Contemporary Trends and Predictors of pT0 in Radical Cystectomy Specimens Among Non-Muscle and Muscle-Invasive Bladder Cancer Patients: A Propensity Score-Matched Analysis from a Single Tertiary Centre in the United Kingdom
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Surgical Procedure
2.3. Statistical Analysis
3. Results
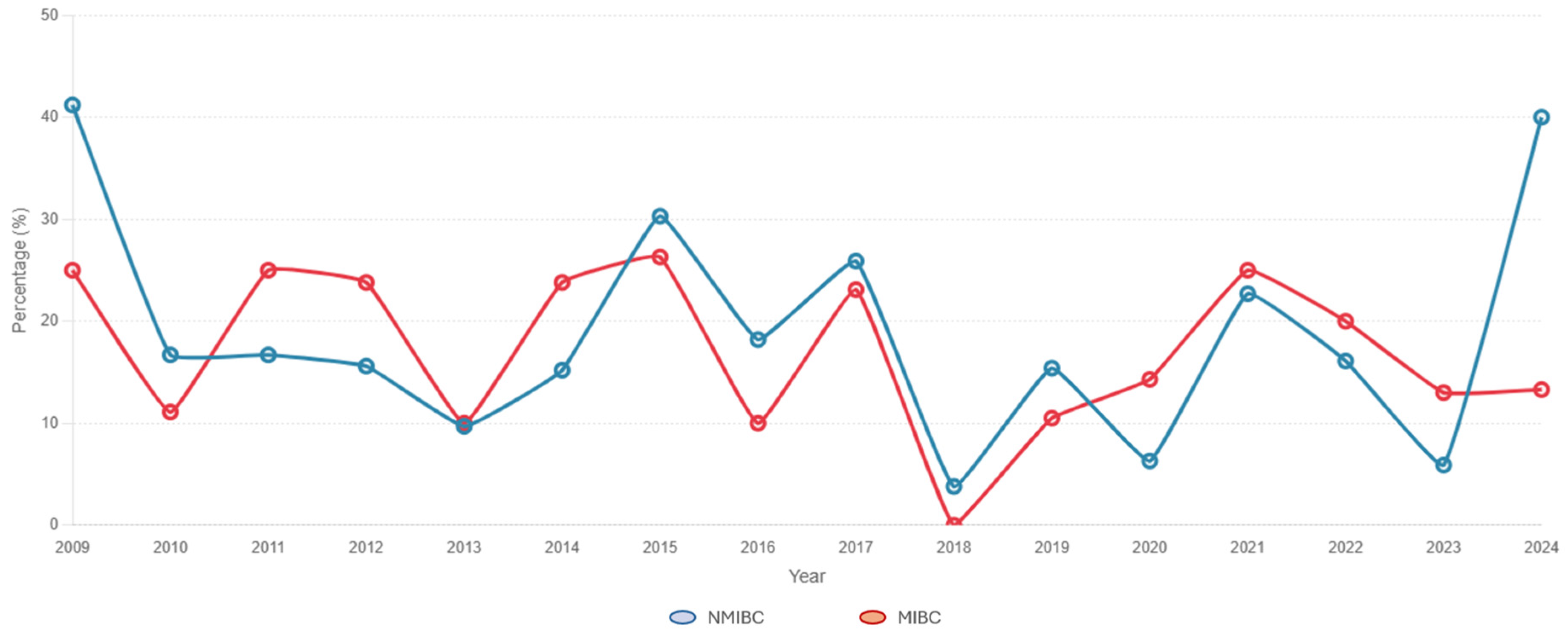
3.1. Description of the Study Cohort
3.2. Predictors of pT0 Status by Clinical Stage Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Roupret, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef]
- Gontero, P.; Birtle, A.; Capoun, O.; Compérat, E.; Dominguez-Escrig, J.L.; Liedberg, F.; Mariappan, P.; Masson-Lecomte, A.; Mostafid, H.A.; Pradere, B.; et al. European Association of Urology Guidelines on Non–muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—A Summary of the 2024 Guidelines Update. Eur. Urol. 2024, 86, 531–549. [Google Scholar] [CrossRef]
- Sylvester, R.J.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting Recurrence and Progression in Individual Patients with Stage Ta T1 Bladder Cancer Using EORTC Risk Tables: A Combined Analysis of 2596 Patients from Seven EORTC Trials. Eur. Urol. 2006, 49, 466–477. [Google Scholar] [CrossRef]
- Lobo, N.; Hensley, P.J.; Bree, K.K.; Nogueras-Gonzalez, G.M.; Navai, N.; Dinney, C.P.; Sylvester, R.J.; Kamat, A.M. Updated European Association of Urology (EAU) Prognostic Factor Risk Groups Overestimate the Risk of Progression in Patients with Non–muscle-invasive Bladder Cancer Treated with Bacillus Calmette-Guérin. Eur. Urol. Oncol. 2022, 5, 84–91. [Google Scholar] [CrossRef]
- Sylvester, R.J.; Rodríguez, O.; Hernández, V.; Turturica, D.; Bauerová, L.; Bruins, H.M.; Bründl, J.; van der Kwast, T.H.; Brisuda, A.; Rubio-Briones, J.; et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non–muscle-invasive Bladder Cancer (NMIBC) Incorporating the WHO 2004/2016 and WHO 1973 Classification Systems for Grade: An Update from the EAU NMIBC Guidelines Panel. Eur. Urol. 2021, 79, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Scilipoti, P.; Longoni, M.; de Angelis, M.; Zaurito, P.; Ślusarczyk, A.; Soria, F.; Pradere, B.; Krajewski, W.; D’ANdrea, D.; Mari, A.; et al. Outcomes of BCG vs upfront radical cystectomy for high-risk non-muscle-invasive bladder cancer. BJU Int. 2025, 136, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Diamant, E.; Roumiguié, M.; Ingels, A.; Parra, J.; Vordos, D.; Bajeot, A.-S.; Chartier-Kastler, E.; Soulié, M.; de la Taille, A.; Rouprêt, M.; et al. Effectiveness of Early Radical Cystectomy for High-Risk Non-Muscle Invasive Bladder Cancer. Cancers 2022, 14, 3797. [Google Scholar] [CrossRef]
