Primary Aggressive Oral Lymphomas (PAOL): A Narrative Review of Diagnosis, Molecular Features, Therapeutic Approaches, and the Integrated Role of Dentists and Hematologists
Simple Summary
Abstract
1. Introduction
2. Epidemiology
3. Histopathologic and Immunophenotypic Features
3.1. Diffuse Large B-Cell Lymphoma (DLBCL)
3.2. Plasmablastic Lymphoma (PBL)
3.3. Burkitt Lymphoma (BL)
3.4. T-Cell Lymphomas
4. Molecular Pathogenesis
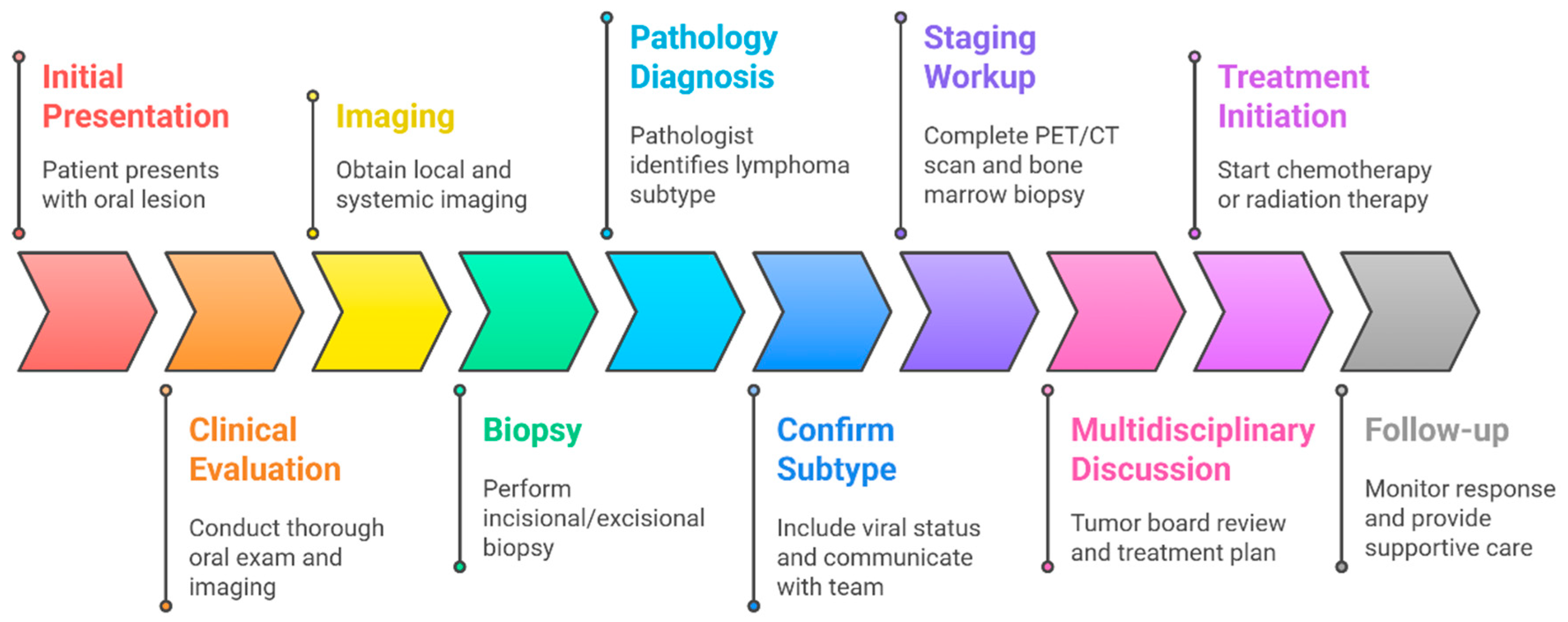
5. Diagnostic Approach
5.1. Clinical Suspicion and Initial Evaluation
5.2. Imaging of the Lesion
5.3. Biopsy and Pathologic Confirmation
5.4. Staging Workup
5.5. Multidisciplinary Case Review
6. Differential Diagnosis
6.1. Squamous Cell Carcinoma (SCC)
6.2. Plasma Cell Neoplasms
6.3. Leukemic Infiltrates (Granulocytic Sarcoma)
6.4. Indolent Lymphomas and Reactive Lymphoid Lesions
6.5. Granulomatous Infections
6.6. Inflammatory and Autoimmune Conditions
6.7. Other Malignant Mimickers
7. Treatment Strategies
7.1. Stage I_E Diffuse Large B-Cell Lymphoma (DLBCL)
7.2. Stage I_E Plasmablastic Lymphoma (PBL)
7.3. Stage I_E Burkitt Lymphoma (BL)
7.4. Stage I_E Peripheral T-Cell Lymphomas (PTCL)
8. Prognosis
8.1. DLBCL
8.2. PBL
8.3. BL
8.4. T-Cell Lymphomas
9. Integrated Role of Dentists and Hematologists
9.1. Pre-Treatment Dental Assessment
9.1.1. Treatment of Infection Foci
9.1.2. Timing of Invasive Procedures
9.1.3. Preventive Measures
9.1.4. Coordination with Oncology Team
9.2. Oral Care During Cancer Therapy
9.2.1. Preventive Oral Care Measures
9.2.2. Management of Mucositis and Pain
9.2.3. Infection Prophylaxis and Management
9.3. Post-Treatment Follow-Up and Long-Term Oral Care
9.3.1. Xerostomia and Salivary Gland Dysfunction
9.3.2. Radiation-Related Sequelae
9.3.3. Chronic GVHD Effects
9.3.4. Secondary Malignancies or Late Complications
9.3.5. Dental Rehabilitation
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barone, S.; Buffone, C.; Ferrillo, M.; Pasqua, F.; Parrotta, S.; Salviati, M.; Bennardo, F.; Antonelli, A. Oral Malignant Non-Hodgkin Lymphoma: A Retrospective Single-Center Study. Int. J. Environ. Res. Public Health 2022, 19, 2605. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silva, T.D.; Ferreira, C.B.; Leite, G.B.; de Menezes Pontes, J.R.; Antunes, H.S. Oral manifestations of lymphoma: A systematic review. Ecancermedicalscience 2016, 10, 665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kijowska, J.; Grzegorczyk, J.; Gliwa, K.; Jędras, A.; Sitarz, M. Epidemiology, Diagnostics, and Therapy of Oral Cancer-Update Review. Cancers 2024, 16, 3156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Richards, A.; Costelloe, M.A.; Eveson, J.W.; Scully, C.; Irvine, G.H.; Rooney, N. Oral mucosal non-Hodgkin’s lymphoma—A dangerous mimic. Oral Oncol. 2000, 36, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Hassona, Y.; Almuhaisen, G.; Almansour, A.; Scully, C. Lymphoma presenting as a toothache: A wolf in sheep’s clothing. BMJ Case Rep. 2017, 2017, bcr2016218686. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bugueno, J.M.; Bouquot, J. Extra-nodal non-Hodgkin’s lymphoma (ENHL) affecting oral cavity misinterpreted as painful dental infection, delaying diagnosis—Case report. J. Oral Med. Oral Surg. 2025, 31, 6. [Google Scholar] [CrossRef]
- Kemp, S.; Gallagher, G.; Kabani, S.; Noonan, V.; O’Hara, C. Oral non-Hodgkin’s lymphoma: Review of the literature and World Health Organization classification with reference to 40 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Epstein, J.D.; Le, N.D.; Gorsky, M. Characteristics of oral and paraoral malignant lymphoma: A population-based review of 361 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2001, 92, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Kolokotronis, A.; Konstantinou, N.; Christakis, I.; Papadimitriou, P.; Matiakis, A.; Zaraboukas, T.; Antoniades, D. Localized B-cell non-Hodgkin’s lymphoma of oral cavity and maxillofacial region: A clinical study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 99, 303–310. [Google Scholar] [CrossRef]
- Triantafillidou, K.; Dimitrakopoulos, J.; Iordanidis, F.; Gkagkalis, A. Extranodal non-hodgkin lymphomas of the oral cavity and maxillofacial region: A clinical study of 58 cases and review of the literature. J. Oral Maxillofac. Surg. 2012, 70, 2776–2785. [Google Scholar] [CrossRef]
- Bibas, M.; Antinori, A.; Mazzotta, V.; Marafioti, T.; Castillo, J.J. The Genetic and Epigenetic Alterations of Plasmablastic Lymphoma: A Narrative Review. Cancers 2025, 17, 1914. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bibas, M. Plasmablastic Lymphoma. A State-of-the-Art Review: Part 1-Epidemiology, Pathogenesis, Clinicopathologic Characteristics, Differential Diagnosis, Prognostic Factors, and Special Populations. Mediterr. J. Hematol. Infect. Dis. 2024, 16, e2024007. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castillo, J.J.; Bibas, M.; Miranda, R.N. The biology and treatment of plasmablastic lymphoma. Blood 2015, 125, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Heyes, R.; Northfelt, D.W.; Lott, D.G. Posttransplant Lymphoproliferative Disorder: Otolaryngological Manifestations and Management. Otolaryngol. Head Neck Surg. 2017, 157, 750–759. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.M.; de Cáceres, C.V.B.L.; Fernandes-Rodrigues, C.I.; Penafort, P.V.M.; Legarrea, J.M.A.; Gomes, N.R.; Pontes, H.A.R.; Vargas, P.A.; Júnior, J.N.R.A.; Soares, C.D.; et al. Oral manifestations of peripheral T cell lymphoma, not otherwise specified: Case series and review of the current literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2025, 139, e37–e45. [Google Scholar] [CrossRef] [PubMed]
