1. Introduction
Prostate cancer is one of the most common malignancies among men globally. For localized disease, radical prostatectomy remains a cornerstone of curative treatment alternative to radiotherapy.
The procedure consists of the removal of the prostate and seminal vesicles and the subsequent re-anastomosis of the bladder neck to the urethra. The removal of the obturator and iliac lymph nodes may also be performed simultaneously according to the classification of the risk of lymph node involvement which is evaluated with the use of specific nomograms [
1].
Since the introduction of robotic systems, especially the da Vinci Surgical System, robot-assisted radical prostatectomy (RARP) has increasingly replaced ORP and laparoscopic approaches in clinical practice. The precision and ergonomic advantages of RARP offer potential benefits in perioperative recovery and functional preservation.
We conducted a selective literature review using PubMed, MEDLINE, and key journals.
2. Indications for Radical Prostatectomy
Radical prostatectomy is part of the active treatment management procedures for prostate cancer. It can be performed in patients with a life expectancy, based on comorbidities, greater than 10 years, and if the patient is not eligible for active surveillance (AS) protocols, or in cases where he does not accept them.
Radical prostatectomy is recommended when the disease is localized and presents at least an unfavorable intermediate risk according to the D’ Amico classification of biochemical recurrence risk (BCR) of localized and locally advanced disease updated by EAU 2025 (shown in
Table 1). It may also be performed in cases in which the pathology is localized at favorable intermediate risk but presents a greater number of positive biopsy samples, or if the patient does not accept the greater risk of progression of the disease associated with active surveillance protocols [
2]; see
Table 1.
In case of high risk localized disease, radical prostatectomy is still an option even if it will present a higher risk of PSA failure and metastatic progression, but not all high-risk patients have a uniformly unfavorable prognosis [
3]. So, in these cases, RARP should be offered as part of a conceivable multi-modal therapy.
Currently, the European guidelines do not give strong recommendations but still weakly recommend performing salvage radiotherapy associated with androgen deprivation therapy in cases where, after radical prostatectomy, the patient has a persistent PSA but has no evidence of metastasis [
2].
3. Perioperative Advantages
Multiple studies have shown that RARP is associated with reduced blood loss, lower transfusion rates, shorter hospital stays, and decreased postoperative pain compared to ORP. A systematic review and meta-analysis by Ficarra et al. (2012) reported a mean blood loss reduction of approximately 400 mL with RARP and a significantly lower transfusion rate [
4]. Additionally, RARP is associated with fewer wound complications due to its minimally invasive nature.
In addition, the conversion rates of RARP into open surgery have always been low, but they have also shown a trend in improvement over time, most likely due to an increase in surgical experience in this area, the progressive improvement of instrumentation, and the spread of robotic surgery [
5].
4. Functional Outcomes
Recovery of urinary continence and sexual function are critical considerations.
High-definition 3D visualization and articulating instruments allow for refined dissection around the neurovascular bundles. Tewari et al. (2018) demonstrated that experienced surgeons performing RARP achieved continence rates exceeding 90% at 12 months and better early recovery of erectile function, particularly in younger patients undergoing bilateral nerve-sparing surgery [
6].
A retrospective study based on data from twelve Dutch hospitals noted a lower incidence of erectile dysfunction in the RARP group compared to LRP (67.7% vs. 76.2%;
p = 0.052) [
7].
