (0.72–188.21) | 0.083 | |
| Grade Group | ≤3 | Reference | Reference | ||
| 4 | 1.66 (0.23–11.82) | 0.611 | 6.05 (0.37–97.96) | 0.205 | |
| 5 | 4.12 (0.75–22.54) | 0.102 | 16.59 (0.93–295.08) | 0.056 | |
| Adjuvant hormonal therapy | No | Reference | Reference | ||
| Yes | 2.07 (0.49–8.70) | 0.320 | 0.38 (0.04–3.77) | 0.411 | |
| HDR-BT protocol | 9.5Gy × 2 | Reference | Reference | ||
| 13Gy × 1 | 2.48 (0.61–10.11) | 0.207 | 1.35 (0.21–8.65) | 0.754 | |
| PSA at 5 years (ng/mL) | ≤0.03 | Reference | Reference | ||
| >0.03, ≤0.2 | 1.10 (0.15–7.80) | 0.925 | 1.73 (0.22–13.62) | 0.602 | |
| >0.2 | 10.70 (1.96–58.42) | 0.006 | 117.57 (6.22–2223.37) | 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makino, T.; Sakurai, T.; Takamatsu, S.; Nakagawa, R.; Kamijima, T.; Kano, H.; Naito, R.; Iwamoto, H.; Yaegashi, H.; Shigehara, K.; et al. Seven-Year PSA ≤ 0.2 ng/mL After High-Dose-Rate Brachytherapy Indicates Eligibility for Discontinuing PSA Surveillance in Prostate Cancer. Cancers 2025, 17, 3151. https://doi.org/10.3390/cancers17193151
Makino T, Sakurai T, Takamatsu S, Nakagawa R, Kamijima T, Kano H, Naito R, Iwamoto H, Yaegashi H, Shigehara K, et al. Seven-Year PSA ≤ 0.2 ng/mL After High-Dose-Rate Brachytherapy Indicates Eligibility for Discontinuing PSA Surveillance in Prostate Cancer. Cancers. 2025; 17(19):3151. https://doi.org/10.3390/cancers17193151
Chicago/Turabian StyleMakino, Tomoyuki, Takayuki Sakurai, Shigeyuki Takamatsu, Ryunosuke Nakagawa, Taiki Kamijima, Hiroshi Kano, Renato Naito, Hiroaki Iwamoto, Hiroshi Yaegashi, Kazuyoshi Shigehara, and et al. 2025. "Seven-Year PSA ≤ 0.2 ng/mL After High-Dose-Rate Brachytherapy Indicates Eligibility for Discontinuing PSA Surveillance in Prostate Cancer" Cancers 17, no. 19: 3151. https://doi.org/10.3390/cancers17193151
APA StyleMakino, T., Sakurai, T., Takamatsu, S., Nakagawa, R., Kamijima, T., Kano, H., Naito, R., Iwamoto, H., Yaegashi, H., Shigehara, K., Nohara, T., Izumi, K., & Mizokami, A. (2025). Seven-Year PSA ≤ 0.2 ng/mL After High-Dose-Rate Brachytherapy Indicates Eligibility for Discontinuing PSA Surveillance in Prostate Cancer. Cancers, 17(19), 3151. https://doi.org/10.3390/cancers17193151







