Peptide Receptor Radionuclide Therapy (PRRT) Using Actinium-225- and Ac-225/Lutetium-177-Labeled (TANDEM) Somatostatin Receptor Antagonist DOTA-LM3 in Patients with Neuroendocrine Neoplasm: A Retrospective Study Concerning Safety and Survival
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
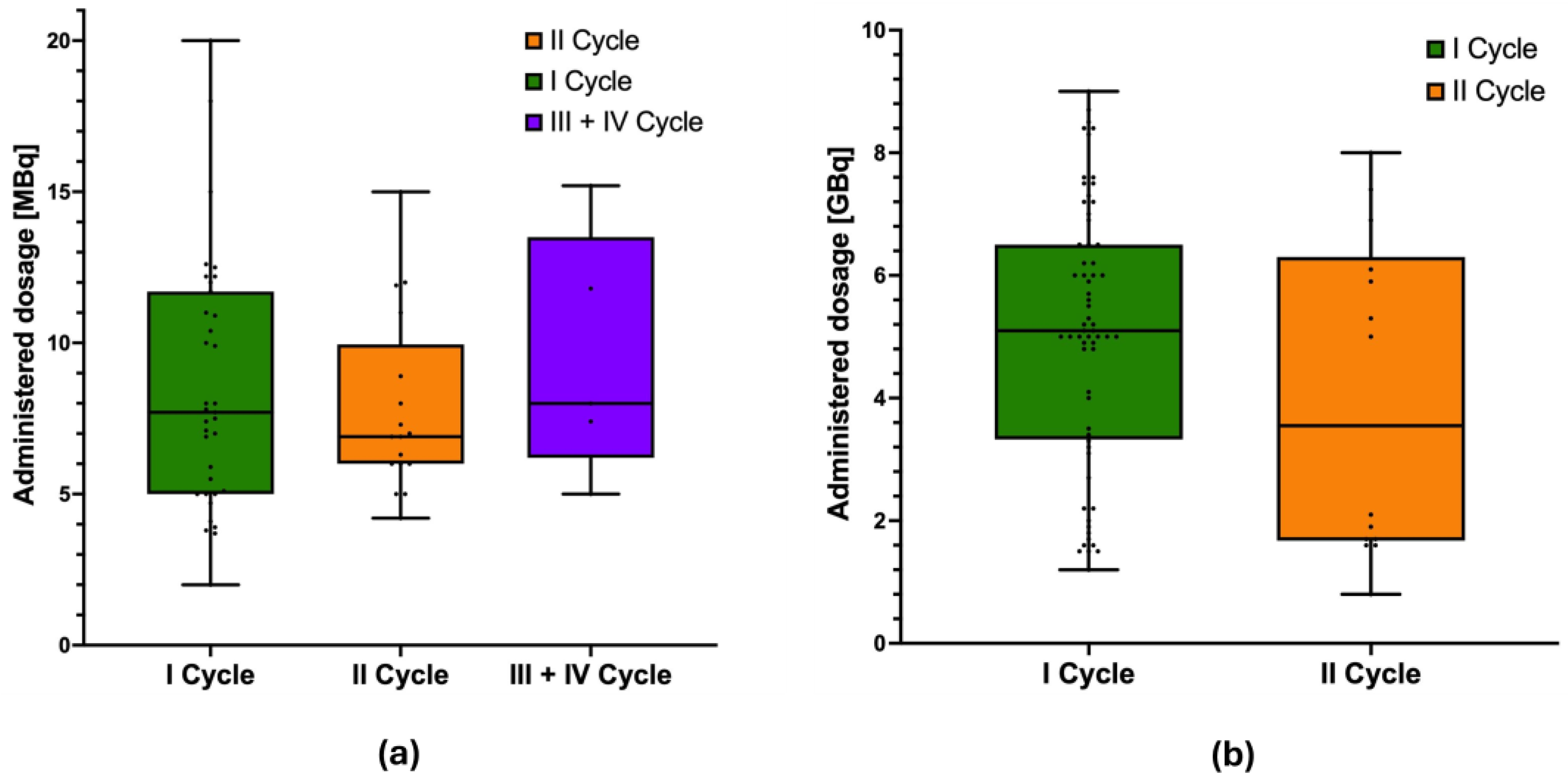
3.1. Patient Cohort and PRRT Data
3.2. Survival Analyses
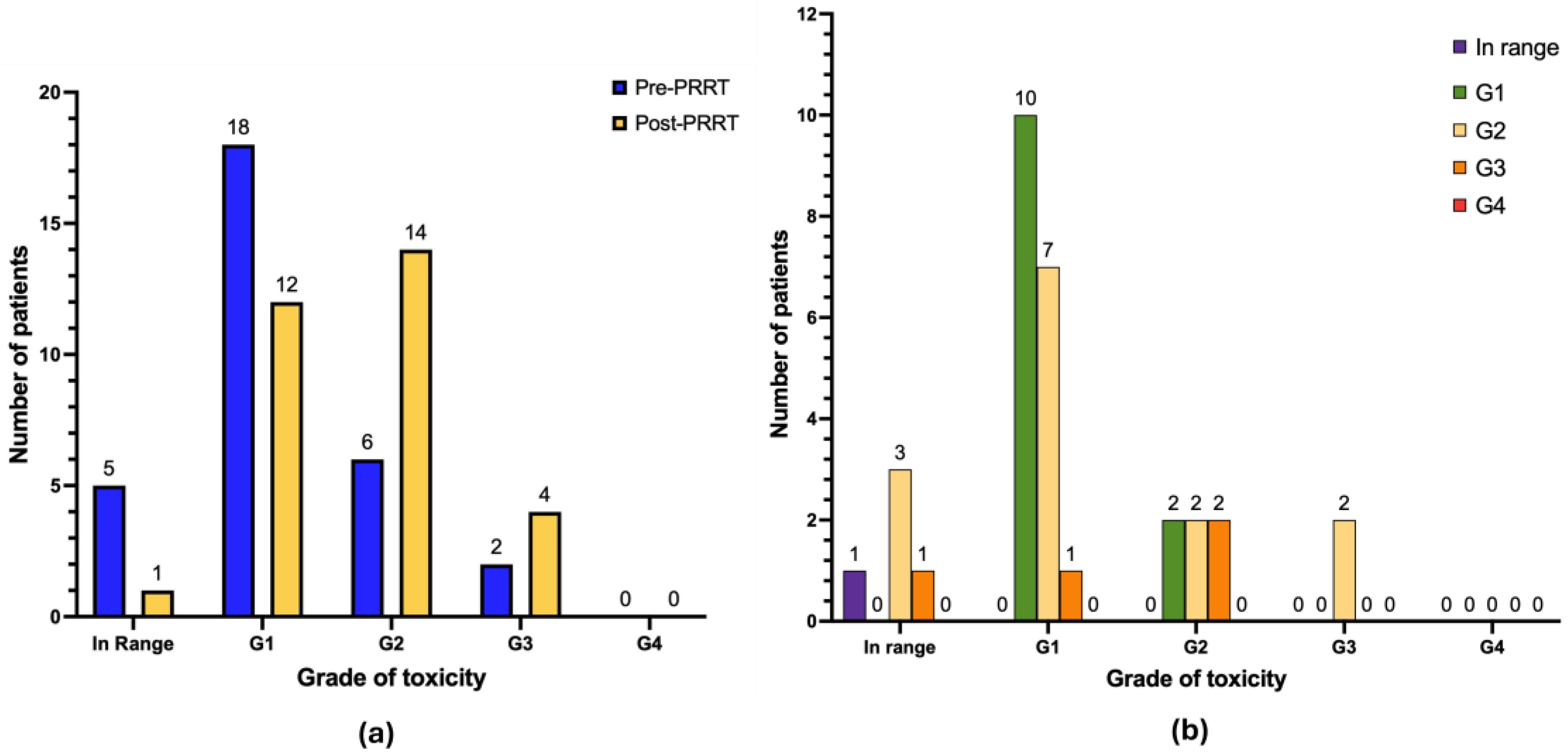
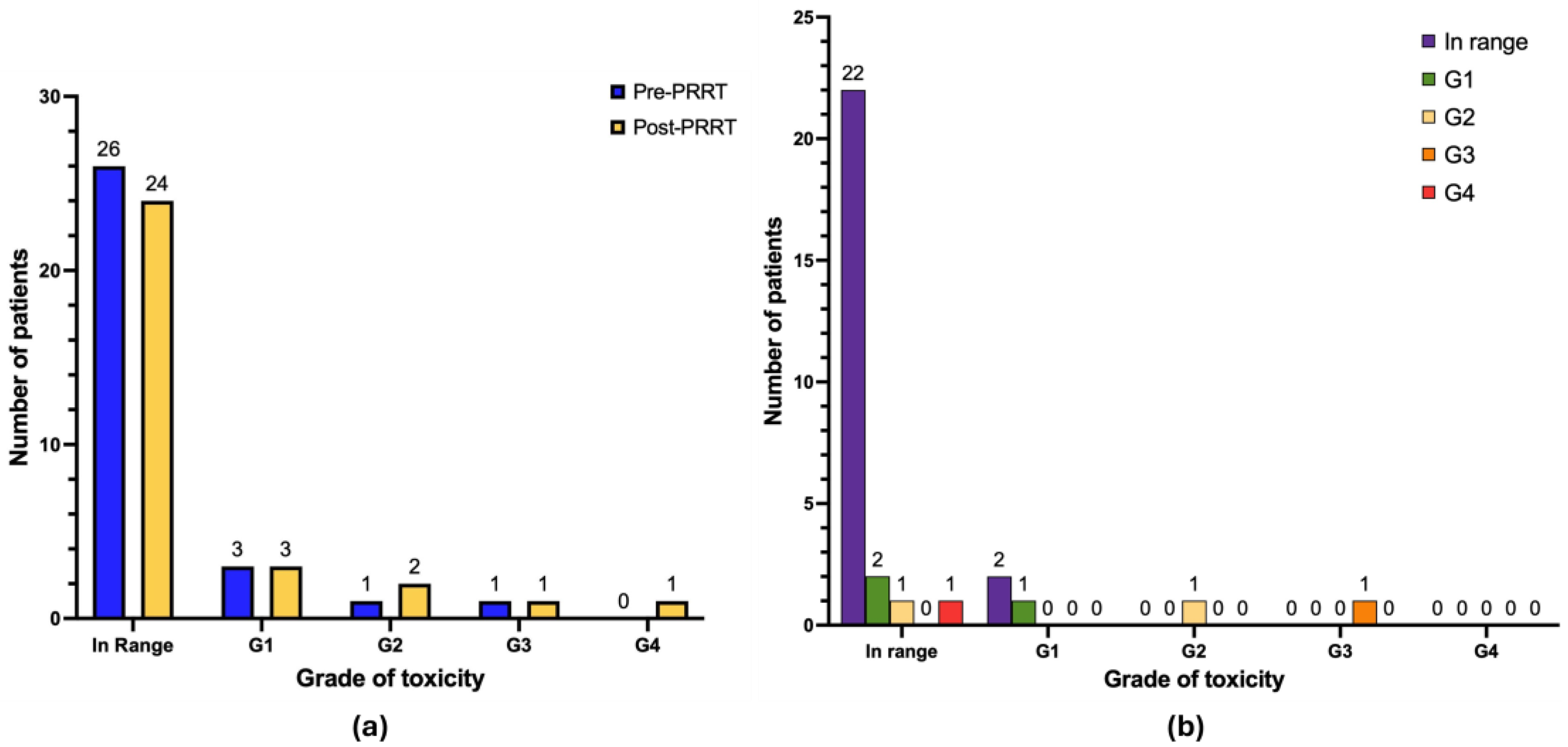
3.3. Hematological Toxicity
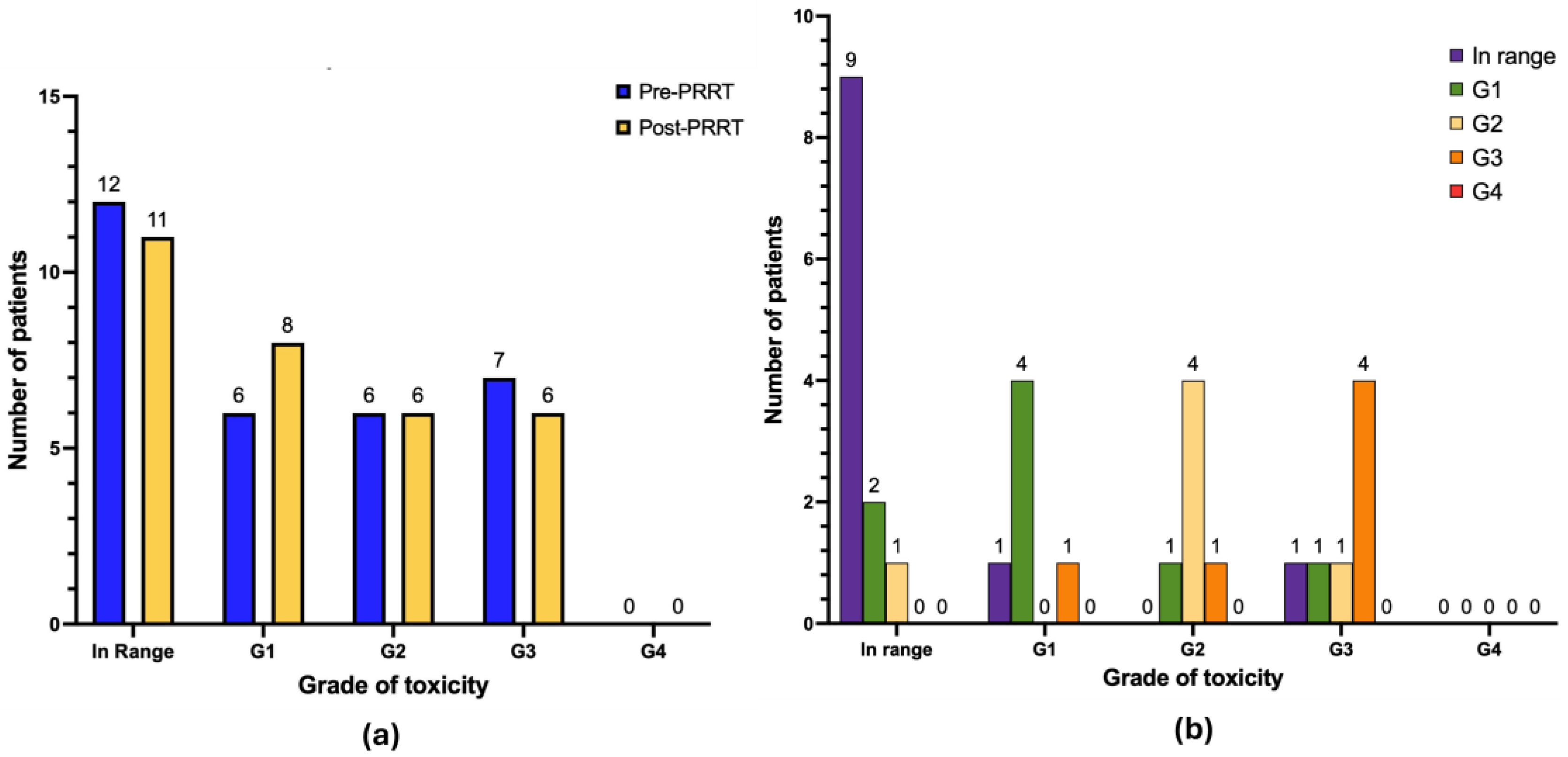
3.4. Nephrotoxicity and Hepatotoxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Modlin, I.M.; Oberg, K.; Chung, D.C.; Jensen, R.T.; de Herder, W.W.; Thakker, R.V.; Caplin, M.; Delle Fave, G.; Kaltsas, G.A.; Krenning, E.P.; et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008, 9, 61–72. [Google