Understanding and Exacerbating the Biological Response of Uveal Melanoma to Proton Beam Therapy
Simple Summary
Abstract
1. Introduction
2. Proton Beam Therapy
3. Evaluating the Clinical Response to Proton Beam Therapy
4. Key Molecular Factors Known to Influence Cellular Response to PBT
4.1. DNA Damage Repair Proficiency
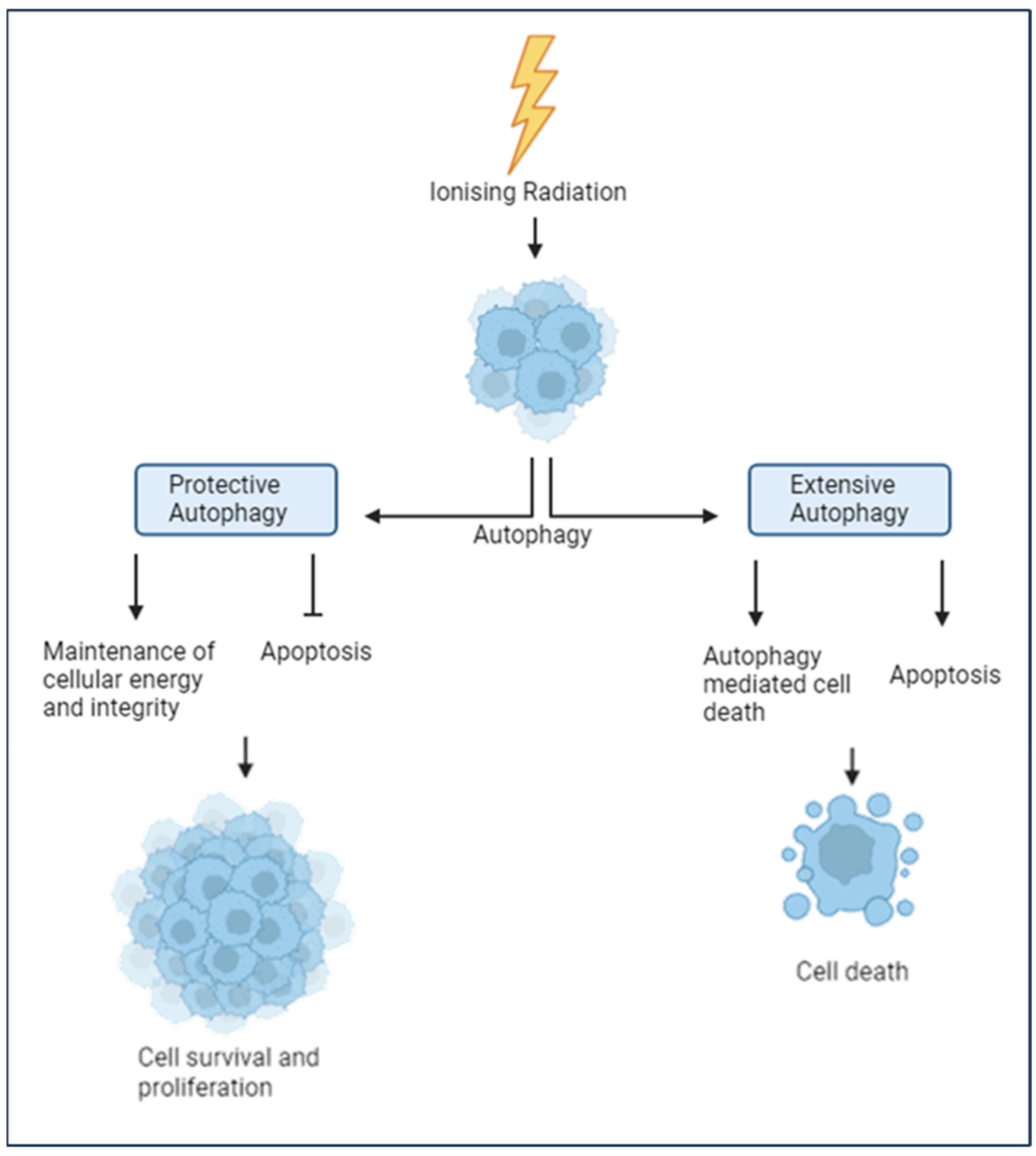
4.2. The Autophagy Response
4.3. Hypoxia and the Tumour Microenvironment
5. Strategies to Exacerbating the PBT Response in UM
5.1. Targeting DNA Damage Repair
5.2. Other Potential Radiosensitisation Targets
5.3. Radiotherapy Advances
5.4. FLASH Radiotherapy
6. Concluding Remarks and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsotridou, E.; Loukovitis, E.; Tsiropoulos, G.N.; Zapsalis, K.; Pentara, I.; Tzima, K.; Eminidou, V.; Anogeianakis, G. Radiation treatment methods in uveal melanoma. Med. Hypothesis Discov. Innov. Ophthalmol. 2021, 10, 32. [Google Scholar] [CrossRef]
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; et al. Uveal melanoma. Nat. Rev. Dis. Primers 2020, 6, 24. [Google Scholar] [CrossRef]
- Khoja, L.; Atenafu, E.G.; Suciu, S.; Leyvraz, S.; Sato, T.; Marshall, E.; Keilholz, U.; Zimmer, L.; Patel, S.P.; Piperno-Neumann, S.; et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: An international rare cancers initiative (IRCI) ocular melanoma study. Ann. Oncol. 2019, 30, 1370–1380. [Google Scholar] [CrossRef]
- Lane, A.M.; Kim, I.K.; Gragoudas, E.S. Survival Rates in Patients After Treatment for Metastasis from Uveal Melanoma. JAMA Ophthalmol. 2018, 136, 981. [Google Scholar] [CrossRef] [PubMed]
- Olofsson Bagge, R.; Nelson, A.; Shafazand, A.; Cahlin, C.; Carneiro, A.; Helgadottir, H.; Levin, M.; Rizell, M.; Ullenhag, G.; Wirén, S.; et al. A phase Ib randomized multicenter trial of isolated hepatic perfusion in combination with ipilimumab and nivolumab for uveal melanoma metastases (SCANDIUM II trial). ESMO Open 2024, 9, 103623. [Google Scholar] [CrossRef] [PubMed]
- Orloff, M. Clinical Trials in Metastatic Uveal Melanoma: Immunotherapy. Ocul. Oncol. Pathol. 2021, 7, 168. [Google Scholar] [CrossRef]
- Bolling, J.P.; Dagan, R.; Rutenberg, M.; Mamalui-Hunter, M.; Buskirk, S.J.; Heckman, M.G.; Hochwald, A.P.; Slopsema, R. Treatment of Uveal Melanoma With Radioactive Iodine 125 Implant Compared with Proton Beam Radiotherapy. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 6, 27. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Sacco, J.J.; Jager, M.J.; Eschelman, D.J.; Olofsson Bagge, R.; Harbour, J.W.; Chieng, N.D.; Patel, S.P.; Joshua, A.M.; Piperno-Neumann, S. Advances in the clinical management of uveal melanoma. Nat. Rev. Clin. Oncol. 2023, 20, 99–115. [Google Scholar] [CrossRef]
- Özcan, G.; Gündüz, A.K.; Mirzayev, İ.; Oysul, K.; Uysal, H. Early results of stereotactic radiosurgery in uveal melanoma and risk factors for radiation retinopathy. Turk. J. Ophthalmol. 2020, 50, 156–162. [Google Scholar] [CrossRef]
- Damato, B.; Kacperek, A.; Errington, D.; Heimann, H. Proton beam radiotherapy of uveal melanoma. Saudi J. Ophthalmol. 2013, 27, 151. [Google Scholar] [CrossRef]
- Davies, C.; Brown, S.L.; Fisher, P.; Hope-Stone, L.; Fisher, D.; Morgan, A.; Cherry, M.G. Predictors of emotional distress in uveal melanoma survivors: A systematic review. Eye 2022, 37, 907–924. [Google Scholar] [CrossRef]
- Brown, S.L.; Fisher, P.L.; Hope-Stone, L.; Hussain, R.N.; Heimann, H.; Damato, B.; Cherry, M.G. Predictors of long-term anxiety and depression in uveal melanoma survivors: A cross-lagged five-year analysis. Psychooncology 2020, 29, 1864–1873. [Google Scholar] [CrossRef]
