Histotripsy: Recent Advances, Clinical Applications, and Future Prospects
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
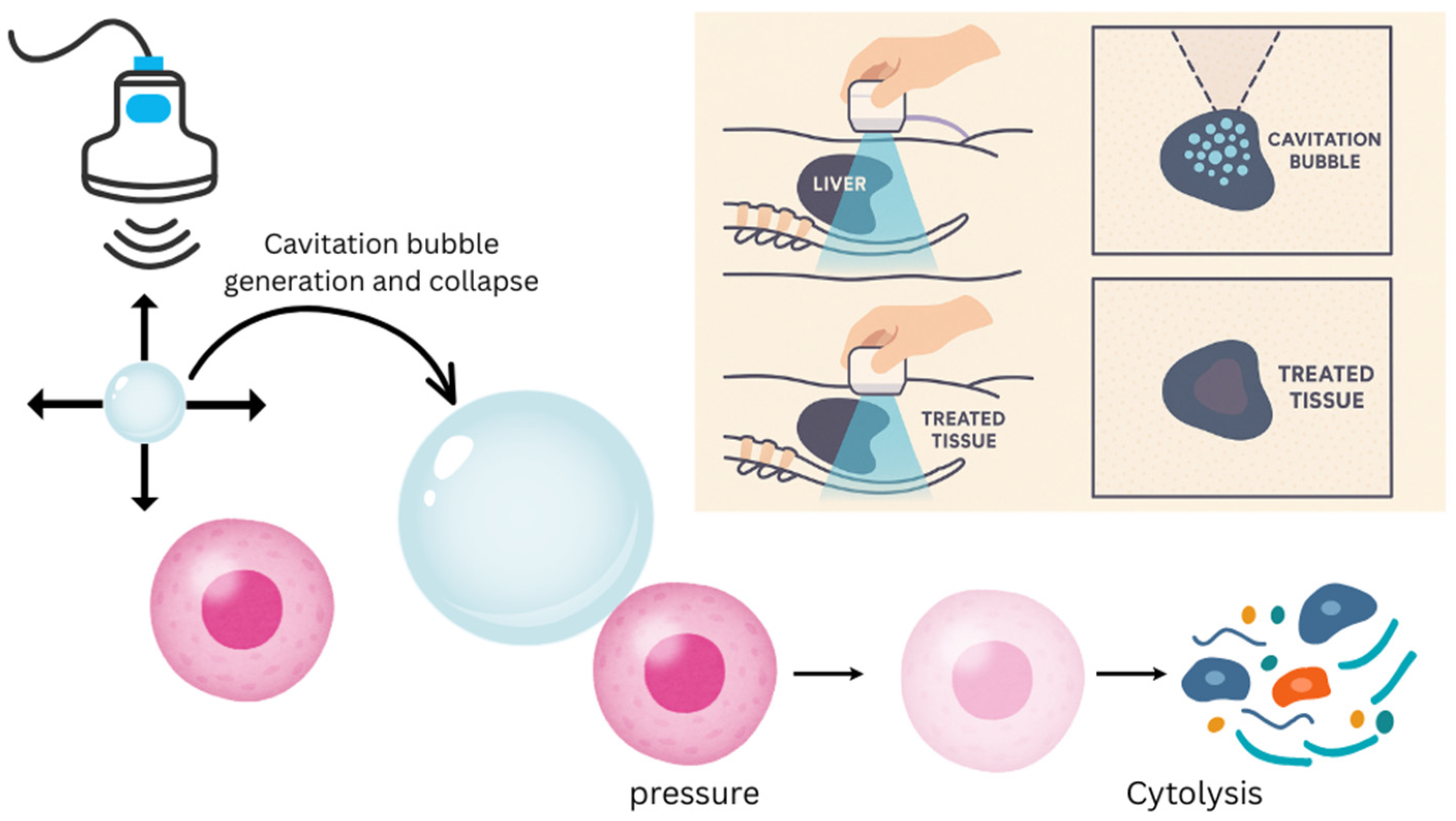
3. Novelty and Innovation of Histotripsy
4. Mechanisms and Bioeffects of Histotripsy
5. Liver
5.1. Current Applications
5.2. Future Directions
6. Pancreas
7. Kidney
8. Brain
9. Cardiovascular
10. Limitations
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular Carcinoma |
| RFA | Radiofrequency Ablation |
| MWA | Microwave Ablation |
| IRE | Irreversible Electroporation |
| DAMPs | Damage Associated Molecular Patterns |
| HIFU | High-Intensity Focused Ultrasound |
| RCC | Renal Cell Carcinoma |
| CAIN | The Clinical Ablation Using Histotripsy for Noninvasive Treatment of Renal Tumors |
| BBB | Blood–Brain Barrier |
References
- Bhat, S.A.; Farooq, Z.; Ismail, H.; Corona-Avila, I.; Khan, W. Unraveling the Sweet Secrets of HCC: Glucometabolic Rewiring in Hepatocellular Carcinoma. Technol. Cancer Res. Treat. 2023, 22, 15330338231219434. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya Thandra, K.; Barsouk, A.; Saginala, K.; Sukumar Aluru, J.; Barsouk, A. Epidemiology of Lung Cancer. Wspolczesna Onkol. Oncol. 2021, 25, 45–52. [Google Scholar] [CrossRef]
- Terris, M.; Klaassen, Z.; Kabaria, R. Renal Cell Carcinoma: Links and Risks. Int. J. Nephrol. Renov. Dis. 2016, 25, 45–52. [Google Scholar] [CrossRef]
- Makary, M.S.; Khandpur, U.; Cloyd, J.M.; Mumtaz, K.; Dowell, J.D. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers 2020, 12, 1914. [Google Scholar] [CrossRef]
- Friedman, M.; Mikityansky, I.; Kam, A.; Libutti, S.K.; Walther, M.M.; Neeman, Z.; Locklin, J.K.; Wood, B.J. Radiofrequency Ablation of Cancer. Cardiovasc. Interv. Radiol. 2004, 27, 427–434. [Google Scholar] [CrossRef]
- Campbell, W.A.; Makary, M.S. Advances in Image-Guided Ablation Therapies for Solid Tumors. Cancers 2024, 16, 2560. [Google Scholar] [CrossRef]
- Patel, N.; King, A.J.; Breen, D.J. Percutaneous Image-Guided Cryoablation of Small Renal Masses. Abdom. Radiol. 2016, 41, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Sakorafas, G.H.; Nissotakis, C.; Peros, G. Abdominal Desmoid Tumors. Surg. Oncol. 2007, 16, 131–142. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Bärnighausen, T.; et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories From 2020 to 2050. JAMA Oncol. 2023, 9, 465. [Google Scholar] [CrossRef]
- Zane, K.E.; Nagib, P.B.; Jalil, S.; Mumtaz, K.; Makary, M.S. Emerging Curative-Intent Minimally-Invasive Therapies for Hepatocellular Carcinoma. World J. Hepatol. 2022, 14, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Siripongsakun, S.; Bahrami, S.; Raman, S.S.; Sayre, J.; Lu, D.S. Microwave Ablation of Liver Tumors: Degree of Tissue Contraction as Compared to RF Ablation. Abdom. Radiol. 2016, 41, 659–666. [Google Scholar] [CrossRef]
- Pillai, K.; Akhter, J.; Chua, T.C.; Shehata, M.; Alzahrani, N.; Al-Alem, I.; Morris, D.L. Heat Sink Effect on Tumor Ablation Characteristics as Observed in Monopolar Radiofrequency, Bipolar Radiofrequency, and Microwave, Using Ex Vivo Calf Liver Model. Medicine 2015, 94, e580. [Google Scholar] [CrossRef]
