Dynamic Integrative Immune Profiling Reveals Early Biomarkers of Response and Prognosis in Advanced Gastric Cancer Treated with Nivolumab Plus Chemotherapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Study Design
2.2. Treatment Regimen
2.3. Blood Sampling and Immune Monitoring
2.4. Definition of Groups and Cutoff Values
2.5. Statistical Analysis
3. Results
3.1. Participants Characteristics
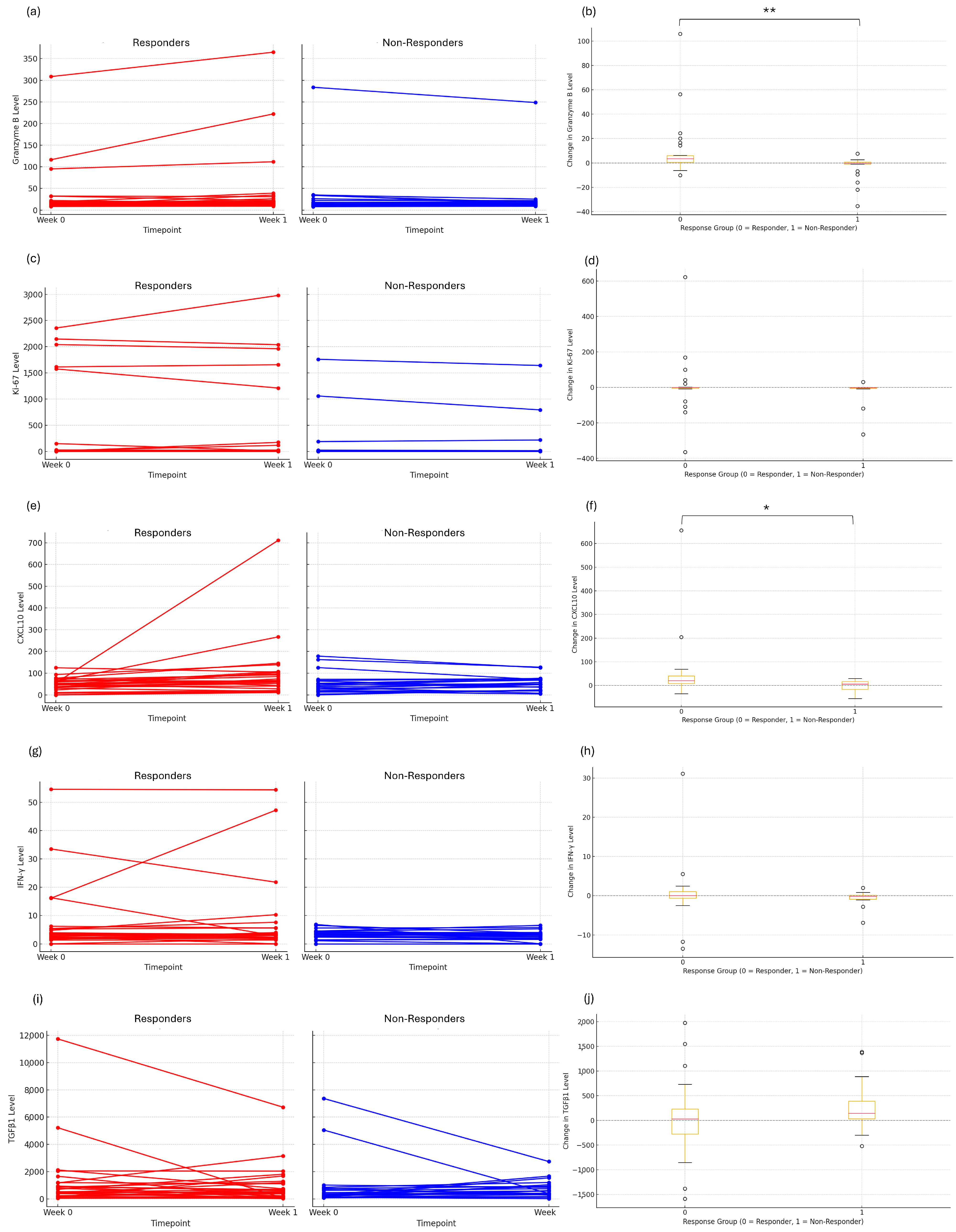
3.2. Early Immune Response and Dynamic Changes in Cytotoxicity
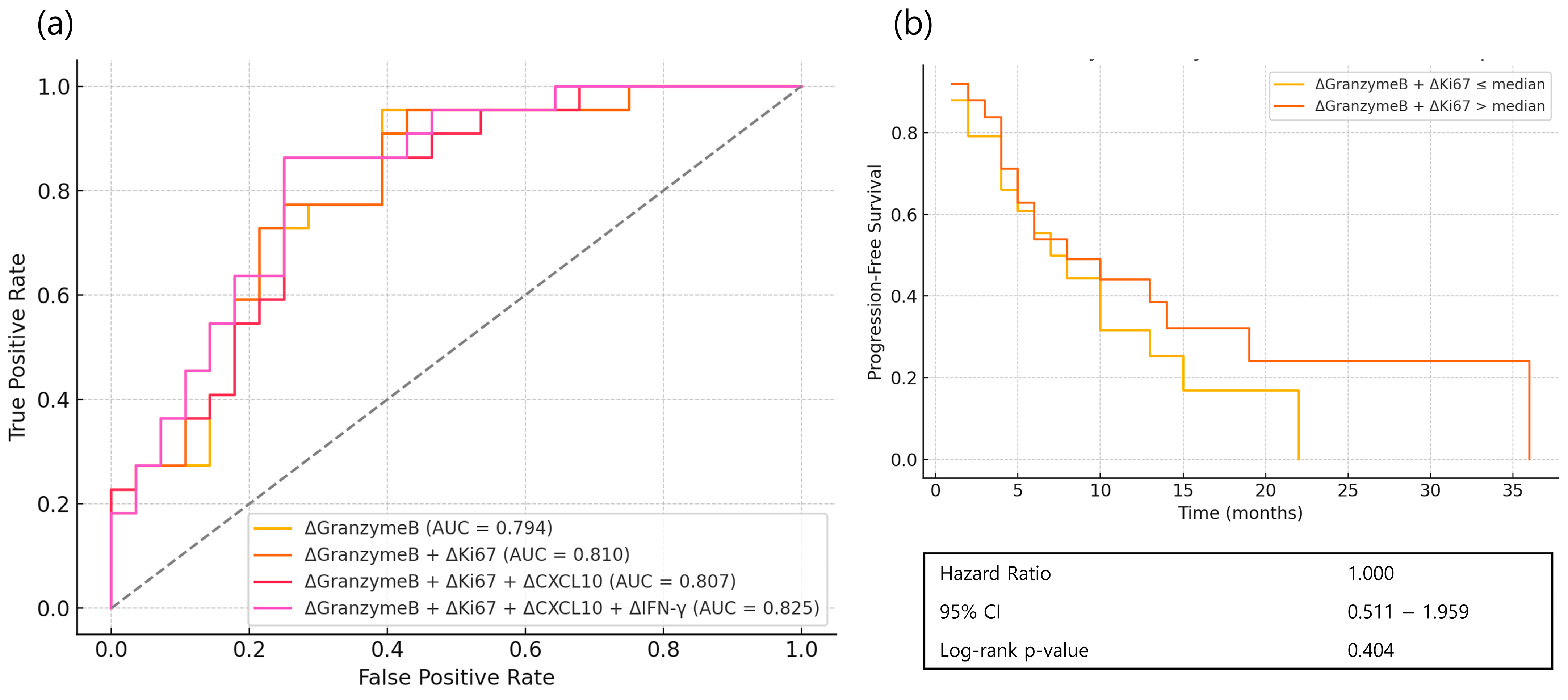
3.3. Prediction for Initial Immune Response
3.4. Prognostic Implications of Classic Immune Markers
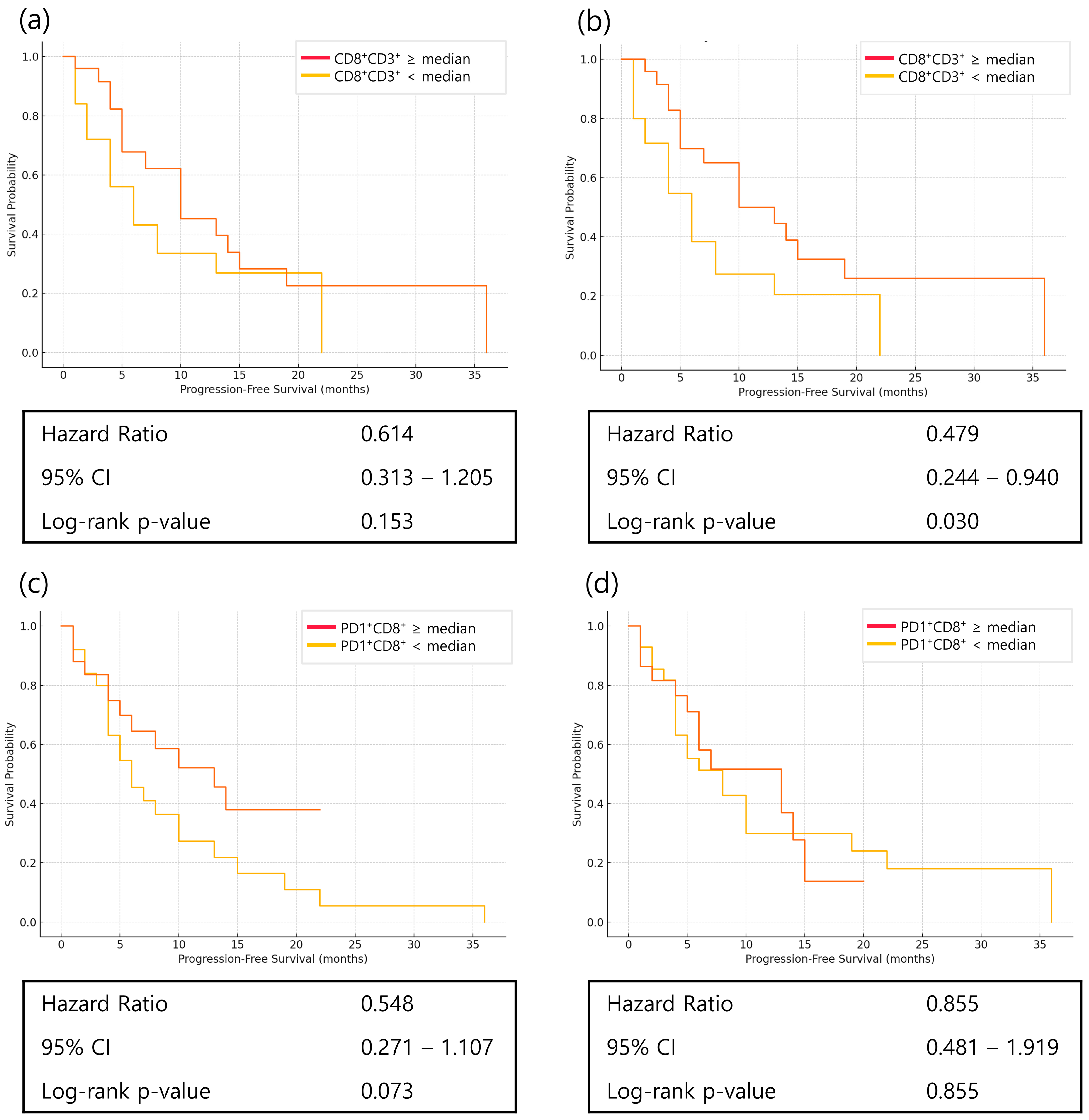
3.5. Prognostic Role of PD1+CD8+ T Cells and Dynamics of CD8+ T-Cell Subsets
3.6. Prognostic Role of Immune Checkpoint Markers in Treatment Outcomes
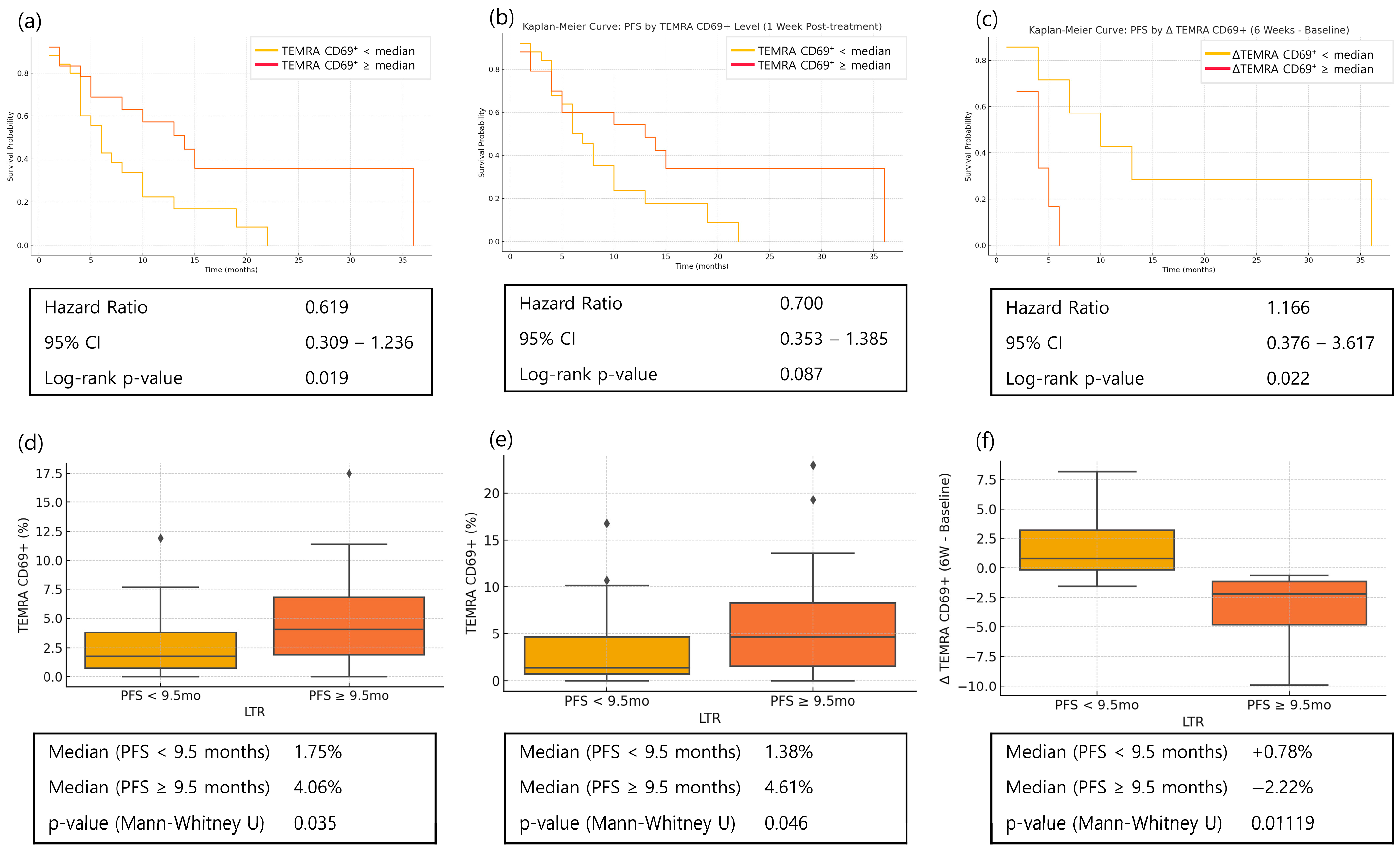
3.7. Memory T Cells in Long-Term Responders
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Qi, Y.-K.; Zheng, J.-S.; Liu, L. Mirror-image protein and peptide drug discovery through mirror-image phage display. Chem 2024, 10, 2390–2407. [Google Scholar] [CrossRef]
- Kim, I.H.; Kang, S.J.; Choi, W.; Seo, A.N.; Eom, B.W.; Kang, B.; Kim, B.J.; Min, B.H.; Tae, C.H.; Choi, C.I.; et al. Korean Practice Guidelines for Gastric Cancer 2024: An Evidence-based, Multidisciplinary Approach (Update of 2022 Guideline). J. Gastric Cancer 2025, 25, 5–114. [Google Scholar] [CrossRef]