- Nathan, P.; Cohen, V.; Coupland, S.; Curtis, K.; Damato, B.; Evans, J.; Fenwick, S.; Kirkpatrick, L.; Li, O.; Marshall, E.; et al. Uveal Melanoma UK National Guidelines. Eur. J. Cancer 2015, 51, 2404–2412. [Google Scholar] [CrossRef]
- Hussain, R.N.; Chiu, A.; Pittam, B.; Taktak, A.; Damato, B.E.; Kacperek, A.; Errington, D.; Cauchi, P.; Chadha, V.; Connolly, J.; et al. Proton beam radiotherapy for choroidal and ciliary body melanoma in the UK—National audit of referral patterns of 1084 cases. Eye 2022, 37, 1033–1036. [Google Scholar] [CrossRef]
- Kent, D.; Noonan, C.P.; Damato, B.E. Management of Irish patients with intraocular melanoma referred to Liverpool, England. Acta Ophthalmol. Scand. 1998, 76, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Daftari, I.K.; Renner, T.R.; Verhey, L.J.; Singh, R.P.; Nyman, M.; Petti, P.L.; Castro, J.R. New UCSF proton ocular beam facility at the Crocker Nuclear Laboratory Cyclotron (UC Davis). Nucl. Instrum. Methods Phys. Res. A 1996, 380, 597–612. [Google Scholar] [CrossRef]
- Sayan, M.; Mamidanna, S.; Oncel, D.; Jan, I.; Vergalasova, I.; Weiner, J.; Ohri, N.; Acikalin, B.; Chundury, A. Clinical management of uveal melanoma: A comprehensive review with a treatment algorithm. Radiat. Oncol. J. 2020, 38, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Dendale, R.; Lumbroso-Le Rouic, L.; Noel, G.; Feuvret, L.; Levy, C.; Delacroix, S.; Meyer, A.; Nauraye, C.; Mazal, A.; Mammar, H.; et al. Proton beam radiotherapy for uveal melanoma: Results of Curie Institut–Orsay Proton Therapy Center (ICPO). Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 780–787. [Google Scholar] [CrossRef]
- Wang, S.; Gonzalez, G.; Sun, L.; Xu, Y.; Pandey, P.; Chen, Y.; Xiang, S. (Liangzhong). Real-time tracking of the Bragg peak during proton therapy via 3D protoacoustic Imaging in a clinical scenario. NPJ Imaging 2024, 2, 34. [Google Scholar] [CrossRef]
- Byun, H.K.; Han, M.C.; Yang, K.; Kim, J.S.; Yoo, G.S.; Koom, W.S.; Kim, Y.B. Physical and Biological Characteristics of Particle Therapy for Oncologists. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2021, 53, 611. [Google Scholar] [CrossRef]
- Wilkinson, B.; Hill, M.A.; Parsons, J.L. The Cellular Response to Complex DNA Damage Induced by Ionising Radiation. Int. J. Mol. Sci. 2023, 24, 4920. [Google Scholar] [CrossRef]
- Jarczak, J.; Karska-Basta, I.; Romanowska-Dixon, B. Deterioration of Visual Acuity after Brachytherapy and Proton Therapy of Uveal Melanoma, and Methods of Counteracting This Complication Based on Recent Publications. Medicina 2023, 59, 1131. [Google Scholar] [CrossRef]
- Damato, B.E. Treatment selection for uveal melanoma. Dev. Ophthalmol. 2012, 49, 16–26. [Google Scholar] [CrossRef]
- Chan, A.W.; Lin, H.; Yacoub, I.; Chhabra, A.M.; Choi, J.I.; Simone, C.B., 2nd. Proton Therapy in Uveal Melanoma. Cancers 2024, 16, 3497. [Google Scholar] [CrossRef]
- Kosydar, S.; Robertson, J.C.; Woodfin, M.; Mayr, N.A.; Sahgal, A.; Timmerman, R.D.; Lo, S.S. Systematic Review and Meta-Analysis on the Use of Photon-based Stereotactic Radiosurgery Versus Fractionated Stereotactic Radiotherapy for the Treatment of Uveal Melanoma. Am. J. Clin. Oncol. 2021, 44, 32–42. [Google Scholar] [CrossRef]
- Wang, Z.; Nabhan, M.; Schild, S.E.; Stafford, S.L.; Petersen, I.A.; Foote, R.L.; Murad, M.H. Charged particle radiation therapy for uveal melanoma: A systematic review and meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 18–26. [Google Scholar] [CrossRef]
- Bekkering, G.E.; Rutjes, A.W.S.; Vlassov, V.V.; Aebersold, D.M.; Von Bremen, K.; Jüni, P.; Kleijnen, J. The effectiveness and safety of proton radiation therapy for indications of the eye: A systematic review. Strahlenther. Und Onkol. 2009, 185, 211–221. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Sari, S.Y.; Zorlu, F.; Yazici, G. External Beam Radiotherapy in the Management of Uveal Melanoma. Curr. Treat. Options Oncol. 2024, 25, 932. [Google Scholar] [CrossRef] [PubMed]