- de Arruda, J.A.A.; Schuch, L.F.; Conte Neto, N.; de Souza, L.L.; Rodrigues-Fernandes, C.I.; Abreu, L.G.; Soares, C.D.; de Carvalho, M.G.F.; Agostini, M.; de Andrade, B.A.B.; et al. Oral and oropharyngeal lymphomas: A multi-institutional collaborative study. J. Oral Pathol. Med. 2021, 50, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Kusuke, N.; Custódio, M.; de Sousa, S.C.O.M. Oral lesion as the primary diagnosis of non-Hodgkin’s lymphoma: A 20-year experience from an oral pathology service and review of the literature. Eur. Arch. Otorhinolaryngol. 2019, 276, 2873–2879. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.M.; de Cáceres, C.V.B.L.; Santos-Silva, A.R.; Vargas, P.A.; Lopes, M.A.; Pontes, H.A.R.; Pontes, F.S.C.; Mesquita, R.A.; de Sousa, S.F.; Abreu, L.G.; et al. Clinical diagnostic approach for oral lymphomas: A multi-institutional, observational study based on 107 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2023, 136, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Wang, W.C.; Chen, C.Y.; Hsu, H.J.; Chen, Y.K. Clinical manifestations of oral lymphomas—Retrospective study of 15 cases in a Taiwanese population and a review of 592 cases from the literature. J. Formos. Med. Assoc. 2021, 120 Pt 2, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.; De, O.; Berti, E.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campo, E.; Jaffe, E.S.; Cook, J.R.; Quintanilla-Martinez, L.; Swerdlow, S.H.; Anderson, K.C.; Brousset, P.; Cerroni, L.; de Leval, L.; Dirnhofer, S.; et al. The International Consensus Classification of Mature Lymphoid Neoplasms: A report from the Clinical Advisory Committee. Blood 2022, 140, 1229–1253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenthal, A.; Younes, A. High grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6: Double hit and triple hit lymphomas and double expressing lymphoma. Blood Rev. 2017, 31, 37–42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ye, Q.; Xu-Monette, Z.Y.; Tzankov, A.; Deng, L.; Wang, X.; Manyam, G.C.; Visco, C.; Montes-Moreno, S.; Zhang, L.; Dybkær, K.; et al. Prognostic impact of concurrent MYC and BCL6 rearrangements and expression in de novo diffuse large B-cell lymphoma. Oncotarget 2016, 7, 2401–2416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friedberg, J.W. How I treat double-hit lymphoma. Blood 2017, 130, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Cucco, F.; Barrans, S.; Sha, C.; Clipson, A.; Crouch, S.; Dobson, R.; Chen, Z.; Thompson, J.S.; Care, M.A.; Cummin, T.; et al. Distinct genetic changes reveal evolutionary history and heterogeneous molecular grade of DLBCL with MYC/BCL2 double-hit. Leukemia 2020, 34, 1329–1341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iorgulescu, J.B.; Medeiros, L.J.; Patel, K.P. Predictive and prognostic molecular biomarkers in lymphomas. Pathology 2024, 56, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Alfaifi, A.; Bahashwan, S.; Alsaadi, M.; Ageel, A.H.; Ahmed, H.H.; Fatima, K.; Malhan, H.; Qadri, I.; Almehdar, H. Advancements in B-Cell Non-Hodgkin’s Lymphoma: From Signaling Pathways to Targeted Therapies. Adv. Hematol. 2024, 2024, 5948170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sánchez-Beato, M.; Méndez, M.; Guirado, M.; Pedrosa, L.; Sequero, S.; Yanguas-Casás, N.; de la Cruz-Merino, L.; Gálvez, L.; Llanos, M.; García, J.F.; et al. A genetic profiling guideline to support diagnosis and clinical management of lymphomas. Clin. Transl. Oncol. 2024, 26, 1043–1062. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patil, S.; Rajput, S.; Patil, S.; Mhaiskar, A. B-cell lymphoma: Advances in pathogenesis, diagnosis, and targeted therapies. Pathol. Res. Pract. 2025, 271, 156036. [Google Scholar] [CrossRef] [PubMed]
- Bibas, M.; Antinori, A. EBV and HIV-Related Lymphoma. Mediterr. J. Hematol. Infect. Dis. 2009, 1, e2009032. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alibrahim, M.N.; Gloghini, A.; Carbone, A. Immune Deficiency/Dysregulation-Associated EBV-Positive Classic Hodgkin Lymphoma. Cancers 2025, 17, 1433. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiao, Q.; Liu, Y.; Li, T.; Wang, C.; He, S.; Zhai, L.; Yang, Z.; Zhang, X.; Wu, Y.; Liu, Y. Viral oncogenesis in cancer: From mechanisms to therapeutics. Signal Transduct. Target. Ther. 2025, 10, 151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chabay, P. Understanding EBV infection and EBV-associated lymphomas in children. Virology 2025, 608, 110544. [Google Scholar] [CrossRef]