Scholar] [CrossRef]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Günther, T.; Tulipano, G.; Dournaud, P.; Bousquet, C.; Csaba, Z.; Kreienkamp, H.J.; Lupp, A.; Korbonits, M.; Castaño, J.P.; Wester, H.J.; et al. International Union of Basic and Clinical Pharmacology. CV. Somatostatin receptors: Structure, function, ligands, and new nomenclature. Pharmacol. Rev. 2018, 70, 763–835. [Google Scholar] [CrossRef] [PubMed]
- Fahey, F.H.; Grant, F.D.; Thrall, J.H. Saul Hertz, MD, and the birth of radionuclide therapy. EJNMMI Phys. 2017, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Kopka, K. Lutathera®: The first FDA- and EMA-approved radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Scheinberg, D.A.; McDevitt, M.R. Actinium-225 in targeted alpha-particle therapeutic applications. Curr. Radiopharm. 2011, 4, 306–320. [Google Scholar] [CrossRef]
- Pouget, J.P.; Constanzo, J. Revisiting the radiobiology of Targeted Alpha Therapy. Front. Med. 2021, 8, 692436. [Google Scholar] [CrossRef]
- Shi, M.; Jakobsson, V.; Greifenstein, L.; Khong, P.L.; Chen, X.; Baum, R.P.; Zhang, J. Alpha-peptide receptor radionuclide therapy using actinium-225 labeled somatostatin receptor agonists and antagonists. Front. Med. 2022, 9, 1034315. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.P.; Mansi, R.; McDougall, L.; Kaufmann, J.; Bouterfa, H.; Wild, D.; Fani, M. Biodistribution, pharmacokinetics, and dosimetry of 177Lu-, 90Y-, and 111In-labeled somatostatin receptor antagonist OPS201 in comparison to the agonist 177Lu-DOTATATE: The mass effect. J. Nucl. Med. 2017, 58, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Zhang, J.; Schuchardt, C.; Müller, D.; Mäcke, H. First-in-humans