- Ghidini, M.; Petrillo, A.; Botticelli, A.; Trapani, D.; Parisi, A.; La Salvia, A.; Sajjadi, E.; Piciotti, R.; Fusco, N.; Khakoo, S. How to Best Exploit Immunotherapeutics in Advanced Gastric Cancer: Between Biomarkers and Novel Cell-Based Approaches. J. Clin. Med. 2021, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.; Postow, M.A.; Orlowski, R.J.; Mick, R.; Bengsch, B.; Manne, S.; Xu, W.; Harmon, S.; Giles, J.R.; Wenz, B.; et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017, 545, 60–65. [Google Scholar] [CrossRef]
- Kamphorst, A.O.; Pillai, R.N.; Yang, S.; Nasti, T.H.; Akondy, R.S.; Wieland, A.; Sica, G.L.; Yu, K.; Koenig, L.; Patel, N.T.; et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc. Natl. Acad. Sci. USA 2017, 114, 4993–4998. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Kim, J.; Park, S.J.; Kim, H.; Lee, M.A.; Kim, O.; Park, J.; Kang, N.; Kim, I.H. Early Increase in Circulating PD-1(+)CD8(+) T Cells Predicts Favorable Survival in Patients with Advanced Gastric Cancer Receiving Chemotherapy. Cancers 2023, 15, 3955. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, J.; Fan, X.; Zheng, Z.; Jiang, X.; Chen, D.; Lu, Y.; Li, Y.; Wang, M.; Hu, M.; et al. Immune Profiling in Gastric Cancer Reveals the Dynamic Landscape of Immune Signature Underlying Tumor Progression. Front. Immunol. 2022, 13, 935552. [Google Scholar] [CrossRef]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, M.; Yuan, M.; Ye, J.; Zhang, W.; Xu, L.; Wu, X.; Hui, B.; Yang, Y.; Wei, B.; et al. CXCL10-armed oncolytic adenovirus promotes tumor-infiltrating T-cell chemotaxis to enhance anti-PD-1 therapy. Oncoimmunology 2022, 11, 2118210. [Google Scholar] [CrossRef]