- Necchi, A.; Roumiguié, M.; Kamat, A.M.; Shore, N.D.; Boormans, J.L.; Esen, A.A.; Lebret, T.; Kandori, S.; Bajorin, D.F.; Krieger, L.E.M.; et al. Pembrolizumab monotherapy for high-risk non-muscle-invasive bladder cancer without carcinoma in situ and unresponsive to BCG (KEYNOTE-057): A single-arm, multicentre, phase 2 trial. Lancet Oncol. 2024, 25, 720–730. [Google Scholar] [CrossRef]
- McFadden, J.; Tachibana, I.; Adra, N.; Collins, K.; Cary, C.; Koch, M.; Kaimakliotis, H.; Masterson, T.; Rice, K. Impact of variant histology on upstaging and survival in patients with nonmuscle invasive bladder cancer undergoing radical cystectomy. Urol. Oncol. Semin. Orig. Investig. 2024, 42, 69.e11–69.e16. [Google Scholar] [CrossRef] [PubMed]
- May, M.; Bastian, P.J.; Burger, M.; Bolenz, C.; Trojan, L.; Herrmann, E.; Wülfing, C.; Müller, S.C.; Ellinger, J.; Buchner, A.; et al. Multicenter evaluation of the prognostic value of pT0 stage after radical cystectomy due to urothelial carcinoma of the bladder. BJU Int. 2011, 108, E278–E283. [Google Scholar] [CrossRef]
- Choi, H.; Park, J.Y.; Bae, J.H.; Tae, B.S. Health-related quality of life after radical cystectomy. Transl. Androl. Urol. 2020, 9, 2997–3006. [Google Scholar] [CrossRef]
- Tilki, D.; Svatek, R.S.; Novara, G.; Seitz, M.; Godoy, G.; Karakiewicz, P.I.; Kassouf, W.; Fradet, Y.; Fritsche, H.-M.; Sonpavde, G.; et al. Stage pT0 at Radical Cystectomy Confers Improved Survival: An International Study of 4,430 Patients. J. Urol. 2010, 184, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Tholomier, C.; Souhami, L.; Kassouf, W. Bladder-sparing protocols in the treatment of muscle-invasive bladder cancer. Transl. Androl. Urol. 2020, 9, 2920–2937. [Google Scholar] [CrossRef]
- Del Giudice, F.; Abu-Ghanem, Y.; Nair, R.; Mensah, E.; Kam, J.; Ibrahim, Y.; Gad, M.; Chatterton, K.; Amery, S.; Alao, R.; et al. Contemporary Trends and Predictors Associated with Adverse Pathological Upstaging Among Non-Metastatic Localized Clinical T2 Muscle-Invasive Bladder Cancers Undergoing Radical Cystectomy: Outcomes from a Single Tertiary Centre in the United Kingdom. Cancers 2025, 17, 1477. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, V.; Carino, D.; Corvino, R.; Salciccia, S.; De Berardinis, E.; Krajewski, W.; Nowak, Ł.; Łaszkiewicz, J.; Szydełko, T.; Nair, R.; et al. Surgical Technique and Perioperative Outcomes of the “Sapienza” Urology Residency Program’s Trocar Placement Configuration During Robotic-Assisted Radical Prostatectomy (RARP): A Retrospective, Single-Centre Observational Study Comparing Experienced Attendings vs. Post-Graduate Year I–III Residents as Bedside Assistants. Cancers 2024, 17, 20. [Google Scholar] [CrossRef]