- Egger, E.; Zografos, L.; Schalenbourg, A.; Beati, D.; Böhringer, T.; Chamot, L.; Goitein, G. Eye retention after proton beam radiotherapy for uveal melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Damato, B.; Kacperek, A.; Chopra, M.; Campbell, I.R.; Errington, R.D. Proton beam radiotherapy of choroidal melanoma: The Liverpool-Clatterbridge experience. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Caujolle, J.P.; Mammar, H.; Chamorey, E.; Pinon, F.; Herault, J.; Gastaud, P. Proton beam radiotherapy for uveal melanomas at nice teaching hospital: 16 years’ experience. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 98–103. [Google Scholar] [CrossRef]
- Macdonald, E.C.A.; Cauchi, P.; Kemp, E.G. Proton beam therapy for the treatment of uveal melanoma in Scotland. Br. J. Ophthalmol. 2011, 95, 1691–1695. [Google Scholar] [CrossRef]
- Tran, E.; Ma, R.; Paton, K.; Blackmore, E.; Pickles, T. Outcomes of Proton Radiation Therapy for Peripapillary Choroidal Melanoma at the BC Cancer Agency. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1425–1431. [Google Scholar] [CrossRef]
- Seibel, I.; Cordini, D.; Rehak, M.; Hager, A.; Riechardt, A.I.; Böker, A.; Heufelder, J.; Weber, A.; Gollrad, J.; Besserer, A.; et al. Local Recurrence After Primary Proton Beam Therapy in Uveal Melanoma: Risk Factors, Retreatment Approaches, and Outcome. Am. J. Ophthalmol. 2015, 160, 628–636. [Google Scholar] [CrossRef]
- Bensoussan, E.; Thariat, J.; Maschi, C.; Delas, J.; Schouver, E.D.; Hérault, J.; Baillif, S.; Caujolle, J.P. Outcomes After Proton Beam Therapy for Large Choroidal Melanomas in 492 Patients. Am. J. Ophthalmol. 2016, 165, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, L.; Groenewald, C.; Coupland, S.E.; Damato, B. Trans-scleral local resection of toxic choroidal melanoma after proton beam radiotherapy. Br. J. Ophthalmol. 2014, 98, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Damato, B.; Duke, C.; Coupland, S.E.; Hiscott, P.; Smith, P.A.; Campbell, I.; Douglas, A.; Howard, P. Cytogenetics of Uveal Melanoma: A 7-Year Clinical Experience. Ophthalmology 2007, 114, 1925–1931.e1. [Google Scholar] [CrossRef]
- Dogrusöz, M.; Bagger, M.; Van Duinen, S.G.; Kroes, W.G.; Ruivenkamp, C.A.L.; Böhringer, S.; Andersen, K.K.; Luyten, G.P.M.; Kiilgaard, J.F.; Jager, M.J. The prognostic value of AJCC staging in uveal melanoma is enhanced by adding chromosome 3 and 8q status. Invest. Ophthalmol. Vis. Sci. 2017, 58, 833–842. [Google Scholar] [CrossRef]
- Dogrusöz, M.; Jager, M.J. Genetic prognostication in uveal melanoma. Acta Ophthalmol. 2018, 96, 331–347. [Google Scholar] [CrossRef]
- Rodriguez-Vidal, C.; Fernandez-Diaz, D.; Fernandez-Marta, B.; Lago-Baameiro, N.; Pardo, M.; Silva, P.; Paniagua, L.; Blanco-Teijeiro, M.J.; Piñeiro, A.; Bande, M. Treatment of Metastatic Uveal Melanoma: Systematic Review. Cancers 2020, 12, 2557. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Shih, J.; Yau, C.; Gibb, E.A.; Oba, J.; Mungall, K.L.; Hess, J.M.; Uzunangelov, V.; Walter, V.; Danilova, L.; et al. Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer Cell 2017, 32, 204. [Google Scholar] [CrossRef]
- Thornton, S.; Coupland, S.E.; Heimann, H.; Hussain, R.; Groenewald, C.; Kacperek, A.; Damato, B.; Taktak, A.; Eleuteri, A.; Kalirai, H. Effects of plaque brachytherapy and proton beam radiotherapy on prognostic testing: A comparison of uveal melanoma genotyped by microsatellite analysis. Br. J. Ophthalmol. 2020, 104, 1462–1466. [Google Scholar] [CrossRef]
