Bladder Cancer: Uncovering the Predictive Role of NOTCH as an Emerging Candidate Biomarker for Therapeutic Strategies
Simple Summary
Abstract
1. Introduction
2. Classification, Clinical Aspects, and Therapy
3. Biomarker in Bladder Cancer
4. Molecular Mutations in NMIBC
5. Molecular Mutations in MIBC and Metastatic Disease
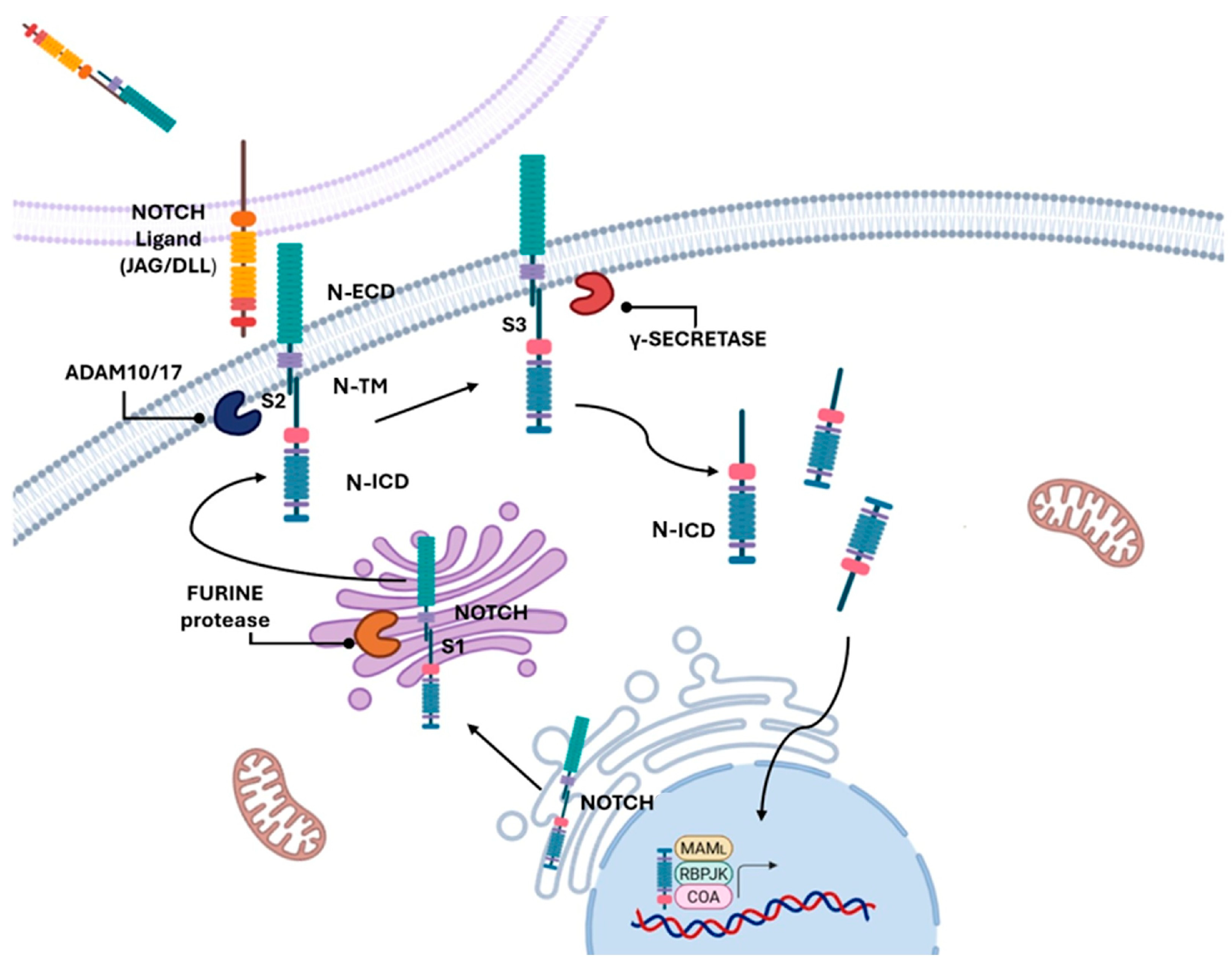
6. The NOTCH Pathway as a Biomarker and Therapeutic Target
7. NOTCH1
8. NOTCH2
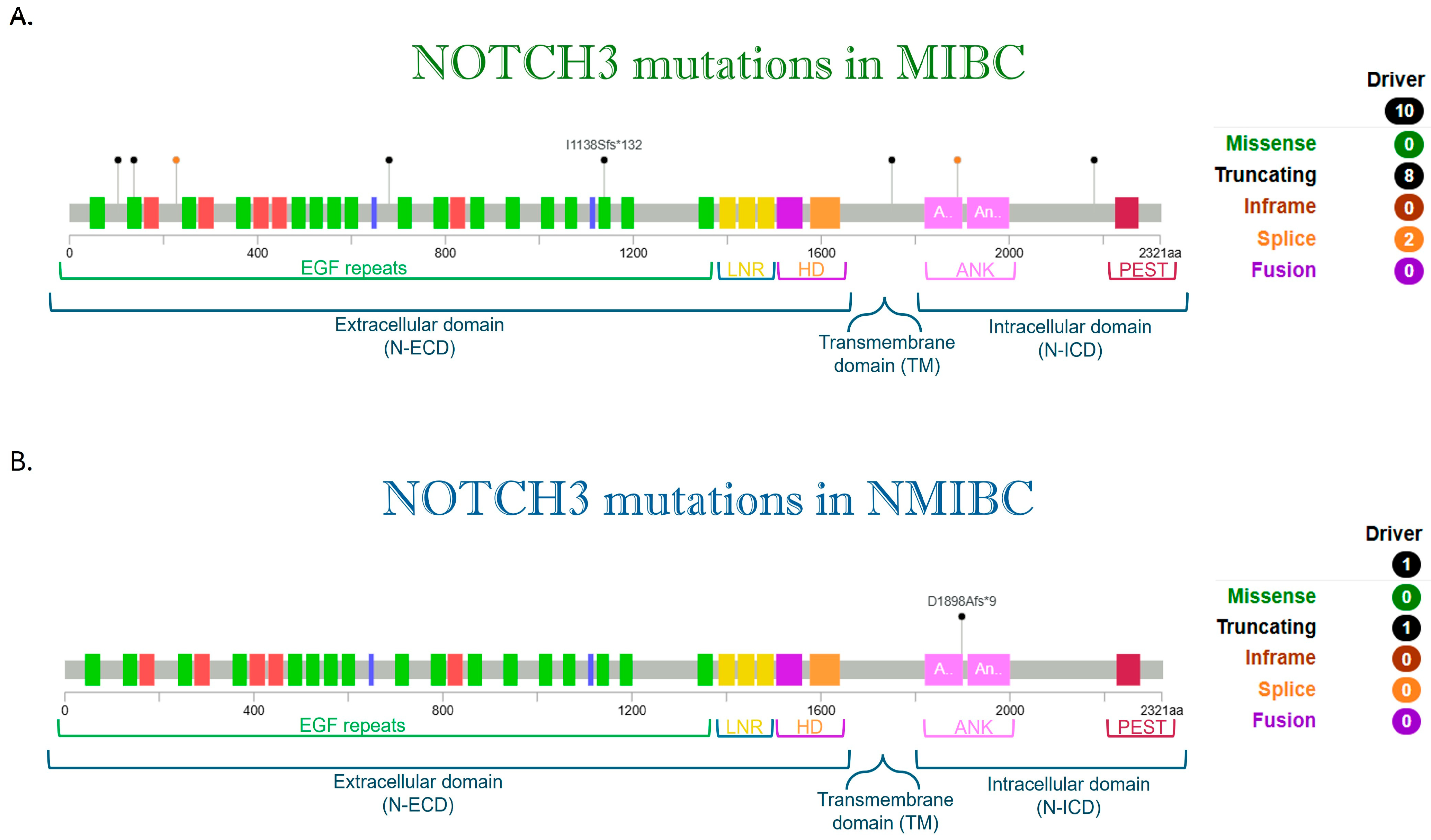
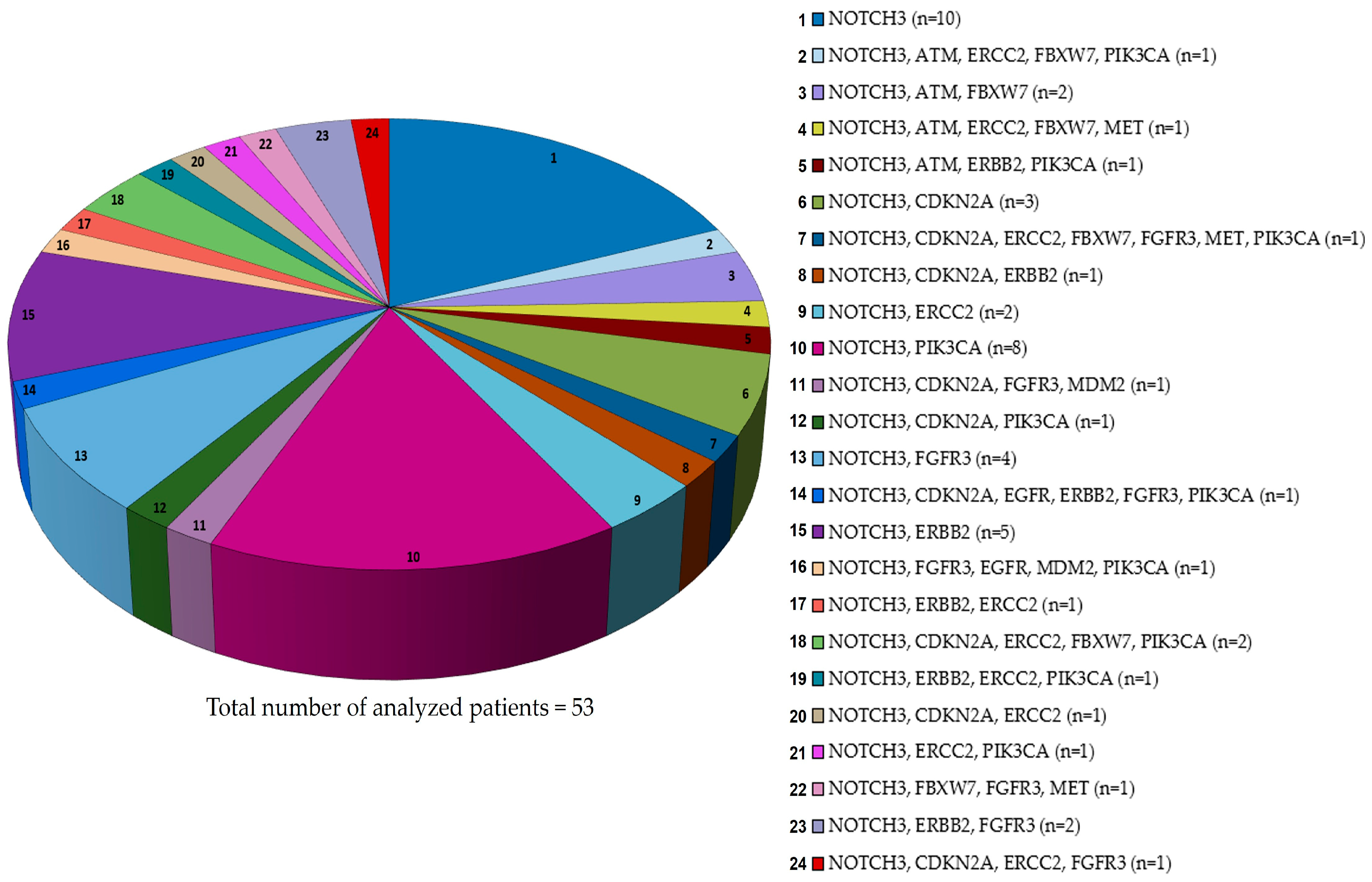
9. NOTCH3
10. NOTCH 4
11. NOTCH-Combined Therapy in Bladder Cancer
12. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Freedman, N.D.; Silverman, D.T.; Holllenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Association Between Smoking and Risk of Bladder Cancer Among Men and Women. JAMA 2011, 306, 737. [Google Scholar] [CrossRef]
- Seyyedsalehi, M.S.; Bonetti, M.; Shah, D.; De Stefano, V.; Boffetta, P. Occupational Benzene Exposure and Risk of Kidney and Bladder Cancers: A Systematic Review and Meta-Analysis. Eur. J. Cancer Prev. 2025, 34, 205–213. [Google Scholar] [CrossRef]
- Alouini, S. Risk Factors Associated with Urothelial Bladder Cancer. Int. J. Environ. Res. Public Health 2024, 21, 954. [Google Scholar] [CrossRef]
- Mantica, G.; Terrone, C.; Van Der Merwe, A. Bladder Cancer and Associated Risk Factors: The African Panorama. Eur. Urol. 2021, 79, 568–570. [Google Scholar] [CrossRef]
- Dobruch, J. Bladder Cancer: Current Challenges and Future Directions. Medicina 2021, 57, 749. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.E.; Adamo, M.P.; Sun, L.; Deorah, S. Bladder Cancer Collaborative Stage Variables and Their Data Quality, Usage, and Clinical Implications: A Review of SEER Data, 2004–2010. Cancer 2014, 120 (Suppl. 23), 3815–3825. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulos, A.D.; Kochergin, M.; Colalillo, G.; Fahmy, O.; Hassan, F.; Renninger, M.; Gallioli, A.; Gavrilov, P.; Gakis, G. New Intravesical Agents for BCG-Unresponsive High-Risk Non-Muscle Invasive Bladder Cancer Bladder. Bladder Cancer 2023, 9, 237–251. [Google Scholar]
- van Rhijn, B.W.; Hentschel, A.E.; Brundl, J.; Comperat, E.M.; Hernandez, V.; Capoun, O.; Bruins, H.M.; Cohen, D.; Roupret, M.; Shariat, S.; et al. Prognostic Value of the WHO1973 and WHO2004/2016 Classification Systems for Grade in Primary Ta/T1 Non-Muscle-Invasive Bladder Cancer: A Multicenter European Association of Urology Non-Muscle-Invasive Bladder Cancer Guidelines Panel Study. Eur. Urol. Oncol. 2021, 4, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, R.J.; van der Meijden, A.P.M.; Oosterlinck, W.; Witjes, J.A.; Denis, L.; Newling, D.W.; Kurth, K. Predicting Recurrence and Progression in Individual Patients with Stage TaT1 Bladder Cancer Using EORTC Risk Tables: A Combined Analysis of 2596 Patients from Seven EORTC Trials. Eur. Urol. 2006, 49, 466–477. [Google Scholar] [CrossRef]
- Palou, J.; Rodriguez-Rubio, F.; Millan, F.; Algaba, F.; Rodriguez-Faba, O.; Huguet, J.; Villavicencio, H. Recurrence at Three Months and High-Grade Recurrence as Prognostic Factor of Progression in Multivariate Analysis of T1G2 Bladder Tumors. Urology 2009, 73, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gomez, J.; Madero, R.; Solsona, E.; Unda, M.; Martinez-Pineiro, L.; Gonzalez, M.; Portillo, J.; Ojea, A.; Pertusa, C.; Rodriguez-Molina, G. Predicting Nonmuscle Invasive Bladder Cancer Recurrence and Progression in Patients Treated with Bacillus Calmette-Guerin: The CUETO Scoring Model. J. Urol. 2009, 182, 2195–2203. [Google Scholar] [CrossRef]
- Cambier, S.; Gontero, P.; Oddens, J. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-Specific and Overall Survival in Non-Muscle-Invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1–3 Years of Maintenance Bacillus Calmette-Guerin. Eur. Urol. 2016, 69, e123–e124. [Google Scholar] [CrossRef]
- Ślusarczyk, A.; Zapała, P.; Zapała, Ł.; Borkowski, T.; Radziszewski, P. Cancer-Specific Survival of Patients with Non-Muscle-Invasive Bladder Cancer: A Population-Based Analysis. Ann. Surg. Oncol. 2023, 30, 7892–7902. [Google Scholar] [CrossRef]
- Asimakopoulos, A.D.; Colalillo, G.; Telesca, R.; Mauriello, A.; Miano, R.; Di Stasi, S.M.; Germani, S.; Finazzi Agrò, E.; Petrozza, V.; Caruso, G.; et al. T1 Bladder Cancer: Comparison of the Prognostic Impact of Two Substaging Systems on Disease Recurrence and Progression and Suggestion of a Novel Nomogram. Front. Surg. 2021, 8, 704902. [Google Scholar] [CrossRef]