study of the SSTR antagonist 177Lu-DOTA-LM3 for Peptide Receptor Radionuclide Therapy in patients with metastatic neuroendocrine neoplasms: Dosimetry, safety, and efficacy. J. Nucl. Med. 2021, 62, 1571–1581. [Google Scholar] [CrossRef]
- Kanellopoulos, P.; Nock, B.A.; Greifenstein, L.; Baum, R.P.; Roesch, F.; Maina, T. 68Ga-DATA5m-LM4, a PET radiotracer in the diagnosis of SST2R-positive tumors: Preclinical and first clinical results. Int. J. Mol. Sci. 2022, 23, 14590. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Phase 1b/3 Global, Randomized, Controlled, Open-Label Trial Comparing Treatment with RYZ101 to Standard of Care Therapy in Subjects with Inoperable, Advanced, SSTR+, Well-Differentiated GEP-NETs that Have Progressed Following Prior 177Lu-SSA Therapy. Available online: https://clinicaltrials.gov/study/NCT05477576 (accessed on 3 May 2025).
- Singh, S.; Halperin, D.; Myrehaug, S.; Herrmann, K.; Pavel, M.; Kunz, P.L.; Chasen, B.; Tafuto, S.; Lastoria, S.; Capdevila, J.; et al. 177Lu-DOTA-TATE plus long-acting octreotide versus high-dose long-acting octreotide for the treatment of newly diagnosed, advanced grade 2-3, well-differentiated, gastroenteropancreatic neuroendocrine tumours (NETTER-2): An open-label, randomised, phase 3 study. Lancet 2024, 403, 2807–2817. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. A Phase III Multi-Center, Randomized, Open-Label Study to Evaluate the Efficacy and Safety of [177Lu]Lu-DOTA-TATE in Patients Newly Diagnosed with Grade 1 and Grade 2 (Ki-67 <10%) Advanced GEP-NET with High Disease Burden (NETTER-3). Available online: https://clinicaltrials.gov/study/NCT06784752 (accessed on 3 May 2025).
