Sentinel Lymph Node Biopsy Versus Elective Neck Dissection in Carcinoma of the Tongue and Floor of the Mouth
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design/Sample
2.2. Variables
2.3. Surgical Management
2.4. Covariates
2.5. Data Collection Methods
2.6. Data Analyses
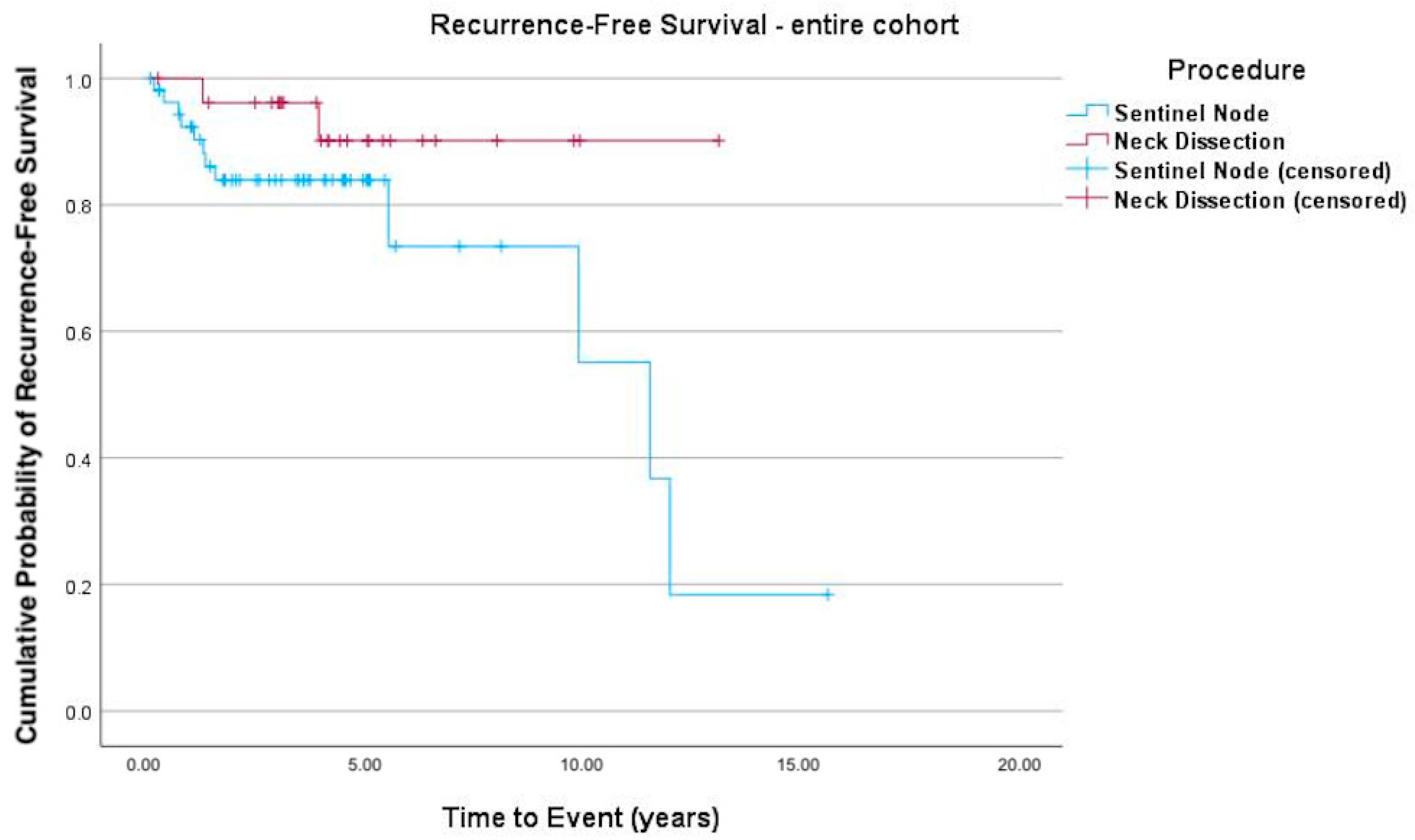
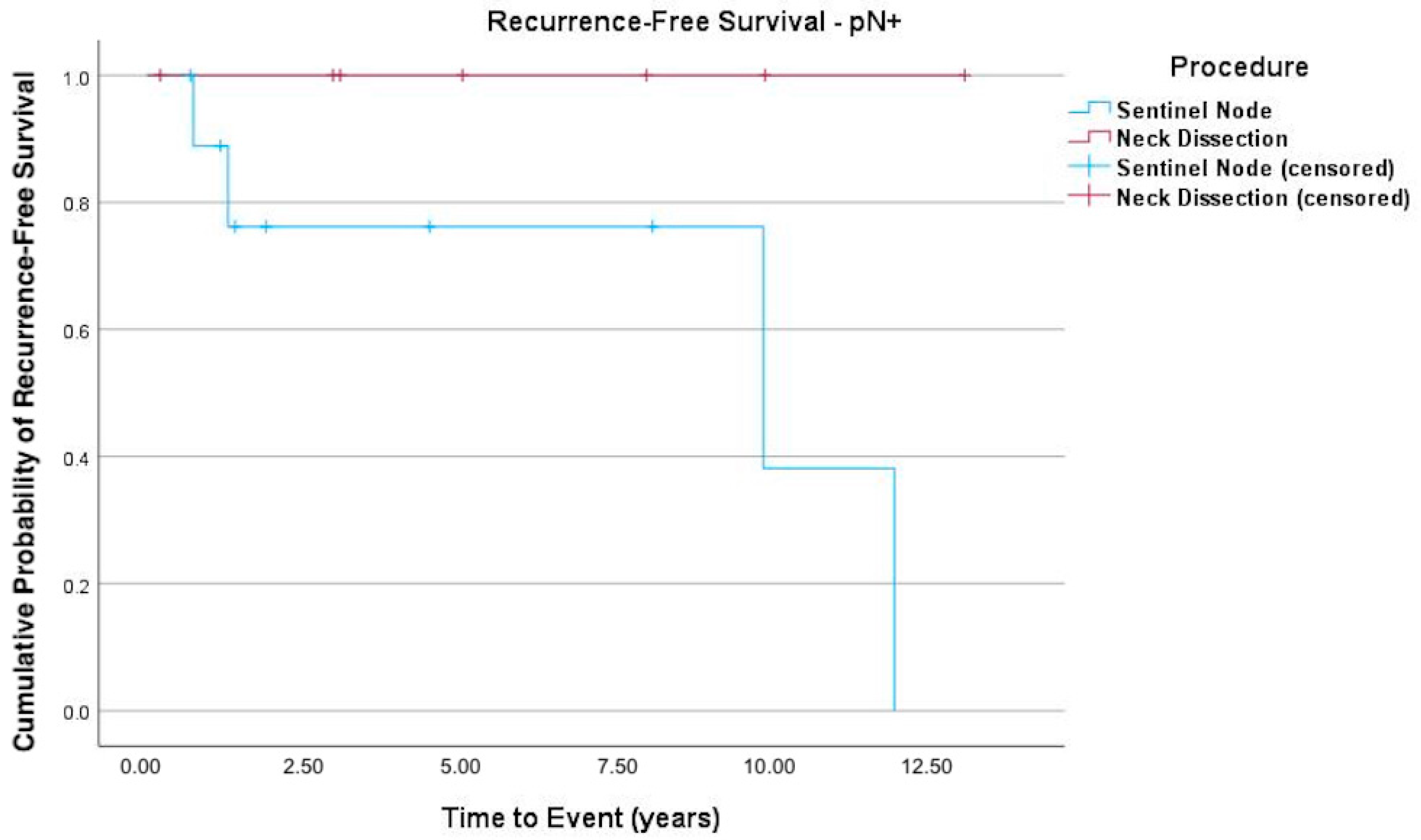
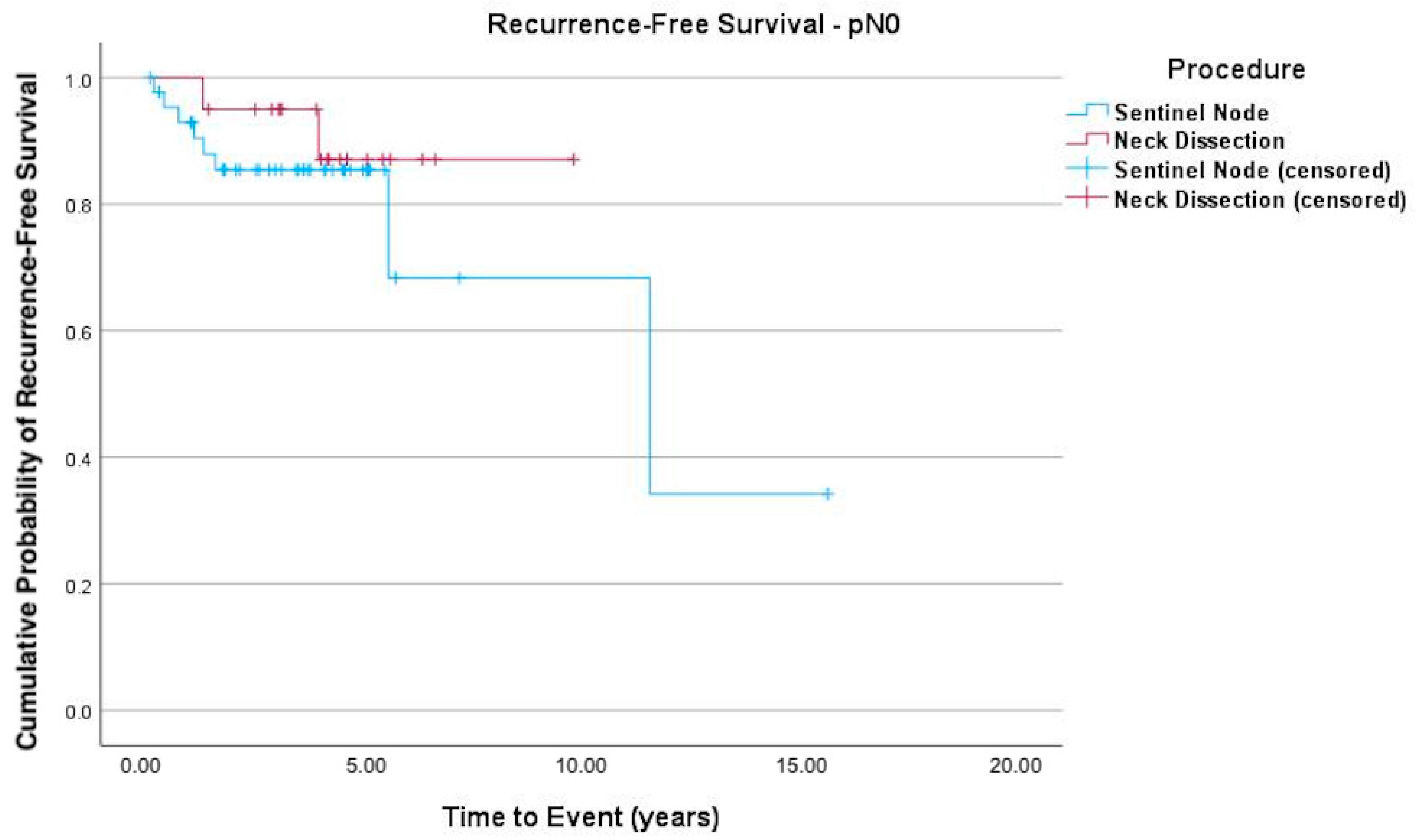
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, J.H.; Iyer, N.G.; Tan, M.H.; Edgren, G. Changing epidemiology of oral squamous cell carcinoma of the tongue: A global study. Head Neck 2017, 39, 297–304. [Google Scholar] [CrossRef]
- Mamelle, G.; Pampurik, J.; Luboinski, B.; Lancar, R.; Lusinchi, A.; Bosq, J. Lymph node prognostic factors in head and neck squamous cell carcinomas. Am. J. Surg. 1994, 168, 494–498. [Google Scholar] [CrossRef]
- Hori, Y.; Kubota, A.; Yokose, T.; Furukawa, M.; Matsushita, T.; Takita, M.; Mitsunaga, S.; Mizoguchi, N.; Nonaka, T.; Nakayama, Y.; et al. Predictive significance of tumor depth and budding for late lymph node metastases in patients with clinical N0 early oral tongue carcinoma. Head Neck 2017, 11, 477–486. [Google Scholar]
- Sharma, A.; Kim, J.W.; Paeng, J.Y. Clinical analysis of neck node metastasis in oral cavity cancer. J. Korean Assoc. Oral Maxillofac. Surg. 2018, 44, 282–288. [Google Scholar] [CrossRef]