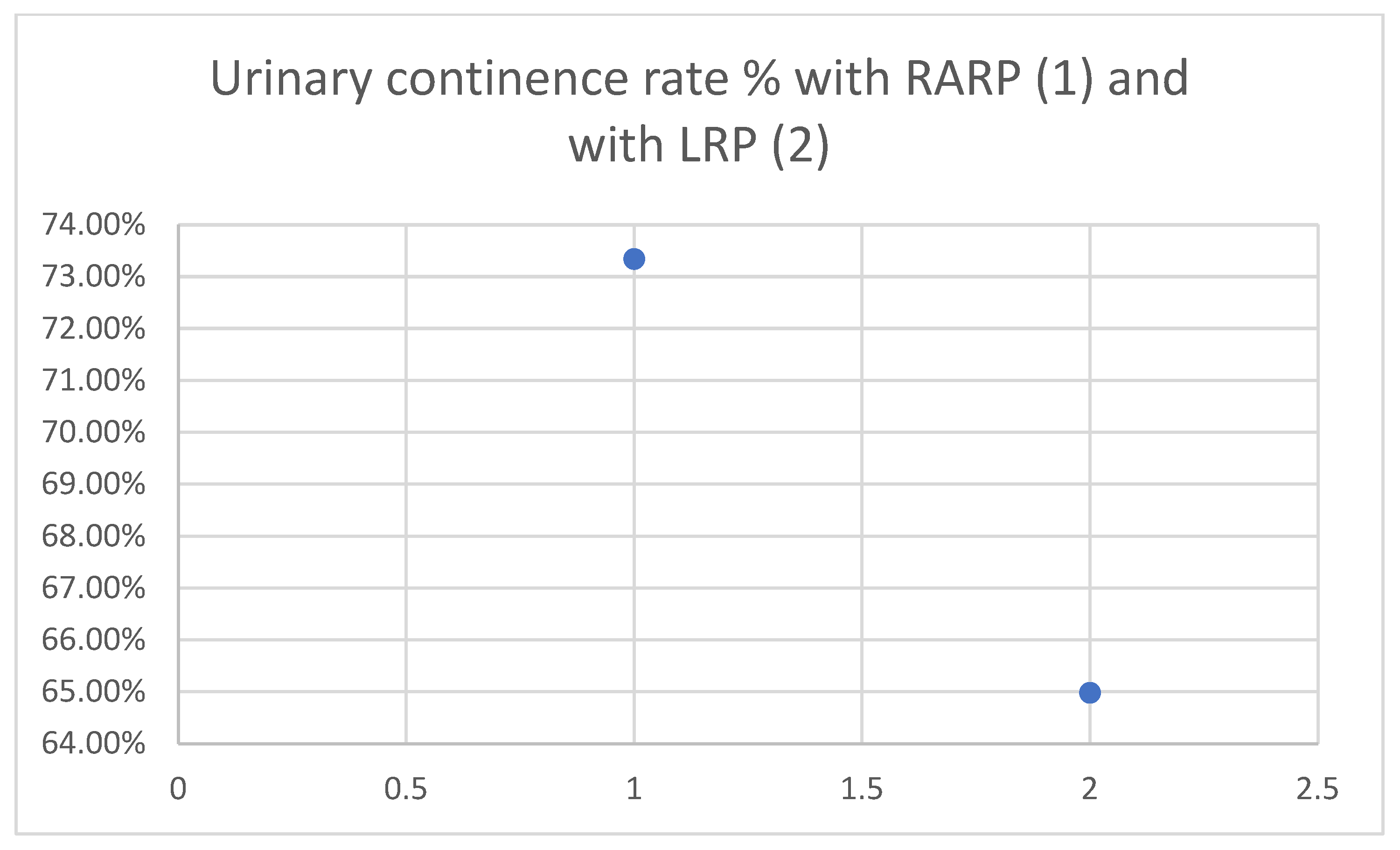
RARP is associated with improved urinary continence outcomes. As shown in
Figure 1, a national retrospective study reported better urinary function scores for RARP compared to LRP (mean EPIC score: 73.34 vs. 64.98;
p = 0.002), with a higher proportion of patients achieving continence [
8].
5. Nerve-Sparing RARP
Nerve-sparing (NS) RARP aims to preserve erectile function and urinary continence without compromising oncological control. Since the original description by Walsh and Donker in the early 1980s, NS techniques have evolved significantly with the advent of minimally invasive approaches, in particular RARP [
9].
The bilateral neurovascular bundles (NVBs), running posterolaterally to the prostate, are critical structures that must be meticulously preserved during surgery to maintain postoperative potency.
A careful preoperative assessment is essential for identifying candidates for NS RARP. Key considerations include preoperative erectile function (for example, using the International Index of Erectile Function), tumor characteristics (clinical stage, PSA, biopsy Gleason grade group), prostatic MRI (particularly concerning extraprostatic extension), and laterality of disease.