- Fazlollahi, F.; Makary, M.S. Precision Oncology: The Role of Minimally-Invasive Ablation Therapy in the Management of Solid Organ Tumors. World J. Radiol. 2025, 17, 98618. [Google Scholar] [CrossRef]
- Aarts, B.M.; Klompenhouwer, E.G.; Rice, S.L.; Imani, F.; Baetens, T.; Bex, A.; Horenblas, S.; Kok, M.; Haanen, J.B.A.G.; Beets-Tan, R.G.H.; et al. Cryoablation and Immunotherapy: An Overview of Evidence on Its Synergy. Insights Into Imaging 2019, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.S.; Bozer, J.; Miller, E.D.; Diaz, D.A.; Rikabi, A. Long-Term Clinical Outcomes of Yttrium-90 Transarterial Radioembolization for Hepatocellular Carcinoma: A 5-Year Institutional Experience. Acad. Radiol. 2024, 31, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Domini, J.; Makary, M.S. Single-Center Analysis of Percutaneous Ablation in the Treatment of Hepatocellular Carcinoma: Long-Term Outcomes of a 7-Year Experience. Abdom. Radiol. 2023, 48, 1173–1180. [Google Scholar] [CrossRef]
- Puijk, R.S.; Ruarus, A.H.; Vroomen, L.G.P.H.; Van Tilborg, A.A.J.M.; Scheffer, H.J.; Nielsen, K.; De Jong, M.C.; De Vries, J.J.J.; Zonderhuis, B.M.; Eker, H.H.; et al. Colorectal Liver Metastases: Surgery versus Thermal Ablation (COLLISION)—A Phase III Single-Blind Prospective Randomized Controlled Trial. BMC Cancer 2018, 18, 821. [Google Scholar] [CrossRef] [PubMed]
- Criss, C.R.; Makary, M.S. Recent Advances in Image-Guided Locoregional Therapies for Primary Liver Tumors. Biology 2023, 12, 999. [Google Scholar] [CrossRef]
- Xu, Z.; Ludomirsky, A.; Eun, L.Y.; Hall, T.L.; Tran, B.C.; Fowlkes, J.B.; Cain, C.A. Controlled Ultrasound Tissue Erosion. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 726–736. [Google Scholar] [CrossRef]
- Roberts, W.W. Development and Translation of Histotripsy: Current Status and Future Directions. Curr. Opin. Urol. 2014, 24, 104–110. [Google Scholar] [CrossRef]
- Xu, Z.; Hall, T.L.; Vlaisavljevich, E.; Lee, F.T. Histotripsy: The First Noninvasive, Non-Ionizing, Non-Thermal Ablation Technique Based on Ultrasound. Int. J. Hyperth. 2021, 38, 561–575. [Google Scholar] [CrossRef]
- Xu, Z.; Khokhlova, T.D.; Cho, C.S.; Khokhlova, V.A. Histotripsy: A Method for Mechanical Tissue Ablation with Ultrasound. Annu. Rev. Biomed. Eng. 2024, 26, 141–167. [Google Scholar] [CrossRef]
- Sandilos, G.; Butchy, M.V.; Koneru, M.; Gongalla, S.; Sensenig, R.; Hong, Y.K. Histotripsy—Hype or Hope? Review of Innovation and Future Implications. J. Gastrointest. Surg. 2024, 28, 1370–1375. [Google Scholar] [CrossRef]
- Wah, T.M.; Pech, M.; Thormann, M.; Serres, X.; Littler, P.; Stenberg, B.; Lenton, J.; Smith, J.; Wiggermann, P.; Planert, M.; et al. A Multi-Centre, Single Arm, Non-Randomized, Prospective European Trial to Evaluate the Safety and Efficacy of the HistoSonics System in the Treatment of Primary and Metastatic Liver Cancers (#HOPE4LIVER). Cardiovasc. Interv. Radiol. 2023, 46, 259–267. [Google Scholar] [CrossRef]