- Cioc, A.M.; Allen, C.; Kalmar, J.R.; Suster, S.; Baiocchi, R.; Nuovo, G.J. Oral plasmablastic lymphomas in AIDS patients are associated with human herpesvirus 8. Am. J. Surg. Pathol. 2004, 28, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Oksenhendler, E.; Meignin, V. HHV-8 associated lymphoma. Curr. Opin. Oncol. 2022, 34, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, K.A.; Shingala, J.; Evens, A.; Birmann, B.M.; Giovannucci, E.; Michaud, D.S. Periodontal disease and risk of non-Hodgkin lymphoma in the Health Professionals Follow-Up Study. Int. J. Cancer 2017, 140, 1020–1026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barton, M.K. Evidence accumulates indicating periodontal disease as a risk factor for colorectal cancer or lymphoma. CA Cancer J. Clin. 2017, 67, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D. Staging and response assessment in lymphomas: The new Lugano classification. Chin. Clin. Oncol. 2015, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.J. The International Prognostic Index in aggressive B-cell lymphoma. Haematologica 2023, 108, 2874–2879. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Imbesi Bellantoni, M.; Picciolo, G.; Pirrotta, I.; Irrera, N.; Vaccaro, M.; Vaccaro, F.; Squadrito, F.; Pallio, G. Oral Cavity Squamous Cell Carcinoma: An Update of the Pharmacological Treatment. Biomedicines 2023, 11, 1112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaur, H.; Mishra, D.; Roychoudhury, A.; Bhalla, A.S.; Ramteke, P.P.S.; Kumar, L. Plasma cells in oral lesion: A clue to diagnosis or a diagnostic dilemma. J. Oral Maxillofac. Pathol. 2022, 26, 591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martínez Nieto, M.; González Gómez, L.A.; Gómez Mireles, J.C.; Lomelí Martínez, S.M. Diagnostic and therapeutic challenges of myeloid sarcoma in the oral cavity. World J. Clin. Cases 2024, 12, 6526–6533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soares, S.C.; Roux, L.J.D.; Castro, A.R.; Silva, C.C.; Rodrigues, R.; Macho, V.M.P.; Silva, F.; Costa, C. Oral Manifestations: A Warning-Sign in Children with Hematological Disease Acute Lymphocytic Leukemia. Hematol. Rep. 2023, 15, 491–502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lodolo, M.; Jordan, R.; Villa, A. An unusual oral manifestation of chronic lymphocytic leukemia: A case report and review of the literature. J. Am. Dent. Assoc. 2025, 156, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Chelius, M.; Chau, K.; Yang, J.; Hajj, C.; Imber, B.; Yahalom, J. Low grade, indolent lymphomas of the head and neck: Comparative toxicity of standard versus very low dose radiation therapy. Hematol. Oncol. 2021, 39, 304–312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krahl, D.; Altenburg, A.; Zouboulis, C.C. Reactive hyperplasias, precancerous and malignant lesions of the oral mucosa. J. Dtsch. Dermatol. Ges. 2008, 6, 217–232, (In English and German). [Google Scholar] [CrossRef] [PubMed]
- Alamoudi, W.A.; Abdelsayed, R.A.; Sollecito, T.P.; Alhassan, G.A.; Kulkarni, R.; Bindakhil, M.A. Causes of Oral Granulomatous Disorders: An Update and Narrative Review of the Literature. Head Neck Pathol. 2024, 18, 72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Villa-Forte, A. Eosinophilic granulomatosis with polyangiitis. Postgrad. Med. 2023, 135 (Suppl. 1), 52–60. [Google Scholar] [CrossRef] [PubMed]
- Suresh, L.; Radfar, L. Oral sarcoidosis: A review of literature. Oral Dis. 2005, 11, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Faustino, I.S.P.; Fernandes, P.M.; Pontes, H.A.R.; Mosqueda-Taylor, A.; Santos-Silva, A.R.; Vargas, P.A.; Lopes, M.A. Langerhans cell histiocytosis in the oral and maxillofacial region: An update. J. Oral Pathol. Med. 2021, 50, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, I.M.; DiTommaso, L.E.; Tsoukas, M.M. Oral Kaposi Sarcoma. JAMA Dermatol. 2019, 155, 370. [Google Scholar] [CrossRef] [PubMed]
- de Castro, M.S.; Reis, B.S.A.; Nogueira, D.A.; de Carli, M.L.; Hanemann, J.A.C.; Pereira, A.A.C.; Almeida, O.P.; Sperandio, F.F. Primary oral melanoma: A clinicopathologic review and case presentation. Quintessence Int. 2017, 48, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.S.; Patil, S.; Sanketh, D.; Amrutha, N. Metastatic tumors of the oral cavity. J. Contemp. Dent. Pract. 2014, 15, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, E.A.; Barraclough, A.; Sehn, L.H. Limited-stage diffuse large B-cell lymphoma. Blood 2022, 139, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Plastaras, J.P.; Ng, A.K.; Kelsey, C.R. The Evolving Role of Radiation Therapy in DLBCL: From Early-Stage to Refractory Disease. Oncology 2022, 36, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.; Koh, Y.K.; Kang, S.; Kim, H.; Koh, K.N.; Im, H.J. Clinical characteristics and treatment outcomes of children and adolescents with aggressive mature B-cell lymphoma: A single-center analysis. Blood Res. 2022, 57, 41–50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shieh, K.; Brem, E. CNS prophylaxis is (mostly) futile in DLBCL. BJC Rep. 2024, 2, 74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, T.P.; Dahlberg, S.; Cassady, J.R.; Adelstein, D.J.; Spier, C.M.; Grogan, T.M.; LeBlanc, M.; Carlin, S.; Chase, E.; Fisher, R.I. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. N. Engl. J. Med. 1998, 339, 21–26. [Google Scholar] [CrossRef]
