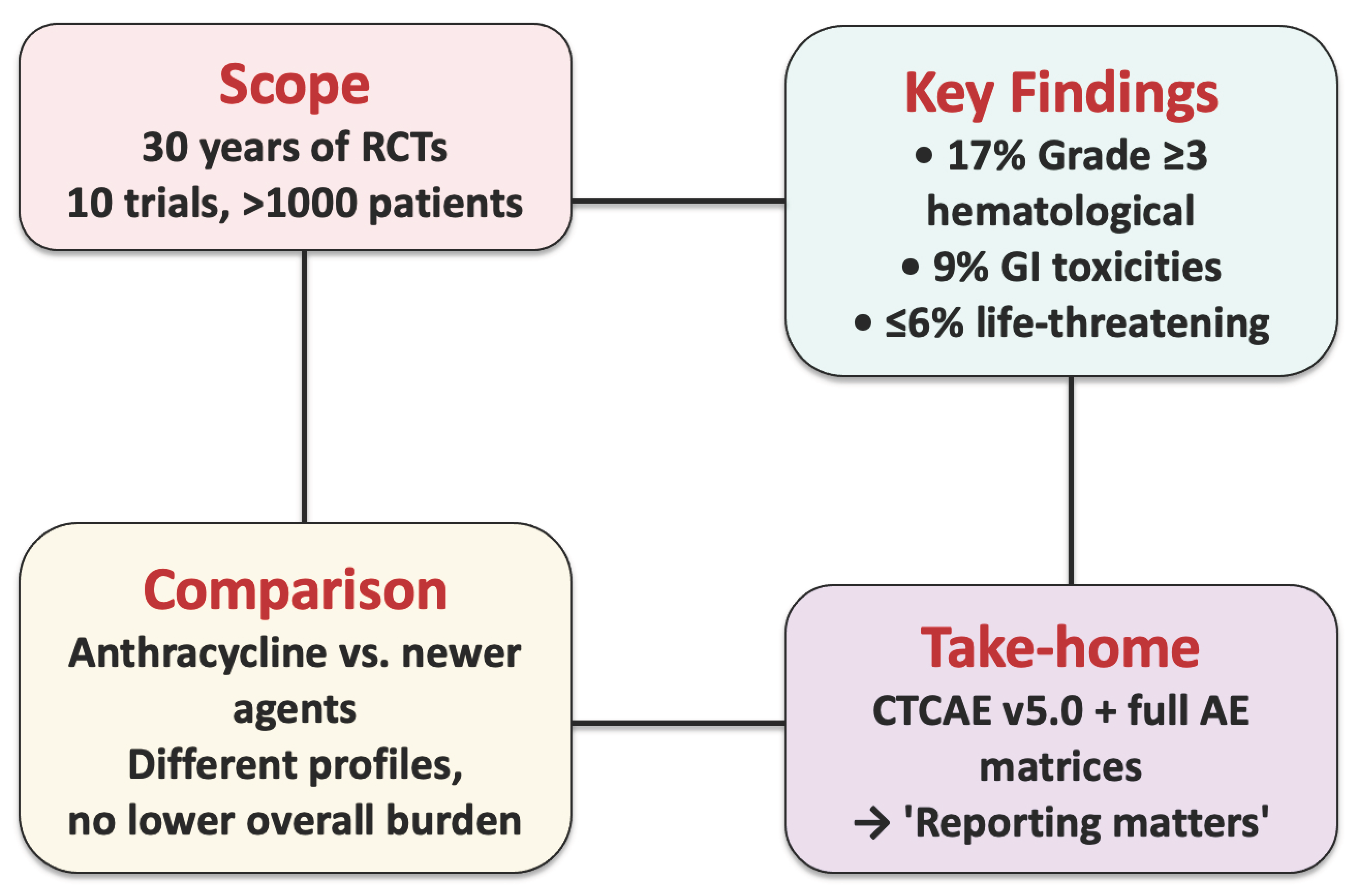
Reporting Matters: Severe Adverse Events in Soft Tissue Sarcoma Therapy—A 30-Year Systematic Review of Placebo- and Non-Systemic-Controlled Randomized Trials
Simple Summary
Abstract
1. Introduction
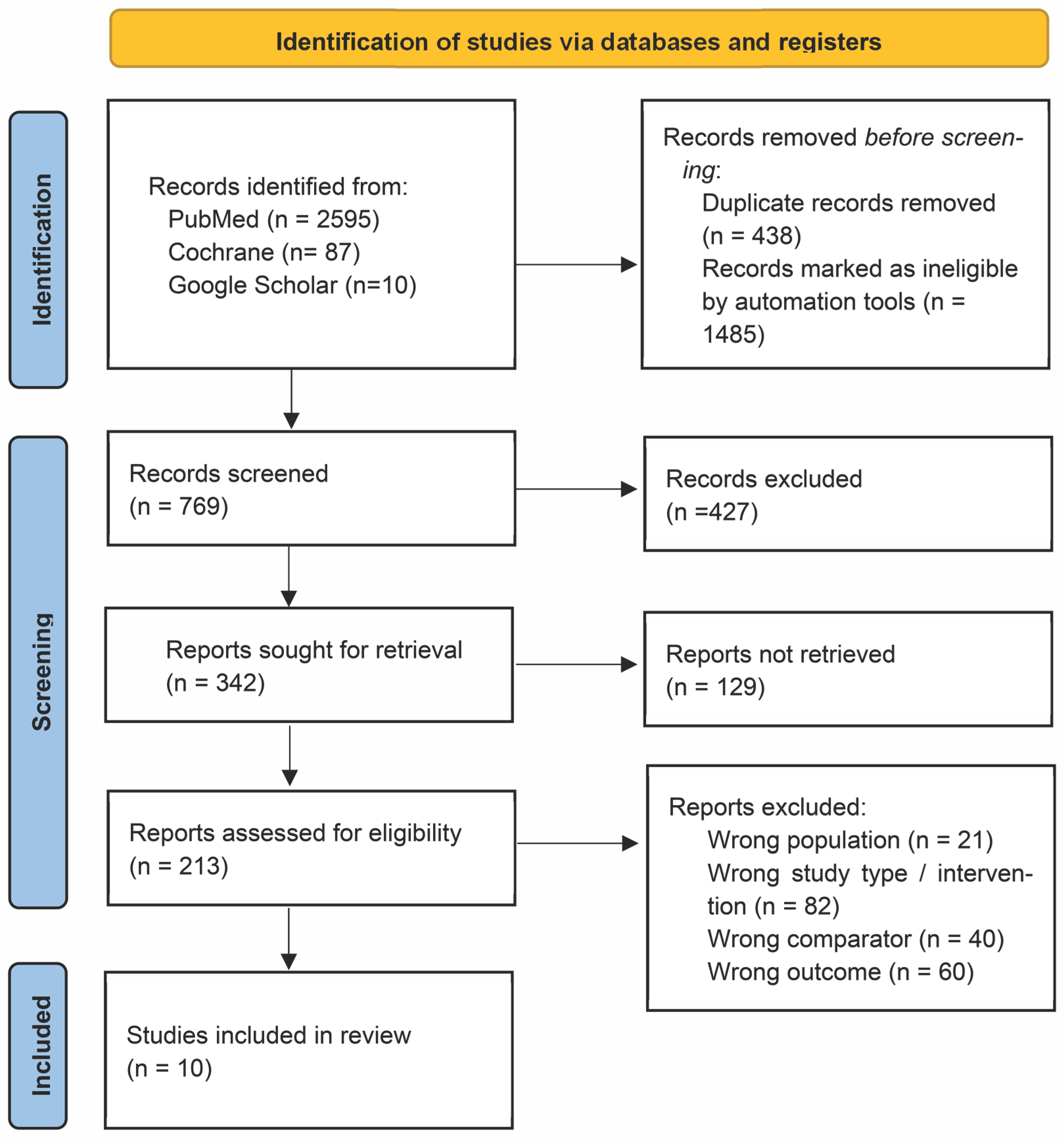
2. Materials and Methods
- Patient/Population: patients with histologically confirmed soft tissue sarcoma (STS) of any subtype or age.