- Vidal-Jove, J.; Serres, X.; Vlaisavljevich, E.; Cannata, J.; Duryea, A.; Miller, R.; Merino, X.; Velat, M.; Kam, Y.; Bolduan, R.; et al. First-in-Man Histotripsy of Hepatic Tumors: The THERESA Trial, a Feasibility Study. Int. J. Hyperth. 2022, 39, 1115–1123. [Google Scholar] [CrossRef]
- Mendiratta-Lala, M.; Wiggermann, P.; Pech, M.; Serres-Créixams, X.; White, S.B.; Davis, C.; Ahmed, O.; Parikh, N.D.; Planert, M.; Thormann, M.; et al. The #HOPE4LIVER Single-Arm Pivotal Trial for Histotripsy of Primary and Metastatic Liver Tumors. Radiology 2024, 312, e233051. [Google Scholar] [CrossRef] [PubMed]
- Imran, K.M.; Ganguly, A.; Paul, T.; Powar, M.; Vlaisavljevich, E.; Cho, C.S.; Allen, I.C. Magic Bubbles: Utilizing Histotripsy to Modulate the Tumor Microenvironment and Improve Systemic Anti-Tumor Immune Responses. Int. J. Hyperth. 2023, 40, 2244206. [Google Scholar] [CrossRef] [PubMed]
- Hendley, S.A.; Paul, J.D.; Maxwell, A.D.; Haworth, K.J.; Holland, C.K.; Bader, K.B. Clot Degradation Under the Action of Histotripsy Bubble Activity and a Lytic Drug. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2942–2952. [Google Scholar] [CrossRef]
- Worlikar, T.; Hall, T.; Zhang, M.; Mendiratta-Lala, M.; Green, M.; Cho, C.S.; Xu, Z. Insights from in Vivo Preclinical Cancer Studies with Histotripsy. Int. J. Hyperth. 2024, 41, 2297650. [Google Scholar] [CrossRef] [PubMed]
- Knott, E.A.; Zlevor, A.M.; Hinshaw, J.L.; Laeseke, P.F.; Longhurst, C.; Frank, J.; Bradley, C.W.; Couillard, A.B.; Rossebo, A.E.; Xu, Z.; et al. A Comparison Study of Microwave Ablation vs. Histotripsy for Focal Liver Treatments in a Swine Model. Eur. Radiol. 2022, 33, 1050–1062. [Google Scholar] [CrossRef]
- Wehrle, C.J.; Burns, K.; Ong, E.; Couillard, A.; Parikh, N.D.; Caoili, E.; Kim, J.; Aucejo, F.; Schlegel, A.; Knott, E.; et al. The First International Experience with Histotripsy: A Safety Analysis of 230 Cases. J. Gastrointest. Surg. 2025, 29, 102000. [Google Scholar] [CrossRef]
- Ziemlewicz, T.J.; Critchfield, J.J.; Mendiratta-Lala, M.; Wiggermann, P.; Pech, M.; Serres-Créixams, X.; Lubner, M.; Wah, T.M.; Littler, P.; Davis, C.R.; et al. The #HOPE4LIVER Single-Arm Pivotal Trial for Histotripsy of Primary and Metastatic Liver Tumors: 1-Year Update of Clinical Outcomes. Ann. Surg. 2025. [Google Scholar] [CrossRef]
- Falk, K.L.; Laeseke, P.F.; Kisting, M.A.; Zlevor, A.M.; Knott, E.A.; Smolock, A.R.; Bradley, C.; Vlaisavljevich, E.; Lee, F.T.; Ziemlewicz, T.J. Clinical Translation of Abdominal Histotripsy: A Review of Preclinical Studies in Large Animal Models. Int. J. Hyperth. 2023, 40, 2272065. [Google Scholar] [CrossRef] [PubMed]
- Longo, K.C.; Zlevor, A.M.; Laeseke, P.F.; Swietlik, J.F.; Knott, E.A.; Rodgers, A.C.; Mao, L.; Zhang, X.; Xu, Z.; Wagner, M.G.; et al. Histotripsy Ablations in a Porcine Liver Model: Feasibility of Respiratory Motion Compensation by Alteration of the Ablation Zone Prescription Shape. Cardiovasc. Interv. Radiol. 2020, 43, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Yeats, E.; Lu, N.; Sukovich, J.R.; Xu, Z.; Hall, T.L. Soft Tissue Aberration Correction for Histotripsy Using Acoustic Emissions From Cavitation Cloud Nucleation and Collapse. Ultrasound Med. Biol. 2023, 49, 1182–1193. [Google Scholar] [CrossRef]