- Ditonno, F.; Veccia, A.; Montanaro, F.; Pettenuzzo, G.; Franco, A.; Manfredi, C.; Triggiani, L.; De Nunzio, C.; De Sio, M.; Cerruto, M.; et al. Trimodal therapy vs radical cystectomy in patients with muscle-invasive bladder cancer: A systematic review and meta-analysis of comparative studies. BJU Int. 2024, 134, 684–695. [Google Scholar] [CrossRef]
- Barbos, V.; Feciche, B.; Latcu, S.; Croitor, A.; Dema, V.; Bardan, R.; Faur, F.I.; Mateescu, T.; Novacescu, D.; Bogdan, G.; et al. The Assessment of SF-36 Survey for Quality-of-Life Measurement after Radical Cystectomy for Muscle-Invasive Bladder Cancer: A Systematic Review. Diseases 2024, 12, 56. [Google Scholar] [CrossRef]
- Akdemir, E.; Stuiver, M.M.; van de Kamp, M.W.; van der Hulst, J.B.-.; Mertens, L.S.; Hendricksen, K.; van Harten, W.H.; May, A.M.; Sweegers, M.G. Long-term quality of life in patients with bladder cancer following radical cystectomy. BJU Int. 2024, 135, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Månsson, Å.; Davidsson, T.; Hunt, S.; Månsson, W. The quality of life in men after radical cystectomy with a continent cutaneous diversion or orthotopic bladder substitution: Is there a difference? BJU Int. 2002, 90, 386–390. [Google Scholar] [CrossRef]
- Hedgepeth, R.C.; Gilbert, S.M.; He, C.; Lee, C.T.; Wood, D.P., Jr. Body Image and Bladder Cancer Specific Quality of Life in Patients with Ileal Conduit and Neobladder Urinary Diversions. Urology 2010, 76, 671–675. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Carrión, A.; Cathomas, R.; Compérat, E.; Efstathiou, J.A.; Fietkau, R.; Gakis, G.; Lorch, A.; Martini, A.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2023 Guidelines. Eur. Urol. 2023, 85, 17–31. [Google Scholar] [CrossRef]
- Mediavilla, E.; Portillo, J.A.; Truán, D.; Campos-Juanatey, F.; Gala, L.; Fuentes, J.; Carrión, C.; Gutiérrez, J.L. Predictive factors of evolution of patients after pT0 radical cystectomy due to bladder cancer. Arch. Esp. Urol. 2014, 67, 764–769. [Google Scholar]
- Boeri, L.; Soligo, M.; Frank, I.; Boorjian, S.A.; Thompson, R.H.; Tollefson, M.; Tarrel, R.; Quevedo, F.J.; Cheville, J.C.; Karnes, R.J. Clinical predictors and survival outcome of patients receiving suboptimal neoadjuvant chemotherapy and radical cystectomy for muscle-invasive bladder cancer: A single-center experience. World J. Urol. 2019, 37, 2409–2418. [Google Scholar] [CrossRef]