- Shi, H.; Chen, S.; Chi, H. Immunometabolism of CD8(+) T cell differentiation in cancer. Trends Cancer 2024, 10, 610–626. [Google Scholar] [CrossRef]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—A target for novel cancer therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef]
- Zumwalt, T.J.; Arnold, M.; Goel, A.; Boland, C.R. Active secretion of CXCL10 and CCL5 from colorectal cancer microenvironments associates with GranzymeB+ CD8+ T-cell infiltration. Oncotarget 2015, 6, 2981–2991. [Google Scholar] [CrossRef]
- Baessler, A.; Vignali, D.A.A. T Cell Exhaustion. Annu. Rev. Immunol. 2024, 42, 179–206. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, C.; Wang, H. Immune-checkpoint inhibitor resistance in cancer treatment: Current progress and future directions. Cancer Lett. 2023, 562, 216182. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, J.; Wang, J.; Chen, X.; Cai, C.; Zhou, F.; Xiong, A. Prognostic role of dynamic changes in inflammatory indicators in patients with non-small cell lung cancer treated with immune checkpoint inhibitors-a retrospective cohort study. Transl. Lung Cancer Res. 2024, 13, 1975–1987. [Google Scholar] [CrossRef]
- Xu-Monette, Z.Y.; Zhang, M.; Li, J.; Young, K.H. PD-1/PD-L1 Blockade: Have We Found the Key to Unleash the Antitumor Immune Response? Front. Immunol. 2017, 8, 1597. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Humeau, J.; Buqué, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef]