- Sylvester, R.J.; Rodriguez, O.; Hernandez, V.; Turturica, D.; Bauerova, L.; Bruins Harman, M.; Brundl, J.; van der Kwast, T.H.; Brisuda, A.; Rubio-Briones, J.; et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non-Muscle-Invasive Bladder Cancer (NMIBC) Incorporating the WHO 2004/2016 and WHO 1973 Classification Systems for Grade: An Update from the EAU NMIBC Guidelines Panel. Eur. Urol. 2021, 79, 480–488. [Google Scholar] [CrossRef]
- Bishr, M.; Lattouf, J.-B.; Latour, M.; Saad, F. Tumour Stage on Re-Staging Transurethral Resection Predicts Recurrence and Progression-Free Survival of Patients with High-Risk Non-Muscle Invasive Bladder Cancer. Can. Urol. Assoc. J. 2014, 8, E306–E310. [Google Scholar] [CrossRef]
- Palou, G.; Sylvester, R.J.; Rodriguex Faba, O.; Parada, R.; Pena, J.A.; Algaba, F.; Villavicencio, H. Female Gender and Carcinoma in Situ in the Prostatic Urethra Are Prognostic Factors for Recurrence, Progression, and Disease-Specific Mortality in T1G3 Bladder Cancer Patients Treated with Bacillus Calmette-Guerin. Eur. Urol. 2012, 62, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, H.; Sun, G.; Zhang, X.; Zhao, J.; Liu, J.; Shen, P.; Shi, M.; Zeng, H. Comparison of the Prognosis of Primary and Progressive Muscle-Invasive Bladder Cancer after Radical Cystectomy: A Systematic Review and Meta-Analysis. Int. J. Surg. 2018, 52, 214–220. [Google Scholar] [CrossRef]
- Ge, P.; Wang, L.; Lu, M.; Mao, L.; Li, W.; Wen, R.; Lin, J.; Wang, J.; Chen, J. Oncological Outcome of Primary and Secondary Muscle-Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 7543. [Google Scholar] [CrossRef] [PubMed]
- Ditonno, F.; Veccia, A.; Montanaro, F.; Pettenuzzo, G.; Franco, A.; Manfredi, C.; Triggiani, L.; De Nunzio, C.; De Sio, M.; Cerruto, M.; et al. Trimodal Therapy vs Radical Cystectomy in Patients with Muscle-Invasive Bladder Cancer: A Systematic Review and Meta-Analysis of Comparative Studies. BJU Int. 2024, 134, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Vlaming, M.; Kiemeney, L.A.L.M.; Van Der Heijden, A.G. Survival after Radical Cystectomy: Progressive versus De Novo Muscle Invasive Bladder Cancer. Cancer Treat. Res. Commun. 2020, 25, 100264. [Google Scholar] [CrossRef]
- Fahmy, O.; Khairul-Asri, M.G.; Schubert, T.; Renninger, M.; Malek, R.; Kubler, H.; Stenzl, A.; Gakis, G. A Systematic Review and Meta-Analysis on the Oncological Long-Term Outcomes after Trimodality Therapy and Radical Cystectomy with or without Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer. Urol. Oncol. 2018, 36, 43–53. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Qin, C.; Zhang, C.; Du, Y.; Xu, T. Three versus Four Cycles of Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. Ann. Med. 2023, 55, 2281654. [Google Scholar] [CrossRef]
- Advanced Bladder Cancer Meta-analysis Collaboration. Neo-adjuvant Chemotherapy for Invasive Bladder Cancer. Cochrane Database Syst. Rev. 2004, 2004, CD005246. [Google Scholar] [CrossRef]
- Schuettfort, V.M.; Pradere, B.; Quhal, F.; Mostafaei, H.; Laukhtina, E.; Mori, K.; Motlagh, R.S.; Fisch, M.; D’Andrea, D.; Rink, M. Incidence and Outcome of Salvage Cystectomy after Bladder Sparing Therapy for Muscle Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. World J. Urol. 2021, 39, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Soukup, V.; Babjuk, M.; Bellmunt, J.; Dalbagni, G.; Giannarini, G.; Hakenberg, O.W.; Herr, H.; Lechevallier, E.; Ribal, M.J. Follow-up After Surgical Treatment of Bladder Cancer: A Critical Analysis of the Literature. Eur. Urol. 2012, 62, 290–302. [Google Scholar] [CrossRef]
- Donat, S.M. Staged Based Directed Surveillance of Invasive Bladder Cancer Following Radical Cystectomy: Valuable and Effective? World J. Urol. 2006, 24, 557–564. [Google Scholar] [CrossRef]
- Vrooman, O.P.J.; Witjes, J.A. Follow-up of Patients after Curative Bladder Cancer Treatment: Guidelines vs. Practice. Curr. Opin. Urol. 2010, 20, 437–442. [Google Scholar] [CrossRef]
- Novakova, V.Z.; Kuniakova, M.; Ziaran, S.; Harsanyi, S. Molecular Biomarkers of Bladder Cancer: A Mini-Review. Physiol. Res. 2023, 72, S247–S256. [Google Scholar] [CrossRef] [PubMed]
- Lindskrog, S.V.; Prip, F.; Lamy, P.; Taber, A.; Groeneveld, C.S.; Birkenkamp-Demtroder, K.; Bierggaard Jensen, J.; Strandgaard, T.; Nordentorft, I.; Christensen, E.; et al. An Integrated Multi-Omics Analysis Identifies Prognostic Molecular Subtypes of Non-Muscle-Invasive Bladder Cancer. Nat. Commun. 2021, 12, 2301. [Google Scholar] [CrossRef]
- Hedegaard, J.; Lamy, P.; Nordentorft, I.; Algaba, F.; Hoyer, S.; Parm Ulhoi, B.; Vang, S.; Reinert, T.; Hermann, G.G.; Mogensen, K.; et al. Comprehensive Transcriptional Analysis of Early-Stage Urothelial Carcinoma. Cancer Cell 2016, 30, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Hurst, C.D.; Cheng, G.; Platt, F.M.; Castro, M.A.; Marzouka, N.A.D.S.; Eriksson, P.; Black, E.V.; Alder, O.; Lawson, A.R.; Lindskrog, S.V.; et al. Stage-Stratified Molecular Profiling of Non-Muscle-Invasive Bladder Cancer Enhances Biological, Clinical, and Therapeutic Insight. Cell Rep. Med. 2021, 2, 100472. [Google Scholar] [CrossRef]