The robotic approach of NS has facilitated enhanced visualization and precision, supporting more refined NS techniques.
There are three main planes of NVB dissection:
Intrafascial: maximal preservation, suitable for low-risk disease with favorable anatomy;
Figure 2 shows an intrafascial dissection phase;
Interfascial: partial preservation, balances functional and oncological outcomes;
Figure 3 shows a frame during interfascial dissection;
Extrafascial: non-NS dissection, reserved for high risk or extracapsular disease [
10].
Figure 2 and
Figure 3.
High anterior release, Veil of Aphrodite technique, and incremental nerve-sparing grading systems (Tewari’s grades 1–4) are examples of refined techniques that allow customization based on tumor risk and anatomic variations [
11].
Oncologic safety is maintained in appropriately selected patients. Studies have shown that NS does not increase the risk of positive surgical margins when careful perioperative and intraoperative judgment is applied [
12].
6. Lateral Pelvic Fascia Preservation Technique
Growing anatomical insight into the multilayered lateral pelvic fascia (LPF)—rich in neurovascular and smooth muscle fibers—has led to targeted preservation techniques postulated to support continence and potency through retention of periurethral innervation [
13].
The lateral pelvic fascia comprises a fibrovascular multilayer covering the antero-lateral prostate capsule, harboring nerve fibers contributing to the membranous urethral sphincter and autonomic support structures [
13]. In practice, the technique involves intrafascial or interfascial dissection to leave the LPF intact (
Figure 2 and
Figure 3). A specific approach, the modified apical dissection with lateral fascia preservation (mod-RARP), avoids aggressive apical dissection and maintains periurethral tissue around the urethral stump, preserving the NVB and lateral fascia envelope [
14].
A retrospective comparison of lateral pelvic fascia preservation (LPFP) versus standard RARP (68 vs. 71 patients) demonstrated significantly faster continence recovery, with shorter time to continence, reduced operative time, and lower intraoperative blood loss; oncologic outcomes were similar between groups [
15].
A separate study of endopelvic fascia preservation vs. standard RARP in 138 patients reported 97.1% continence at 12 months in the preservation group vs. 88.4% in controls (
p < 0.05), and preservation was the only independent predictor in multivariate analysis [
16].
Another single-surgeon series comparing fascia-sparing PFS-RARP (n = 239) to standard RARP (n = 102) found time to full continence reduced from 261 to 91 days and a 66% reduced risk of incontinence (0–1 pad), with no significant difference in biochemical recurrence [
17].
Regarding sexual function, a feasibility series (35 potent men) undergoing prostatic fascia preservation reported that 97% retained erections sufficient for vaginal penetration at 12 months [
13].
Unlike previous evidence, a randomized controlled trial of 158 men at Tampere University Hospital, comparing endopelvic fascia preservation vs. standard RARP, reported no significant difference in urinary continence or sexual function at 12 months [
18].
So, applying intrafascial or interfascial that preserve lateral pelvic fascia and periurethral tissues can accelerate urinary continence recovery. Modified apical techniques further spare NVB and supporting fascia. Outcomes appear better in high-volume centers and in experienced hands, particularly for more refined LPF preservation techniques.
7. Posterior Reconstruction and Anterior Suspension Techniques
Posterior reconstruction involves approximating Denonivilliers’ fascia to the posterior aspect of the rhabdosphincter and/or anchoring to the bladder neck. This cranially repositioning of the urethral sphincter allows to improve pelvic support and reduces tension to the vesico-urethral anastomosis [
19].
Several studies report different surgical techniques for posterior reconstruction, with the aim of improving continence recovery.
Nguyen et al. reported continence at 3 days (34% vs. 3%) and 6 weeks (56% vs. 17%), favoring posterior reconstruction, with average restoration of membranous urethral length by 2 mm [
20].
By 3 and 12 months, differences tend to diminish, with many studies showing no long-term benefit compared to standard technique [
19].
Anterior suspension, following ligation of the dorsal venous complex, secures the rhabdosphincter to peri-pubic structures (pubic bone, cooper’s ligament) providing an anterior hammock support [
19].