- Han, J.; Khatwani, N.; Searles, T.G.; Turk, M.J.; Angeles, C.V. Memory CD8(+) T cell responses to cancer. Semin. Immunol. 2020, 49, 101435. [Google Scholar] [CrossRef] [PubMed]
- Luoma, A.M.; Suo, S.; Wang, Y.; Gunasti, L.; Porter, C.B.M.; Nabilsi, N.; Tadros, J.; Ferretti, A.P.; Liao, S.; Gurer, C.; et al. Tissue-resident memory and circulating T cells are early responders to pre-surgical cancer immunotherapy. Cell 2022, 185, 2918–2935.e2929. [Google Scholar] [CrossRef]
- Virassamy, B.; Caramia, F.; Savas, P.; Sant, S.; Wang, J.; Christo, S.N.; Byrne, A.; Clarke, K.; Brown, E.; Teo, Z.L.; et al. Intratumoral CD8(+) T cells with a tissue-resident memory phenotype mediate local immunity and immune checkpoint responses in breast cancer. Cancer Cell 2023, 41, 585–601.e8. [Google Scholar] [CrossRef] [PubMed]
- Oja, A.E.; Piet, B.; Helbig, C.; Stark, R.; van der Zwan, D.; Blaauwgeers, H.; Remmerswaal, E.B.M.; Amsen, D.; Jonkers, R.E.; Moerland, P.D.; et al. Trigger-happy resident memory CD4(+) T cells inhabit the human lungs. Mucosal Immunol. 2018, 11, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J.; Teichgräber, V.; Becker, T.C.; Masopust, D.; Kaech, S.M.; Antia, R.; von Andrian, U.H.; Ahmed, R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003, 4, 225–234. [Google Scholar] [CrossRef]