- Li, H.; Hu, J.; Zu, X.; Chen, M.; Chen, J.; Zou, Y.; Deng, R.; Qin, G.; Li, W.; Tang, J.; et al. A novel signature to predict the neoadjuvant chemotherapy response of bladder carcinoma: Results from a territory multicenter real-world study. Front. Genet. 2022, 13, 1047481. [Google Scholar] [CrossRef] [PubMed]
- Catarino, R.; Alves, L.; Pereira, D.; Costa, G.; Pereira, J.; Cardoso, A.; Braga, I.; Freitas, R.; Correia, T.; Cerqueira, M.; et al. Neoadjuvant chemotherapy for muscle-invasive bladder cancer: Does variant histology matter? Int. Urol. Nephrol. 2022, 54, 3163–3169. [Google Scholar] [CrossRef] [PubMed]
- Lin-Brande, M.; Zainfeld, D.; Ghodoussipour, S.; Cai, J.; Miranda, G.; Djaladat, H.; Schuckman, A.; Sadeghi, S.; Dorff, T.; Quinn, D.; et al. MP47-06 Impact of Variant Histology on Response to Neoadjuvant Chemotherapy for Urothelial Bladder Cancer. J. Urol. 2018, 199, e617. [Google Scholar] [CrossRef][Green Version]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Escrig, J.L.D.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non–muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef]
- D’ANdrea, D.; Black, P.C.; Zargar, H.; Zargar-Shoshtari, K.; Zehetmayer, S.; Fairey, A.S.; Mertens, L.S.; Dinney, C.P.; Mir, M.C.; Krabbe, L.-M.; et al. Impact of sex on response to neoadjuvant chemotherapy in patients with bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 639.e1–639.e9. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Iwata, T.; Abufaraj, M.; Janisch, F.; D’aNdrea, D.; Moschini, M.; Al-Rawashdeh, B.; Fajkovic, H.; Seebacher, V.; Egawa, S.; et al. Impact of Gender on Chemotherapeutic Response and Oncologic Outcomes in Patients Treated with Radical Cystectomy and Perioperative Chemotherapy for Bladder Cancer: A Systematic Review and Meta-Analysis. Clin. Genitourin. Cancer 2020, 18, 78–87. [Google Scholar] [CrossRef]
- Fajkovic, H.; Halpern, J.A.; Cha, E.K.; Bahadori, A.; Chromecki, T.F.; Karakiewicz, P.I.; Breinl, E.; Merseburger, A.S.; Shariat, S.F. Impact of gender on bladder cancer incidence, staging, and prognosis. World J. Urol. 2011, 29, 457–463. [Google Scholar] [CrossRef]
- Bahlburg, H.; Reicherz, A.; Reike, M.; Bach, P.; Butea-Bocu, M.C.; Tully, K.H.; Roghmann, F.; Noldus, J.; Müller, G. A prospective evaluation of quality of life, psychosocial distress, and functional outcomes two years after radical cystectomy and urinary diversion in 842 German bladder cancer patients. J. Cancer Surviv. 2024, 19, 1102–1110. [Google Scholar] [CrossRef]