Anterior suspension according to Patel demonstrated improved continence at 3 months (92.8% vs. 83%,
p = 0.013) vs. controls [
19].
This is a combination that reconstructs Denonvilliers’ fascia and the posterior bladder wall first, performs tension-free anastomosis, then suspends the bladder neck to arcus tendineus/puboprostatic plate with continuous suturing. This respects anatomical alignment and enhances both anterior and posterior support [
21].
Systematic reviews claim that total reconstruction shortens time to continence and increases early continence rates more than posterior or anterior technique alone, with comparable complication or positive-margin rates [
22].
A large volume but monocentric study reports a statistically significant better return to urinary continence, even in the long term (up to 48 months), in patients undergoing RARP with both anterior and posterior reconstructions compared to those undergoing RARP without any type of reconstruction [
23].
8. Bladder Neck Preservation Technique
The internal sphincter at the bladder neck plays a critical role in passive urinary continence. Bladder neck preservation (BNP) in RARP aims to retain the internal urethral sphincter and urothelial coaptation zone, potentially accelerating urinary continence recovery.
In a prospective, randomized, single-blind trial involving 208 men, full bladder neck preservation let to significantly lower urine loss at 3, 6, and 12 months, higher social continence rates (84.2% vs. 55.3% at 3 months, rising to 94.7% vs. 81.4% at 12 months; all
p < 0.05), and better quality of life scores. There was no significant difference in positive surgical margin rates between groups (12.5% vs. 14.7%,
p = 0.65) [
24].
A single-surgeon cohort of 233 RARP patients with bladder neck sparing showed a median blood loss of 75 mL, early catheter removal, and at six weeks, 69% were pad-free with quality of life improving significantly; surgical margin negativity stood at 85% [
25].
Meta-analytic data indicate that BNP accelerates continence recovery, especially within the first 3–6 months, without increasing margin positivity. While long-term (12 months) continence rates eventually converge, early benefit is clear [
26].
European association of urology (EAU) guidelines suggest that bladder neck preservation should be performed routinely when the cancer is distant from the base. However, bladder neck preservation cannot be performed in the presence of a large median lobe or a previous transurethral surgery procedure of the prostate [
2].
9. Oncological Efficacy
While RARP offers clear perioperative advantages, oncologic outcomes are paramount. Several large cohort studies, including data from the SEER-Medicare database, have shown that RARP achieves similar positive surgical margin (PSM) rates and long-term biochemical recurrence-free survival compared to ORP. A prospective by Yaxley et al. (2016) found no significant difference in oncologic outcomes between RARP and ORP at 24 months [
27].
Notably, in patients with higher-stage disease (pT3/4), RARP has been associated with a lower risk of PSA recurrence [
8].
A recent network meta-analysis encompassing 80 studies found that RARP had a significantly lower PSM rate compared to ORP (relative risks (RR) 0.893, 95% credible intervals (Crl) 0.807–0.985).
The same meta-analysis reported lower BCR rate for RARP compared to ORP (RR 0.713, 95% Crl 0.587–0.869) and LRP (RR 0.672, 95% Crl 0.505–0.895), indicating improved oncological control with RARP [
28].
10. Limitations and Learning Curve
Despite its advantages, RARP is not without limitations. High capital and maintenance costs may restrict its availability, particularly in low-resource settings. Additionally, outcomes are highly operator-dependent. Proficiency in RARP requires a steep learning curve, often estimated at 150–250 cases for consistent oncologic and functional outcomes [
29]. Moreover, the learning curve for RARP depends on surgical volume at both individual and institutional levels. High-volume institutions demonstrate significantly improved perioperative metrics (short operative time, estimated blood loss) and more favorable oncological outcomes [
30]. Low-volume centers often require more cases to reach comparable results; structured mentorship and previous laparoscopic experience can mitigate the disparity [
31].
11. Future Directions
Technological advancements, including image-guided surgery, augmented reality, and real-time intraoperative margin assessment, continue to evolve. Additionally, several manufacturers have developed new robotic platforms, and their diffusion may reduce costs and increase accessibility, but there is still a need for time and studies that demonstrate the comparable effectiveness of these new robotic platforms with the da Vinci Surgical system.