- Intervention: systemic therapy (e.g., cytotoxic chemotherapy, kinase inhibitors, or immune checkpoint inhibitors) administered in the adjuvant, neoadjuvant, or palliative setting.
- Comparison: no systemic therapy, i.e., surgery ± radiotherapy, best supportive care, or placebo.
- Outcome: incidences of grade ≥ 3 adverse events (AEs); secondary outcomes: overall survival and treatment-related mortality.
- Study type: randomized controlled trials.
3. Results
3.1. CTCAE Categories and Denominator Definitions
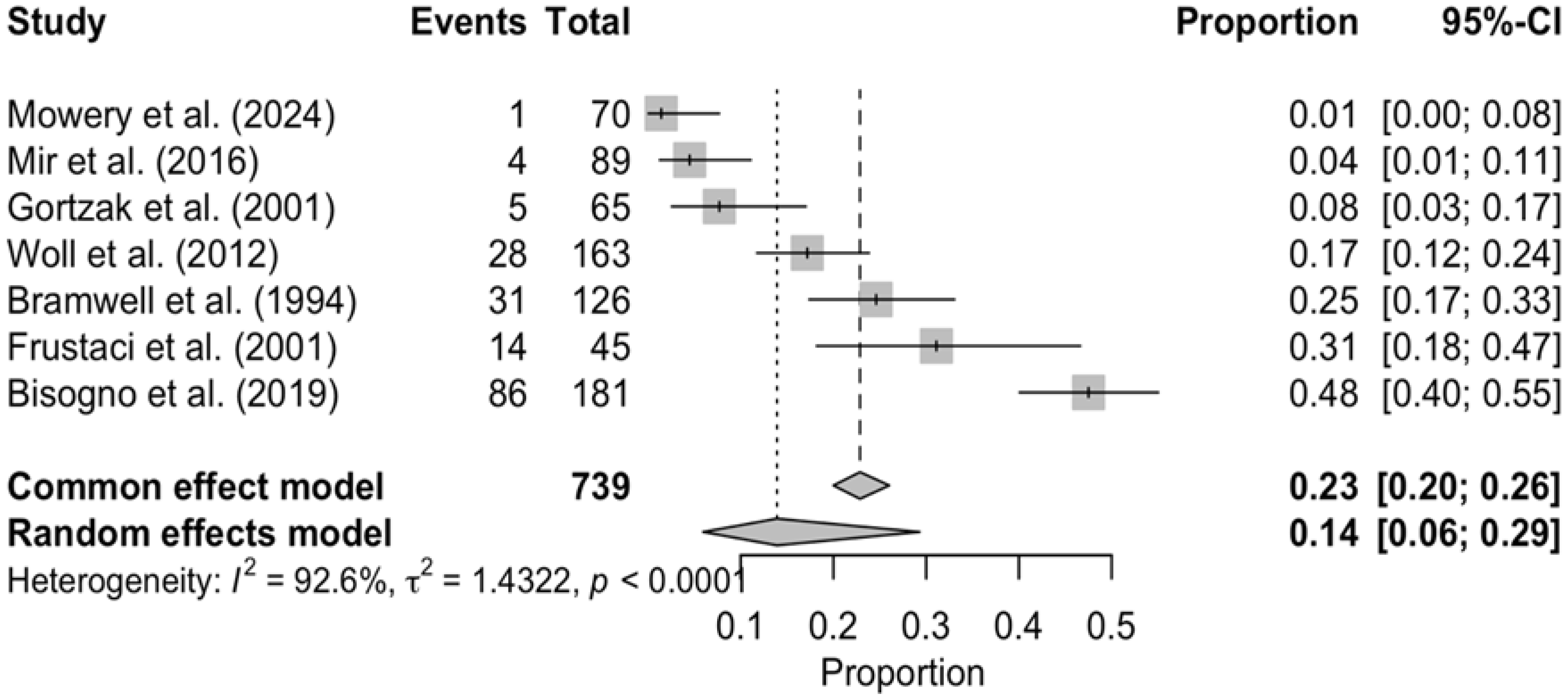
3.2. Frequency of CTCAE Grade 3–4 Adverse Events Across Trials
3.3. Toxicity-Related Deaths and Treatment Discontinuation
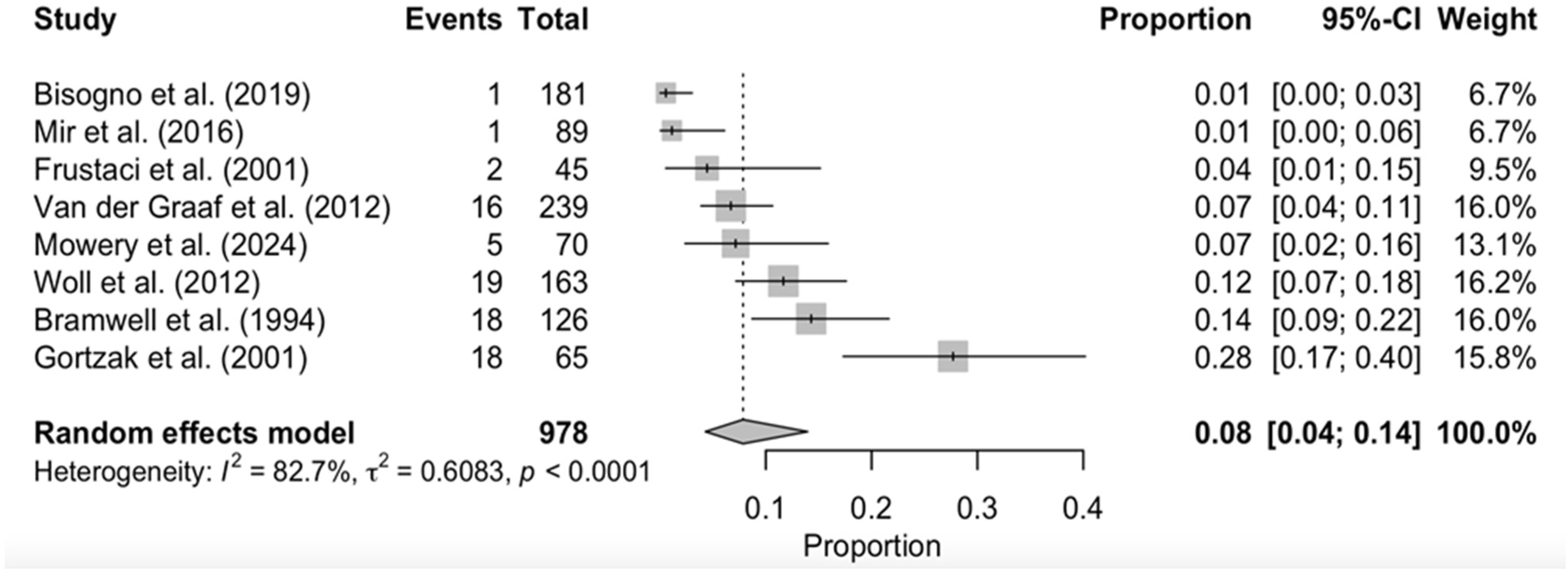
3.4. Persisting/Late-Term Toxicities
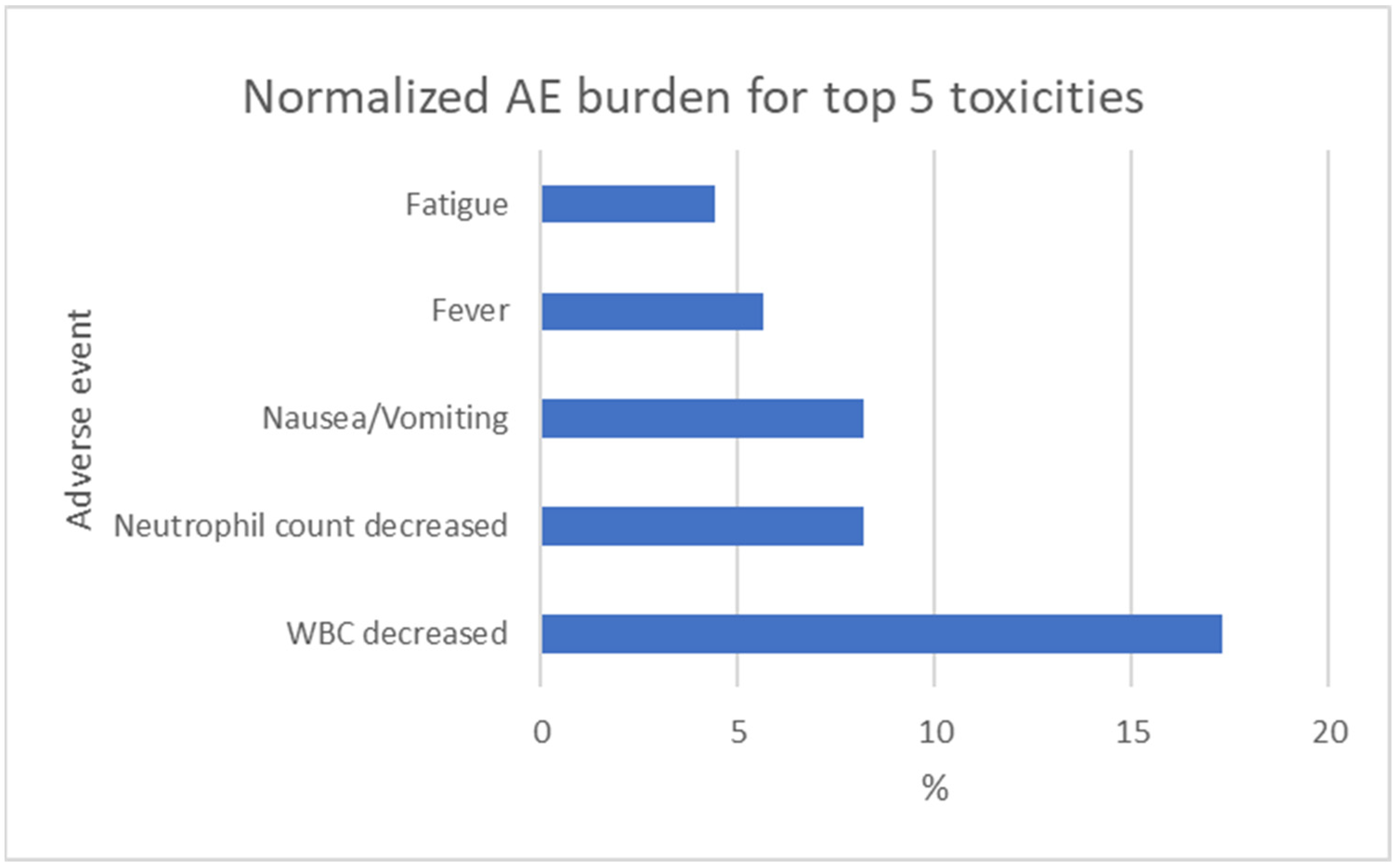
3.5. Normalized Grade 3 Toxicity Burden
3.6. Treatment-Versus-Control Comparison of Toxicities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| STS | Soft tissue sarcoma |
| RCT | Randomized controlled trial |
| AE | Adverse event |
| CTCAE | Common terminology criteria for adverse events |
| MFS | Metastatic free survival |
| PFS | Progression free survival |
| OS | Overall survival |
| HR | Hazard ratio |
References
- Hui, J.Y. Epidemiology and Etiology of Sarcomas. Surg. Clin. N. Am. 2016, 96, 901–914. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Stiller, C.A.; Trama, A.; Serraino, D.; Rossi, S.; Navarro, C.; Chirlaque, M.D.; Casali, P.G.; Group, R.W. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur. J. Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef]
- Chen, C.; Wang, C.; Li, S.; Zheng, X.; Yang, Y. Global, regional, and national burden of soft tissue and extraosseous sarcomas from 1990 to 2021. Prev. Med. Rep. 2024, 47, 102903. [Google Scholar] [CrossRef]
- Bourcier, K.; Le Cesne, A.; Tselikas, L.; Adam, J.; Mir, O.; Honore, C.; de Baere, T. Basic Knowledge in Soft Tissue Sarcoma. Cardiovasc. Intervent. Radiol. 2019, 42, 1255–1261. [Google Scholar] [CrossRef]
- Tierney, J.F.; Mosseri, V.; Stewart, L.A.; Souhami, R.L.; Parmar, M.K. Adjuvant chemotherapy for soft-tissue sarcoma: Review and meta-analysis of the published results of randomised clinical trials. Br. J. Cancer 1995, 72, 469–475. [Google Scholar] [CrossRef]
- Ratan, R.; Patel, S.R. Chemotherapy for soft tissue sarcoma. Cancer 2016, 122, 2952–2960. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Xu, G.; Zhao, L.; Zhang, T. Effect of anthracyclines/ifosfamide-based adjuvant chemotherapy for soft tissue sarcoma: A conventional and network Meta-analysis. J. Chemother. 2021, 33, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Tap, W.D.; Wagner, A.J.; Schoffski, P.; Martin-Broto, J.; Krarup-Hansen, A.; Ganjoo, K.N.; Yen, C.C.; Abdul Razak, A.R.; Spira, A.; Kawai, A.; et al. Effect of Doxorubicin Plus Olaratumab vs Doxorubicin Plus Placebo on Survival in Patients With Advanced Soft Tissue Sarcomas: The ANNOUNCE Randomized Clinical Trial. JAMA 2020, 323, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Le Cesne, A.; Ouali, M.; Leahy, M.G.; Santoro, A.; Hoekstra, H.J.; Hohenberger, P.; Van Coevorden, F.; Rutkowski, P.; Van Hoesel, R.; Verweij, J.; et al. Doxorubicin-based adjuvant chemotherapy in soft tissue sarcoma: Pooled analysis of two STBSG-EORTC phase III clinical trials. Ann. Oncol. 2014, 25, 2425–2432. [Google Scholar] [CrossRef]
- van der Graaf, W.T.; Blay, J.Y.; Chawla, S.P.; Kim, D.W.; Bui-Nguyen, B.; Casali, P.G.; Schoffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Braik, D.; Lemieux, C.; Wilson, B.E.; Salawu, A.; Abdul Razak, A.R. Clinical benefit and fragility evaluation of systemic therapy trials for advanced soft tissue sarcoma. Cancer 2025, 131, e35564. [Google Scholar] [CrossRef] [PubMed]