- Andrews, L.P.; Cillo, A.R.; Karapetyan, L.; Kirkwood, J.M.; Workman, C.J.; Vignali, D.A.A. Molecular Pathways and Mechanisms of LAG3 in Cancer Therapy. Clin. Cancer Res. 2022, 28, 5030–5039. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Hu, Y.; Yang, Z.; Li, J.; Liu, X.; Deng, L.; Wang, Y.; Zhang, X.; Jiang, T.; et al. Targeting PD-1 and Tim-3 Pathways to Reverse CD8 T-Cell Exhaustion and Enhance Ex Vivo T-Cell Responses to Autologous Dendritic/Tumor Vaccines. J. Immunother. 2016, 39, 171–180. [Google Scholar] [CrossRef]
- Long, G.V.; Lipson, E.J.; Hodi, F.S.; Ascierto, P.A.; Larkin, J.; Lao, C.; Grob, J.J.; Ejzykowicz, F.; Moshyk, A.; Garcia-Horton, V.; et al. First-Line Nivolumab Plus Relatlimab Versus Nivolumab Plus Ipilimumab in Advanced Melanoma: An Indirect Treatment Comparison Using RELATIVITY-047 and CheckMate 067 Trial Data. J. Clin. Oncol. 2024, 42, 3926–3934. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Hegewisch-Becker, S.; Mendez, G.; Chao, J.; Nemecek, R.; Feeney, K.; Van Cutsem, E.; Al-Batran, S.E.; Mansoor, W.; Maisey, N.; Pazo Cid, R.; et al. First-Line Nivolumab and Relatlimab Plus Chemotherapy for Gastric or Gastroesophageal Junction Adenocarcinoma: The Phase II RELATIVITY-060 Study. J. Clin. Oncol. 2024, 42, 2080–2093. [Google Scholar] [CrossRef]
- Seghers, S.; Domen, A.; Prenen, H. Challenges and prospects of LAG-3 inhibition in advanced gastric and gastroesophageal junction cancer: Insights from the RELATIVITY-060 trial. J. Gastrointest. Oncol. 2024, 15, 2735–2738. [Google Scholar] [CrossRef]