- Psychogios, G.; Mantsopoulos, K.; Bohr, C.; Koch, M.; Zenk, J.; Iro, H. Incidence of occult cervical metastasis in head and neck carcinomas: Development over time. J. Surg. Oncol. 2013, 107, 384–387. [Google Scholar] [CrossRef]
- Lim, Y.C.; Lee, J.S.; Koo, B.S.; Kim, S.H.; Kim, Y.H.; Choi, E.C. Treatment of contralateral N0 neck in early squamous cell carcinoma of the oral tongue: Elective neck dissection versus observation. Laryngoscope 2006, 116, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Broglie, M.A.; Haerle, S.K.; Huber, G.F.; Haile, S.R.; Stoeckli, S.J. Occult metastases detected by sentinel node biopsy in patients with early oral and oropharyngeal squamous cell carcinomas: Impact on survival. Head Neck 2013, 35, 660–666. [Google Scholar] [PubMed]
- Kelner, N.; Vartanian, J.G.; Pinto, C.A.; Coutinho-Camillo, C.M.; Kowalski, L.P. Does elective neck dissection in T1/T2 carcinoma of the oral tongue and floor of the mouth influence recurrence and survival rates? Br. J. Oral Maxillofac. Surg. 2014, 52, 590–597. [Google Scholar] [CrossRef] [PubMed]
- de Bree, R.; de Keizer, B.; Civantos, F.J.; Takes, R.P.; Rodrigo, J.P.; Hernandez-Prera, J.C.; Halmos, G.B.; Rinaldo, A.; Ferlito, A. What is the role of sentinel lymph node biopsy in the management of oral cancer in 2020? Eur. Arch. Otorhinolaryngol. 2021, 278, 3181–3191. [Google Scholar]
- Stoeckli, S.J.; Pfaltz, M.; Ross, G.L.; Steinert, H.C.; MacDonald, D.G.; Wittekind, C.; Soutar, D.S. The second international conference on sentinel node biopsy in mucosal head and neck cancer. Ann. Surg. Oncol. 2005, 12, 919–924. [Google Scholar] [CrossRef]