- Dyrskjøt, L.; Hansel, D.E.; Efstathiou, J.A.; Knwoles, M.A.; Galsky, M.D.; Teoh, J.; Theodorescu, D. Bladder Cancer. Nat. Rev. Dis. Primers 2023, 9, 58. [Google Scholar] [CrossRef]
- Keegan, K.; Johnson, D.E.; Williams, T.; Hayman, M. Isolation of an Additional Member of the Fibroblast Growth Factor Receptor Family, FGFR-3. Proc. Natl. Acad. Sci. USA 1991, 88, 1095–1099. [Google Scholar] [CrossRef]
- Harsanyi, S.; Novakova, Z.V.; Bevizova, K.; Danisovic, L.; Ziaran, S. Biomarkers of Bladder Cancer: Cell-Free DNA, Epigenetic Modifications and Non-Coding RNAs. Int. J. Mol. Sci. 2022, 23, 13206. [Google Scholar] [CrossRef] [PubMed]
- Helsten, T.; Schwaederle, M.; Kurzrock, R. Fibroblast Growth Factor Receptor Signaling in Hereditary and Neoplastic Disease: Biologic and Clinical Implications. Cancer Metastasis Rev. 2015, 34, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Ascione, C.M.; Napolitano, F.; Esposito, D.; Servetto, A.; Belli, S.; Santaniello, A.; Scagliarini, S.; Crocetto, F.; Bianco, R.; Formisano, L. Role of FGFR3 in Bladder Cancer: Treatment Landscape and Future Challenges. Cancer Treat. Rev. 2023, 115, 102530. [Google Scholar] [CrossRef]
- AACR Project GENIE Consortium. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831. [Google Scholar] [CrossRef]
- Al-Obaidy, K.I.; Cheng, L. Fibroblast Growth Factor Receptor (FGFR) Gene: Pathogenesis and Treatment Implications in Urothelial Carcinoma of the Bladder. J. Clin. Pathol. 2021, 74, 491–495. [Google Scholar] [CrossRef]
- Noeraparast, M.; Krajina, C.; Pichler, R.; Niedersub-Beke, D.; Shariat, S.F.; Grunwald, V.; Ahyai, S.; Pichler, M. FGFR3 Alterations in Bladder Cancer: Sensitivity and Resistance to Targeted Therapies. Cancer Commun. 2024, 44, 1189–1208. [Google Scholar] [CrossRef] [PubMed]
- Van Rhijn, B.W.G.; Mertens, L.S.; Mayr, R.; Bostrom, P.J.; Real, F.X.; Zwarthoff, E.C.; Boormans, J.L.; Abas, C.; Van Leenders, G.J.L.H.; Götz, S.; et al. FGFR3 Mutation Status and FGFR3 Expression in a Large Bladder Cancer Cohort Treated by Radical Cystectomy: Implications for Anti-FGFR3 Treatment? Eur. Urol. 2020, 78, 682–687. [Google Scholar] [CrossRef]
- Piao, C.; Cui, X.; BoZhan; Li, J.; Li, Z.; Liu, X.; Bi, J.; Zhang, Z.; Kong, C. Inhibition of Stearoyl CoA Desaturase-1 Activity Suppresses Tumour Progression and Improves Prognosis in Human Bladder Cancer. J. Cell Mol. Med. 2019, 23, 2064–2076. [Google Scholar] [CrossRef]
- Pandith, A.A.; Hussain, A.; Khan, M.S.; Shah, Z.A.; Wani, M.S.; Siddiqi, M.A. Oncogenic Activation of Fibroblast Growth Factor Receptor-3 and RAS Genes as Non-Overlapping Mutual Exclusive Events in Urinary Bladder Cancer. Asian Pac. J. Cancer Prev. 2016, 17, 2787–2793. [Google Scholar]
- Jebar, A.H.; Hurst, C.D.; Tomlinson, D.C.; Johnston, C.; Taylor, C.F.; Knowles, M.A. FGFR3 and Ras Gene Mutations Are Mutually Exclusive Genetic Events in Urothelial Cell Carcinoma. Oncogene 2005, 24, 5218–5225. [Google Scholar] [CrossRef] [PubMed]
- Juanpere, N.; Agel, L.; Lorenzo, M.; de Muga, S.; Lopez-Vilaro, L.; Murillo, R.; Mojal, S.; Serrano, S.; Lorente, J.A.; Lloreta, J.; et al. Mutations in FGFR3 and PIK3CA, Singly or Combined with RAS and AKT1, Are Associated with AKT but Not with MAPK Pathway Activation in Urothelial Bladder Cancer. Hum. Pathol. 2012, 43, 1573–1582. [Google Scholar] [CrossRef]
- Pecori, R.; Di Giorgio, S.; Lorenzo, J.P.; Papavasiliou, F.N. Functions and Consequences of AID/APOBEC-Mediated DNA and RNA Deamination. Nat. Rev. Genet. 2022, 23, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Naguib, E.M.; Ismail, E.; Badran, D.; Sherief, M.; El-Abaseri, T. Clinicopathological Significance of C-MET and HER2 Altered Expression in Bladder Cancer. J. Egypt. Natl. Canc. Inst. 2024, 36, 42. [Google Scholar] [CrossRef]
- Gordon, N.S.; Humayun-Zakaria, N.; Goel, A.; Abbotts, B.; Zeegers, M.P.; Cheng, K.K.; James, N.D.; Arnold, R.; Bryan, R.T.; Ward, D.G. STAG2 Protein Expression in Non–Muscle-Invasive Bladder Cancer: Associations with Sex, Genomic and Transcriptomic Changes, and Clinical Outcomes. Eur. Urol. Open Sci. 2022, 38, 88–95. [Google Scholar] [CrossRef]
- Bernasconi-Elias, P.; Hu, T.; Jenkins, D.; Firestone, B.; Gans, S.; Kurth, E.; Capodieci, P.; Deplazes-Lauber, J.; Petropoulos, K.; Thiel, P.; et al. Characterization of Activating Mutations of NOTCH3 in T-Cell Acute Lymphoblastic Leukemia and Anti-Leukemic Activity of NOTCH3 Inhibitory Antibodies. Oncogene 2016, 35, 6077–6086. [Google Scholar] [CrossRef]