Between the various platforms that are gaining ground are Hugo RAS (by Medtronic) and Versius (by CMR).
Hugo RAS is a modular system that offers the ability to operate using up to four bogies per arm, an approach that can offer greater flexibility and adaptability.
Also, Versius is a modular system and features an open console that allows the surgeon to adopt an ergonomic position both while standing and sitting for the entire duration of the operation; this allows the operator to decide how he wants to work and helps reduce the physical effort required to perform an operation.
The meta-analysis of Chen SY et al. (2025) aggregates perioperative and early postoperative data, including four studies totaling 145 patients undergoing Versius-assisted RARP, and shows perioperative safety and oncologic validity comparable to conventional RARP benchmarks (e.g., those derived from da Vinci-based case series), at least in low-volume, early-stage cohorts [
32]. It showed a continence recovery by 3 months that appears favorable, aligning with current multiportal robotic surgery standards, and a positive surgical margins rate within expected ranges for RARP.
In addition, the study of Gavi F. et al. (2025) concluded that the Hugo RAS robotic system allows a successful and safe RARP, and that prior experience with robotic surgery, specifically the da Vinci system, positively influences perioperative outcomes in robotic-assisted radical prostatectomy [
33]. Indeed, surgeons with more extensive experience exhibited shorter operative times, reduced blood loss, a quicker recovery, and fewer complications.
Therefore, there is already evidence, but there is a need for further and extensive studies that allow these new platforms to be compared with the most widespread (da Vinci surgical system).
Another emerging minimally invasive surgical technique is Single-port robot-assisted radical prostatectomy (SP-RARP), using a single access incision. This da Vinci system obtained the Food and Drug Administration (FDA) approval in 2018 and is available in Europe from 2024 [
34]. Early clinical experiences suggest that SP-RARP is feasible and safe, with perioperative outcomes comparable to traditional multi-port approaches, while potentially offering benefits in reduced pain, shorter hospital stays, and improved cosmesis [
35]. However, limitations include a learning curve impacting initial operative time and margins, limited long-term data, and equipment cost [
36]. However, widespread adoption awaits more robust long-term data, cost-effectiveness assessments, and broader surgical proficiency. Technological enhancements and collaborative training programs will be key to optimizing outcomes.
12. Conclusions
RARP represents a safe and effective treatment for localized prostate cancer, offering excellent perioperative benefits, and at least an equivalent oncologic control to traditional approaches. These overall benefits position RARP as a favorable option for patients undergoing radical prostatectomy for localized prostate cancer. With ongoing innovations and surgeon training, RARP will likely continue to play a central role in the surgical management of prostate cancer.
Author Contributions
Conceptualization: M.R.; Methodology, M.R.; software, M.R.; validation, M.R. and A.C.; formal analysis, M.R.; investigation, M.R. and A.C.; resources, M.R., A.A., A.P. and A.C.; data curation, M.R.; writing—original draft preparation, M.R.; writing—review and editing, M.R. and A.C.; visualization, M.R. and A.C.; supervision, S.D.L., A.P. and A.C.; project administration, M.R.; funding acquisition, M.R., S.D.L., A.A., A.P. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received “exDM2004” funding from the Ministry of University and Research to cover publication costs.
Conflicts of Interest
None of the contributing authors have any conflict of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.
References
- Gandaglia, G.; Ploussard, G.; Valerio, M.; Mattei, A.; Fiori, C.; Fossati, N.; Stabile, A.; Beauval, J.-B.; Malavaud, B.; Roumiguié, M.; et al. A novel nomogram to identify candidates for extended pelvic lymph node dissection among patients with clinically localized prostate cancer diagnosed with magnetic resonance imaging-targeted and systematic biopsies. Eur. Urol. 2019, 75, 506514. [Google Scholar] [CrossRef] [PubMed]
- EAU. Guidelines on Prostate Cancer. 2025. Available online: http://www.uroweb.org (accessed on 3 September 2025).