- National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v 5.0. 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 3 May 2025).
- Goncalves, I.; Burbury, K.; Michael, M.; Iravani, A.; Ravi Kumar, A.S.; Akhurst, T.; Tiong, I.S.; Blombery, P.; Hofman, M.S.; Westerman, D.; et al. Characteristics and outcomes of therapy-related myeloid neoplasms after peptide receptor radionuclide/chemoradionuclide therapy (PRRT/PRCRT) for metastatic neuroendocrine neoplasia: A single-institution series. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1902–1910. [Google Scholar] [CrossRef]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177Lu-Dotatate in the phase III NETTER-1 trial. J. Clin. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. A phase III Multi-Center, Randomized, Open-Label Study to Evaluate the Efficacy and Safety of Lutathera in Patients with Grade 2 and Grade 3 Advanced GEP-NET. Available online: https://clinicaltrials.gov/study/NCT03972488 (accessed on 3 May 2025).
- Kratochwil, C.; Apostolidis, L.; Rathke, H.; Apostolidis, C.; Bicu, F.; Bruchertseifer, F.; Choyke, P.L.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Dosing 225Ac-DOTATOC in patients with somatostatin-receptor-positive solid tumors: 5-year follow-up of hematological and renal toxicity. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 54–63. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Li, H.; Zhang, Y.; Feng, Y.; Yang, X.; Chen, Y. Efficacy and safety of 225Ac-DOTATATE in the treatment of neuroendocrine neoplasms with high SSTR expression. Clin. Nucl. Med. 2024, 49, 505–512. [Google Scholar] [CrossRef]
- Ginj, M.; Zhang, H.; Waser, B.; Cescato, R.; Wild, D.; Wang, X.; Erchegyi, J.; Rivier, J.; Mäcke, H.R.; Reubi, J.C. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc. Natl. Acad. Sci. USA 2006, 103, 16436–16441. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Nicolas, G.P.; Wild, D. Somatostatin receptor antagonists for imaging and therapy. J. Nucl. Med. 2017, 58 (Suppl. S2), 61S–66S. [Google Scholar] [CrossRef]
- Bodei, L.; Weber, W.A. Somatostatin receptor imaging of neuroendocrine tumors: From agonists to antagonists. J. Nucl. Med. 2018, 59, 907–908. [Google Scholar] [CrossRef]
- Wild, D.; Fani, M.; Fischer, R.; Del Pozzo, L.; Kaul, F.; Krebs, S.; Fischer, R.; Rivier, J.E.; Reubi, J.C.; Maecke, H.R.; et al. Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: A pilot study. J. Nucl. Med. 2014, 55, 1248–1252. [Google Scholar] [CrossRef]
- Handula, M.; Beekman, S.; Konijnenberg, M.; Stuurman, D.; de Ridder, C.; Bruchertseifer, F.; Morgenstern, A.; Denkova, A.; de Blois, E.; Seimbille, Y. First preclinical evaluation of 225Ac-DOTA-JR11 and comparison with 177Lu-DOTA-JR11, alpha versus beta radionuclide therapy of NETs. EJNMMI Radiopharm. Chem. 2023, 8, 13. [Google Scholar] [CrossRef]