- Australian Pancreatic Cancer Genome Initiative; ICGC Breast Cancer Consortium; ICGC MMML-Seq Consortium; ICGC PedBrain; Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; et al. Signatures of Mutational Processes in Human Cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef]
- Meeks, J.J.; Al-Ahmadie, H.; Faltas, B.M.; Taylor, J.A.; Flaig, T.W.; DeGraff, D.J.; Christensen, E.; Woolbright, B.L.; McConkey, D.J.; Dyrskjøt, L. Genomic Heterogeneity in Bladder Cancer: Challenges and Possible Solutions to Improve Outcomes. Nat. Rev. Urol. 2020, 17, 259–270. [Google Scholar] [CrossRef]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2018, 174, 1033. [Google Scholar] [CrossRef]
- Miyata, Y.; Sagara, Y.; Kanda, S.; Hayashi, T.; Kanetake, H. Phosphorylated Hepatocyte Growth Factor Receptor/c-Met Is Associated with Tumor Growth and Prognosis in Patients with Bladder Cancer: Correlation with Matrix Metalloproteinase-2 and -7 and E-Cadherin. Hum. Pathol. 2009, 40, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Zhdanovskaya, N.; Firrincieli, M.; Lazzari, S.; Pace, E.; Scribani Rossi, P.; Felli, M.P.; Talora, C.; Screpanti, I.; Palermo, R. Targeting Notch to Maximize Chemotherapeutic Benefits: Rationale, Advanced Strategies, and Future Perspectives. Cancers 2021, 13, 5106. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Damish, A.W.; Frazier, Z.; Liu, D.; Reznichenko, E.; Kamburov, A.; Bell, A.; Zhao, H.; Jordan, E.J.; Gao, S.P.; et al. ERCC2 Helicase Domain Mutations Confer Nucleotide Excision Repair Deficiency and Drive Cisplatin Sensitivity in Muscle-Invasive Bladder Cancer. Clin. Cancer Res. 2019, 25, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Imanirad, P.; Sreevalsan, S.; Nair, V.; Jutooru, I. Transcription Factor Sp1, Also Known as Specificity Protein 1 as a Therapeutic Target. Expert. Opin. Ther. Targets 2014, 18, 759–769. [Google Scholar] [CrossRef]
- Jiang, N.Y.; Woda, B.A.; Banner, B.F.; Whalen, G.F.; Dresser, K.A.; Lu, D. Sp1, a New Biomarker That Identifies a Subset of Aggressive Pancreatic Ductal Adenocarcinoma. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1648–1652. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, Z.; Zhou, F.; Cui, J.; Wang, L. High Co-Expression of Sp1 and HER-2 Is Correlated with Poor Prognosis of Gastric Cancer Patients. Surg. Oncol. 2015, 24, 220–225. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, Z.; Ke, M.; Cai, X. Sp1 Is Overexpressed and Associated with Progression and Poor Prognosis in Bladder Urothelial Carcinoma Patients. Int. Urol. Nephrol. 2022, 54, 1505–1512. [Google Scholar] [CrossRef]
- Pietzak, E.J.; Bagrodia, A.; Cha, E.K.; Drill, E.N.; Iyer, G.; Isharwal, S.; Ostrovnaya, I.; Baez, P.; Li, Q.; Berger, M.F.; et al. Next-Generation Sequencing of Nonmuscle Invasive Bladder Cancer Reveals Potential Biomarkers and Rational Therapeutic Targets. Eur. Urol. 2017, 72, 952–959. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Comprehensive Molecular Characterization of Urothelial Bladder Carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef]
- Goriki, A.; Seiler, R.; Wyatt, A.W.; Contreras-Sanz, A.; Bhat, A.; Matsubara, A.; Hayashi, T.; Black, P.C. Unravelling Disparate Roles of NOTCH in Bladder Cancer. Nat. Rev. Urol. 2018, 15, 345–357. [Google Scholar] [CrossRef]
- Tsaouli, G.; Barbarulo, A.; Vacca, A.; Screpanti, I.; Felli, M.P. Molecular Mechanisms of Notch Signaling in Lymphoid Cell Lineages Development: NF-κB and Beyond. Adv. Exp. Med. Biol. 2020, 1227, 145–164. [Google Scholar] [PubMed]
- Ranganathan, P.; Weaver, K.L.; Capobianco, A.J. Notch Signalling in Solid Tumours: A Little Bit of Everything but Not All the Time. Nat. Rev. Cancer 2011, 11, 338–351. [Google Scholar] [CrossRef]
- Talora, C.; Campese, A.F.; Bellavia, D.; Felli, M.P.; Vacca, A.; Gulino, A.; Screpanti, I. Notch Signaling and Diseases: An Evolutionary Journey from a Simple Beginning to Complex Outcomes. Biochim. Et. Biophys. Acta (BBA) Mol. Basis Dis. 2008, 1782, 489–497. [Google Scholar] [CrossRef]
- Weng, A.P.; Ferrando Adolfo, A.; Lee, W.; Morris, J.P., IV; Silverman, L.B.; Sanchez-Irizarry, C.; Blacklow, S.C.; Look, A.T.; Aster, J.C. Activating Mutations of NOTCH1 in Human T Cell Acute Lymphoblastic Leukemia. Science 2004, 306, 269–271. [Google Scholar] [CrossRef]
- Ellisen, L.W.; Bird, J.; West, C.; Soreng, A.L.; Reynolds, T.C.; Smith, S.D.; Sklar, J. TAN-l, the Human Homolog of the Drosophila Notch Gene, Is Broken by Chromosomal Translocations in T Lymphoblastic Neoplasms. Cell 1991, 66, 649–661. [Google Scholar] [CrossRef]
- Sergio, I.; Varricchio, C.; Patel, S.K.; Del Gaizo, M.; Russo, E.; Orlando, A.; Peruzzi, G.; Ferrandino, F.; Tsaouli, G.; Coni, S.; et al. Notch3-Regulated microRNAs Impair CXCR4-Dependent Maturation of Thymocytes Allowing Maintenance and Progression of T-ALL. Oncogene 2024, 43, 2535–2547. [Google Scholar] [CrossRef] [PubMed]