- Hermanek, P.; Hutter, R.V.; Sobin, L.H.; Wittekind, C. International union against cancer. classification of isolated tumor cells and micrometastasis. Cancer 1999, 86, 2668–2673. [Google Scholar] [PubMed]
- Gera, R.; Kasem, A.; Mokbel, K. Can complete axillary node dissection be safely omitted in patients with early breast cancer when the sentinel node biopsy is positive for malignancy? An update for clinical practice. In Vivo 2018, 32, 1301–1307. [Google Scholar] [CrossRef]
- Park, K.U.; Caudle, A. Management of the axilla in the patient with breast cancer. Surg. Clin. N. Am. 2018, 98, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Kumar Tyagi, N.; Dhesy-Thirnd, S. Clinical practice guidelines in breast cancer. Curr. Oncol. 2018, 25 (Suppl. 1), 151–160. [Google Scholar] [CrossRef]
- Holden, A.M.; Sharma, D.; Schilling, C.; Gnanasegaran, G.; Odell, E.; Sassoon, I.; McGurk, M. Biopsy of the sentinel lymph node in oral squamous cell carcinoma: Analysis of error in 100 consecutive cases. Br. J. Oral Maxillofac. Surg. 2018, 56, 615–620. [Google Scholar] [CrossRef]
- Sloan, P. Head and neck sentinel lymph node biopsy: Current state of the art. Head Neck Pathol. 2009, 3, 231–237. [Google Scholar] [CrossRef]