- Haas, R.L.; Gronchi, A.; van de Sande, M.A.J.; Baldini, E.H.; Gelderblom, H.; Messiou, C.; Wardelmann, E.; Le Cesne, A. Perioperative Management of Extremity Soft Tissue Sarcomas. J. Clin. Oncol. 2018, 36, 118–124. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Mir, O.; Brodowicz, T.; Italiano, A.; Wallet, J.; Blay, J.Y.; Bertucci, F.; Chevreau, C.; Piperno-Neumann, S.; Bompas, E.; Salas, S.; et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1732–1742. [Google Scholar] [CrossRef]
- Colosia, A.; Khan, S.; Hackshaw, M.D.; Oglesby, A.; Kaye, J.A.; Skolnik, J.M. A Systematic Literature Review of Adverse Events Associated with Systemic Treatments Used in Advanced Soft Tissue Sarcoma. Sarcoma 2016, 2016, 3597609. [Google Scholar] [CrossRef]
- Woll, P.J.; Reichardt, P.; Le Cesne, A.; Bonvalot, S.; Azzarelli, A.; Hoekstra, H.J.; Leahy, M.; Van Coevorden, F.; Verweij, J.; Hogendoorn, P.C.; et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): A multicentre randomised controlled trial. Lancet Oncol. 2012, 13, 1045–1054. [Google Scholar] [CrossRef]
- Frustaci, S.; Gherlinzoni, F.; De Paoli, A.; Bonetti, M.; Azzarelli, A.; Comandone, A.; Olmi, P.; Buonadonna, A.; Pignatti, G.; Barbieri, E.; et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: Results of the Italian randomized cooperative trial. J. Clin. Oncol. 2001, 19, 1238–1247. [Google Scholar] [CrossRef]
- Mowery, Y.M.; Ballman, K.V.; Hong, A.M.; Schuetze, S.M.; Wagner, A.J.; Monga, V.; Heise, R.S.; Attia, S.; Choy, E.; Burgess, M.A.; et al. Safety and efficacy of pembrolizumab, radiation therapy, and surgery versus radiation therapy and surgery for stage III soft tissue sarcoma of the extremity (SU2C-SARC032): An open-label, randomised clinical trial. Lancet 2024, 404, 2053–2064. [Google Scholar] [CrossRef]
- Gortzak, E.; Azzarelli, A.; Buesa, J.; Bramwell, V.H.; van Coevorden, F.; van Geel, A.N.; Ezzat, A.; Santoro, A.; Oosterhuis, J.W.; van Glabbeke, M.; et al. A randomised phase II study on neo-adjuvant chemotherapy for ’high-risk’ adult soft-tissue sarcoma. Eur. J. Cancer 2001, 37, 1096–1103. [Google Scholar] [CrossRef]
- Bramwell, V.; Rouesse, J.; Steward, W.; Santoro, A.; Schraffordt-Koops, H.; Buesa, J.; Ruka, W.; Priario, J.; Wagener, T.; Burgers, M.; et al. Adjuvant CYVADIC chemotherapy for adult soft tissue sarcoma--reduced local recurrence but no improvement in survival: A study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J. Clin. Oncol. 1994, 12, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Fakhrai, N.; Ebm, C.; Kostler, W.J.; Jantsch, M.; Abdolvahab, F.; Dominkus, M.; Pokrajac, B.; Kauer-Dorner, D.; Zielinski, C.C.; Brodowicz, T.; et al. Intensified adjuvant IFADIC chemotherapy in combination with radiotherapy versus radiotherapy alone for soft tissue sarcoma: Long-term follow-up of a prospective randomized feasibility trial. Wien. Klin. Wochenschr. 2010, 122, 614–619. [Google Scholar] [CrossRef]
- Lerner, H.J.; Amato, D.A.; Savlov, E.D.; DeWys, W.D.; Mittleman, A.; Urtasun, R.C.; Sobel, S.; Shiraki, M. Eastern Cooperative Oncology Group: A comparison of adjuvant doxorubicin and observation for patients with localized soft tissue sarcoma. J. Clin. Oncol. 1987, 5, 613–617. [Google Scholar] [CrossRef]
- Bisogno, G.; De Salvo, G.L.; Bergeron, C.; Gallego Melcon, S.; Merks, J.H.; Kelsey, A.; Martelli, H.; Minard-Colin, V.; Orbach, D.; Glosli, H.; et al. Vinorelbine and continuous low-dose cyclophosphamide as maintenance chemotherapy in patients with high-risk rhabdomyosarcoma (RMS 2005): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019, 20, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H.; Eriksson, M.; Sundby Hall, K.; Hartmann, J.T.; Pink, D.; Schutte, J.; Ramadori, G.; Hohenberger, P.; Duyster, J.; Al-Batran, S.E.; et al. One vs. three years of adjuvant imatinib for operable gastrointestinal stromal tumor: A randomized trial. JAMA 2012, 307, 1265–1272. [Google Scholar] [CrossRef]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schoffski, P.; Blay, J.Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Istl, A.C.; Ruck, J.M.; Morris, C.D.; Levin, A.S.; Meyer, C.F.; Johnston, F.M. Call for improved design and reporting in soft tissue sarcoma studies: A systematic review and meta-analysis of chemotherapy and survival outcomes in resectable STS. J. Surg. Oncol. 2019, 119, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Goh, M.H.; Gonzalez, M.R.; Heiling, H.M.; Mazzola, E.; Connolly, J.J.; Choy, E.; Cote, G.M.; Spentzos, D.; Lozano-Calderon, S.A. Adjuvant chemotherapy in localized, resectable extremity and truncal soft tissue sarcoma and survival outcomes—A systematic review and meta-analysis of randomized controlled trials. Cancer 2025, 131, e35792. [Google Scholar] [CrossRef]
- Cooper, K.L.; Madan, J.; Whyte, S.; Stevenson, M.D.; Akehurst, R.L. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: Systematic review and meta-analysis. BMC Cancer 2011, 11, 404. [Google Scholar] [CrossRef]
- Herrstedt, J.; Clark-Snow, R.; Ruhlmann, C.H.; Molassiotis, A.; Olver, I.; Rapoport, B.L.; Aapro, M.; Dennis, K.; Hesketh, P.J.; Navari, R.M.; et al. 2023 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting. ESMO Open 2024, 9, 102195. [Google Scholar] [CrossRef]
- Atkinson, T.M.; Ryan, S.J.; Bennett, A.V.; Stover, A.M.; Saracino, R.M.; Rogak, L.J.; Jewell, S.T.; Matsoukas, K.; Li, Y.; Basch, E. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): A systematic review. Support. Care Cancer 2016, 24, 3669–3676. [Google Scholar] [CrossRef]
- Bonvalot, S.; Gronchi, A.; Le Pechoux, C.; Swallow, C.J.; Strauss, D.; Meeus, P.; van Coevorden, F.; Stoldt, S.; Stoeckle, E.; Rutkowski, P.; et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1366–1377. [Google Scholar] [CrossRef]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C.; et al. Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients With Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef]
- Berclaz, L.M.; Jurinovic, V.; Burkhard-Meier, A.; Abdel-Rahman, S.; Albertsmeier, M.; Klein, A.; Durr, H.R.; Schmidt-Hegemann, N.S.; Knosel, T.; Kunz, W.G.; et al. Doxorubicin Plus Dacarbazine Versus Doxorubicin Plus Ifosfamide in Combination With Regional Hyperthermia in Patients With Advanced Leiomyosarcoma: A Propensity Score-Matched Analysis. Cancer Med. 2025, 14, e70655. [Google Scholar] [CrossRef] [PubMed]
- Alvegard, T.A.; Sigurdsson, H.; Mouridsen, H.; Solheim, O.; Unsgaard, B.; Ringborg, U.; Dahl, O.; Nordentoft, A.M.; Blomqvist, C.; Rydholm, A.; et al. Adjuvant chemotherapy with doxorubicin in high-grade soft tissue sarcoma: A randomized trial of the Scandinavian Sarcoma Group. J. Clin. Oncol. 1989, 7, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Palmerini, E.; Quagliuolo, V.; Martin Broto, J.; Lopez Pousa, A.; Grignani, G.; Brunello, A.; Blay, J.Y.; Tendero, O.; Diaz Beveridge, R.; et al. Neoadjuvant Chemotherapy in High-Risk Soft Tissue Sarcomas: Final Results of a Randomized Trial From Italian (ISG), Spanish (GEIS), French (FSG), and Polish (PSG) Sarcoma Groups. J. Clin. Oncol. 2020, 38, 2178–2186. [Google Scholar] [CrossRef]
- Pratt, C.B.; Pappo, A.S.; Gieser, P.; Jenkins, J.J.; Salzbergdagger, A.; Neff, J.; Rao, B.; Green, D.; Thomas, P.; Marcus, R.; et al. Role of adjuvant chemotherapy in the treatment of surgically resected pediatric nonrhabdomyosarcomatous soft tissue sarcomas: A Pediatric Oncology Group Study. J. Clin. Oncol. 1999, 17, 1219. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.R.; Chen, Y.L.; Scharschmidt, T.J.; Xue, W.; Gao, Z.; Black, J.O.; Choy, E.; Davis, J.L.; Fanburg-Smith, J.C.; Kao, S.C.; et al. Outcomes After Preoperative Chemoradiation With or Without Pazopanib in Non-Rhabdomyosarcoma Soft Tissue Sarcoma: A Report From Children’s Oncology Group and NRG Oncology. J. Clin. Oncol. 2023, 41, 4842–4848. [Google Scholar] [CrossRef]