- Kelly, R.J.; Landon, B.V.; Zaidi, A.H.; Singh, D.; Canzoniero, J.V.; Balan, A.; Hales, R.K.; Voong, K.R.; Battafarano, R.J.; Jobe, B.A.; et al. Neoadjuvant nivolumab or nivolumab plus LAG-3 inhibitor relatlimab in resectable esophageal/gastroesophageal junction cancer: A phase Ib trial and ctDNA analyses. Nat. Med. 2024, 30, 1023–1034. [Google Scholar] [CrossRef] [PubMed]




| Characteristic | Group | n (%) |
|---|---|---|
| Total | 50 (100) | |
| Sex | Female | 22 (44.0) |
| Male | 28 (56.0) | |
| Age | <65 years | 38 (76.0) |
| ≥65 years | 12 (24.0) | |
| Histology | Adenocarcinoma | 32 (64.0) |
| Poorly cohesive carcinoma | 18 (36.0) | |
| Peritoneal carcinomatosis | No | 19 (38.0) |
| Yes | 31 (62.0) | |
| MSI-H | No | 41 (82.0) |
| Yes | 7 (14.0) | |
| Unknown | 2 (4.0) | |
| HER2 | Negative | 21 (42.0) |
| Low | 29 (58.0)) | |
| Positive | 0 (0.0) | |
| EBV | Negative | 46 (92.0) |
| Positive | 4 (8.0) | |
| PD-L1 CPS | <5 | 20 (40.0) |
| ≥5 | 28 (56.0) | |
| Unknown | 2 (4.0) | |
| Treatment | Nivolumab + XELOX | 42 (84.0) |
| Nivolumab + FOLFOX | 8 (16.0) | |
| Best response | CR or PR | 28 (56.0) |
| SD or PD | 22 (44.0) |
| Marker | Responder (Mean Change) | Non-Responder (Mean Change) | p-Value |
|---|---|---|---|
| ΔGranzyme B | +9.81 | −3.44 | <0.01 |
| ΔKi-67 | +8.35 | −18.13 | 0.17 |
| ΔCXCL10 | +48.68 | −1.17 | 0.02 |
| ΔIFNγ | +0.56 | −0.51 | 0.25 |
| ΔTGFβ1 | +53.22 | +268.68 | 0.16 |
| Timepoint | Group | Mean | Median | IQR (25–75%) | Max | p-Value |
|---|---|---|---|---|---|---|