- Donohue, J.F.; Bianco, F.J., Jr.; Kuroiwa, K.; Vickers, A.J.; Wheeler, T.M.; Scardino, P.T.; Reuter, V.A.; Eastham, J.A. Poorly differentiated prostate cancer treated with radical prostatectomy: Long-term outcome and incidence of pathological downgrading. J. Urol. 2006, 176, 991–995. [Google Scholar] [CrossRef]
- Ficarra, V.; Novara, G.; Rosen, R.C.; Artibani, W.; Carroll, P.R.; Costello, A.; Menon, M.; Montorsi, F.; Patel, V.R.; Stolzenburg, J.-U.; et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur. Urol. 2012, 62, 405–417. [Google Scholar] [CrossRef]
- Luzzago, S.; Rosiello, G.; Pecoraro, A.; Deuker, M.; Stolzenbach, F.; Mistretta, F.A.; Tian, Z.; Musi, G.; Montanari, E.; Shariat, S.F.; et al. Contemporary rates and predictors of open conversion during minimally invasive radical prostatectomy for nonmetastatic prostate cancer. J. Endourol. 2020, 34, 600–607. [Google Scholar] [CrossRef]
- Tewari, A.; Srivastava, A.; Sooriakumaran, P. Technique of nerve-sparing robotic prostatectomy. BJU Int. 2018, 121, 382–388. [Google Scholar]
- Lindenberg, M.; Retèl, V.; Kieffer, J.; Wijburg, C.; Fossion, L.; van der Poel, H.; van Harten, W. Long-term functional outcomes after robot-assisted prostatectomy compared to laparoscopic prostatectomy: Results from a national retrospective cluster study. Eur. J. Surg. Oncol. 2021, 47, 2658–2666. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yang, Z.; Qi, L.; Chen, M. Robot-assisted and laparoscopic vs open radical prostatectomy in clinically localized prostate cancer: Perioperative, functional and oncological outcomes: A systematic review and meta-analysis. Medicine 2019, 98, e15770. [Google Scholar] [CrossRef]
- Walsh, P.C.; Donker, P.J. Impotence following radical prostatectomy: Insight into etiology and prevention. J. Urol. 1982, 128, 492–497. [Google Scholar] [CrossRef]
- Gandaglia, G.; Briganti, A.; Suardi, N.; Gallina, A.; Cucchiara, V.; Vizziello, D.; Zaffuto, E.; Moschini, M.; Montorsi, F. Fascial layers in Nerve Sparing Robot-Assisted Radical Prostatectomy. Urol. Pract. 2014, 1, 86–91. [Google Scholar] [CrossRef]
- Tewari, A.K.; Srivastava, A.; Huang, M.W.; Robinson, B.D.; Shevchuk, M.M.; Durand, M.; Sooriakumaran, P.; Grover, S.; Yadav, R.; Mishra, N.; et al. Anatomical grades of nerve sparing: A risk-stratified approach to neural-hammock sparing during robot-assisted radical prostatectomy (RARP). BJU Int. 2011, 108 Pt 2, 984–992. [Google Scholar] [CrossRef]
- Montorsi, F.; Wilson, T.G.; Rosen, R.C.; Ahlering, T.E.; Artibani, W.; Carroll, P.R.; Costello, A.; Eastham, J.A.; Ficarra, V.; Guazzoni, G.; et al. Best practices in robot-assisted radical prostatectomy: Recommendations of the Pasedana Consensus Panel. Eur. Urol. 2012, 62, 368–381. [Google Scholar] [CrossRef]
- Kaul, S.; Bhandari, A.; Hemal, A.; Savera, A.; Shrivastava, A.; Menon, M. Robotic radical prostatectomy with preservation of the prostatic fascia: A feasibility study. Urology 2005, 66, 1261–1265. [Google Scholar] [CrossRef]
- Moschovas, M.C.; Bhat, S.; Onol, F.F.; Rogers, T.; Roof, S.; Mazzone, E.; Mottrie, A.; Patel, V. Modified apical dissection and lateral prostatic fascia preservation improves early postoperative functional recovery in robotic-assisted laparoscopic radical prostatectomy: Results from a propensity score-matched analysis. Eur. Urol. 2020, 78, 875–884. [Google Scholar] [CrossRef]