- Hingsammer, L.; Seier, T.; Zweifel, D.; Huber, G.; Rücker, M.; Bredell, M.; Lanzer, M. Sentinel lymph node biopsy for early stage tongue cancer—A 14-year single-centre experience. Int. J. Oral Maxillofac. Surg. 2018, 48, 437–442. [Google Scholar] [CrossRef]
- Flach, G.B.; Bloemena, E.; Klop, W.M.; van Es, R.J.; Schepman, K.-P.; Hoekstra, O.S.; Castelijns, J.A.; Leemans, C.R.; de Bree, R. Sentinel lymph node biopsy in clinically N0 T1-T2 staged oral cancer: The Dutch multicenter trial. Oral Oncol. 2014, 50, 1020–1024. [Google Scholar] [CrossRef]
- Schilling, C.; Steockli, S.J.; Haerle, S.K.; Broglie, M.A.; Huber, G.F.; Sorensen, J.A.; Bakholdt, V.; Krogdahl, A.; von Buchwald, C.; Bilde, A.; et al. Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer. Eur. J. Cancer 2015, 51, 2777–2784. [Google Scholar] [CrossRef]
- Govers, T.M.; Hannink, G.; Merkx, M.A.; Takes, R.P.; Rovers, M.M. Sentinel node biopsy for squamous cell carcinoma of the oral cavity and oropharynx: A diagnostic meta-analysis. Oral Oncol. 2013, 49, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Loree, J.T.; Popat, S.R.; Burke, M.S.; Frustino, J.; Grewal, J.S.; Loree, T.R. Sentinel lymph node biopsy for management of the N0 neck in oral cavity squamous cell carcinoma. J. Surg. Oncol. 2019, 120, 101–108. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.J.; Yang, X.; Peng, H. Diagnostic efficacy of sentinel lymph node biopsy in early oral squamous cell carcinoma: A meta-analysis of 66 studies. PLoS ONE 2017, 12, e0170322. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Li, Y.; Pan, X.; Xuan, M.; Xie, H.; Wang, X. Entinel lymph node biopsy versus elective neck dissection in squamous cell carcinoma of the oral cavity with a clinically N0 neck: Systematic review and meta-analysis of prospective studies. Head Neck 2010, 10, 3185–3198. [Google Scholar]
- den Toom, I.J.; Boeve, K.; Lobeek, D.; Bloemena, E.; Donswijk, M.L.; de Keizer, B.; Klop, W.M.C.; Leemans, C.R.; Willems, S.M.; Takes, R.P.; et al. Elective neck dissection or sentinel lymph node biopsy in early stage oral cavity cancer patients: The Dutch experience. Cancers 2020, 12, 1783. [Google Scholar] [CrossRef]
- Thomsen, J.B.; Sørensen, J.A.; Krogdahl, A. Sentinel lymph nodes in cancer of the oral cavity—Isolated tumour cells. J. Oral Pathol. Med. 2005, 34, 65–69. [Google Scholar] [CrossRef]
- Dhawan, I.; Sandhu, S.V.; Bhandari, R.; Sood, N.; Bhullar, R.K.; Sethi, N. Detection of cervical lymph node micrometastasis and isolated tumor cells in oral squamous cell carcinoma using immunohistochemistry and serial sectioning. J. Oral Maxillofac. Pathol. 2016, 20, 436–444. [Google Scholar] [CrossRef]
- Montpellier, U.H. Randomized, Open-Label Economic and Medical Study on the Lymph Node Management of Squamous Cell Carcinoma of the Oral Cavity and Oropharynx Tumor Stage 1 or 2, Nodes 0 (T1-T2 N0) Operable (SentiMERORL). National Library of Medicine. 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02855723 (accessed on 26 November 2018).