- Sahlgren, C.; Gustafsson, M.V.; Jin, S.; Poellinger, L.; Lendhal, U. Notch Signaling Mediates Hypoxia-Induced Tumor Cell Migration and Invasion. Proc. Natl. Acad. Sci. USA 2008, 105, 6392–6397. [Google Scholar] [CrossRef]
- Roesch, A.; Fukunaga-Kalabis, M.; Schmidt, E.C.; Zabierowski, S.E.; Brafford, P.A.; Vultur, A.; Basu, D.; Gimotty, P.; Vogt, T.; Herlyn, M. A Temporarily Distinct Subpopulation of Slow-Cycling Melanoma Cells Is Required for Continuous Tumor Growth. Cell 2010, 141, 583–594. [Google Scholar] [CrossRef]
- Zheng, Y.; de la Cruz, C.C.; Sayles, L.C.; Alleyne-Chin, C.; Vaka, D.; Knaak, T.D.; Bigos, M.; Xu, Y.; Hoang, C.D.; Shrager, J.B.; et al. A Rare Population of CD24+ITGB4+Notchhi Cells Drives Tumor Propagation in NSCLC and Requires Notch3 for Self-Renewal. Cancer Cell 2013, 24, 59–74. [Google Scholar] [CrossRef]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretić, L.; Kong, G.; Leenders, F.; Lu, X.; Fernández-Cuesta, L.; Bosco, G.; et al. Comprehensive Genomic Profiles of Small Cell Lung Cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef]
- Lobry, C.; Ntziachristos, P.; Ndiaye-Lobry, D.; Oh, P.; Cimmino, L.; Zhu, N.; Araldi, E.; Hu, W.; Freund, J.; Abdel-Wahab, O.; et al. Notch Pathway Activation Targets AML-Initiating Cell Homeostasis and Differentiation. J. Exp. Med. 2013, 210, 301–319. [Google Scholar] [CrossRef]
- Van Es, J.H.; Van Gijn, M.E.; Riccio, O.; Van Den Born, M.; Vooijs, M.; Begthel, H.; Cozijnsen, M.; Robine, S.; Winton, D.J.; Radtke, F.; et al. Notch/γ-Secretase Inhibition Turns Proliferative Cells in Intestinal Crypts and Adenomas into Goblet Cells. Nature 2005, 435, 959–963. [Google Scholar] [CrossRef]
- Wu, Y.; Cain-Hom, C.; Choy, L.; Hagenbeek, T.J.; De Leon, G.P.; Chen, Y.; Finkle, D.; Venook, R.; Wu, X.; Ridgway, J.; et al. Therapeutic Antibody Targeting of Individual Notch Receptors. Nature 2010, 464, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Mazur, P.K.; Einwächter, H.; Lee, M.; Sipos, B.; Nakhai, H.; Rad, R.; Zimber-Strobl, U.; Strobl, L.J.; Radtke, F.; Klöppel, G.; et al. Notch2 Is Required for Progression of Pancreatic Intraepithelial Neoplasia and Development of Pancreatic Ductal Adenocarcinoma. Proc. Natl. Acad. Sci. USA 2010, 107, 13438–13443. [Google Scholar] [CrossRef]
- Hayashi, T.; Gust, K.M.; Wyatt, A.W.; Goriki, A.; Jäger, W.; Awrey, S.; Li, N.; Oo, H.Z.; Altamirano-Dimas, M.; Buttyan, R.; et al. Not All NOTCH Is Created Equal: The Oncogenic Role of NOTCH2 in Bladder Cancer and Its Implications for Targeted Therapy. Clin. Cancer Res. 2016, 22, 2981–2992. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, M.; Feng, Y.; Huang, Y.-F.; Xu, Y.-F.; Che, J.-P.; Wang, G.-C. High Expression of Notch Ligand Jagged2 Is Associated with the Metastasis and Recurrence in Urothelial Carcinoma of Bladder. Int. J. Clin. Exp. Pathol. 2013, 6, 2430. [Google Scholar]
- Rampias, T.; Vgenopoulou, P.; Avgeris, M.; Polizo, A.; Stravodimos, K.; Valavanis, C.; Scorilas, A.; Klinakis, A. A New Tumor Suppressor Role for the Notch Pathway in Bladder Cancer. Nat. Med. 2014, 20, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Xu, H.; Wei, J.; Xing, A.; Ma, X.; Wang, B.; Ju, Z.; Zhang, G.; Wang, C.; Wu, Z.; et al. Association of Low Expression of Notch-1 and Jagged-1 in Human Papillary Bladder Cancer and Shorter Survival. J. Urol. 2008, 180, 361–366. [Google Scholar] [CrossRef]
- Greife, A.; Jankowiak, S.; Steinbring, J.; Nikpour, P.; Niegisch, G.; Hoffmann, M.J.; Schulz, W.A. Canonical Notch Signalling Is Inactive in Urothelial Carcinoma. BMC Cancer 2014, 14, 628. [Google Scholar] [CrossRef]
- Maraver, A.; Fernandez-Marcos, P.J.; Cash, T.P.; Mendez-Pertuz, M.; Dueñas, M.; Maietta, P.; Martinelli, P.; Muñoz-Martin, M.; Martínez-Fernández, M.; Cañamero, M.; et al. NOTCH Pathway Inactivation Promotes Bladder Cancer Progression. J. Clin. Investig. 2015, 125, 824–830. [Google Scholar] [CrossRef]
- Fernandez-Marcos, P.J.; Serrano, M.; Maraver, A. Bladder Cancer and the Notch Pathway. Oncotarget 2015, 6, 1346–1347. [Google Scholar] [CrossRef] [PubMed]
- García-Cao, I.; Duran, A.; Collado, M.; Carrascosa, M.J.; Martín-Caballero, J.; Flores, J.M.; Diaz-Meco, M.T.; Moscat, J.; Serrano, M. Tumour-suppression Activity of the Proapoptotic Regulator Par4. EMBO Rep. 2005, 6, 577–583. [Google Scholar] [CrossRef]
- Schulz, G.B.; Elezkurtaj, S.; Börding, T.; Schmidt, E.M.; Elmasry, M.; Stief, C.G.; Kirchner, T.; Karl, A.; Horst, D. Therapeutic and Prognostic Implications of NOTCH and MAPK Signaling in Bladder Cancer. Cancer Sci. 2021, 112, 1987–1996. [Google Scholar] [CrossRef]