- Shiota, M.; Tsukahara, S.; Ueda, S.; Mutaguchi, J.; Goto, S.; Kobayashi, S.; Matsumoto, T.; Blas, L.; Monji, K.; Inokuchi, J.; et al. Improved urinary continence recovery after robot-assisted radical prostatectomy with lateral pelvic fascia preservation. J. Robot. Surg. 2023, 17, 2721–2728. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Lee, J.N.; Kim, H.T.; Kim, T.-H.; Kim, B.W.; Choi, G.-S.; Kwon, T.G. Endoplevic fascia prservation during robot-assisted laparoscopic radical prostatectomy: Does it affect urinary incontinence? Scand. J. Urol. 2014, 48, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Dall, C.P.; Mason, J.B.; Choudhury, E.; Mora-Garijo, B.; Egan, J.; Hu, J.C.; Kowalczyk, K.J. Long-term outcomes of pelvic-fascia sparing robotic-assisted radical prostatectomy versus standard technique: Superior urinary function and quality of life without compromising oncologic efficacy in a single-surgeon series. Urol. Oncol. 2024, 42, 67.e17–67.e24. [Google Scholar] [CrossRef] [PubMed]
- Siltari, A.; Riikonen, J.; Murtola, T.J. Preservation of endopelvic fascia: Effects on postoperative incontinence and sexual function—A randomized clinical trial. J. Sex. Med. 2021, 18, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Katsimperis, S.; Juliebø-Jones, P.J.-J.; Ta, A.T.; Tandogdu, Z.T.; Al-Bermani, O.A.-B.; Bellos, T.B.; Esperto, F.E.; Tonyali, S.T.; Mitsogiannis, I.M.; Skolarikos, A.S.; et al. Surgical techniques to preserve continence after robot-assisted radical prostatectomy. Front. Surg. 2023, 10, 1289765. [Google Scholar] [CrossRef] [PubMed]
- Coelho, R.F.; Chauhan, S.; Orvieto, M.A.; Sivaraman, A.; Palmer, K.J.; Coughlin, G.; Patel, V.R. Influence of modified posterior reconstruction of the rhabdosphincter on early recovery of continence and anastomotic leakage rates after robot-assisted radical prostatectomy. Eur. Urol. 2011, 59, 72–80. [Google Scholar] [CrossRef]
- Dovey, Z.S.; Tewari, A.K. Anatomical robotic prostatectomy: Technical factors to achive superb continence and erectile function. Transl. Androl. Urol. 2020, 9, 887–897. [Google Scholar] [CrossRef]
- Vis, A.N.; van der Poel, H.G.; Ruiter, A.E.; Hu, J.C.; Tewari, A.K.; Rocco, B.; Patel, V.R.; Razdan, S.; Nieuwenhuijzen, J.A. Posterior, anterior, and periurethral surgical reconstruction of urinary continence mechanisms in robot-assisted radical prostatectomy: A description and video compilation of commonly performed surgical techniques. Eur. Urol. 2019, 76, 814–822. [Google Scholar] [CrossRef]
- Rinaldi, M.; Porreca, A.; Di Lena, S.; Di Gianfrancesco, L.; Zazzara, M.; Scarcia, M.; Ludovico, G.M. A matched-analysis on short-term and long-term (up to 5 years of follow-up) urinary incontinence outcomes after robot-assisted radical prostatectomy with and without anterior and posterior reconstruction: Data on 1358 patients. Int. Urol. Nephrol. 2024, 56, 121–127. [Google Scholar] [CrossRef]
- Nyarangi-Dix, J.N.; Radtke, J.P.; Hadaschik, B.; Pahernik, S.; Hohenfellner, M. Impact of complete bladder neck preservation on urinary continence, quality of life and surgical margins after radical prostatectomy: A randomized, controlled, single blind trial. J. Urol. 2013, 189, 891–898. [Google Scholar] [CrossRef]
- Xiao, G.; Motoo, A.; Carson, W. Continence outcomes after bladder neck preservation during robot-assisted laparoscopic prostatectomy (RALP). Minim. Invasive Ther. Allied Technol. 2015, 24, 364–371. [Google Scholar] [CrossRef]