- Eilber, F.R.; Giuliano, A.E.; Huth, J.F.; Morton, D.L. A randomized prospective trial using postoperative adjuvant chemotherapy (adriamycin) in high-grade extremity soft-tissue sarcoma. Am. J. Clin. Oncol. 1988, 11, 39–45. [Google Scholar] [CrossRef]




| Study Title or Registration Number | Trial Type | Setting | Chemotherapy | Dose or Schedule | Patient Count (Treatment/Control) | Median Follow-Up, Months | Evaluated Outcomes |
|---|---|---|---|---|---|---|---|
| Pazopanib for metastatic soft-tissue sarcoma (PALETTE) [11] | Randomized, double-blind, placebo-controlled phase 3 trial | Adjuvant | Pazopanib | 246/123 | 14.9 | PFS, OS, response rate, safety and quality of life | |
| EORTC 62931 [17] | Multicenter randomized controlled trial | Adjuvant | Doxorubicin, Ifosfamide, Lenograstim | 800 mg daily | 175/176 | 95.88 | PFS, OS, toxic effects |
| Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles [18] | Randomized cooperative trial | Adjuvant | Epidoxorubicin, Ifosfamide | Five cycles of doxorubicin 75 mg/m2, ifosfamide 5 g/m2, and lenograstim every 3 weeks | 53/51 | 59 | DFS, OS, toxicity |
| SU2C-SARC032 [19] | Randomized clinical trial | Neoadjuvant | Pembrolizumab | 5 cycles of 4′-epidoxorubicin 60 mg/m2 days 1 and 2 and ifosfamide 1.8 g/m2 days 1 through 5, with hydration, mesna, and granulocyte colony-stimulating factor | 64/63 | 43 | DFS, OS, grade 3 or higher adverse events |
| Neo-adjuvant chemotherapy for ‘high-risk’ adult soft tissue sarcoma [20] | Randomized phase II study | Neoadjuvant | Doxorubicin and Ifosfamide | 200 mg i.v. every 3 weeks | 67/67 | 88 | Estimated 5-year DFS, 5-year OS, toxicity |
| Adjuvant CYVADIC chemotherapy for adult soft tissue sarcoma [21] | Randomized controlled trial | Adjuvant | Cyclophosphamide, Vincristine, Doxorubicin, Dacarbazine | 3 cycles of 3-weekly doxorubicin 50 mg/m2 intravenous (i.v.) bolus and ifosfamide 5 g/m2 (24 h infusion) | 145/172 | 80 | RFS, OS, local recurrence, distant metastases, toxicity |
| Intensified adjuvant IFADIC chemotherapy in combination with radiotherapy versus radiotherapy alone for soft tissue sarcoma: long-term follow-up of a prospective randomized feasibility trial [22] | Randomized controlled trial | Adjuvant | Ifosfamide, Dacarbazin, Doxorubicin | Ifosfamide (1500 mg/m2, days 1–4), DTIC (200 mg/m2, days 1–4) and doxorubicin (25 mg/m2, days 1 and 2) i.v., in a 14-day cycle. | 31/28 | 97 | RFS, time to local failure, time to distant failure, OS |
| Eastern Cooperative Oncology Group: a comparison of adjuvant doxorubicin and observation for patients with localized soft tissue sarcoma [23] | Randomized controlled trial | Adjuvant | Doxorubicin | 70 mg/m2 (slow push, every 3 weeks for seven courses for a maximum of 550 mg/m2) | 17/13 | 30 | DFS, OS |
| Vinorelbine and continuous low-dose cyclophosphamide in patients with high-risk rhabdomyosarcoma [24] | Multicenter, open-label, randomized, phase 3 trial | Maintenance Therapy | Vinorelbine, Cyclophosphamide | Cyclophosphamide 500 mg/m2 intravenously (i.v.) bolus on day 1, vincristine 1.4 mg/m2 i.v. bolus on day 1, doxorubicin (Adriamycin; Adria Laboratories, Columbus, OH, USA) 50 mg/m2 i.v. bolus on day 1, and dacarbazine (DTIC) 400 mg/m2 by 1-h infusion on days 1 to 3 (CYVADIC) cycles repeated every 28 days for eight courses | 185/186 | 60.3 | 5-year DFS, 5-year OS, toxicity |
| Safety and efficacy pf regorafenib in patients with advanced soft tissue sarcoma [15] | Randomized, double-blind, placebo-controlled, phase 2 trial | Regorafenib | 6 cycles of i.v. vinorelbine 25 mg/m2 on days 1, 8, and 15, and daily oral cyclophosphamide 25 mg/m2, on days 1–28 | 90/92 | 20 | PFS, grade 3 or higher adverse events |