- Persky, D.O.; Unger, J.M.; Spier, C.M.; Stea, B.; LeBlanc, M.; McCarty, M.J.; Rimsza, L.M.; Fisher, R.I.; Miller, T.P.; Southwest Oncology Group. Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J. Clin. Oncol. 2008, 26, 2258–2263. [Google Scholar] [CrossRef] [PubMed]
- Lamy, T.; Damaj, G.; Soubeyran, P.; Gyan, E.; Cartron, G.; Bouabdallah, K.; Gressin, R.; Cornillon, J.; Banos, A.; Le Du, K.; et al. R-CHOP 14 with or without radiotherapy in nonbulky limited-stage diffuse large B-cell lymphoma. Blood 2018, 131, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Poeschel, V.; Held, G.; Ziepert, M.; Witzens-Harig, M.; Holte, H.; Thurner, L.; Borchmann, P.; Viardot, A.; Soekler, M.; Keller, U.; et al. Four versus six cycles of CHOP chemotherapy in combination with six applications of rituximab in patients with aggressive B-cell lymphoma with favourable prognosis (FLYER): A randomised, phase 3, non-inferiority trial. Lancet 2019, 394, 2271–2281. [Google Scholar] [CrossRef]
- Davis, J.A.; Shockley, A.; Herbst, A.; Hendrickson, L. Polatuzumab Vedotin for the Front-Line Treatment of Diffuse Large B-Cell Lymphoma: A New Standard of Care? J. Adv. Pract. Oncol. 2023, 14, 67–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): B-Cell Lymphomas. Version 2.2025. Available online: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/b-cell_blocks.pdf (accessed on 10 February 2025).
- Minghan, Q.; Shan, W.; Xinrui, C.; Huaqing, W. Update on diffuse large B-cell lymphoma: Highlights from the 2022 ASCO Annual Meeting. Cancer Biol. Med. 2022, 19, 1117–1120. [Google Scholar] [CrossRef]
- Ramirez-Gamero, A.; Martínez-Cordero, H.; Beltrán, B.E.; Florindez, J.; Malpica, L.; Castillo, J.J. Plasmablastic lymphoma: 2024 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2024, 99, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.T.; Giri, A.; Park, Y.; Patel, K.K.; Link, B.K.; Nowakowski, G.S.; Maliske, S.M.; Fortin, S.; Chavez, J.C.; Saeed, H.; et al. Outcomes of patients with limited-stage plasmablastic lymphoma: A multi-institutional retrospective study. Am. J. Hematol. 2023, 98, 300–308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bibas, M. Plasmablastic Lymphoma. A State-of-the-Art Review: Part 2-Focus on Therapy. Mediterr. J. Hematol. Infect. Dis. 2024, 16, e2024015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ribrag, V.; Bron, D.; Rymkiewicz, G.; Hoelzer, D.; Jørgensen, J.; de Armas-Castellano, A.; Trujillo-Martín, M.; Fenaux, P.; Malcovati, L.; Bolaños, N.; et al. Diagnosis and treatment of Burkitt lymphoma in adults: Clinical practice guidelines from ERN-EuroBloodNet. Lancet Haematol. 2025, 12, e138–e150. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Jin, L.; Yang, J.; Duan, Y.L.; Zhang, M.; Zhou, C.J.; Zhang, Y.H. Treatment outcome in children with central nervous system-positive Burkitt lymphoma using only intrathecal and systemic chemotherapy combined with rituximab. Chin. Med. J. (Engl.) 2021, 134, 1329–1334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- d’Amore, F.; Federico, M.; de Leval, L.; Ellin, F.; Hermine, O.; Kim, W.S.; Lemonnier, F.; Vermaat, J.S.P.; Wulf, G.; Buske, C.; et al. Peripheral T- and natural killer-cell lymphomas: ESMO-EHA Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2025, 36, 626–644. [Google Scholar] [CrossRef] [PubMed]
- Sorigue, M.; Kuittinen, O. Controversies in the Front-Line Treatment of Systemic Peripheral T Cell Lymphomas. Cancers 2022, 15, 220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ludvigsen Al-Mashhadi, A.; Cederleuf, H.; Kuhr Jensen, R.; Holm Nielsen, T.; Bjerregård Pedersen, M.; Bech Mortensen, T.; Relander, T.; Jerkeman, M.; Ortved Gang, A.; Kristensen, A.L.; et al. Outcome of limited-stage peripheral T-Cell lymphoma after CHOP(-like) therapy: A population based study of 239 patients from the Nordic lymphoma epidemiology group. Am. J. Hematol. 2023, 98, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Illidge, T.; Fanale, M.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double-blind, randomised, phase 3 trial. Lancet 2019, 393, 229–240. [Google Scholar] [CrossRef]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Trümper, L.; Iyer, S.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. The ECHELON-2 Trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann. Oncol. 2022, 33, 288–298. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): T-Cell Lymphomas. Version 2.2025. Available online: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/t-cell_blocks.pdf (accessed on 28 May 2025).