- Davis, A.J.; Chen, D.J. Complex DSBs: A need for resection. Cell Cycle 2014, 13, 3796. [Google Scholar] [CrossRef]
- Melia, E.; Parsons, J.L. DNA damage and repair dependencies of ionising radiation modalities. Biosci. Rep. 2023, 43, BSR20222586. [Google Scholar] [CrossRef]
- Collins, A.R.; Ai-guo, M.; Duthie, S.J. The kinetics of repair of oxidative DNA damage (strand breaks and oxidised pyrimidines) in human cells. Mutat. Res.-DNA Repair 1995, 336, 69–77. [Google Scholar] [CrossRef]
- Dianov, G.L.; Hübscher, U. Mammalian Base Excision Repair: The Forgotten Archangel. Nucleic Acids Res. 2013, 41, 3483. [Google Scholar] [CrossRef]
- Caldecott, K.W. Causes and consequences of DNA single-strand breaks. Trends Biochem. Sci. 2024, 49, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Gohil, D.; Sarker, A.H.; Roy, R. Base Excision Repair: Mechanisms and Impact in Biology, Disease, and Medicine. Int. J. Mol. Sci. 2023, 24, 14186. [Google Scholar] [CrossRef] [PubMed]
- Vitti, E.T.; Parsons, J.L. The Radiobiological Effects of Proton Beam Therapy: Impact on DNA Damage and Repair. Cancers 2019, 11, 946. [Google Scholar] [CrossRef]
- Symington, L.S.; Gautier, J. Double-strand break end resection and repair pathway choice. Annu. Rev. Genet. 2011, 45, 247–271. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Yu, T.; Yi, Q.; Du, Y.; Zhou, L.; Zhao, Y.; Wu, Y.; Wu, L.; Wang, T.; Bian, P. High-complexity of DNA double-strand breaks is key for alternative end-joining choice. Commun. Biol. 2024, 7, 936. [Google Scholar] [CrossRef] [PubMed]
- Bétermier, M.; Bertrand, P.; Lopez, B.S. Is Non-Homologous End-Joining Really an Inherently Error-Prone Process? PLoS Genet. 2014, 10, e1004086. [Google Scholar] [CrossRef]
- Sadoughi, F.; Mirsafaei, L.; Dana, P.M.; Hallajzadeh, J.; Asemi, Z.; Mansournia, M.A.; Montazer, M.; Hosseinpour, M.; Yousefi, B. The role of DNA damage response in chemo- and radio-resistance of cancer cells: Can DDR inhibitors sole the problem? DNA Repair 2021, 101, 103074. [Google Scholar] [CrossRef]
- Moynahan, M.E.; Jasin, M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat. Rev. Mol. Cell Biol. 2010, 11, 196–207. [Google Scholar] [CrossRef]
- Fabbrizi, M.R.; Nickson, C.M.; Hughes, J.R.; Robinson, E.A.; Vaidya, K.; Rubbi, C.P.; Kacperek, A.; Bryant, H.E.; Helleday, T.; Parsons, J.L. Targeting OGG1 and PARG radiosensitises head and neck cancer cells to high-LET protons through complex DNA damage persistence. Cell Death Dis. 2024, 15, 150. [Google Scholar] [CrossRef]
- Carter, R.J.; Nickson, C.M.; Thompson, J.M.; Kacperek, A.; Hill, M.A.; Parsons, J.L. Characterisation of Deubiquitylating Enzymes in the Cellular Response to High-LET Ionizing Radiation and Complex DNA Damage. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 656–665. [Google Scholar] [CrossRef]
- Nickoloff, J.A.; Sharma, N.; Taylor, L. Clustered DNA Double-Strand Breaks: Biological Effects and Relevance to Cancer Radiotherapy. Genes 2020, 11, 99. [Google Scholar] [CrossRef]
- Bright, S.J.; Manandhar, M.; Flint, D.B.; Kolachina, R.; Ben Kacem, M.; Martinus, D.K.J.; Turner, B.X.; Qureshi, I.; McFadden, C.H.; Marinello, P.C.; et al. ATR inhibition radiosensitizes cells through augmented DNA damage and G2 cell cycle arrest abrogation. JCI Insight 2024, 9, e179599. [Google Scholar] [CrossRef] [PubMed]
- Gerelchuluun, A.; Zhu, J.; Su, F.; Asaithamby, A.; Chen, D.J.; Tsuboi, K. Homologous recombination pathway may play a major role in high-LET radiation-induced DNA double-strand break repair. J. Radiat. Res. 2014, 55, i83. [Google Scholar] [CrossRef]