- Bawiec, C.R.; Khokhlova, T.D.; Sapozhnikov, O.A.; Rosnitskiy, P.B.; Cunitz, B.W.; Ghanem, M.A.; Hunter, C.; Kreider, W.; Schade, G.R.; Yuldashev, P.V.; et al. A Prototype Therapy System for Boiling Histotripsy in Abdominal Targets Based on a 256-Element Spiral Array. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 1496–1510. [Google Scholar] [CrossRef]
- Stocker, G.E.; Lundt, J.E.; Sukovich, J.R.; Miller, R.M.; Duryea, A.P.; Hall, T.L.; Xu, Z. A Modular, Kerf-Minimizing Approach for Therapeutic Ultrasound Phased Array Construction. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 2766–2775. [Google Scholar] [CrossRef]
- Khokhlova, T.D.; Wang, Y.-N.; Simon, J.C.; Cunitz, B.W.; Starr, F.; Paun, M.; Crum, L.A.; Bailey, M.R.; Khokhlova, V.A. Ultrasound-Guided Tissue Fractionation by High Intensity Focused Ultrasound in an in Vivo Porcine Liver Model. Proc. Natl. Acad. Sci. USA 2014, 111, 8161–8166. [Google Scholar] [CrossRef]
- Khokhlova, T.D.; Schade, G.R.; Wang, Y.-N.; Buravkov, S.V.; Chernikov, V.P.; Simon, J.C.; Starr, F.; Maxwell, A.D.; Bailey, M.R.; Kreider, W.; et al. Pilot in Vivo Studies on Transcutaneous Boiling Histotripsy in Porcine Liver and Kidney. Sci. Rep. 2019, 9, 20176. [Google Scholar] [CrossRef]
- Thomas, G.P.L.; Khokhlova, T.D.; Sapozhnikov, O.A.; Wang, Y.-N.; Totten, S.I.; Khokhlova, V.A. In Vivo Aberration Correction for Transcutaneous HIFU Therapy Using a Multielement Array. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 2955–2964. [Google Scholar] [CrossRef]
- Qu, S.; Worlikar, T.; Felsted, A.E.; Ganguly, A.; Beems, M.V.; Hubbard, R.; Pepple, A.L.; Kevelin, A.A.; Garavaglia, H.; Dib, J.; et al. Non-Thermal Histotripsy Tumor Ablation Promotes Abscopal Immune Responses That Enhance Cancer Immunotherapy. J. Immunother. Cancer 2020, 8, e000200. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Jove, J.; Serres-Creixams, X.; Ziemlewicz, T.J.; Cannata, J.M. Liver Histotripsy Mediated Abscopal Effect—Case Report. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 3001–3005. [Google Scholar] [CrossRef]
- Hendricks-Wenger, A.; Arnold, L.; Gannon, J.; Simon, A.; Singh, N.; Sheppard, H.; Nagai-Singer, M.A.; Imran, K.M.; Lee, K.; Clark-Deener, S.; et al. Histotripsy Ablation in Preclinical Animal Models of Cancer and Spontaneous Tumors in Veterinary Patients: A Review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Gannon, J.; Imran, K.M.; Hendricks-Wenger, A.; Edwards, M.; Covell, H.; Ruger, L.; Singh, N.; Nagai-Singer, M.; Tintera, B.; Eden, K.; et al. Ultrasound-Guided Noninvasive Pancreas Ablation Using Histotripsy: Feasibility Study in an in Vivo Porcine Model. Int. J. Hyperth. 2023, 40, 2247187. [Google Scholar] [CrossRef]
- Hendricks-Wenger, A.; Sereno, J.; Gannon, J.; Zeher, A.; Brock, R.M.; Beitel-White, N.; Simon, A.; Davalos, R.V.; Coutermarsh-Ott, S.; Vlaisavljevich, E.; et al. Histotripsy Ablation Alters the Tumor Microenvironment and Promotes Immune System Activation in a Subcutaneous Model of Pancreatic Cancer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2987–3000. [Google Scholar] [CrossRef]