- Lin, H.; Fu, L.; Zhou, X.; Yu, A.; Chen, Y.; Liao, W.; Shu, G.; Zhang, L.; Tan, L.; Liang, H.; et al. LRP1 Induces Anti-PD-1 Resistance by Modulating the DLL4-NOTCH2-CCL2 Axis and Redirecting M2-like Macrophage Polarisation in Bladder Cancer. Cancer Lett. 2024, 593, 216807. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Li, X.; Wang, J.; Li, K.; Wan, S.; Yang, J.; Wang, H.; Cao, J.; Wang, C.; et al. BIN1 Inhibited Tumor Growth, Metastasis and Stemness by ALDH1/NOTCH Pathway in Bladder Carcinoma. Hereditas 2025, 162, 29. [Google Scholar] [CrossRef] [PubMed]
- Nagumo, Y.; Hattori, K.; Kimura, T.; Sekino, Y.; Naiki, T.; Kobayashi, Y.; Matsumoto, T.; Osawa, T.; Kita, Y.; Takemura, M.; et al. Combined Molecular Subclass and Immune Phenotype Correlate to Atezolizumab Plus Radiation Therapy Response in Invasive Bladder Cancer: BPT-ART Phase 2 Study. Int. J. Radiat. Oncol. Biol. Phys. 2025, 122, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Weimann, A.; Stolzenburg, J.-U.; Neuhaus, J.; Berndt-Paetz, M. Notch2/3-DLL4 Interaction in Urothelial Cancer Cell Lines Supports a Tumorigenic Role of Notch Signaling Pathways in Bladder Carcinoma. PLoS ONE 2025, 20, e0317709. [Google Scholar] [CrossRef]
- Pietzak, E.J.; Zabor, E.C.; Bagrodia, A.; Armenia, J.; Hu, W.; Zehir, A.; Funt, S.; Audenet, F.; Barron, D.; Maamouri, N.; et al. Genomic Differences Between “Primary” and “Secondary” Muscle-Invasive Bladder Cancer as a Basis for Disparate Outcomes to Cisplatin-Based Neoadjuvant Chemotherapy. Eur. Urol. 2019, 75, 231–239. [Google Scholar] [CrossRef]
- Iyer, G.; Tangen, C.M.; Sarfaty, M.; Regazzi, A.M.; Lee, I.-L.; Fong, M.; Choi, C.W.; Dinney, C.P.; Flaig, T.W.; Thompson, I.M., Jr.; et al. DNA Damage Response Alterations Predict for Neoadjuvant Chemotherapy Sensitivity in Muscle-Invasive Bladder Cancer: A Correlative Analysis of the SWOG S1314 Trial. JCO Precis. Oncol. 2024, 8, e2400287. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Liu, C.; Pan, J.; Lu, G.; Zhou, Z.; Chen, Z.; Qian, C. Notch3 Overexpression Enhances Progression and Chemoresistance of Urothelial Carcinoma. Oncotarget 2017, 8, 34362–34373. [Google Scholar] [CrossRef] [PubMed]
- Tottone, L.; Zhdanovskaya, N.; Carmona Pestaña, Á.; Zampieri, M.; Simeoni, F.; Lazzari, S.; Ruocco, V.; Pelullo, M.; Caiafa, P.; Felli, M.P.; et al. Histone Modifications Drive Aberrant Notch3 Expression/Activity and Growth in T-ALL. Front. Oncol. 2019, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Ristic Petrovic, A.; Stokanović, D.; Stojnev, S.; Potić Floranović, M.; Krstić, M.; Djordjević, I.; Skakić, A.; Janković Veličković, L. The Association between NOTCH3 Expression and the Clinical Outcome in the Urothelial Bladder Cancer Patients. Bosn. J. Basic. Med. Sci. 2022, 24, 523. [Google Scholar] [CrossRef] [PubMed]
- Bin, Y.; Guikang, L.; Jin, H.; Xue, Z.; Ruihan, W.; Jianchao, Z. Notch Signaling Pathway-Based Classification of Bladder Cancer in Relation to Tumor Immune Infiltration. Cent. Eur. J. Immunol. 2023, 48, 274–289. [Google Scholar] [CrossRef]
- Liu, C.; Ge, H.; Shen, C.; Hu, D.; Zhao, X.; Qin, R.; Wang, Y. NOTCH3 Promotes Malignant Progression of Bladder Cancer by Directly Regulating SPP1 and Activating PI3K/AKT Pathway. Cell Death Dis. 2024, 15, 840. [Google Scholar] [CrossRef]
- Nedjadi, T.; Ahmed, M.E.; Ansari, H.R.; Aouabdi, S.; Al-Maghrabi, J. Identification of SPP1 as a Prognostic Biomarker and Immune Cells Modulator in Urothelial Bladder Cancer: A Bioinformatics Analysis. Cancers 2023, 15, 5704. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ashida, S.; Nao, T.; Murakami, K.; Iga, R.; Shimamoto, T.; Fukuhara, H.; Shimizu, N.; Fukata, S.; Kurabayashi, A.; et al. Comprehensive Genomic Profiling of Primary Bladder Mucinous Adenocarcinoma, a Rare Genitourinary Cancer: A Case Report. Urol. Case Rep. 2025, 58, 102892. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ye, F.; Cui, M.; Lee, P.; Wei, C.; Hao, Y.; Wang, X.; Wang, Y.; Lu, Z.; Galsky, M.; et al. Protein Profiling of Bladder Urothelial Cell Carcinoma. PLoS ONE 2016, 11, e0161922. [Google Scholar] [CrossRef]
- Long, J.; Wang, D.; Yang, X.; Wang, A.; Lin, Y.; Zheng, M.; Zhang, H.; Sang, X.; Wang, H.; Hu, K.; et al. Identification of NOTCH4 Mutation as a Response Biomarker for Immune Checkpoint Inhibitor Therapy. BMC Med. 2021, 19, 154. [Google Scholar] [CrossRef]
- He, P.; Dai, Q.; Wu, X. New Insight in Urological Cancer Therapy: From Epithelial-Mesenchymal Transition (EMT) to Application of Nano-Biomaterials. Environ. Res. 2023, 229, 115672. [Google Scholar] [CrossRef]