- Kashyap, S.; Jha, J.; Singh, M.K.; Singh, L.; Sen, S.; Kaur, J.; Bajaj, M.S.; Pushker, N. DNA damage response proteins and its role in tumor progression of uveal melanoma with patient outcome. Clin. Transl. Oncol. 2020, 22, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Dogrusöz, M.; Trasel, A.R.; Cao, J.; Çolak, S.; van Pelt, S.I.; Kroes, W.G.M.; Teunisse, A.F.A.S.; Alsafadi, S.; van Duinen, S.G.; Luyten, G.P.M.; et al. Differential Expression of DNA Repair Genes in Prognostically-Favorable versus Unfavorable Uveal Melanoma. Cancers 2019, 11, 1104. [Google Scholar] [CrossRef]
- Jha, J.; Singh, M.K.; Singh, L.; Pushker, N.; Bajaj, M.S.; Sen, S.; Kashyap, S. Prognostic relevance of ATM protein in uveal melanoma and its association with clinicopathological factors. Int. J. Clin. Oncol. 2019, 24, 1526–1535. [Google Scholar] [CrossRef]
- Hussain, R.N.; Coupland, S.E.; Khzouz, J.; Kalirai, H.; Parsons, J.L. Inhibition of ATM Increases the Radiosensitivity of Uveal Melanoma Cells to Photons and Protons. Cancers 2020, 12, 1388. [Google Scholar] [CrossRef]
- Lühr, A.; von Neubeck, C.; Pawelke, J.; Seidlitz, A.; Peitzsch, C.; Bentzen, S.M.; Bortfeld, T.; Debus, J.; Deutsch, E.; Langendijk, J.A.; et al. “Radiobiology of Proton Therapy”: Results of an international expert workshop. Radiother. Oncol. 2018, 128, 56–67. [Google Scholar] [CrossRef]
- Roy, A.; Bera, S.; Saso, L.; Dwarakanath, B.S. Role of autophagy in tumor response to radiation: Implications for improving radiotherapy. Front. Oncol. 2022, 12, 957373. [Google Scholar] [CrossRef]
- Wang, J.Z.; Paulus, P.; Niu, Y.; Zhu, L.; Morisseau, C.; Rawling, T.; Murray, M.; Hammock, B.D.; Zhou, F. The Role of Autophagy in Human Uveal Melanoma and the Development of Potential Disease Biomarkers and Novel Therapeutic Paradigms. Biomedicines 2024, 12, 462. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, M.; Bhatt, A.N.; Das, A.; Dwarakanath, B.S.; Sharma, K. Radiation-induced autophagy: Mechanisms and consequences. Free Radic. Res. 2016, 50, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.M.; Fok, M.; Grundy, G.; Parsons, J.L.; Rocha, S. The Role of Autophagy in Hypoxia-Induced Radioresistance. Radiother. Oncol. 2023, 189, 109951. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.H.; Sohal, S.S.; Manjili, M.H.; Harrell, J.C.; Gewirtz, D.A. The Roles of Autophagy and Senescence in the Tumor Cell Response to Radiation. Radiat. Res. 2020, 194, 103. [Google Scholar] [CrossRef]
- Li, L.; Liu, W.-L.; Su, L.; Lu, Z.-C.; He, X.-S. The Role of Autophagy in Cancer Radiotherapy. Curr. Mol. Pharmacol. 2019, 13, 31–40. [Google Scholar] [CrossRef]
- Liu, S.Z.; Yao, S.J.; Yang, H.; Liu, S.J.; Wang, Y.J. Autophagy: Regulator of cell death. Cell Death Dis. 2023, 14, 648. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Hirata, N.; Tanaka, T.; Suizu, F.; Nakajima, H.; Chiorini, J.A. Autophagy as a modulator of cell death machinery. Cell Death Dis. 2020, 11, 517. [Google Scholar] [CrossRef]
- Giatromanolaki, A.N.; St Charitoudis, G.; Bechrakis, N.E.; Kozobolis, V.P.; Koukourakis, M.I.; Foerster, M.H.; Sivridis, E.L. Autophagy patterns and prognosis in uveal melanomas. Mod. Pathol. 2011, 24, 1036–1045. [Google Scholar] [CrossRef]
- Truong, A.; Yoo, J.H.; Scherzer, M.T.; Sanchez, J.M.S.; Dale, K.J.; Kinsey, C.G.; Richards, J.R.; Shin, D.; Ghazi, P.C.; Onken, M.D.; et al. Chloroquine sensitizes GNAQ/11-mutated melanoma to MEK1/2 inhibition. Clin. Cancer Res. 2020, 26, 6374–6386. [Google Scholar] [CrossRef]
- Zhao, J.; Yi, Q.; Li, K.; Chen, L.; Dai, L.; Feng, J.; Li, Y.; Zhou, M.; Sun, J. A multi-omics deep learning model for hypoxia phenotype to predict tumor aggressiveness and prognosis in uveal melanoma for rationalized hypoxia-targeted therapy. Comput. Struct. Biotechnol. J. 2022, 20, 3182–3194. [Google Scholar] [CrossRef]