- Costa, L.J.; Xavier, A.C.; Wahlquist, A.E.; Hill, E.G. Trends in survival of patients with Burkitt lymphoma/leukemia in the USA: An. analysis of 3691 cases. Blood 2013, 121, 4861–4866. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Evens, A.M.; Danilov, A.; Jagadeesh, D.; Sperling, A.; Kim, S.H.; Vaca, R.; Wei, C.; Rector, D.; Sundaram, S.; Reddy, N.; et al. Burkitt lymphoma in the modern era: Real-world outcomes and prognostication across 30 US cancer centers. Blood 2021, 137, 374–386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andreou, A.; Thermos, G.; Sklavounou-Andrikopoulou, A. Extranodal NK/T Cell Lymphoma, Nasal Type with Palatal Involvement: A Rare Case Report and Literature Review. Head Neck Pathol. 2021, 15, 621–627. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toljanic, J.A.; Bedard, J.F.; Larson, R.A.; Fox, J.P. A prospective pilot study to evaluate a new dental assessment and treatment paradigm for patients scheduled to undergo intensive chemotherapy for cancer. Cancer 1999, 85, 1843–1848. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Thariat, J.; Bensadoun, R.J.; Barasch, A.; Murphy, B.A.; Kolnick, L.; Popplewell, L.; Maghami, E. Oral complications of cancer and cancer therapy: From cancer treatment to survivorship. CA Cancer J. Clin. 2012, 62, 400–422. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.; Dorna Mojdami, Z.; Oladega, A.; Hope, A.; Glogauer, M.; Canadian Dental Oncology Network Consensus Group. Clinical practice guidelines for dental management prior to radiation for head and neck cancer. Oral Oncol. 2021, 123, 105604. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.T.; Woo, S.B.; Lockhart, P.B. Dental treatment planning and management in the patient who has cancer. Dent. Clin. N. Am. 2008, 52, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, S.; Liu, A.; Hull, K. A retrospective cohort study of the oral healthcare needs of cancer patients. Support. Care Cancer 2025, 33, 314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schuurhuis, J.M.; Stokman, M.A.; Witjes, M.J.; Dijkstra, P.U.; Vissink, A.; Spijkervet, F.K. Evidence supporting pre-radiation elimination of oral foci of infection in head and neck cancer patients to prevent oral sequelae. A systematic review. Oral Oncol. 2015, 51, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.P.; Rouleau, T.; Cheng, K.; Yarom, N.; Kandwal, A.; Joy, J.; Bektas Kayhan, K.; van de Wetering, M.; Brito-Dellan, N.; Kataoka, T.; et al. Systematic review of antimicrobials, mucosal coating agents, anesthetics, and analgesics for the management of oral mucositis in cancer patients and clinical practice guidelines. Support. Care Cancer 2020, 28, 2473–2484. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.H.L.; Gueiros, L.A.; Fulton, J.S.; Cheng, K.K.F.; Kandwal, A.; Galiti, D.; Fall-Dickson, J.M.; Johansen, J.; Ameringer, S.; Kataoka, T.; et al. Systematic review of basic oral care for the management of oral mucositis in cancer patients and clinical practice guidelines. Support. Care Cancer 2019, 27, 3949–3967. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.; The Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Villa, J.F.; Strang, A.; Owolabi, A.; Ramirez, M.F. Addressing Pain in Oral Mucositis: Narrative Review of Current Practices and Emerging Treatments. J. Pain Res. 2025, 18, 3723–3741. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cronshaw, M.; Parker, S.; Anagnostaki, E.; Mylona, V.; Lynch, E.; Grootveld, M. Photobiomodulation and Oral Mucositis: A Systematic Review. Dent. J. 2020, 8, 87. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al Beesh, F.A.; Martini, N.; Suleiman, S.; Aljoujou, A. Oral ulcers in hematological malignancy patients undergoing chemotherapy: Is it chemotherapy or neutropenia?: A case report and review of the literature. J. Med. Case Rep. 2025, 19, 119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohammadi, R.; Foroughifar, E. Candida infections among neutropenic patients. Casp. J. Intern. Med. 2016, 7, 71–77. [Google Scholar] [PubMed] [PubMed Central]
- Kishimoto, M.; Akashi, M.; Tsuji, K.; Kusumoto, J.; Furudoi, S.; Shibuya, Y.; Inui, Y.; Yakushijin, K.; Kawamoto, S.; Okamura, A.; et al. Intensity and duration of neutropenia relates to the development of oral mucositis but not odontogenic infection during chemotherapy for hematological malignancy. PLoS ONE 2017, 12, e0182021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mawardi, H.; Treister, N.; Felemban, O.; Alamoudi, W.; Algohary, G.; Alsultan, A.; Alshehri, N.; Tazi, I.; Shaheen, M.; Alsharani, M.; et al. Current Practice of Oral Care for Hematopoietic Stem Cell Transplant Patients: A Survey of the Eastern Mediterranean Blood and Marrow Transplantation Group. Hematol. Oncol. Stem Cell Ther. 2023, 16, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Nakagaki, M.; Kennedy, G.A.; Gavin, N.C.; Clavarino, A.; Whitfield, K. The incidence of severe oral mucositis in patients undergoing different conditioning regimens in haematopoietic stem cell transplantation. Support. Care Cancer 2022, 30, 9141–9149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hosseini, M.S.; Sanaie, S.; Mahmoodpoor, A.; Jabbari Beyrami, S.; Jabbari Beyrami, H.; Fattahi, S.; Jahanshahlou, F.; Zarei, M.; Rahimi Mamaghani, A.; Kuchaki Rafsanjani, M. Cancer treatment-related xerostomia: Basics, therapeutics, and future perspectives. Eur. J. Med. Res. 2024, 29, 571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Louise Kent, M.; Brennan, M.T.; Noll, J.L.; Fox, P.C.; Burri, S.H.; Hunter, J.C.; Lockhart, P.B. Radiation-induced trismus in head and neck cancer patients. Support. Care Cancer 2008, 16, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Huryn, J.M.; Kronstadt, K.L.; Yom, S.K.; Randazzo, J.R.; Estilo, C.L. Osteoradionecrosis of the jaw: A mini review. Front. Oral Health 2022, 3, 980786. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fall-Dickson, J.M.; Pavletic, S.Z.; Mays, J.W.; Schubert, M.M. Oral Complications of Chronic Graft-Versus-Host Disease. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz007. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thiagarajan, A.; Iyer, N.G. Radiation-induced sarcomas of the head and neck. World J. Clin. Oncol. 2014, 5, 973–981. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cafro, A.M.; Barbarano, L.; Nosari, A.M.; D’Avanzo, G.; Nichelatti, M.; Bibas, M.; Gaglioti, D.; Taroni, A.; Riva, F.; Morra, E.; et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: Definition and management of the risk related to zoledronic acid. Clin. Lymphoma Myeloma 2008, 8, 111–116, Erratum in Clin. Lymphoma Myeloma 2008, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- AlRowis, R.; Aldawood, A.; AlOtaibi, M.; Alnasser, E.; AlSaif, I.; Aljaber, A.; Natto, Z. Medication-Related Osteonecrosis of the Jaw (MRONJ): A Review of Pathophysiology, Risk Factors, Preventive Measures and Treatment Strategies. Saudi Dent. J. 2022, 34, 202–210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
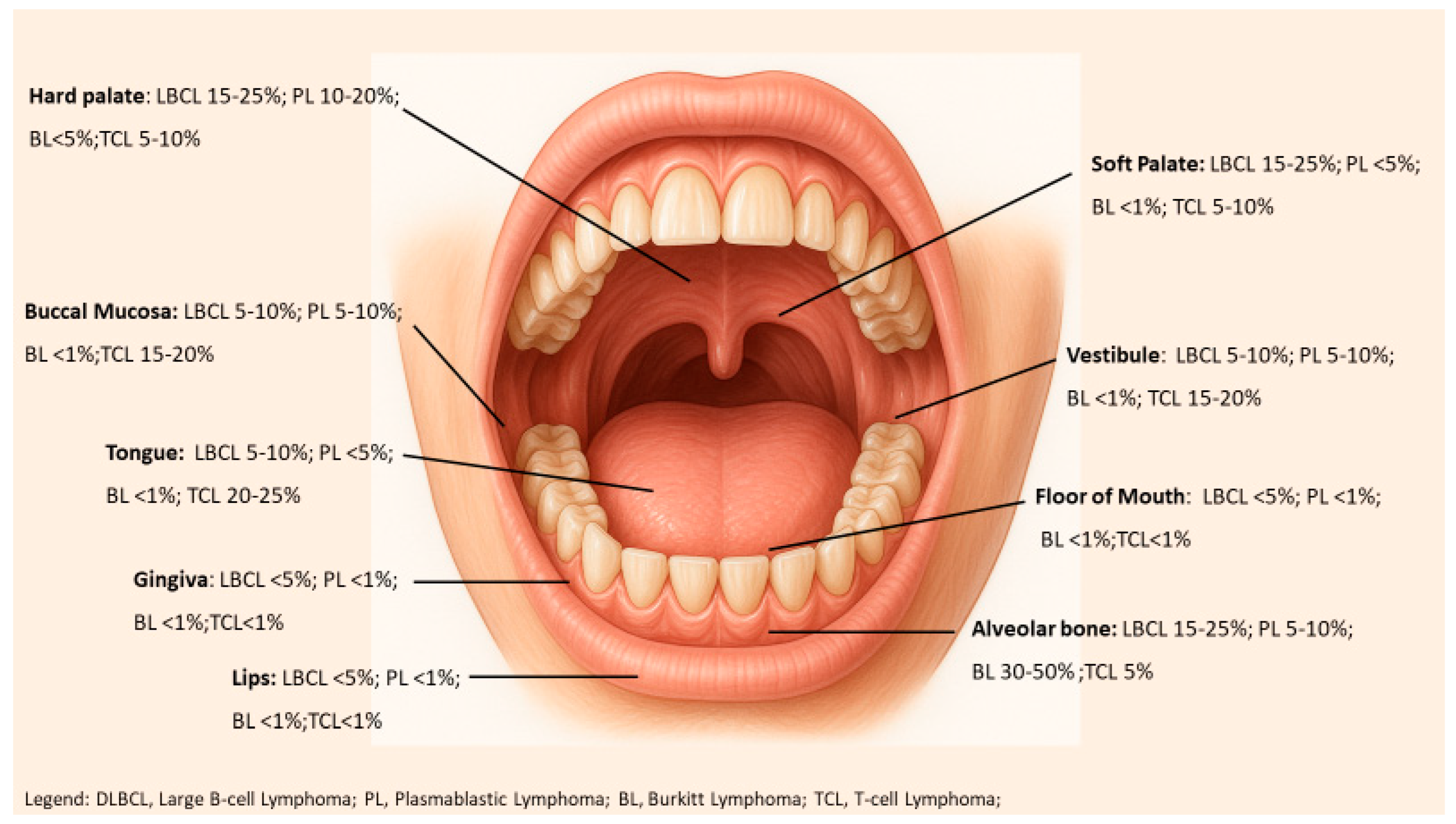
| Oral Site | DLBCL | PBL | BL | PTCL |
|---|---|---|---|---|
| Lips | Rare. DLBCL uncommon. | Rare. Only isolated case reports | Rare. | Rare. |
| Tongue | Occasional. The tongue is involved in a minority of oral DLBCL cases. | Rare. Involvement by PBL is infrequent. | Rare. BL rarely presents in the mobile tongue. | Common. Tongue is one of the most frequent sites for oral PTCL, reported in 25% of cases. |
| Hard Palate | Common. The hard palate is a frequent site. | Common. usually present as soft-tissue masses on the hard palate. | Rare. BL seldom presents in the palate. | Occasional–Common. Palatal involvement (often destructive in nature). |
| Soft Palate | Frequent. Lymphomas of the tonsils/soft palate are very common for DLBCL. | Rare. PBL only rarely involves the soft palate specifically. | Rare. Primary BL of the soft palate/tonsil is very uncommon. | Occasional. PTCL can involve soft palate region, but this is not a predominant site for TCL. |
| Gingiva | Frequent. This is the single most common site of oral lymphoma overall. | Frequent. The gingiva is the most commonly affected site in PBL HIV-positive patients. | Common. involves the jaw and presents with gingival swelling. In non-endemic BL = 15%. | Occasional. Gingival involvement by PTCL is reported but not as predominant as for B-cell lymphomas. |
| Alveolar Bone | Common. Intraosseous jaw involvement is seen in many oral DLBCL cases. | Occasional. PBL can extend into or originate in jaw bones. | Frequent. Presents as destructive tumors. | Rare. Primary PTCL in the intraoral bones is very uncommon. |
| Buccal Mucosa | Occasional. Buccal mucosa is a less common site for oral lymphomas. | Occasional. PBL can involve the buccal mucosa, though it is not among the top sites. | Rare. BL rarely presents in the buccal soft tissue. | Common. Buccal mucosa is one of the more frequent sites for PTCL in the oral cavity. |
| Vestibule | Occasional. Lymphomas can present in the gingivobuccal sulcus. | Occasional. PBL is possible but uncommon. | Rare. BL in the vestibule is not typical. | Rare. PTCL involving the oral vestibule is very rare. |
| Floor of Mouth | Rare. Extremely uncommon. | Rare. No significant occurrences of PBL. | Rare. | Rare. PTCL is unreported. |
| Lymphoma | Key Markers Positive | Key Markers Negative | EBV/HHV8 |
|---|---|---|---|
| DLBCL | CD20, CD79a, PAX5, +/- BCL6, +/- CD10 (GCB type). | Surface Ig often present; lacks plasma markers (CD138–). May express BCL2 (variable). | EBV positive in subset (EBV+ DLBCL of elderly); HHV8−. |
| PBL | CD38, CD138, MUM1/IRF4, PRDM1(Blimp1), Ig light chain. Often CD79a+, CD30+. Ki-67 ~100%. | Pan-B markers CD20, CD19, PAX5 usually negative (or dim). Often CD45–. Usually CD10–, BCL6– (not GC-derived). | EBV positive in ~70%; HHV8 negative. |
| BL | CD20, CD10, BCL6, surface IgM, Ki-67 ~100%. MYC translocation present in all cases. | BCL2 typically negative. (CD138–, CD30–). | EBV positive in ~90% (endemic); ~30% in sporadic; HHV8−. |
| ENK/TL | CD3ε (cytoplasmic), CD56, cytotoxic granule proteins (TIA-1, Granzyme B). EBER+ in tumor cells. | Surface CD3 often−; usually CD5−, CD4−CD8− (“double negative”). B-cell markers all −. | EBV positive in >95%; HHV8−. |
| PTCL-NOS | CD3, CD5 (often partial), CD4 or CD8 (commonly CD4+). Clonal TCR gene rearrangement+. | May lose pan-T markers (e.g., CD5 or CD7−). No lineage-specific single marker. | EBV usually negative. |