- Proietti, F.; Flammia, R.S.; Licari, L.C.; Bologna, E.; Bove, A.M.; Brassetti, A.; Tuderti, G.; Mastroianni, R.; Tufano, A.; Simone, G.; et al. Impacts of Neoadjuvant Chemotherapy on Perioperative Outcomes in Patients with Bladder Cancer Treated with Radical Cystectomy: A Single High-Volume Center Experience. J. Pers. Med. 2024, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, S.; Jeong, S.-H.; Ku, J.H.; Kim, K.H.; Kil Nam, J.; Lim, B.; Hong, B.S.; Nam, W.; Kang, S.G.; et al. The effect of neoadjuvant chemotherapy on survival outcomes subsequent to radical cystectomy in pathological T0 bladder cancer patients: A multicenter large-scale analysis. Investig. Clin. Urol. 2025, 66, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.C.; Harris, W.; Cheng, H.H.; Shenoi, J.; Zhao, S.; Wang, J.; Champion, T.; Izard, J.; Gore, J.L.; Porter, M.; et al. Pathologic Response Rates of Gemcitabine/Cisplatin versus Methotrexate/Vinblastine/Adriamycin/Cisplatin Neoadjuvant Chemotherapy for Muscle Invasive Urothelial Bladder Cancer. Adv. Urol. 2013, 2013, 317190. [Google Scholar] [CrossRef]
- Miyagi, H.; Kwenda, E.; Ramnaraign, B.H.; Chatzkel, J.A.; Brisbane, W.G.; O’malley, P.; Crispen, P.L. Predicting Complete Response to Neoadjuvant Chemotherapy in Muscle-Invasive Bladder Cancer. Cancers 2022, 15, 168. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Meghani, K.; Cooley, L.F.; McLaughlin, K.A.; Fall, L.A.; Yu, Y.; Castro, M.A.A.; Groeneveld, C.S.; de Reyniès, A.; Nazarov, V.I.; et al. Expression-based subtypes define pathologic response to neoadjuvant immune-checkpoint inhibitors in muscle-invasive bladder cancer. Nat. Commun. 2023, 14, 2126. [Google Scholar] [CrossRef]
- García-Rayo, C.; Juste-Álvarez, S.; Gómez-Cañizo, C.; Hernández-Arroyo, M.; Velasco, G.; Castellano, D.; Rodríguez-Antolín, A.; Guerrero-Ramos, F. The Dynamic Field of Perioperative Treatment for Localized Muscle-Invasive Bladder Cancer: A Review of the Current Research Landscape. J. Clin. Med. 2025, 14, 5653. [Google Scholar] [CrossRef]
| Variable | Total N = 655 |
|---|---|
| Sex | |
| -Male | 498 (76%) |
| -Female | 157 (24%) |
| Age | 69.6 (62.7–75.3) |
| BMI | 27.5 (24.2–30.7) |
| ACCI | 5 (3–6) |
| Ethnicity | |
| -White | 508 (77.53%) |
| -Black | 28 (4.3%) |
| -Asian | 21 (3.21%) |
| -Other | 98 (14.96%) |
| EGFR | 73 (60–86) |
| Creatinine (mg/L) | 86 (75–102) |
| Discussed in Pelvic MDM | |
| -No | 255 (39%) |
| -Yes | 400 (61%) |
| CT | |
| -No | 28 (4.3%) |
| -Yes | 627 (95.7%) |
| MRI | |
| -No | 563 (86%) |
| -Yes | 92 (14%) |
| Grade | |
| -LG | 34 (5.2%) |
| -HG | 621 (94.8%) |
| MIBC vs. NMIBC | |
| -NMIBC | 373 (57%) |