- Wang, X.; Qiao, G.; Jiang, N.; Morse, M.A.; Zhou, X.; Wang, S.; Wu, J.; Song, Y.; Zhao, Y.; Zhou, L.; et al. Serial Assessment of Circulating T Lymphocyte Phenotype and Receptor Repertoire During Treatment of Non-Muscle Invasive Bladder Cancer with Adoptive T Cell Immunotherapy. Am. J. Cancer Res. 2021, 11, 1709. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, G.; Zhang, X.; Zhou, X.; Liu, Z.; Huang, L.; Liu, R.; Lang, B.; Xu, X.; Liu, W.; et al. γ-Secretase Inhibitor Inhibits Bladder Cancer Cell Drug Resistance and Invasion by Reducing Epithelial-Mesenchymal Transition. Mol. Med. Rep. 2015, 12, 2821–2827. [Google Scholar] [CrossRef]
- Gai, J.-W.; Wahafu, W.; Hsieh, Y.-C.; Liu, M.; Zhang, L.; Li, S.W.; Zhang, B.; He, Q.; Guo, H.; Jin, J. Inhibition of Presenilins Attenuates Proliferation and Invasion in Bladder Cancer Cells through Multiple Pathways. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 36.e19–36.e25. [Google Scholar] [CrossRef]
- Chen, J.; Ding, H.; Liu, B.; Zhou, X.; Lin, Z.; Yang, F.; Zhan, H.; Xiao, H. Notch1 Signaling Contributes to Mechanical Allodynia Associated with Cyclophosphamide-Induced Cystitis by Promoting Microglia Activation and Neuroinflammation. Mediat. Inflamm. 2021, 2021, 1791222. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, H.; Xu, H.; Xiong, H.; Chu, Q.; Yu, S.; Wu, G.S.; Wu, K. Notch Signaling: An Emerging Therapeutic Target for Cancer Treatment. Cancer Lett. 2015, 369, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Dobranowski, P.; Ban, F.; Contreras-Sanz, A.; Cherkasov, A.; Black, P.C. Perspectives on the Discovery of NOTCH2-Specific Inhibitors. Chem. Biol. Drug Des. 2018, 91, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C.; Chugh, R.; Patnaik, A.; Papadopoulos, K.P.; Wang, M.; Kapoun, A.M.; Xu, L.; Dupont, J.; Stagg, R.J.; Tolcher, A. A Phase 1 Dose Escalation and Expansion Study of Tarextumab (OMP-59R5) in Patients with Solid Tumors. Invest. New Drugs 2019, 37, 722–730. [Google Scholar] [CrossRef]
- Rosen, L.S.; Wesolowski, R.; Baffa, R.; Liao, K.-H.; Hua, S.Y.; Gibson, B.L.; Pirie-Shepherd, S.; Tolcher, A.W. A Phase I, Dose-Escalation Study of PF-06650808, an Anti-Notch3 Antibody–Drug Conjugate, in Patients with Breast Cancer and Other Advanced Solid Tumors. Invest. New Drugs 2020, 38, 120–130. [Google Scholar] [CrossRef]
- Sergio, I.; Varricchio, C.; Squillante, F.; Cantale Aeo, N.M.; Campese, A.F.; Felli, M.P. Notch Inhibitors and BH3 Mimetics in T-Cell Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2024, 25, 12839. [Google Scholar] [CrossRef]
- Ribal, M.J.; Rosenberg, J.; Ajami, T.; Vilaseca, A.; Xia, L.; Sternschuss, M.; Schuckman, A.K. Advancing Perioperative Treatment Options for Localized Muscle-Invasive Bladder Cancer: A Step Forward. Am. Soc. Clin. Oncol. Educ. Book. 2025, 45, e472822. [Google Scholar]
- Pagliaro, L.; Marchesini, M.; Roti, G. Targeting Oncogenic Notch Signaling with SERCA Inhibitors. J. Hematol. Oncol. 2021, 14, 8. [Google Scholar] [CrossRef]
- Weir, S.J.; Dandawate, P.; Standing, D.; Bhattacharyya, S.; Ramamoorthy, P.; Rangarajan, P.; Wood, R.; Brinker, A.E.; Woolbright, B.L.; Tanol, M.; et al. Fosciclopirox Suppresses Growth of High-Grade Urothelial Cancer by Targeting the γ-Secretase Complex. Cell Death Dis. 2021, 12, 562. [Google Scholar] [CrossRef]
- Peng, W.; Zhang, H.; Yin, M.; Kong, D.; Kang, L.; Teng, X.; Wang, J.; Chu, Z.; Sun, Y.; Long, P.; et al. Combined Inhibition of PI3K and STAT3 Signaling Effectively Inhibits Bladder Cancer Growth. Oncogenesis 2024, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Loriot, Y.; Matsubara, N.; Park, S.H.; Huddart, R.A.; Burgess, E.F.; Houede, N.; Banek, S.; Guadalupi, V.; Ku, J.H.; Valderrama, B.P.; et al. Erdafitinib or Chemotherapy in Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2023, 389, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Kim, D.; Ho, J.-N.; Le, V.-H.; Lee, S. Crizotinib Inhibits Viability, Migration, and Invasion by Suppressing the c-Met/PI3K/Akt Pathway in the Three-Dimensional Bladder Cancer Spheroid Model. Curr. Oncol. 2025, 32, 236. [Google Scholar]
- Chu, C.E.; Sjöström, M.; Egusa, E.A.; Gibb, E.A.; Badura, M.L.; Zhu, J.; Koshkin, V.S.; Stohr, B.A.; Meng, M.V.; Pruthi, R.S.; et al. Heterogeneity in NECTIN4 Expression Across Molecular Subtypes of Urothelial Cancer Mediates Sensitivity to Enfortumab Vedotin. Clin. Cancer Res. 2021, 27, 5123–5130. [Google Scholar] [CrossRef]
- Hong, X.; Chen, X.; Wang, H.; Xu, Q.; Xiao, K.; Zhang, Y.; Chi, Z.; Liu, Y.; Liu, G.; Li, H.; et al. A HER2-targeted Antibody-Drug Conjugate, RC48-ADC, Exerted Promising Antitumor Efficacy and Safety with Intravesical Instillation in Preclinical Models of Bladder Cancer. Adv. Sci. 2023, 10, 2302377. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Yang, D.; Liu, T.; Liu, S.; Lu, X.; Chen, L. Laminin-Integrin A6b4 Interaction Activates Notch Signaling to Facilitate Bladder Cancer Development. BMC Cancer 2022, 22, 558. [Google Scholar] [CrossRef]