- Beckers, C.; Pruschy, M.; Vetrugno, I. Tumor hypoxia and radiotherapy: A major driver of resistance even for novel radiotherapy modalities. Semin. Cancer Biol. 2024, 98, 19–30. [Google Scholar] [CrossRef]
- Boulefour, W.; Rowinski, E.; Louati, S.; Sotton, S.; Wozny, A.S.; Moreno-Acosta, P.; Mery, B.; Rodriguez-Lafrasse, C.; Magne, N. A Review of the Role of Hypoxia in Radioresistance in Cancer Therapy. Med. Sci. Monit. 2021, 27, e934116-1. [Google Scholar] [CrossRef]
- D-Kondo, N.; Masilela, T.A.M.; Shin, W.G.; Faddegon, B.; LaVerne, J.; Schuemann, J.; Ramos-Mendez, J. Modeling the oxygen effect in DNA strand break induced by gamma-rays with TOPAS-nBio. Phys. Med. Biol. 2024, 69, 215028. [Google Scholar] [CrossRef]
- Sun, J.; Ding, J.; Yue, H.; Xu, B.; Sodhi, A.; Xue, K.; Ren, H.; Qian, J. Hypoxia-induced BNIP3 facilitates the progression and metastasis of uveal melanoma by driving metabolic reprogramming. Autophagy 2024, 21, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Lee, D.; Lee, S.A. BAP1 as a guardian of genome stability: Implications in human cancer. Exp. Mol. Med. 2023, 55, 745–754. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Yang, L.; Li, H. Hypoxia, a key factor in the immune microenvironment. Biomed. Pharmacother. 2022, 151, 113068. [Google Scholar] [CrossRef]
- Chen, S.; Sang, N. Hypoxia-Inducible Factor-1: A critical player in the survival strategy of stressed cells. J. Cell Biochem. 2016, 117, 267. [Google Scholar] [CrossRef]
- Paraskeva, E.; Haj Zen, A.; Zuheir Bakleh, M.; Al Haj Zen, A. The Distinct Role of HIF-1α and HIF-2α in Hypoxia and Angiogenesis. Cells 2025, 14, 673. [Google Scholar] [CrossRef]
- Asnaghi, L.; Lin, M.H.; Lim, K.S.; Lim, K.J.; Tripathy, A.; Wendeborn, M.; Merbs, S.L.; Handa, J.T.; Sodhi, A.; Bar, E.E.; et al. Hypoxia Promotes Uveal Melanoma Invasion through Enhanced Notch and MAPK Activation. PLoS ONE 2014, 9, e105372. [Google Scholar] [CrossRef]
- Zemba, M.; Dumitrescu, O.M.; Gheorghe, A.G.; Radu, M.; Ionescu, M.A.; Vatafu, A.; Dinu, V. Ocular Complications of Radiotherapy in Uveal Melanoma. Cancers 2023, 15, 333. [Google Scholar] [CrossRef]
- Lane, A.M.; Hartley, C.; Go, A.K.; Wu, F.; Gragoudas, E.S.; Kim, I.K. Survival of patients with recurrent uveal melanoma after treatment with radiation therapy. Br. J. Ophthalmol. 2024, 108, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.X.; Zhou, P.K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target. Ther. 2020, 5, 60. [Google Scholar] [CrossRef]
- Blackford, A.N.; Jackson, S.P. ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol. Cell 2017, 66, 801–817. [Google Scholar] [CrossRef] [PubMed]
- Doherty, R.E.; Bryant, H.E.; Valluru, M.K.; Rennie, I.G.; Sisley, K. Increased Non-Homologous End Joining Makes DNA-PK a Promising Target for Therapeutic Intervention in Uveal Melanoma. Cancers 2019, 11, 1278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, H.; Hao, J.; Wu, Y.; Yang, B. Inhibition of DNA-PKcs activity re-sensitizes uveal melanoma cells to radio- and chemotherapy. Biochem. Biophys. Res. Commun. 2020, 522, 639–646. [Google Scholar] [CrossRef]
- Rose, M.; Burgess, J.T.; O’Byrne, K.; Richard, D.J.; Bolderson, E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front. Cell Dev. Biol. 2020, 8, 564601. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, H.; Lord, C.J.; Tutt, A.H.J.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005, 434, 913–917. [Google Scholar] [CrossRef]
- Louie, B.H.; Kurzrock, R. BAP1: Not just a BRCA1-associated protein. Cancer Treat. Rev. 2020, 90, 102091. [Google Scholar] [CrossRef] [PubMed]