| ALK + TL | CD30++, ALK-1 protein++, EMA+, often CD4+. | Usually CD3− (or weak), CD5−, CD8−, CD15−. | EBV− (typically). HHV8−. |
| Subtype | Key Genetic Alterations | Viral Associations |
|---|---|---|
| DLBCL | MYC translocation in ~10%; BCL2 translocation in subset (esp. double-hit cases); BCL6 translocation in ~20%. TP53 mutations in subset (correlating with high risk). | EBV+ in EBV+ DLBCL, NOS (often in immunosenescence or immunosuppression); no direct HHV-8 link. |
| PBL | MYC translocations in ~50% (often IgH partner); Complex karyotypes common. PRDM1 (BLIMP1) inactivating mutations in up to 50%; other mutations: TP53 (~20–30%), JAK3, STAT3 (esp. HIV+ cases). Often exhibits MYC overexpression (even if no translocation) via NF-κB/STAT3 activation. | EBV frequently positive (~70%) (Latency I program typical). HHV-8 negative in classic PBL; but oral HHV-8+ cases occur in HIV (solid PEL variant). |
| BL | MYC-Ig translocation in ~100% (t(8;14) or variants). Often additional 13q or 1q abnormalities. Very few other mutations (germinal center B-cell derived): can have ID3, TCF3 mutations in sporadic BL. TP53 mutations in ~30%. | EBV+ in nearly all endemic BL; ~20% of sporadic BL (higher if HIV+). No HHV-8 involvement. |
| NK/TL | No pathognomonic translocation; commonly mutated genes: TP53, Janus kinase pathway (e.g., JAK3), STAT5b, DDX3X, BCOR. Also, 6q21 deletions (PRDM1, etc.) common. LOH at 17p (TP53) frequent. | EBV universal (clonal EBV in tumor cells). EBV likely causative; expresses EBV latent genes (EBNA1, LMP1 variably) and EBV miRNAs. No association with HHV-8. |
| ALK + TL | t(2;5)(p23;q35) NPM1-ALK in most; variant ALK translocations (partner genes) in others. ALK fusion protein. | Usually EBV−. HHV-8−. (Not virus-driven, unlike B-PTLD). |
| Red Flag | Description |
|---|---|
| Non-healing ulcer | Lasting > 2 weeks; indurated margins, irregular surface, not responding to topical therapy. |
| Red or white patches | Persistent adherent lesions that can be precancerous or malignant. |
| Intraoral swelling or palpable mass | Localized or infiltrative lesion without trauma or obvious cause; may include neck lymph node enlargement due to metastasis. |
| Unexplained tooth mobility | Tooth loosening not related to periodontal disease or trauma; often associated with underlying bone destruction. |
| Persistent pain or unusual sensations | Ineffective response to analgesics; may include deep pain or paresthesia (numbness, tingling in tongue, lip, or chin). |
| Functional difficulties | Dysphagia, speech changes, difficulty chewing or opening the mouth without clear cause. |
| Ear pain (referred otalgia) | Typical of tumors in the tongue or base of the mouth; absence of local ear infection. |
| Persistent halitosis and weight loss | Not explained by oral hygiene or diet; often a sign of advanced disease. |
| Persistent cervical lymph nodes | Enlarged, hard, progressive, non-tender nodes suggestive of metastasis. |
| Condition | Clinical Clues | Pathology/IHC Clues |
|---|---|---|
| Squamous Cell Carcinoma | Irregular ulcer or mass with raised rolled border; surface texturing (keratosis), common on lateral tongue, FOM, gingiva. | Malignant epithelial cells forming nests/pearls; IHC: Cytokeratin+, p63+. No diffuse CD45+ lymphoid infiltrate as in lymphoma. Dysplastic epithelium adjacent often present. |
| Plasmacytoma/ Myeloma | Often involves bone can have jaw pain; patient may have systemic signs (anemia, renal issues). Soft tissue plasmacytomas can appear as violaceous submucosal masses in older adults. | Sheets of plasma cells, eccentric nuclei. IHC: CD138+, CD56+ (common), MUM1+, monoclonal light chain in tumor; CD20–. EBER– (unlike many PBL). Serum/urine monoclonal protein usually detectable. Bone marrow involvement common in MM. |
| Reactive Plasma Cell Gingivitis (benign) | Diffuse gingival redness/enlargement often due to hypersensitivity. Generalized involvement rather than a focal mass; bleeds easily but patient well. | Plasma cell infiltrate polyclonal (mixed κ/λ by IHC). No atypia or destructive growth. Will lack clonal light chain restriction seen in neoplastic plasma cell lesions. Responds to removal of irritant or steroids, unlike lymphoma. |
| Chronic Periodontitis/ Osteomyelitis | Common; deep periodontal pockets, calculus, tooth mobility due to bone loss. Osteomyelitis can cause fistula, purulent discharge. | Periodontitis: granulation tissue with polymorphous and bone resorption; no monoclonal population. Osteomyelitis: necrotic bone with bacterial colonies, granulocytes; culture positive |
| Granulomatous Infection (TB, deep fungus) | Chronic non-healing ulcer or nodule. May have sinus tract (in TB of jaw), or systemic B symptoms. Regional lymph nodes often involved (scrofula). In histo/blasto, often pulmonary signs too. | Granulomas with central necrosis (TB) or organisms visible. Special stains: AFB stain+ for TB (or PCR), GMS+ for fungi. No monoclonal B-cell population; IHC CD20/CD3 will show mixed T and B without light chain restriction. Cultures confirm pathogen. |
| NK/T-cell Lymphoma (Nasal type) | Ulcerative lesion on midline palate often with perforation; nasal congestion/epistaxis common. Rapid progression, systemic symptoms. | Angiocentric atypical lymphoid infiltrate with necrosis. IHC: CD3ε+, CD56+, cytotoxic markers+; EBER positive (virtually 100%). |