| Adverse Event | Van der Graaf et al. (2012) [11] | Woll et al. (2012) [17] | Frustaci et al. (2001) [18] | Mowery et al. (2024) [19] |
|---|---|---|---|---|
| General disorders [%] | 13 | 1 | 0 | 6 |
| Fatigue | 30 | nr | 0 | 1 |
| Fever | nr | 1 | 0 | 0 |
| Pain | nr | nr | 0 | 3 |
| Gastrointestinal disorders [%] | 13 | 15 | 16 | 10 |
| Diarrhea | 11 | 0 | 0 | 2 |
| Nausea/vomiting | 16 | 19 | 2 | 5 |
| Oral mucositis | 3 | 5 | 5 | 0 |
| Investigations [%] | 0 | 43 | 38 | 1 |
| Weight loss | 0 | nr | 0 | 0 |
| Increased creatinine | nr | 0 | 0 | 0 |
| Increased Alanine aminotransferase | nr | 2 | 0 | 0 |
| Increased aspartate aminotransferase | nr | 0 | 0 | 0 |
| Increased blood bilirubin | nr | 1 | 0 | nr |
| Decreased white blood cell count | nr | 28 | 14 | 1 |
| Decreased neutrophil count | nr | 13 | nr | nr |
| Decreased platelet count | nr | 19 | 3 | nr |
| Vascular disorders [%] | 7 | nr | 0 | 9 |
| Hypertension | 16 | nr | 0 | 6 |
| Blood and lymphatic system disorders [%] | nr | 7 | 17 | 10 |
| Anemia | nr | 11 | 7 | 7 |
| Infections and infestations [%] | nr | 7 | 0 | 13 |
| Wound infection | nr | 0 | 0 | 7 |
| Infection, general | nr | 12 | 0 | 2 |
| Metabolism and nutrition disorders [%] | 6 | nr | 0 | 0 |
| Anorexia | 14 | nr | 0 | 0 |
| Dysgeusia | 0 | nr | 0 | 0 |
| Skin and subcutaneous tissue disorders [%] | 1 | nr | 0 | 3 |
| Rash/desquamation | 1 | nr | 0 | 2 |
| Nervous system disorders [%] | nr | 1 | 0 | 0 |
| Cardiac disorders [%] | 1 | 1 | 0 | 0 |
| Adverse Event | Gortzak et al. (2001) [20] | Bramwell et al. (1994) [21] | Bisogno et al. (2019) [24] | Mir et al. (2016) [15] |
|---|---|---|---|---|
| General disorders [%] | nr | nr | 29 | 22 |
| Fatigue | nr | nr | nr | 12 |
| Fever | nr | nr | 53 | 1 |
| Pain | nr | nr | nr | 7 |
| Gastrointestinal disorders [%] | 28 | 14 | 6 | 10 |
| Diarrhea | nr | nr | 9 | 4 |
| Nausea/vomiting | 18 | 18 | 1 | 1 |
| Oral mucositis | nr | nr | nr | 4 |
| Investigations [%] | 8 | 31 | 85 | 8 |
| Weight loss | nr | nr | nr | nr |
| Increased creatinine | 0 | nr | nr | 0 |
| Increased Alanine aminotransferase | nr | 0 | nr | nr |
| Increased aspartate aminotransferase | nr | 0 | nr | 1 |
| Increased blood bilirubin | nr | 0 | nr | nr |
| Decreased white blood cell count | 5 | 31 | 86 | 4 |
| Decreased neutrophil count | nr | nr | 66 | 1 |
| Decreased platelet count | 0 | 8 | 1 | 1 |
| Vascular disorders [%] | nr | nr | nr | 18 |
| Hypertension | nr | nr | nr | 16 |
| Blood and lymphatic system disorders [%] | nr | nr | 9 | 3 |
| Anemia | nr | nr | 16 | 3 |
| Infections and infestations [%] | 0 | 2 | 2 | 3 |
| Wound infection | 0 | nr | nr | nr |
| Infection, general | 0 | 3 | 3 | 3 |
| Metabolism and nutrition disorders [%] | nr | 5 | nr | 3 |
| Anorexia | nr | 6 | nr | 3 |
| Dysgeusia | nr | nr | nr | nr |
| Skin and subcutaneous tissue disorders [%] | nr | nr | 1 | 4 |
| Rash/desquamation | nr | nr | 1 | 4 |
| Nervous system disorders [%] | 1 | 5 | 1 | 1 |
| Cardiac disorders [%] | 1 | 2 | 0 | 1 |
| Adverse Event | Van der Graaf et al. (2012) [11] | Woll et al. (2012) [17] | Frustaci et al. (2001) [18] | Mowery et al. (2024) [19] | Gortzak et al. (2001) [20] | Bramwell et al. (1994) [21] | Bisogno et al. (2019) [24] | Mir et al. (2016) [15] |
|---|---|---|---|---|---|---|---|---|
| Fatigue | 30 | nr | 0 | 1 | nr | nr | nr | nr |
| Diarrhea | 11 | 0 | 0 | 2 | nr | nr | 9 | 4 |
| Nausea/vomiting | 16 | 19 | 2 | 5 | 18 | 18 | 1 | 1 |
| Weight loss | 0 | nr | 0 | 0 | nr | nr | nr | nr |
| Hypertension | 16 | nr | 0 | 6 | nr | nr | nr | 16 |
| Anorexia | 14 | nr | 0 | 0 | nr | 6 | nr | 3 |
| Dysgeusia | 0 | nr | 0 | 0 | nr | nr | nr | nr |
| Rash/desquamation | 1 | nr | 0 | 2 | nr | nr | 1 | nr |
| Mucositis | 1 | 5 | 5 | 0 | nr | nr | nr | 4 |
| Fever | nr | 1 | 0 | 0 | nr | nr | 53 | 1 |