- Perrone, E.; Ghai, K.; Eismant, A.; Andreassen, M.; Langer, S.W.; Knigge, U.; Kjaer, A.; Baum, R.P. Impressive response to TANDEM Peptide Receptor Radionuclide Therapy with 177Lu/225Ac-DOTA-LM3 somatostatin receptor antagonist in a patient with therapy-refractory, rapidly progressive neuroendocrine neoplasm of the pancreas. Diagnostics 2024, 14, 907. [Google Scholar] [CrossRef] [PubMed]
- Speicher, T.; Burgard, C.; Bastian, M.; Rosar, F.; Bartholomä, M.; Maus, S.; Ezziddin, S. First successful in-human application of 225Ac-DOTA-LM3 mono-PRRT for the treatment of an otherwise therapy-resistant Neuroendocrine Tumor (NET G3). Clin. Nucl. Med. 2025, 50, 101–102. [Google Scholar] [CrossRef]
- Kong, G.; Buteau, J.P.; Hofman, M.S. Is 161Tb really happening? J. Nucl. Med. 2024, 65, 686–687. [Google Scholar] [CrossRef]
- Borgna, F.; Haller, S.; Rodriguez, J.M.M.; Ginj, M.; Grundler, P.V.; Zeevaart, J.R.; Köster, U.; Schibli, R.; van der Meulen, N.P.; Müller, C. Combination of terbium-161 with somatostatin receptor antagonists-a potential paradigm shift for the treatment of neuroendocrine neoplasms. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Fricke, J.; Westerbergh, F.; McDougall, L.; Favaretto, C.; Christ, E.; Nicolas, G.P.; Geistlich, S.; Borgna, F.; Fani, M.; Bernhardt, P.; et al. First-in-human administration of terbium-161-labelled somatostatin receptor subtype 2 antagonist (161Tb-DOTA-LM3) in a patient with a metastatic neuroendocrine tumour of the ileum. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2517–2519. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R. Is 212Pb really happening? The post-177Lu/225Ac blockbuster? J. Nucl. Med. 2024, 65, 176–177. [Google Scholar] [CrossRef]
- Delpassand, E.S.; Tworowska, I.; Esfandiari, R.; Torgue, J.; Hurt, J.; Shafie, A.; Núñez, R. Targeted α-emitter therapy with 212Pb-DOTAMTATE for the treatment of metastatic SSTR-expressing neuroendocrine tumors: First-in-humans dose-escalation clinical trial. J. Nucl. Med. 2022, 63, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Winter, R.C.; Amghar, M.; Wacker, A.S.; Bakos, G.; Taş, H.; Roscher, M.; Kelly, J.M.; Benešová-Schäfer, M. Future treatment strategies for cancer patients combining targeted alpha therapy with pillars of cancer treatment: External beam radiation therapy, checkpoint inhibition immunotherapy, cytostatic chemotherapy, and brachytherapy. Pharmaceuticals 2024, 17, 1031. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Steenblock, C.; Fischer, A.; Lemm, S.; Ziegler, C.G.; Bechmann, N.; Nölting, S.; Pietzsch, J.; Ullrich, M. Improving susceptibility of neuroendocrine tumors to radionuclide therapies: Personalized approaches towards complementary treatments. Theranostics 2024, 14, 17–32. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Tripathi, M.; Sahoo, R.K.; Bal, C. Survival outcomes in metastatic gastroenteropancreatic neuroendocrine tumor patients receiving concomitant 225Ac-DOTATATE targeted alpha therapy and capecitabine: A real-world scenario management based long-term outcome study. J. Nucl. Med. 2022, 64, 211–218. [Google Scholar] [CrossRef] [PubMed]




| Before Treatment | After Treatment | |||
|---|---|---|---|---|