- Yao, S.; Shen, P.; Dai, F.; Deng, L.; Qiu, X.; Zhao, Y.; Gao, M.; Zhang, H.; Zheng, X.; Yu, X.; et al. Thyroid Cancer Central Lymph Node Metastasis Risk Stratification Based on Homogeneous Positioning Deep Learning. Research 2024, 7, 0432. [Google Scholar] [CrossRef] [PubMed]
- Bark, R.; Kolev, A.; Elliot, A.; Piersiala, K.; Näsman, A.; Grybäck, P.; Georén, S.K.; Wendt, M.; Cardell, L.O.; Margolin, G.; et al. Sentinel node-assisted neck dissection in advanced oral squamous cell carcinoma—A new protocol for staging and treatment. Cancer Med. 2023, 12, 12524–12534. [Google Scholar] [CrossRef] [PubMed]
| SLNB (n = 55) | END (n = 27) | Total (n = 82) | ||
|---|---|---|---|---|
| Age at surgery | 57.2 (47.2–70.8) | 59.9 (53.7–72.6) | 58.9 (51.4–71.5) | |
| Male | 31 (56.4%) | 15 (55.6%) | 46 (56.1%) | |
| Cancer location | Floor of the mouth | 13 (23.6%) | 18 (66.7%) | 31 (37.8%) |
| Tongue | 42 (76.4%) | 9 (33.3%) | 51 (62.2%) | |
| Total | 55 (100%) | 27 (100%) | 82 (100%) | |
| Touching midline | 13 (23.6%) | 9 (33.3%) | 22 (26.8%) | |
| Laterality | Left | 25 (45.5%) | 12 (44.4%) | 37 (45.1%) |
| Center | 4 (7.3%) | 1 (3.7%) | 5 (6.1%) | |
| Right | 26 (47.3%) | 14 (51.9%) | 40 (48.8%) | |
| pT classification | pT1 | 44 (80.0%) | 17 (63.0%) | 61 (74.4%) |
| pT2 | 11 (20.0%) | 10 (37.0%) | 21 (25.6%) | |
| pN classification | pN0 | 45 (81.8%) | 20 (74.1%) | 65 (79.3%) |
| pN1 | 4 (7.3%) | 3 (11.1%) | 7 (8.5%) | |
| pN2a | 1 (1.8%) | 0 | 1 (1.2%) | |
| pN2b | 3 (5.5%) | 4 (14.8%) | 7 (8.5%) | |
| pN2c | 1 (1.8%) | 0 | 1 (1.2%) | |
| NA | 1 (1.8%) | 0 | 1 (1.2%) | |
| R0 resection | 53 (96.4%) | 27 (100%) | 80 (97.6%) | |
| Grade | G1 | 17 (30.9%) | 1 (3.7%) | 18 (22.0%) |
| G2 | 30 (54.6%) | 17 (63.0%) | 47 (57.3%) | |
| G3 | 8 (14.5%) | 9 (33.3%) | 17 (20.7%) | |
| Postoperative radiotherapy | 3 (5.5%) | 14 (51.9%) | 17 (20.7%) | |
| Total recurrence | 13 (23.6%) | 2 (7.4%) | 15 (18.3%) | |
| Recurrence in lymph nodes | 3 (5.5%) | 1 (3.7%) | 4 (4.9%) | |
| Time to recurrence (d) | 1240 (476–1807) | 1506 (1094–2035) | 1326 (631–1847) | |
| Sex | Date of Surgery | Procedure (1 = Sentinel; 2 = ND) | pT | pN | Time to Recurrence (Months) | Location of Recurrence |
|---|---|---|---|---|---|---|
| m | 20.02.2014 | 1 | 1 | 0 | 8.3 | tongue |
| m | 19.10.2009 | 1 | 1 | 0 | 66.4 | floor of the mouth |