| -MIBC | 282 (43%) |
| Clinical T Stage | |
| -T0 | 3 (0.5%) |
| -Ta | 74 (11.3%) |
| -Tis | 30 (4.6%) |
| -T1 | 266 (40.5%) |
| -T2 | 272 (41.5%) |
| -T3 | 9 (1.4%) |
| -T4 | 1 (0.2%) |
| Clinical N Stage | |
| -Nx | 62 (9.5%) |
| -N0 | 586 (89.5%) |
| -N1 | 7 (1%) |
| BCG Failure | |
| -No | 355 (54.2%) |
| -Yes | 300 (45.8%) |
| NAC | |
| -No | 493 (75.3%) |
| -Yes | 162 (24.7%) |
| Pathological T Stage | |
| -T0 | 117 (17.9%) |
| ->T0 | 538 (82.1%) |
| Variable | Total n = 228 | NMIBC n = 130 | MIBC n = 98 | ||||
|---|---|---|---|---|---|---|---|
| >pT0 n = 65 | pT0 n = 65 | p Value | >pT0 n = 49 | pT0 n = 49 | p Value | ||
| Sex | 0.027 | 0.170 | |||||
| -Male | 169 (74.1%) | 43 (66.2%) | 54 (83.1%) | 33 (67.3%) | 39 (79.6%) | ||
| -Female | 59 (25.9%) | 22 (33.8%) | 11 (16.9%) | 16 (32.7%) | 10 (20.4%) | ||
| Ethnicity | 0.4 | 0.5 | |||||
| -White | 182 (79.8%) | 51 (78.5%) | 52 (80%) | 37 (73.5%) | 42 (85.7%) | ||
| -Black | 10 (4.4%) | 2 (3.1%) | 4 (6.2%) | 3 (6.1%) | 1 (2%) | ||
| -Asian | 7 (3.1%) | 1 (1.5%) | 4 (6.2%) | 1 (2%) | 1 (2%) | ||
| -Other | 29 (12.7%) | 11 (16.9%) | 5 (7.7%) | 8 (16.3%) | 5 (10.2%) | ||
| Age | 69.3 (62.7–75.1) | 69.1 (62.3–76.8) | 70.6 (63.2–76.1) | 0.7 | 66 (56.7–74.3) | 70 (63.5–75.2) | 0.05 |
| BMI | 27.5 (24–30.7) | 27.7 (23.7–32.3) | 27.4 (24.7–29.9) | 0.44 | 27.2 (23.4–29.9) | 27.3 (24–32.5) | 0.17 |
| ACCI | 5 (3–6) | 5 (2.5–6) | 5 (4–6) | 0.75 | 5 (2–6) | 5 (3–6.26) | 0.6 |
| EGFR | 75 (62.5–87) | 73 (63–86.5) | 77 (63–98.5) | 0.13 | 75 (66–86.75) | 71 (55–87) | 0.07 |
| Creatinine (mg/L) | 84 (73–96) | 87 (77–96) | 83 (67–91) | 0.04 | 83.5 (75–96.25) | 90 (69–106) | 0.18 |
| Discussed in Pelvic MDM | 0.16 | 0.5 | |||||
| -No | 64 (28.1%) | 14 (21.5%) | 21 (32.3%) | 16 (32.7%) | 13 (26.5%) | ||
| -Yes | 164 (71.9%) | 51 (78.5%) | 44 (67.7%) | 33 (67.3%) | 36 (73.5%) | ||
| BCG failure | 0.018 | 0.18 | |||||
| -No | 117 (51.3%) | 17 (26.2%) | 30 (46.2%) | 32 (65.3%) | 38 (71.4%) | ||
| -Yes | 111 (48.7%) | 48 (73.8%) | 35 (53.8%) | 17 (34.7%) | 11 (22.4%) | ||
| NAC | 0.3 | 0.04 | |||||
| -No | 169 (74.1%) | 64 (98.5%) | 62 (95.4%) | 27 (55.1%) | 16 (32.7%) | ||
| -Yes | 59 (25.9%) | 1 (1.5%) | 3 (4.6%) | 22 (44.9%) | 33 (67.3%) | ||
| Technique | 0.16 | 0.6 | |||||
| -Open | 61 (26.8%) | 21 (32.3%) | 14 (21.5%) | 13 (26.5%) | 13 (26.5%) | ||
| -Robotic | 166 (72.8%) | 44 (67.7%) | 51 (78.5%) | 36 (73.5%) | 35 (71.4%) | ||
| Laparoscopic | 1 (0.4%) | 0 | 0 | 0 | 1 (2%) | ||
| Diversion Type | 0.56 | 0.045 | |||||
| -UCS | 1 (0.4%) | 1 (1.5%) | 0 | ||||
| -IC | 210 (92.1%) | 61 (93.8%) | 4 (6.2%) | 47 (95.9%) | 41 (83.7%) | ||