| Anemia (Grading) | Number (n) | Percent (%) | Number (n) | Percent (%) |
| G0 | 5 | 16.1 | 1 | 3.2 |
| G1 | 18 | 58.0 | 12 | 38.7 |
| G2 | 6 | 19.3 | 14 | 45.1 |
| G3 | 2 | 6.4 | 4 | 12.9 |
| G4 | 0 | 0.0 | 0 | 0.0 |
| Leukocytopenia (Grading) | Number (n) | Percent (%) | Number (n) | Percent (%) |
| G0 | 26 | 83.9 | 24 | 77.4 |
| G1 | 3 | 9.7 | 3 | 9.7 |
| G2 | 1 | 3.2 | 2 | 6.4 |
| G3 | 1 | 3.2 | 1 | 3.2 |
| G4 | 0 | 0.0 | 1 | 3.2 |
| Thrombocytopenia (Grading) | Number (n) | Percent (%) | Number (n) | Percent (%) |
| G0 | 24 | 77.4 | 18 | 58 |
| G1 | 6 | 19.3 | 5 | 16.1 |
| G2 | 1 | 3.2 | 1 | 3.2 |
| G3 | 0 | 0.0 | 7 | 22.6 |
| G4 | 0 | 0.0 | 0 | 0.0 |
| Before Treatment | After Treatment | |||
|---|---|---|---|---|
| Renal Function (Grading) | Number (n) | Percent (%) | Number (n) | Percent (%) |
| G0 | 26 | 83.9 | 20 | 64.5 |
| G1 | 4 | 12.9 | 7 | 22.6 |
| G2 | 1 | 3.2 | 2 | 6.4 |
| G3 | 0 | 0.0 | 2 | 6.4 |
| G4 | 0 | 0.0 | 0 | 0.0 |
| Hepatic Function (Grading) | Number (n) | Percent (%) | Number (n) | Percent (%) |
| G0 | 12 | 38.7 | 11 | 35.5 |
| G1 | 6 | 19.3 | 8 | 25.8 |
| G2 | 6 | 19.3 | 6 | 19.3 |
| G3 | 7 | 22.6 | 6 | 19.3 |
| G4 | 0 | 0.0 | 0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrone, E.; Calcagni, M.L.; Leccisotti, L.; Moretti, R.; Ghai, K.; Eismant, A.; Parkar, T.; Greifenstein, L.; Baum, R.P. Peptide Receptor Radionuclide Therapy (PRRT) Using Actinium-225- and Ac-225/Lutetium-177-Labeled (TANDEM) Somatostatin Receptor Antagonist DOTA-LM3 in Patients with Neuroendocrine Neoplasm: A Retrospective Study Concerning Safety and Survival. Cancers 2025, 17, 3070. https://doi.org/10.3390/cancers17183070
Perrone E, Calcagni ML, Leccisotti L, Moretti R, Ghai K, Eismant A, Parkar T, Greifenstein L, Baum RP. Peptide Receptor Radionuclide Therapy (PRRT) Using Actinium-225- and Ac-225/Lutetium-177-Labeled (TANDEM) Somatostatin Receptor Antagonist DOTA-LM3 in Patients with Neuroendocrine Neoplasm: A Retrospective Study Concerning Safety and Survival. Cancers. 2025; 17(18):3070. https://doi.org/10.3390/cancers17183070
Chicago/Turabian StylePerrone, Elisabetta, Maria Lucia Calcagni, Lucia Leccisotti, Roberto Moretti, Kriti Ghai, Aleksandr Eismant, Tanay Parkar, Lukas Greifenstein, and Richard Paul Baum. 2025. "Peptide Receptor Radionuclide Therapy (PRRT) Using Actinium-225- and Ac-225/Lutetium-177-Labeled (TANDEM) Somatostatin Receptor Antagonist DOTA-LM3 in Patients with Neuroendocrine Neoplasm: A Retrospective Study Concerning Safety and Survival" Cancers 17, no. 18: 3070. https://doi.org/10.3390/cancers17183070
APA StylePerrone, E., Calcagni, M. L., Leccisotti, L., Moretti, R., Ghai, K., Eismant, A., Parkar, T., Greifenstein, L., & Baum, R. P. (2025). Peptide Receptor Radionuclide Therapy (PRRT) Using Actinium-225- and Ac-225/Lutetium-177-Labeled (TANDEM) Somatostatin Receptor Antagonist DOTA-LM3 in Patients with Neuroendocrine Neoplasm: A Retrospective Study Concerning Safety and Survival. Cancers, 17(18), 3070. https://doi.org/10.3390/cancers17183070