- de Koning, L.; Decaudin, D.; Botty, R.; Nicolas, A.; Carita, G.; Schuller, M.; Ouine, B.; Cartier, A.; Naguez, A.; Fleury, J.; et al. PARP Inhibition Increases the Response to Chemotherapy in Uveal Melanoma. Cancers 2019, 11, 751. [Google Scholar] [CrossRef]
- Bland, P.; Saville, H.; Wai, P.T.; Curnow, L.; Muirhead, G.; Nieminuszczy, J.; Ravindran, N.; John, M.B.; Hedayat, S.; Barker, H.E.; et al. SF3B1 hotspot mutations confer sensitivity to PARP inhibition by eliciting a defective replication stress response. Nat. Genet. 2023, 55, 1311–1323. [Google Scholar] [CrossRef]
- Zhu, X.; Zou, W.; Meng, X.; Ji, J.; Wang, X.; Shu, H.; Chen, Y.; Pan, D.; Wang, K.; Zhou, F. Elaiophylin Inhibits Tumorigenesis of Human Uveal Melanoma by Suppressing Mitophagy and Inducing Oxidative Stress via Modulating SIRT1/FoxO3a Signaling. Front. Oncol. 2022, 12, 788496. [Google Scholar] [CrossRef] [PubMed]
- Karasic, T.B.; O’Hara, M.H.; Loaiza-Bonilla, A.; Reiss, K.A.; Teitelbaum, U.R.; Borazanci, E.; De Jesus-Acosta, A.; Redlinger, C.; Burrell, J.A.; Laheru, D.A.; et al. Effect of Gemcitabine and nab-Paclitaxel with or Without Hydroxychloroquine on Patients With Advanced Pancreatic Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Anandharaj, A.; Cinghu, S.; Park, W.Y. Rapamycin-mediated mTOR inhibition attenuates survivin and sensitizes glioblastoma cells to radiation therapy. Acta Biochim. Biophys. Sin. 2011, 43, 292–300. [Google Scholar] [CrossRef]
- Kim, E.J.; Jeong, J.H.; Bae, S.; Kang, S.; Kim, C.H.; Lim, Y.B. mTOR inhibitors radiosensitize PTEN-deficient non-small-cell lung cancer cells harboring an EGFR activating mutation by inducing autophagy. J. Cell Biochem. 2013, 114, 1248–1256. [Google Scholar] [CrossRef]
- Li, Q.; Xia, L.; Sun, C.; Zhang, H.; Zheng, M.; Zhang, H.; Lu, H.; Wang, Z. Role of Borneol Induced Autophagy in Enhancing Radiosensitivity of Malignant Glioma. Front. Oncol. 2021, 11, 749987. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, W.M.; Morris, C.G.; Amdur, R.J.; Mendenhall, N.P.; Siemann, D.W. Radiotherapy alone or combined with carbogen breathing for squamous cell carcinoma of the head and neck. Cancer 2005, 104, 332–337. [Google Scholar] [CrossRef]
- Dong, L.; You, S.; Zhang, Q.; Osuka, S.; Devi, N.S.; Kaluz, S.; Ferguson, J.H.; Yang, H.; Chen, G.; Wang, B.; et al. Arylsulfonamide 64B inhibits hypoxia/HIF-induced expression of c-Met and CXCR4 and reduces primary tumor growth and metastasis of uveal melanoma. Clin. Cancer Res. 2018, 25, 2206. [Google Scholar] [CrossRef]
- Cheng, B.; Ma, X.; Zhou, Y.; Liu, J.; Fei, X.; Pan, W.; Peng, X.; Wang, W.; Chen, J. Recent progress in the development of hypoxia-inducible factor 2α (HIF-2α) modulators: Inhibitors, agonists, and degraders (2009–2024). Eur. J. Med. Chem. 2024, 275, 116645. [Google Scholar] [CrossRef]
- Patel, S.A.; Simon, M.C. Biology of Hypoxia-Inducible Factor-2α in Development and Disease. Cell Death Differ. 2008, 15, 628. [Google Scholar] [CrossRef]
- Nystrom, H.; Jensen, M.F.; Nystrom, P.W. Treatment planning for proton therapy: What is needed in the next 10 years? Br. J. Radiol. 2020, 93, 20190304. [Google Scholar] [CrossRef]
- Parker, T.; Rigney, G.; Kallos, J.; Stefko, S.T.; Kano, H.; Niranjan, A.; Green, A.L.; Aziz, T.; Rath, P.; Lunsford, L.D. Gamma knife radiosurgery for uveal melanomas and metastases: A systematic review and meta-analysis. Lancet Oncol. 2020, 21, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Fleury, E.; Pignol, J.P.; Kiliç, E.; Milder, M.; van Rij, C.; Naus, N.; Yavuzyigitoglu, S.; den Toom, W.; Zolnay, A.; Spruijt, K.; et al. Comparison of stereotactic radiotherapy and protons for uveal melanoma patients. Phys. Imaging Radiat. Oncol. 2024, 31, 100605. [Google Scholar] [CrossRef] [PubMed]