| -Neobladder | 17 (7.5%) | 3 (4.6%) | 61 (93.8%) | 2 (4.1%) | 8 (16.3%) | ||
| Lymph Node Excision Technique | 0.5 | 0.2 | |||||
| -Not Performed | 17 (7.5%) | 5 (7.7%) | 9 (13.8%) | 3 (6.1%) | 0 | ||
| -Standard | 166 (72.8%) | 48 (73.8%) | 44 (67.7%) | 36 (73.5%) | 38 (77.6%) | ||
| -Extended | 45 (19.7%) | 12 (18.5%) | 12 (18.5%) | 10 (20.4%) | 11 (22.4%) | ||
| Clinical T Stage | 0.004 | ||||||
| -Ta | 35 (15.4%) | 16 (24.6%) | 19 (29.2%) | 0 | 0 | ||
| -Tis | 8 (3.5%) | 7 (10.8%) | 1 (1.5%) | 0 | 0 | ||
| -T1 | 87 (38.1%) | 42 (64.6%) | 45 (67.7%) | 0 | 0 | ||
| -T2 | 88 (38.6%) | 0 | 39 (79.6%) | 49 (100%) | |||
| -T3 | 9 (3.9%) | 0 | 9 (18.4%) | 0 | |||
| -T4 | 1 (0.4%) | 0 | 1 (2%) | 0 | |||
| Clinical N Stage | 0.29 | 0.6 | |||||
| -Nx | 24 (10.4%) | 10 (15.4%) | 6 (9.2%) | 4 (8.2%) | 4 (8.2%) | ||
| -N0 | 202 (88.6%) | 55 (84.6%) | 58 (89.2%) | 45 (91.8%) | 44 (89.8%) | ||
| -N1 | 3 (0.9%) | 0 | 1 (1.5%) | 0 | 1 (2%) | ||
| Grade | 0.19 | 0.15 | |||||
| -LG | 12 (5.3%) | 3 (4.6%) | 7 (10.8%) | 0 | 2 (4.1%) | ||
| -HG | 216 (94.7%) | 62 (95.4%) | 58 (89.2%) | 49 (100%) | 47 (98%) | ||
| Histological Variant | 0.08 | 0.06 | |||||
| -No | 202 (88.6%) | 58 (89.2%) | 63 (96.9%) | 37 (75.5%) | 44 (89.8%) | ||
| -Yes | 26 (11.4%) | 7 (10.8%) | 2 (3.1%) | 12 (24.5%) | 5 (10.2%) | ||
| Additional CIS | 0.05 | 0.029 | |||||
| -No | 153 (67.1%) | 30 (46.2%) | 41 (63.1%) | 37 (75.5%) | 45 (91.8%) | ||
| -Yes | 75 (32.9%) | 35 (53.8%) | 24 (36.9%) | 12 (24.5%) | 4 (8.2%) | ||
| pT Stage | <0.001 | <0.001 | |||||
| -T0 | 114 (50%) | 0 | 65 (100%) | 0 | 49 (100%) | ||
| -Ta | 15 (6.6%) | 14 (21.5%) | 0 | 1 (2%) | 0 | ||
| -Tis | 20 (8.8%) | 17 (26.2%) | 0 | 3 (6.1%) | 0 | ||
| -T1 | 21 (9.2%) | 18 (27.7%) | 0 | 3 (6.1%) | 0 | ||
| -T2 | 22 (9.6%) | 9 (13.8%) | 0 | 13 (26.5%) | 0 | ||
| -T3 | 28 (12.3%) | 4 (6.2%) | 0 | 24 (49%) | 0 | ||
| -T4 | 8 (3.5%) | 3 (4.6%) | 0 | 5 (10.2%) | 0 | ||
| pN Stage | 0.5 | 0.03 | |||||
| -Nx | 17 (7.5%) | 5 (7.7%) | 6 (9.2%) | 4 (8.2%) | 2 (4.1%) | ||
| -N0 | 191 (83.7%) | 57 (87.7%) | 58 (89.2%) | 30 (61.2%) | 46 (93.8%) | ||
| -N1 | 10 (4.0%) | 2 (3.1%) | 1 (1.5%) | 6 (12.2%) | 1 (2%) | ||
| -N2 | 9 (3.9%) | 1 (1.5%) | 0 | 8 (16.3%) | 0 | ||
| -N3 | 1 (0.4%) | 0 | 0 | 1 (2%) | 0 | ||
| Lymph Node Yield | 17 (12–22) | 17 (13–23) | 15 (10–22) | 0.3 | 17 (12–21) | 17.5 (12.7–21.2) | 0.7 |
| Positive Surgical Margins | 0.3 | 0.006 | |||||
| -No | 220 (96.5%) | 64 (98.5%) | 65 (100%) | 42 (85.7%) | 49 (100%) | ||
| -Yes | 8 (3.5%) | 1 (1.5%) | 0 | 7 (14.3%) | 0 | ||
| Variable | aOR | 95% CI | p Value |