| EORTC 2006 Risk Tables [10] | EAU NMIBC Guidelines 2021 [9] | |
|---|---|---|
| RISK STRATIFICATION | Classified NMIBC into three risk groups: low, intermediate and high-risk | Categorise NMIBC into low, intermediate, high and very high-risk groups |
| RISK FACTORS CONSIDERED | Tumour size, prior recurrence rate, tumour stage (Ta/T1), presence of CIS and grade, number of tumours | Similar parameters with the addition of patient age, tumour size ( > 3 cm), multiplicity and other factors |
| SCORING SYSTEM | Estimate the probability of recurrence and progression at 1 year and 5 years | Referenced but criticised for not estimating the risk of progression |
| LIMITATIONS | Molecular markers are not considered; Overestimates the risk of progression, especially in patients undergoing intravesical instillation with BGC | Acknowledge the limitations of EORTC, suggest combining clinical data and emerging molecular data |
| BIOMARKERS | Not considered | Promotes research on biomarkers to improve risk stratification, but not yet standardised in clinical practice |
| BCG THERAPY GUIDANCE | Not completely integrated into risk models | Risk-adapted use of BCG including for intermediate and high-risk patients |
| CIS MANAGEMENT | Treated as high risk; included in scoring | Lay emphasis on aggressive management due to high risk of progression |
| FOLLOW-UP RECOMMENDATIONS | Based on risk group; Frequent cystoscopies and cytology | More individualised follow-up based on updated risk groups and BCG status |
| MIBC | Status of Clinical Trial | Number of Clinical Trial | Completed with Results |
|---|---|---|---|
| RECRUITING | NCT05241340 | NO | |
| RECRUITING | NCT06305767 | NO | |
| ACTIVE, NOT RECRUITING | NCT02447549 | NO | |
| MET | COMPLETED | NCT00829920 | NO |
| COMPLETED | NCT03702179 | YES | |
| ACTIVE, NOT RECRUITING | NCT02546661 | NO | |
| COMPLETED | NCT04209114 | YES | |
| RECRUITING | NCT05544552 | NO | |
| COMPLETED | NCT01031420 | YES | |
| FGFR | COMPLETED | NCT02177695 | YES |
| ACTIVE, NOT RECRUITING | NCT03775265 | NO | |
| COMPLETED | NCT00380029 | YES | |
| RECRUITING | NCT06511648 | NO | |
| RECRUITING | NCT05316155 | NO |
| Frequency of Mutated Genes | NMIBC | MIBC | Overall BC |
|---|---|---|---|
| NOTCH 1 | 2.9% | 3.4% | 3.8% |
| NOTCH 2 | 1.0% | 3.5% | 3.7% |
| NOTCH 3 | 2.9% | 4.4% | 4.8% |
| NOTCH 4 | 4.8% | 4.0% | 4.3% |
| NUMBER OF PROFILED SAMPLES | 105 | 1200 | 5265 |
| Protein Change | Type | Consequences | VEP Impact | SIFT Impact | PolyPhen Impact |
|---|---|---|---|---|---|
| R169C | Substitution | Missense | MO | DH | PO |
| W1750* | Substitution | Stop Gained | HI | -- | -- |
| H1690Y | Substitution | Missense | MO | DH | BE |
| E1585K | Substitution | Missense | MO | DH | PR |
| R1568Q | Substitution | Missense | MO | DH | BE |
| 1138Sfs*132 | Deletion | Frameshift | HI | -- | -- |
| S834N | Substitution | Missense | MO | TO | BE |
| S1688C | Substitution | Missense | MO | TO | BE |
| S1542L | Substitution | Missense | MO | DH | BE |
| E538K | Substitution | Missense | MO | TO | BE |
| Q214H | Substitution | Missense | MO | DH | PR |
| L1654= | Substitution | Synonymous | LO | -- | -- |
| A1796S | Substitution | Missense | MO | DH | PO |
| V633= | Substitution | Synonymous | LO | -- | -- |
| E1161K | Substitution | Missense | MO | DH | PO |
| R2009W | Substitution | Missense | MO | DH | PR |
| D1587N | Substitution | Missense | MO | DH | PR |
| E309K | Substitution | Missense | MO | TO | PO |
 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cusumano, C.; Squillante, F.; Roma, M.; Miano, R.; Felli, M.P. Bladder Cancer: Uncovering the Predictive Role of NOTCH as an Emerging Candidate Biomarker for Therapeutic Strategies. Cancers 2025, 17, 3078. https://doi.org/10.3390/cancers17183078
Cusumano C, Squillante F, Roma M, Miano R, Felli MP. Bladder Cancer: Uncovering the Predictive Role of NOTCH as an Emerging Candidate Biomarker for Therapeutic Strategies. Cancers. 2025; 17(18):3078. https://doi.org/10.3390/cancers17183078
Chicago/Turabian StyleCusumano, Chiara, Federica Squillante, Marco Roma, Roberto Miano, and Maria Pia Felli. 2025. "Bladder Cancer: Uncovering the Predictive Role of NOTCH as an Emerging Candidate Biomarker for Therapeutic Strategies" Cancers 17, no. 18: 3078. https://doi.org/10.3390/cancers17183078
APA StyleCusumano, C., Squillante, F., Roma, M., Miano, R., & Felli, M. P. (2025). Bladder Cancer: Uncovering the Predictive Role of NOTCH as an Emerging Candidate Biomarker for Therapeutic Strategies. Cancers, 17(18), 3078. https://doi.org/10.3390/cancers17183078