| Wegener’s (Granulomatosis with polyangiitis) | Sinonasal involvement with ulceration can extend to palate; often multiple lesions, crusting, also lung/kidney involvement. c-ANCA positive in ~90%. | Necrotizing granulomas with vasculitis (fibrinoid necrosis in vessel walls). No clonal lymphocyte population; negative for EBV in lesions. PR3-ANCA blood test positive. No atypical T-cells expressing CD56 as in NK/T lymphoma. |
| Peripheral T-cell Lymphoma, NOS | Rare in oral cavity; may present as diffuse ulcerative stomatitis or a tumor. B symptoms present. | Diffuse infiltrate of pleomorphic T-cells. IHC: CD3+, often loss of CD5 or other B-cell markers; no EBV (unless associated with immunosuppression). Diagnosis by exclusion—requires clonality testing. |
| Anaplastic Large Cell Lymphoma | Very rare as primary oral lesion; might present as a solitary ulcerated nodule on the lip or gingiva. ALK+ subtype more in younger, ALK- in older. | Sheets of large anaplastic cells, pleomorphic, CD30 strongly positive. ALK IHC ± (depending on type). |
| Lymphoma | Chemotherapy | Immunotherapy | Radiotherapy | Stem Cell Transplant (Auto/Allo) | Targeted Agents/Novel Drugs | CAR-T Therapy |
|---|---|---|---|---|---|---|
| DLBCL | R-CHOP × 3–4 cycles (non-bulky) or × 6 (bulky/high IPI) | Rituximab (anti-CD20) with all cycles | ISRT 30–36 Gy (if <4 cycles of R-CHOP or residual mass) | Not indicated upfront; ASCT in relapsed refractory; Allo-SCT rarely used | Polatuzumab-vedotin + R-CHP (for high-risk first-line) | For relapsed/refractory cases (CD19+) after ≥2 lines |
| PBL | Dose-adjusted EPOCH or HyperCVAD; CHOP inadequate | Daratumumab or Bortezomib or brentuximab in trials | 30–50 Gy ISRT after chemoCT in localized disease | ASCT in CR1 (fit patients); AlloSCT for R/R cases | Bortezomib, lenalidomide, thalidomide; PD-1 inhibitors | Limited use (CD19 often negative), only experimental |
| BL | CODOX-M/IVAC or HyperCVAD + rituximab; DA-EPOCH-R for older adults; | Rituximab standard (CD20+) | RT not routinely used (only in residual disease) | No role in 1st line; ASCT/AlloSCT in relapsed BL | No standard; investigational agents in R/R BL | CD19 CAR-T (salvage setting) |
| TCL | CHOP or CHOEP × 6; BV-CHP for CD30+ (e.g., ALCL) | Brentuximab-vedotin (CD30+) integrated into BV-CHP | ISRT 30–40 Gy to the oral site post-chemo | ASCT in CR1 (especially PTCL-NOS, ALK– ALCL); AlloSCT for relapsed or refractory cases | Romidepsin, pralatrexate, belinostat; ALK inhibitors (e.g., crizotinib in ALK + ALCL) | No approved CAR-T; CD30 or TRBC1 CAR-T under trial |
| Lymphoma Subtype | 5-Year OS | Notes |
|---|---|---|
| DLBCL | ~75–80% (if low IPI) | R-CHOP cures majority; worse if advanced stage or double-hit (5-yr OS < 30%). |
| PBL | <20% (5-year); median OS ~8–12 months | Very poor despite intensive chemo; high relapse. |
| BL | ~60–70% (5-year) | Children > 85% cured; adults > 60% if fit for intensive chemo. Rapidly fatal if not treated promptly. |
| NK/T-cell Lymphoma | ~50–70% (5-year) | Localized disease can be cured with chemoradiation; disseminated disease <20% 2-year survival. |
| T-cell, ALK-positive Lymphoma | ~70–80% (5-year) | Excellent prognosis in younger patients, especially with addition of brentuximab to chemo. ALK-negative ALCL ~50% 5-year. |
| T-cell, ALK-negative Lymphoma | ~40–50% (5-year) | Generally poor with CHOP; consolidation with transplant improves some outcomes. |
| HIV-associated DLBCL | ~50–60% (3-year) | Approaches as HIV-neg outcomes if CD4 adequate and full-dose chemo given. PBL in HIV very poor (<1 year survival). |
| Treatment Phase | Dental Role and Interventions | Key Considerations |
|---|---|---|
| Pre-Treatment Dental Assessment |
|
|
| During Oncology Treatment |
|
|
| Treatment Planning Considerations |
|
|
| Supportive and Preventive Oral Care During Cancer Therapy |
|
|
| Post-Treatment Follow-Up and Long-Term Oral Care |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bibas, M.; Pilloni, A.; Maggio, E.; Antinori, A.; Mazzotta, V. Primary Aggressive Oral Lymphomas (PAOL): A Narrative Review of Diagnosis, Molecular Features, Therapeutic Approaches, and the Integrated Role of Dentists and Hematologists. Cancers 2025, 17, 3138. https://doi.org/10.3390/cancers17193138
Bibas M, Pilloni A, Maggio E, Antinori A, Mazzotta V. Primary Aggressive Oral Lymphomas (PAOL): A Narrative Review of Diagnosis, Molecular Features, Therapeutic Approaches, and the Integrated Role of Dentists and Hematologists. Cancers. 2025; 17(19):3138. https://doi.org/10.3390/cancers17193138
Chicago/Turabian StyleBibas, Michele, Andrea Pilloni, Edmondo Maggio, Andrea Antinori, and Valentina Mazzotta. 2025. "Primary Aggressive Oral Lymphomas (PAOL): A Narrative Review of Diagnosis, Molecular Features, Therapeutic Approaches, and the Integrated Role of Dentists and Hematologists" Cancers 17, no. 19: 3138. https://doi.org/10.3390/cancers17193138
APA StyleBibas, M., Pilloni, A., Maggio, E., Antinori, A., & Mazzotta, V. (2025). Primary Aggressive Oral Lymphomas (PAOL): A Narrative Review of Diagnosis, Molecular Features, Therapeutic Approaches, and the Integrated Role of Dentists and Hematologists. Cancers, 17(19), 3138. https://doi.org/10.3390/cancers17193138