- Osada, T.; Jiang, X.; Zhao, Y.; Chen, M.; Kreager, B.C.; Wu, H.; Kim, H.; Ren, J.; Snyder, J.; Zhong, P.; et al. The Use of Histotripsy as Intratumoral Immunotherapy beyond Tissue Ablation—The Rationale for Exploring the Immune Effects of Histotripsy. Int. J. Hyperth. 2023, 40, 2263672. [Google Scholar] [CrossRef]
- Khokhlova, T.D.; Wang, Y.-N.; Son, H.; Totten, S.; Whang, S.; Ha Hwang, J. Chronic Effects of Pulsed High Intensity Focused Ultrasound Aided Delivery of Gemcitabine in a Mouse Model of Pancreatic Cancer. Ultrasonics 2023, 132, 106993. [Google Scholar] [CrossRef] [PubMed]
- Mancia, L.; Vlaisavljevich, E.; Yousefi, N.; Rodriguez, M.; Ziemlewicz, T.J.; Lee, F.T.; Henann, D.; Franck, C.; Xu, Z.; Johnsen, E. Modeling Tissue-Selective Cavitation Damage. Phys. Med. Biol. 2019, 64, 225001. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Yang, R.; Liu, X.; Lu, M.; Liu, Y.; Li, R.; Mao, J.; Zhang, Y.; Jing, Y.; Chang, J.; et al. Ultrasound-Guided in Vivo Histotripsy in Rabbit Kidneys Using Millisecond-Length Two-Stage Ultrasound Pulses Combined with Fundamental and Second Harmonic Superposition. Phys. Med. Biol. 2022, 67, 215020. [Google Scholar] [CrossRef]
- Ponomarchuk, E.; Thomas, G.; Song, M.; Wang, Y.-N.; Totten, S.; Schade, G.; Thiel, J.; Bruce, M.; Khokhlova, V.; Khokhlova, T. Advancing Boiling Histotripsy Dose in Ex Vivo And In Vivo Renal Tissues Via Quantitative Histological Analysis and Shear Wave Elastography. Ultrasound Med. Biol. 2024, 50, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Couillard, A.B.; Zlevor, A.M.; Ziemlewicz, T.J.; Kisting, M.A.; Knott, E.; Rossebo, A.E.; White, J.; Lubner, M.G.; Gettle, L.M.; Hinshaw, J.L.; et al. A Comparison of Histotripsy and Percutaneous Cryoablation in a Chronic Healthy Swine Kidney Model. J. Vasc. Interv. Radiol. 2023, 34, 1986–1996. [Google Scholar] [CrossRef]
- Mauch, S.C.; Zlevor, A.M.; Knott, E.A.; Couillard, A.B.; Periyasamy, S.; Williams, E.C.; Swietlik, J.F.; Laeseke, P.F.; Zhang, X.; Xu, Z.; et al. Hepatic and Renal Histotripsy in an Anticoagulated Porcine Model. J. Vasc. Interv. Radiol. 2023, 34, 386–394.e2. [Google Scholar] [CrossRef]
- Kisting, A.L.; Zlevor, A.M.; Falk, K.L.; Kisting, M.A.; Laklouk, I.A.; Wagner, M.G.; White, J.K.; Winterholler, J.E.; Jentink, M.S.; Abel, E.J.; et al. Histotripsy of the Proximal Ureter and Renal Pelvis: Evaluation of Urothelial Injury in a Porcine Survival Model. J. Vasc. Interv. Radiol. 2025, 36, 512–520.e1. [Google Scholar] [CrossRef]
- Wah, T.M.; Amaral, J.F.; Laeseke, P.F. Treatment of Primary Solid Renal Tumours Using Histotripsy: Study Protocol for the CAIN Feasibility Trial. Cardiovasc. Interv. Radiol. 2025, 48, 857–865. [Google Scholar] [CrossRef]
- HistoSonics, Inc. The HistoSonics Investigational System for Treatment of Primary Solid Renal Tumors Using Histotripsy (CAIN). Available online: https://www.clinicaltrials.gov/study/NCT05432232?term=AREA%5BBasicSearch%5D(histosonics)&rank=5 (accessed on 22 August 2025).