|---|---|---|---|
| Male gender | 2.89 | 1.13–7.30 | 0.026 |
| Discussed in Pelvic MDM | 0.71 | 0.32–1.4 | 0.3 |
| BCG Failure | 0.40 | 0.19–0.99 | 0.05 |
| Additional CIS | 0.16 | 0.025–0.97 | 0.04 |
| Histological variant | 0.49 | 0.21–1.08 | 0.08 |
| Variable | aOR | 95% CI | p Value |
|---|---|---|---|
| Male gender | 1.97 | 0.74–5.25 | 0.26 |
| Discussed in Pelvic MDM | 1.14 | 0.41–3.16 | 0.8 |
| Neoadjuvant Chemotherapy | 2.20 | 1.01–6.82 | 0.049 |
| Additional CIS | 0.22 | 0.06–0.80 | 0.02 |
| Histological variant | 0.36 | 0.1–1.21 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Giudice, F.; Santarelli, V.; Spurna, K.; Ali Kirmani, S.G.; Bhatti, N.H.; Abu-Ghanem, Y.; Mensah, E.; Challacombe, B.; Davies, S.J.; Hegazy, M.; et al. Contemporary Trends and Predictors of pT0 in Radical Cystectomy Specimens Among Non-Muscle and Muscle-Invasive Bladder Cancer Patients: A Propensity Score-Matched Analysis from a Single Tertiary Centre in the United Kingdom. Cancers 2025, 17, 3110. https://doi.org/10.3390/cancers17193110
Del Giudice F, Santarelli V, Spurna K, Ali Kirmani SG, Bhatti NH, Abu-Ghanem Y, Mensah E, Challacombe B, Davies SJ, Hegazy M, et al. Contemporary Trends and Predictors of pT0 in Radical Cystectomy Specimens Among Non-Muscle and Muscle-Invasive Bladder Cancer Patients: A Propensity Score-Matched Analysis from a Single Tertiary Centre in the United Kingdom. Cancers. 2025; 17(19):3110. https://doi.org/10.3390/cancers17193110
Chicago/Turabian StyleDel Giudice, Francesco, Valerio Santarelli, Katarina Spurna, Syed Ghazi Ali Kirmani, Noor Huda Bhatti, Yasmin Abu-Ghanem, Elsie Mensah, Benjamin Challacombe, Samuel J. Davies, Mohammad Hegazy, and et al. 2025. "Contemporary Trends and Predictors of pT0 in Radical Cystectomy Specimens Among Non-Muscle and Muscle-Invasive Bladder Cancer Patients: A Propensity Score-Matched Analysis from a Single Tertiary Centre in the United Kingdom" Cancers 17, no. 19: 3110. https://doi.org/10.3390/cancers17193110
APA StyleDel Giudice, F., Santarelli, V., Spurna, K., Ali Kirmani, S. G., Bhatti, N. H., Abu-Ghanem, Y., Mensah, E., Challacombe, B., Davies, S. J., Hegazy, M., Ibrahim, Y., Gad, M., Khan, A., Corvino, R., Crocetto, F., Łaszkiewicz, J., Rocco, B., Chung, B. I., Thuraraja, R., ... Nair, R. (2025). Contemporary Trends and Predictors of pT0 in Radical Cystectomy Specimens Among Non-Muscle and Muscle-Invasive Bladder Cancer Patients: A Propensity Score-Matched Analysis from a Single Tertiary Centre in the United Kingdom. Cancers, 17(19), 3110. https://doi.org/10.3390/cancers17193110