| (Skin) infection | nr | 12 | 0 | 2 | 0 | 3 | 3 | 3 |
| Neurological problem | nr | 1 | 0 | 0 | 1 | 5 | 5 | nr |
| Cardiac | 16 | 1 | 0 | 0 | 1 | 2 | 0 | 1 |
| Pain | nr | nr | 0 | 3 | nr | nr | nr | 7 |
| Wound infection | nr | nr | 0 | 7 | nr | nr | nr | nr |
| Creatinine | nr | 0 | 0 | 0 | 0 | nr | nr | nr |
| Bilirubin | nr | 1 | 0 | nr | nr | 0 | nr | 0 |
| AST elevated | nr | 0 | 0 | 0 | nr | 0 | nr | 1 |
| ALT elevated | nr | 2 | 0 | 0 | nr | 0 | nr | 1 |
| Leucopenia | nr | 28 | 14 | 1 | 5 | 31 | 86 | 4 |
| Neutropenia | nr | 13 | nr | nr | nr | nr | 66 | 1 |
| Thrombocytopenia | nr | 19 | 3 | nr | 0 | 8 | 1 | 1 |
| Anemia | nr | 11 | 5 | 7 | nr | nr | 16 | 3 |
| Adverse Event | Van der Graaf et al. (2012) [11] | Woll et al. (2012) [17] | Frustaci et al. (2001) [18] | Mowery et al. (2024) [19] | Gortzak et al. (2001) [20] | Bramwell et al. (1994) [21] | Bisogno et al. (2019) [24] | Mir et al. (2016) [15] |
|---|---|---|---|---|---|---|---|---|
| Fatigue | 1 | nr | 0 | 0 | nr | nr | nr | 0 |
| Diarrhea | 0 | 0 | 0 | 0 | nr | nr | 0 | 0 |
| Nausea/vomiting | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Weight loss | 0 | nr | 0 | 0 | nr | nr | nr | nr |
| Hypertension | 0 | nr | 0 | 0 | nr | nr | nr | 1 |
| Anorexia | 0 | nr | 0 | 0 | nr | 2 | nr | 0 |
| Dysgeusia | 0 | nr | 0 | 0 | nr | nr | nr | nr |
| Rash/desquamation | 0 | nr | 0 | 0 | nr | nr | 0 | 0 |
| Mucositis | 0 | 0 | 0 | 0 | nr | nr | nr | 0 |
| Fever | nr | 0 | 0 | 1 | nr | nr | 0 | 0 |
| (Skin) infection | nr | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Neurological problem | nr | 0 | 0 | 0 | 1 | 1 | 1 | nr |
| Cardiac | nr | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Pain | nr | nr | 0 | 0 | nr | nr | nr | 0 |
| Wound infection | nr | nr | 0 | 0 | nr | nr | nr | 0 |
| Creatinine | nr | 0 | 0 | 0 | 0 | nr | nr | 0 |
| Bilirubin | nr | 0 | 0 | nr | nr | 0 | nr | nr |
| AST elevated | nr | 0 | 0 | 0 | nr | 0 | nr | 0 |
| ALT elevated | nr | 0 | 0 | 0 | nr | 0 | nr | nr |
| Leucopenia | nr | 41 | 12 | 1 | 0 | 0 | 50 | 0 |
| Neutropenia | nr | 44 | nr | nr | nr | nr | 82 | 0 |
| Thrombocytopenia | nr | 11 | 2 | nr | 0 | 0 | 1 | nr |
| Anemia | nr | 2 | 1 | 0 | nr | nr | 3 | 1 |
| Adverse Event | Van der Graaf et al. (2012) [11] | Woll et al. (2012) [17] | Frustaci et al. (2001) [18] | Mowery et al. (2024) [19] | Gortzak et al. (2001) [20] | Bramwell et al. (1994) [21] | Bisogno et al. (2019) [24] | Mir et al. (2016) [15] |
|---|---|---|---|---|---|---|---|---|
| Fatigue | 13 | nr | 0 | 1 | nr | nr | nr | 13 |
| Diarrhea | 5 | 0 | 0 | 3 | nr | nr | 5 | 4 |
| Nausea/vomiting | 6 | 12 | 3 | 7 | 28 | 14 | 1 | 1 |
| Weight loss | 0 | nr | 0 | 0 | nr | nr | nr | nr |
| Hypertension | 7 | nr | 0 | 9 | nr | nr | nr | 18 |
| Anorexia | 6 | nr | 0 | 0 | nr | 5 | nr | 3 |
| Dysgeusia | 0 | nr | 0 | 0 | nr | nr | nr | nr |
| Rash/desquamation | 1 | nr | 0 | 8 | nr | nr | 1 | nr |
| Mucositis | 1 | 3 | 10 | 0 | nr | nr | nr | 4 |
| Fever | nr | 1 | 0 | 0 | nr | nr | 29 | 1 |
| (Skin) infection | nr | 7 | 0 | 7 | 0 | 2 | 31 | 3 |
| Neurological problem | nr | 1 | 0 | 0 | 1 | 5 | 1 | nr |
| Cardiac | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 1 |
| Pain | nr | nr | 0 | 4 | nr | nr | nr | 8 |
| Wound infection | nr | nr | 0 | 10 | nr | nr | nr | nr |
| Creatinine | nr | 0 | 0 | 0 | 0 | nr | nr | nr |
| Bilirubin | nr | 1 | 0 | nr | nr | 0 | nr | 0 |
| AST elevated | nr | 0 | 0 | 0 | nr | 0 | nr | 0 |
| ALT elevated | nr | 2 | 0 | 0 | nr | 0 | nr | 1 |
| Leucopenia | nr | 19 | 30 | 1 | 8 | 25 | 48 | 4 |
| Neutropenia | nr | 9 | nr | nr | nr | nr | 37 | 1 |
| Thrombocytopenia | nr | 13 | 7 | nr | 0 | 6 | 1 | 1 |
| Anemia | nr | 8 | 17 | 10 | nr | nr | 9 | 3 |