- HistoSonics, Inc. The HistoSonics EdisonTM System for Treatment of Primary Solid Renal Tumors Using Histotripsy (#HOPE4KIDNEY). Available online: https://www.clinicaltrials.gov/study/NCT05820087?term=AREA%5BBasicSearch%5D(histosonics)&rank=7 (accessed on 22 August 2025).
- Eigner, E.; Malshy, K.; Bandari, J.; Fazaa, N.; Nsair, A.; Hines, L.; Atallah, M.; Joseph, J.V.; Rappold, P.M. Histotripsy in the Management of RCC: A New Frontier in Focused Therapies. Clin. Genitourin. Cancer 2025, 23, 102360. [Google Scholar] [CrossRef]
- Miao, K.; Basterrechea, K.F.; Hernandez, S.L.; Ahmed, O.S.; Patel, M.V.; Bader, K.B. Development of Convolutional Neural Network to Segment Ultrasound Images of Histotripsy Ablation. IEEE Trans. Biomed. Eng. 2024, 71, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Gupta, D.; Daou, B.J.; Fox, A.; Choi, D.; Sukovich, J.R.; Hall, T.L.; Camelo-Piragua, S.; Chaudhary, N.; Snell, J.; et al. Transcranial Magnetic Resonance-Guided Histotripsy for Brain Surgery: Pre-Clinical Investigation. Ultrasound Med. Biol. 2022, 48, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Duclos, S.; Camelo-Piragua, S.; Chaudhary, N.; Sukovich, J.; Hall, T.; Pandey, A.; Xu, Z. Histotripsy Treatment of Murine Brain and Glioma: Temporal Profile of Magnetic Resonance Imaging and Histological Characteristics Post-Treatment. Ultrasound Med. Biol. 2023, 49, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Duclos, S.; Golin, A.; Fox, A.; Chaudhary, N.; Camelo-Piragua, S.; Pandey, A.; Xu, Z. Transcranial Histotripsy Parameter Study in Primary and Metastatic Murine Brain Tumor Models. Int. J. Hyperth. 2023, 40, 2237218. [Google Scholar] [CrossRef]
- Lu, N.; Hall, T.L.; Choi, D.; Gupta, D.; Daou, B.J.; Sukovich, J.R.; Fox, A.; Gerhardson, T.I.; Pandey, A.S.; Noll, D.C.; et al. Transcranial MR-Guided Histotripsy System. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2917–2929. [Google Scholar] [CrossRef] [PubMed]
- Ruger, L.; Langman, M.; Farrell, R.; Rossmeisl, J.H.; Prada, F.; Vlaisavljevich, E. Ultrasound-Guided Mechanical High-Intensity Focused Ultrasound (Histotripsy) Through an Acoustically Permeable Polyolefin-Based Cranioplasty Device. IEEE Trans. Biomed. Eng. 2024, 71, 2877–2888. [Google Scholar] [CrossRef]
- Lu, N.; Yeats, E.M.; Sukovich, J.R.; Hall, T.L.; Pandey, A.S.; Xu, Z. Treatment Envelope of Transcranial Histotripsy: Challenges and Strategies to Maximize the Treatment Location Profile. Phys. Med. Biol. 2024, 69, 225006. [Google Scholar] [CrossRef]
- Duclos, S.; Choi, S.W.; Andjelkovic, A.V.; Chaudhary, N.; Camelo-Piragua, S.; Pandey, A.; Xu, Z. Characterization of Blood–Brain Barrier Opening Induced by Transcranial Histotripsy in Murine Brains. Ultrasound Med. Biol. 2024, 50, 639–646. [Google Scholar] [CrossRef]
- Gupta, D.; Choi, D.; Lu, N.; Allen, S.P.; Hall, T.L.; Noll, D.C.; Xu, Z. Magnetic Resonance Thermometry Targeting for Magnetic Resonance–Guided Histotripsy Treatments. Ultrasound Med. Biol. 2023, 49, 1102–1107. [Google Scholar] [CrossRef]