| m | 25.11.2003 | 1 | 1 | 1 | 109 | floor of the mouth |
| m | 19.07.2012 | 2 | 1 | 0 | 47.1 | floor of the mouth |
| m | 19.05.2008 | 1 | 1 | 3 | 144.3 | tongue |
| m | 12.05.2009 | 1 | 1 | 0 | 138.9 | tongue and floor of the mouth |
| m | 17.12.2013 | 1 | 2 | 1 | 9 | lymph node level II |
| m | 06.11.2015 | 1 | 1 | 0 | 12.5 | tongue |
| m | 13.02.2018 | 1 | 2 | 0 | 1.5 | lymph node levels I, II, III, IV, V |
| f | 03.09.2018 | 1 | 1 | 3 | 15.7 | soft tissue level VI/VII |
| m | 21.12.2007 | 1 | 1 | 0 | 4.2 | floor of the mouth |
| f | 20.12.2016 | 2 | 2 | 0 | 15 | lymph node level III |
| f | 21.05.2019 | 1 | 1 | 0 | 18.0 | anterior mandible |
| m | 25.10.2019 | 1 | 1 | 0 | 15.1 | lymph node level Ib |
| SLNB | END | |
| Total recurrence, n (%) | 13 (23.6) | 2 (7.4) |
| Site of first recurrence | ||
| Local (tongue/floor of the mouth) | 6 | 1 |
| Regional/nodal (levels I-V/VI/VII) | 5 | 1 |
| Local + regional | 1 | 0 |
| Distant | 1 | 0 |
| Median time to recurrence (months) | 23.6 (IQR 9–67) | 31.1 (IQR 15–47) |
| Soft tissue level VI/VII |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naegeli-Pullankavumkal, C.; Manser, T.; Mehra, T.; Rupp, N.J.; Gander, T.; Huellner, M.W.; Lanzer, M. Sentinel Lymph Node Biopsy Versus Elective Neck Dissection in Carcinoma of the Tongue and Floor of the Mouth. Cancers 2025, 17, 3098. https://doi.org/10.3390/cancers17193098
Naegeli-Pullankavumkal C, Manser T, Mehra T, Rupp NJ, Gander T, Huellner MW, Lanzer M. Sentinel Lymph Node Biopsy Versus Elective Neck Dissection in Carcinoma of the Tongue and Floor of the Mouth. Cancers. 2025; 17(19):3098. https://doi.org/10.3390/cancers17193098
Chicago/Turabian StyleNaegeli-Pullankavumkal, Carolin, Tamara Manser, Tarun Mehra, Niels Jan Rupp, Thomas Gander, Martin W. Huellner, and Martin Lanzer. 2025. "Sentinel Lymph Node Biopsy Versus Elective Neck Dissection in Carcinoma of the Tongue and Floor of the Mouth" Cancers 17, no. 19: 3098. https://doi.org/10.3390/cancers17193098
APA StyleNaegeli-Pullankavumkal, C., Manser, T., Mehra, T., Rupp, N. J., Gander, T., Huellner, M. W., & Lanzer, M. (2025). Sentinel Lymph Node Biopsy Versus Elective Neck Dissection in Carcinoma of the Tongue and Floor of the Mouth. Cancers, 17(19), 3098. https://doi.org/10.3390/cancers17193098