| Adverse Event | Van der Graaf et al. (2012) [11] | Woll et al. (2012) [17] | Frustaci et al. (2001) [18] | Mowery et al. (2024) [19] | Gortzak et al. (2001) [20] | Bramwell et al. (1994) [21] | Bisogno et al. (2019) [24] | Mir et al. (2016) [15] |
|---|---|---|---|---|---|---|---|---|
| Fatigue | 1 | nr | 0 | 0 | nr | nr | nr | 0 |
| Diarrhea | 0 | 0 | 0 | 0 | nr | nr | 0 | 0 |
| Nausea/vomiting | 0 | 0 | 0 | 0 | 1 | 12 | 0 | 0 |
| Weight loss | 0 | nr | 0 | 0 | nr | nr | nr | nr |
| Hypertension | 0 | nr | 0 | 0 | nr | nr | nr | 1 |
| Anorexia | 0 | nr | 0 | 0 | nr | 2 | nr | 0 |
| Dysgeusia | 0 | nr | 0 | 0 | nr | nr | nr | nr |
| Rash/desquamation | 0 | nr | 0 | 0 | nr | nr | 0 | 0 |
| Mucositis | 0 | 0 | 0 | 0 | nr | nr | nr | 0 |
| Fever | nr | 0 | 0 | 1 | nr | nr | 0 | 0 |
| (Skin) infection | nr | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Neurological problem | nr | 0 | 0 | 0 | 1 | 4 | 1 | nr |
| Cardiac | nr | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Pain | nr | nr | 0 | 0 | nr | nr | nr | 0 |
| Wound infection | nr | nr | 0 | 0 | nr | nr | nr | 0 |
| Creatinine | nr | 0 | 0 | 0 | 0 | nr | nr | 0 |
| Bilirubin | nr | 0 | 0 | nr | nr | 0 | nr | nr |
| AST elevated | nr | 0 | 0 | 0 | nr | 0 | nr | 0 |
| ALT elevated | nr | 0 | 0 | 0 | nr | 0 | nr | nr |
| Leucopenia | nr | 28 | 27 | 1 | 0 | 0 | 28 | 0 |
| Neutropenia | nr | 30 | nr | nr | nr | nr | 45 | 0 |
| Thrombocytopenia | nr | 8 | 7 | nr | 0 | 0 | 1 | nr |
| Anemia | nr | 1 | 7 | 0 | nr | nr | 2 | 1 |
| Adverse Event | Van der Graaf et al. (2012) [11] Treatment Group | Control Group | Mowery et al. (2024) [19] Treatment Group | Control Group | Mir et al. (2016) [15] Treatment Group | Control Group |
|---|---|---|---|---|---|---|
| Fatigue | 30 | 6 | 1 | 0 | 12 | 6 |
| Diarrhea | 11 | 1 | 2 | 1 | 4 | 2 |
| Nausea/vomiting | 16 | 3 | 5 | 0 | 1 | 0 |
| Weight loss | 0 | 0 | 0 | 0 | nr | 0 |
| Hypertension | 16 | 4 | 6 | 3 | 16 | 2 |
| Anorexia | 14 | 0 | 0 | 0 | 3 | 4 |
| Dysgeusia | 0 | 0 | 0 | 0 | nr | nr |
| Rash/desquamation | 1 | 0 | 6 | 4 | nr | nr |
| Mucositis | 1 | 0 | 0 | 0 | 4 | 0 |
| Fever | nr | nr | 0 | 0 | 1 | 1 |
| (Skin) infection | nr | nr | 2 | 0 | 3 | 0 |
| Neurological problem | nr | nr | 0 | 0 | nr | nr |
| Cardiac | 16 | 3 | 0 | 0 | 1 | 0 |
| Pain | nr | nr | 7 | 0 | 7 | 5 |
| Wound infection | nr | nr | 7 | 6 | nr | nr |
| Creatinine | nr | nr | 0 | 0 | 0 | 0 |
| Bilirubin | nr | nr | nr | nr | nr | nr |
| Increased AST | nr | nr | 0 | 0 | 1 | 1 |
| Increased ALT | nr | nr | 0 | 0 | 1 | 1 |
| Leucopenia | nr | nr | 1 | 1 | 4 | 5 |
| Neutropenia | nr | nr | nr | nr | 1 | 0 |
| Thrombocytopenia | nr | nr | nr | nr | 1 | 0 |
| Anemia | nr | nr | 10 | 3 | 3 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aeschbacher, R.; Fuchs, B.; Studer, G.; Heesen, P. Reporting Matters: Severe Adverse Events in Soft Tissue Sarcoma Therapy—A 30-Year Systematic Review of Placebo- and Non-Systemic-Controlled Randomized Trials. Cancers 2025, 17, 3118. https://doi.org/10.3390/cancers17193118
Aeschbacher R, Fuchs B, Studer G, Heesen P. Reporting Matters: Severe Adverse Events in Soft Tissue Sarcoma Therapy—A 30-Year Systematic Review of Placebo- and Non-Systemic-Controlled Randomized Trials. Cancers. 2025; 17(19):3118. https://doi.org/10.3390/cancers17193118
Chicago/Turabian StyleAeschbacher, Rahel, Bruno Fuchs, Gabriela Studer, and Philip Heesen. 2025. "Reporting Matters: Severe Adverse Events in Soft Tissue Sarcoma Therapy—A 30-Year Systematic Review of Placebo- and Non-Systemic-Controlled Randomized Trials" Cancers 17, no. 19: 3118. https://doi.org/10.3390/cancers17193118
APA StyleAeschbacher, R., Fuchs, B., Studer, G., & Heesen, P. (2025). Reporting Matters: Severe Adverse Events in Soft Tissue Sarcoma Therapy—A 30-Year Systematic Review of Placebo- and Non-Systemic-Controlled Randomized Trials. Cancers, 17(19), 3118. https://doi.org/10.3390/cancers17193118