- Gerhardson, T.; Sukovich, J.R.; Pandey, A.S.; Hall, T.L.; Cain, C.A.; Xu, Z. Catheter Hydrophone Aberration Correction for Transcranial Histotripsy Treatment of Intracerebral Hemorrhage: Proof-of-Concept. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 1684–1697. [Google Scholar] [CrossRef]
- Sukovich, J.R.; Macoskey, J.J.; Lundt, J.E.; Gerhardson, T.I.; Hall, T.L.; Xu, Z. Real-Time Transcranial Histotripsy Treatment Localization and Mapping Using Acoustic Cavitation Emission Feedback. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 1178–1191. [Google Scholar] [CrossRef]
- Zhang, X.; Owens, G.E.; Gurm, H.S.; Ding, Y.; Cain, C.A.; Xu, Z. Noninvasive Thrombolysis Using Histotripsy beyond the Intrinsic Threshold (Microtripsy). IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 1342–1355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Owens, G.E.; Cain, C.A.; Gurm, H.S.; Macoskey, J.; Xu, Z. Histotripsy Thrombolysis on Retracted Clots. Ultrasound Med. Biol. 2016, 42, 1903–1918. [Google Scholar] [CrossRef]
- Maxwell, A.D.; Cain, C.A.; Duryea, A.P.; Yuan, L.; Gurm, H.S.; Xu, Z. Noninvasive Thrombolysis Using Pulsed Ultrasound Cavitation Therapy—Histotripsy. Ultrasound Med. Biol. 2009, 35, 1982–1994. [Google Scholar] [CrossRef] [PubMed]
- Bader, K.B.; Flores Basterrechea, K.; Hendley, S.A. In Silico Assessment of Histotripsy-Induced Changes in Catheter-Directed Thrombolytic Delivery. Front. Physiol. 2023, 14, 1225804. [Google Scholar] [CrossRef]
- Miller, R.M.; Kim, Y.; Lin, K.-W.; Cain, C.A.; Owens, G.E.; Xu, Z. Histotripsy Cardiac Therapy System Integrated with Real-Time Motion Correction. Ultrasound Med. Biol. 2013, 39, 2362–2373. [Google Scholar] [CrossRef]
- Owens, G.E.; Miller, R.M.; Owens, S.T.; Swanson, S.D.; Ives, K.; Ensing, G.; Gordon, D.; Xu, Z. Intermediate-Term Effects of Intracardiac Communications Created Noninvasively by Therapeutic Ultrasound (Histotripsy) in a Porcine Model. Pediatr. Cardiol. 2012, 33, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Villemain, O.; Kwiecinski, W.; Bel, A.; Robin, J.; Bruneval, P.; Arnal, B.; Tanter, M.; Pernot, M.; Messas, E. Pulsed Cavitational Ultrasound for Non-Invasive Chordal Cutting Guided by Real-Time 3D Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Lu, X.; Dou, C.; Zhu, Y.I.; Fuller, R.; Fields, K.; Fabiilli, M.L.; Owens, G.E.; Gordon, D.; Kripfgans, O.D. Ultrasonic Cavitation-Enabled Treatment for Therapy of Hypertrophic Cardiomyopathy: Proof of Principle. Ultrasound Med. Biol. 2018, 44, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Winterholler, J.E.; Kisting, M.A.; Falk, K.L.; Kisting, A.L.; Jentink, M.S.; White, J.K.; Lubner, M.G.; Laeseke, P.F.; Knavel Koepsel, E.M.; Swietlik, J.F.; et al. Hepatic Histotripsy: Jet Ventilation Decreases the Effect of Respiratory Motion in A Porcine Liver Model. Cardiovasc. Interv. Radiol. 2025, 48, 1164–1173. [Google Scholar] [CrossRef]
- Smolock, A.R.; Cristescu, M.M.; Vlaisavljevich, E.; Gendron-Fitzpatrick, A.; Green, C.; Cannata, J.; Ziemlewicz, T.J.; Lee, F.T. Robotically Assisted Sonic Therapy as a Noninvasive Nonthermal Ablation Modality: Proof of Concept in a Porcine Liver Model. Radiology 2018, 287, 485–493. [Google Scholar] [CrossRef]