| Baseline | Non-LT | 3.83 | 3.23 | 1.96–4.19 | 20.37 | 0.822 |
| LT | 3.44 | 2.35 | 1.92–4.37 | 10.07 | ||
| Week 1 | Non-LT | 2.93 | 2.76 | 1.95–3.67 | 8.70 | 0.045 |
| LT | 2.14 | 1.73 | 1.33–2.94 | 5.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Shin, K.; Park, S.J.; Lee, M.A.; Park, J.; Kim, O.; Kang, N.; Kim, I.-H. Dynamic Integrative Immune Profiling Reveals Early Biomarkers of Response and Prognosis in Advanced Gastric Cancer Treated with Nivolumab Plus Chemotherapy. Cancers 2025, 17, 3131. https://doi.org/10.3390/cancers17193131
Kim H, Shin K, Park SJ, Lee MA, Park J, Kim O, Kang N, Kim I-H. Dynamic Integrative Immune Profiling Reveals Early Biomarkers of Response and Prognosis in Advanced Gastric Cancer Treated with Nivolumab Plus Chemotherapy. Cancers. 2025; 17(19):3131. https://doi.org/10.3390/cancers17193131
Chicago/Turabian StyleKim, Hyunho, Kabsoo Shin, Se Jun Park, Myung Ah Lee, Juyeon Park, Okran Kim, Nahyeon Kang, and In-Ho Kim. 2025. "Dynamic Integrative Immune Profiling Reveals Early Biomarkers of Response and Prognosis in Advanced Gastric Cancer Treated with Nivolumab Plus Chemotherapy" Cancers 17, no. 19: 3131. https://doi.org/10.3390/cancers17193131
APA StyleKim, H., Shin, K., Park, S. J., Lee, M. A., Park, J., Kim, O., Kang, N., & Kim, I.-H. (2025). Dynamic Integrative Immune Profiling Reveals Early Biomarkers of Response and Prognosis in Advanced Gastric Cancer Treated with Nivolumab Plus Chemotherapy. Cancers, 17(19), 3131. https://doi.org/10.3390/cancers17193131