- Ma, X.; Tang, K.; Yang, C.; Wu, G.; Xu, N.; Wang, M.; Zeng, X.; Hu, Z.; Song, R.; Yuh, B.; et al. Bladder neck preservation improves time to continence after radical prostatectomy: A systematic review and meta-analysis. Oncotarget 2016, 7, 67463–67475. [Google Scholar] [CrossRef] [PubMed]
- Yaxley, J.W.; Coughlin, G.D.; Chambers, S.K.; Occhipinti, S.; Samaratunga, H.; Zajdlewicz, L.; Dunglison, N.; Carter, R.; Payton, D.J.; Williams, S.; et al. Robot-assisted laparoscopic prostatectomy vs open radical retropubic prostatectomy: Early outcomes from a randomized controlled phase 3 study. Lancet 2016, 388, 1057–1066. [Google Scholar] [CrossRef]
- Kim, D.K.; Moon, Y.J.; Chung, D.Y.; Jung, H.D.; Jeon, S.H.; Kang, S.H.; Paick, S.; Korean Society of Endourology and Robotics (KSER) Research Committee. Comparison of Robot-Assisted, Laparoscopic, and Open radical Prostatectomy Outcomes: A Systematic review and network meta-analysis from KSER update series. Medicina 2025, 61, 61. [Google Scholar] [CrossRef]
- Gandi, C.; Totaro, A.; Bientinesi, R.; Marino, F.; Pierconti, F.; Martini, M.; Russo, A.; Racioppi, M.; Bassi, P.; Sacco, E. A multi-surgeon learning curve analysis of overall and site-specific positive surgical margins after RARP and implications for training. J. Robot. Surg. 2022, 16, 1451–1461. [Google Scholar] [CrossRef]
- Perera, S.; Fernando, N.; O’Brien, J.; Murphy, D.; Lawrentschuk, N. Robotic-assisted radical prostatectomy: Learning curves and outcomes from an Australian perspective. Prostate Int. 2022, 11, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Tobias-Machado, M.; Mitre, A.I.; Rubinstein, M.; da Costa, E.F.; Hidaka, A.K. Robotic-assisted radical prostatectomy learningcurve for experienced laparoscopic surgeons: Does it really exist? Int. Braz. J. Urol. 2016, 42, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Ma, Y.; Yang, J.-W.; Liu, H.; Wang, L.; Li, X.-R. Safety and functional outcomes of Versius surgical platform in prostate cancer radical prostatectomy: A single arm meta-analysis. J. Robot. Surg. 2025, 19, 284. [Google Scholar] [CrossRef]
- Gavi, F.; Sighinolfi, M.C.; Fettucciari, D.; Carerj, C.; Marino, F.; Panio, E.; Russo, P.; Foschi, N.; Bientinesi, R.; Gandi, C.; et al. Perioperative, Impact of prior robotic surgical expertise on the results of Hugo RAS radical prostatectomy: A propensity score-matched comparison between Da Vinci-expert and non-Da Vinci-expert surgeons. World J. Urol. 2025, 43, 236. [Google Scholar] [CrossRef] [PubMed]
- Katsimperis, S.; Tzelves, L.; Feretzakis, G.; Bellos, T.; Douroumis, K.; Kostakopoulos, N.; Skolarikos, A. Single-port vs. Multi-port robotic surgery in urologic oncology: A comparative analysis of current evidence and future directions. Cancers 2025, 17, 2847. [Google Scholar] [CrossRef] [PubMed]
- Checcucci, E.; De Cillis, S.; Pecoraro, A.; Peretti, D.; Volpi, G.; Amparore, D.; Piramide, F.; Piana, A.; Manfredi, M.; Fiori, C.; et al. Single-port robot-assisted radical prostatectomy: A systematic review and pooled analysis of the preliminary experiences. BJU Int. 2020, 126, 55–64. [Google Scholar] [CrossRef]
- Lai, A.; Dobbs, R.W.; Talamini, S.; Halgrimson, W.R.; Wilson, J.O.; Vigneswaran, H.T.; Crivellaro, S. Single port robotic radical prostatectomy: A systematic review. Transl. Androl. Urol. 2020, 9, 898–905. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).