- Semeniuk, O.; Yu, E.; Rivard, M.J. Current and Emerging Radiotherapy Options for Uveal Melanoma. Cancers 2024, 16, 1074. [Google Scholar] [CrossRef]
- Hughes, J.R.; Parsons, J.L. FLASH Radiotherapy: Current Knowledge and Future Insights Using Proton-Beam Therapy. Int. J. Mol. Sci. 2020, 21, 6492. [Google Scholar] [CrossRef]
- Di Marco, B.; Sansevero, G.; D’Orsi, B.; De Santis, E.; Salamone, G.; Cavalieri, A.; Masturzo, L.; Celentano, M.; Di Martino, F.; Capaccioli, S.; et al. Unraveling the effects of FLASH and conventional irradiation on retinal pigment epithelial cells: In vitro and in vivo studies. Sci. Rep. 2025, 15, 22938. [Google Scholar] [CrossRef] [PubMed]
- Hrbacek, J.; Kacperek, A.; Beenakker, J.W.M.; Mortimer, L.; Denker, A.; Mazal, A.; Shih, H.A.; Dendale, R.; Slopsema, R.; Heufelder, J.; et al. PTCOG Ocular Statement: Expert Summary of Current Practices and Future Developments in Ocular Proton Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, 1307–1325. [Google Scholar] [CrossRef] [PubMed]
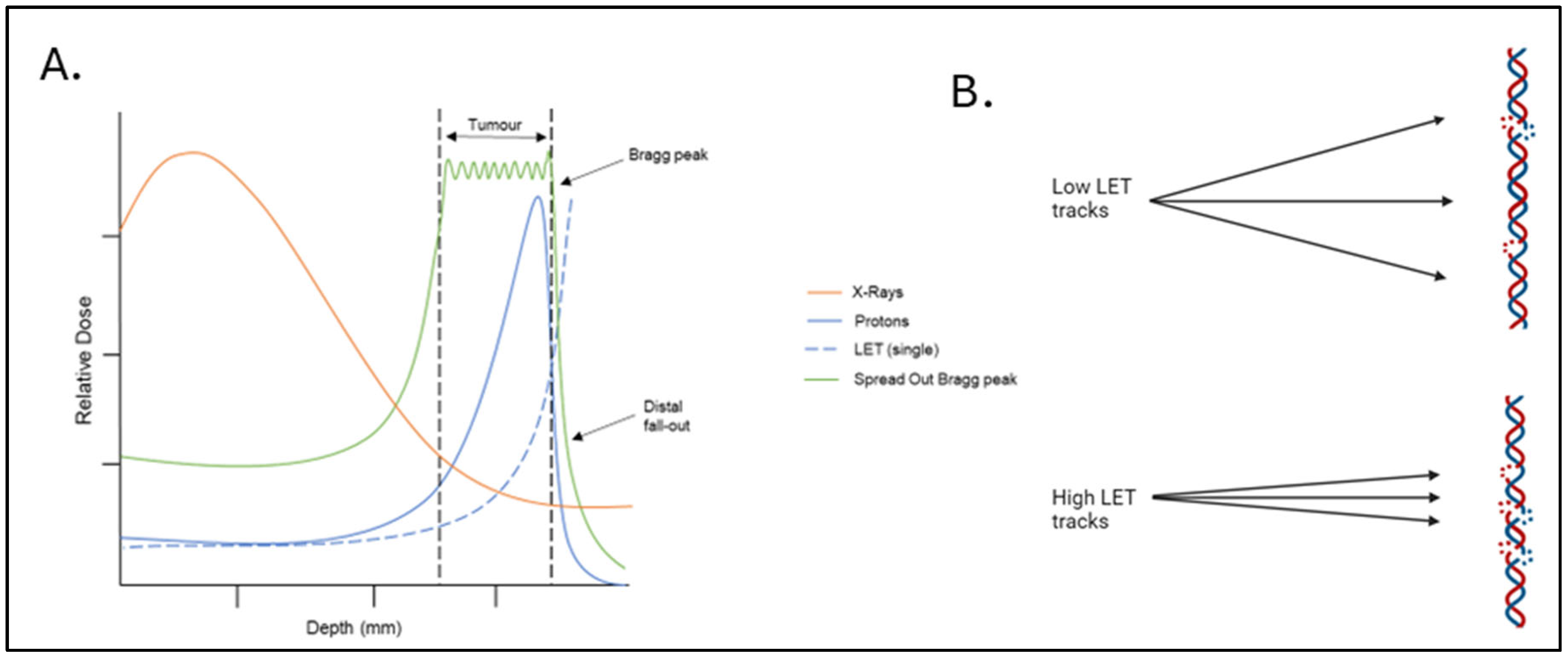


| Time | Patient Numbers | Dose | Median Follow up | Local Control | Eye Preservation | Overall Survival | Toxicity | Ref |
|---|---|---|---|---|---|---|---|---|
| 1984–1999 | 2648 | 60 Gy in 5 fractions | 24 months | 5-year 95.8% 10-year 94.8% | 91.8% | 10-year 73% | [29] | |
| 1993–2003 | 349 | 53.1 Gy in 4 fractions | 37 months | 5-year 96.5% | 92% | 5-year 90% | 17.9% glaucoma 12.8% rubeosis 16.7% pain 9% vitreous haemorrhage 38% retinal detachment | [30] |
| 1991–2001 | 1406 | 60 Gy in 4 fractions | 73 months | 5-year 96% | 92.3% | 5-year 79% | 66.5% maculopathy 23.4% papillopathy 28.6% glaucoma 61.8% cataract 11.5% keratitis 13.9% vitreous haemorrhage | [18] |
| 1991–2007 | 886 | 60 Gy in 4 fractions | 64 months | 5-year 93.9% 10-year 92.1% | 92.2% | 5-year 79.4% 10-year 64.1% | 7% glaucoma 27.5% retinopathy 31.7% cataract | [31] |
| 1993–2008 | 147 | 58 Gy in 4 fractions | 53 months | 5-year 71.3% | 5-year 87.8% | [32] | ||
| 1995–2007 | 59 | 60 Gy in 4 fractions | 125 months | 5-year 91% | 5-year 93.2% | 5-year 85% | 74% radiation retinopathy 64% optic nerve neuropathy 29.6% cataract | [33] |
| 1998–2008 | 982 | 60 Gy in 4 fractions | 60.7 months | 5-year 96% 10-year 94% | 5-year 95% | 5-year 80% 10-year 60% | 12.1% neovascular glaucoma | [34] |
| 1991–2015 | 492 | 60 Gy in 4 fractions | 61.9 months | 5-year 60% 10-year 47% | 80.5% | 39.8% cataracts 27% neovascular glaucoma 23.4% retinopathy | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawkins, L.; Kalirai, H.; Aughton, K.; Hussain, R.N.; Coupland, S.E.; Parsons, J.L. Understanding and Exacerbating the Biological Response of Uveal Melanoma to Proton Beam Therapy. Cancers 2025, 17, 3104. https://doi.org/10.3390/cancers17193104
Hawkins L, Kalirai H, Aughton K, Hussain RN, Coupland SE, Parsons JL. Understanding and Exacerbating the Biological Response of Uveal Melanoma to Proton Beam Therapy. Cancers. 2025; 17(19):3104. https://doi.org/10.3390/cancers17193104
Chicago/Turabian StyleHawkins, Laura, Helen Kalirai, Karen Aughton, Rumana N. Hussain, Sarah E. Coupland, and Jason L. Parsons. 2025. "Understanding and Exacerbating the Biological Response of Uveal Melanoma to Proton Beam Therapy" Cancers 17, no. 19: 3104. https://doi.org/10.3390/cancers17193104
APA StyleHawkins, L., Kalirai, H., Aughton, K., Hussain, R. N., Coupland, S. E., & Parsons, J. L. (2025). Understanding and Exacerbating the Biological Response of Uveal Melanoma to Proton Beam Therapy. Cancers, 17(19), 3104. https://doi.org/10.3390/cancers17193104