- Longo, K.C.; Knott, E.A.; Watson, R.F.; Swietlik, J.F.; Vlaisavljevich, E.; Smolock, A.R.; Xu, Z.; Cho, C.S.; Mao, L.; Lee, F.T.; et al. Robotically Assisted Sonic Therapy (RAST) for Noninvasive Hepatic Ablation in a Porcine Model: Mitigation of Body Wall Damage with a Modified Pulse Sequence. Cardiovasc. Interv. Radiol. 2019, 42, 1016–1023. [Google Scholar] [CrossRef]
- Landry, T.G.; Brown, J.A. Ultrasound Imaging Guided Precision Histotripsy: Effects of Pulse Settings on Ablation Properties in Rat Brain. J. Acoust. Soc. Am. 2024, 155, 2860–2874. [Google Scholar] [CrossRef]
| Technique | Mechanism | Key Features | Limitations |
|---|---|---|---|
| Histotripsy | Nonthermal mechanical tissue fractionation using focused ultrasound-induced cavitation | Noninvasive, precise targeting, minimal collateral damage, real-time imaging, mechanical and immunological efficacy | Emerging clinical data, targeting challenges in mobile anatomy, limited immunologic understanding, limited indications currently |
| Radiofrequency Ablation (RFA) | Thermal injury via high-frequency alternating current | Widely used, effective for small tumors, percutaneous or laparoscopic access | Heat-sink effect, limited precision, risk of thermal damage to adjacent structures |
| Microwave Ablation (MWA) | Dielectric heating through microwave energy | Faster and larger ablation zones than RFA, less susceptible to heat sink | Collateral thermal injury, limited use near heat-sensitive structures |
| High intensity Focused Ultrasound (HIFU) | Thermal ablation via focused ultrasound heating | Noninvasive, MRI-guided, precise heating, acoustic cavitation | Thermal latency, bone interference, skin burns, long treatment time |
| Cryoablation | Cell death via freeze–thaw cycles using cryoprobes | Visible ice-ball on imaging, less pain, potential immune activation | Longer procedure time, risk of hemorrhage, ice-ball unpredictability |
| Irreversible Electroporation (IRE) | Nonthermal cell death by high-voltage electric pulses disrupting membranes | Spares extracellular matrix, useful near vessels and ducts | Requires general anesthesia, cardiac synchronization, limited availability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallumeera, M.; Hong, M.; Giang, J.C.; Makary, M.S. Histotripsy: Recent Advances, Clinical Applications, and Future Prospects. Cancers 2025, 17, 3072. https://doi.org/10.3390/cancers17183072
Pallumeera M, Hong M, Giang JC, Makary MS. Histotripsy: Recent Advances, Clinical Applications, and Future Prospects. Cancers. 2025; 17(18):3072. https://doi.org/10.3390/cancers17183072
Chicago/Turabian StylePallumeera, Mustaqueem, Marcus Hong, Jonathan C Giang, and Mina S Makary. 2025. "Histotripsy: Recent Advances, Clinical Applications, and Future Prospects" Cancers 17, no. 18: 3072. https://doi.org/10.3390/cancers17183072
APA StylePallumeera, M., Hong, M., Giang, J. C., & Makary, M. S. (2025). Histotripsy: Recent Advances, Clinical Applications, and Future Prospects. Cancers, 17(18), 3072. https://doi.org/10.3390/cancers17183072






