Long-Term Outcomes Following Reconstruction of Diaphyseal Defects of the Upper and Lower Extremities Using Diaphyseal Implants: A Retrospective Study with Focus on Fixation Technique
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Patient Groups
2.3. Methodology
2.4. Statistical Methods
3. Results
3.1. Surgical Indications and Comorbidities
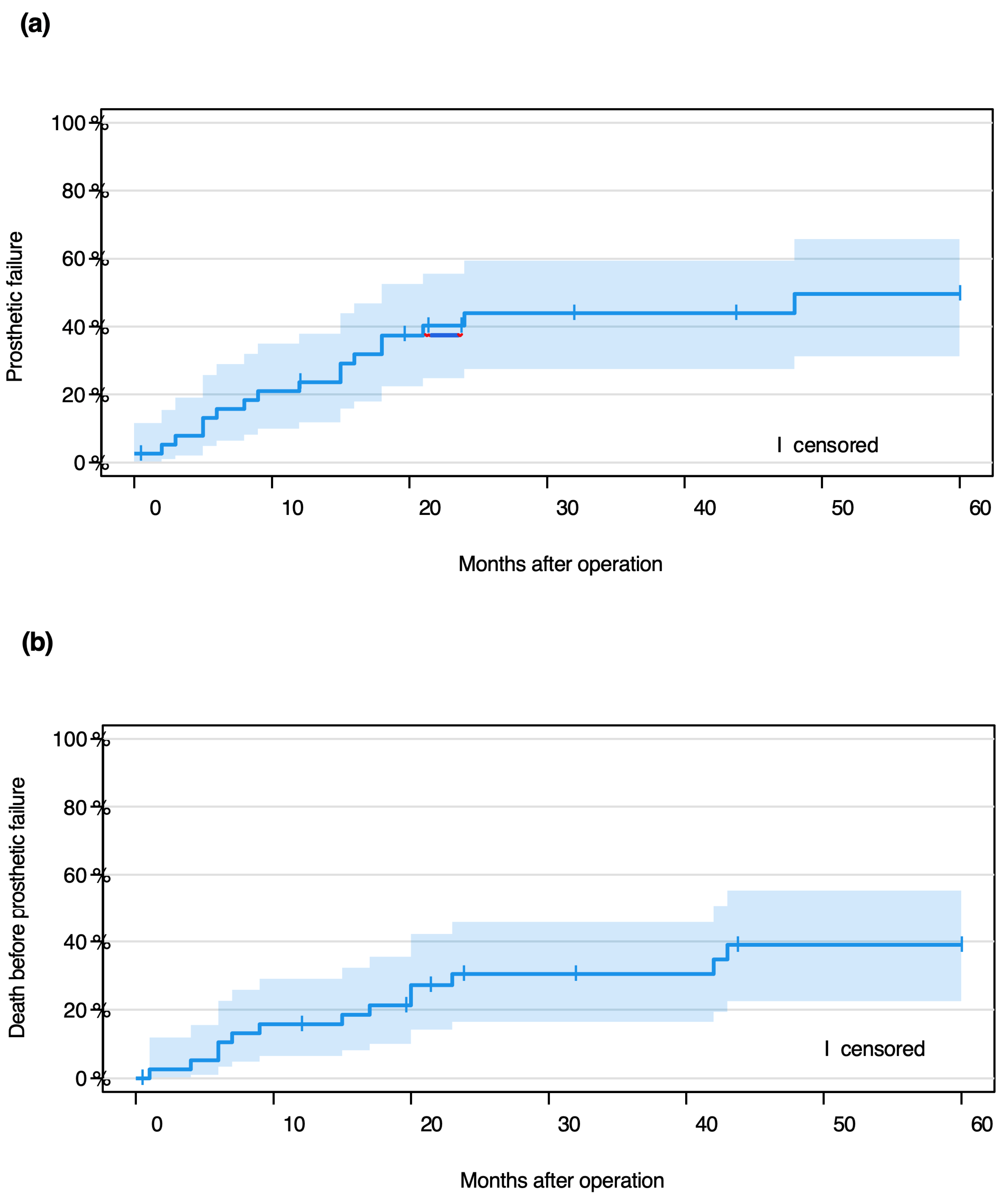
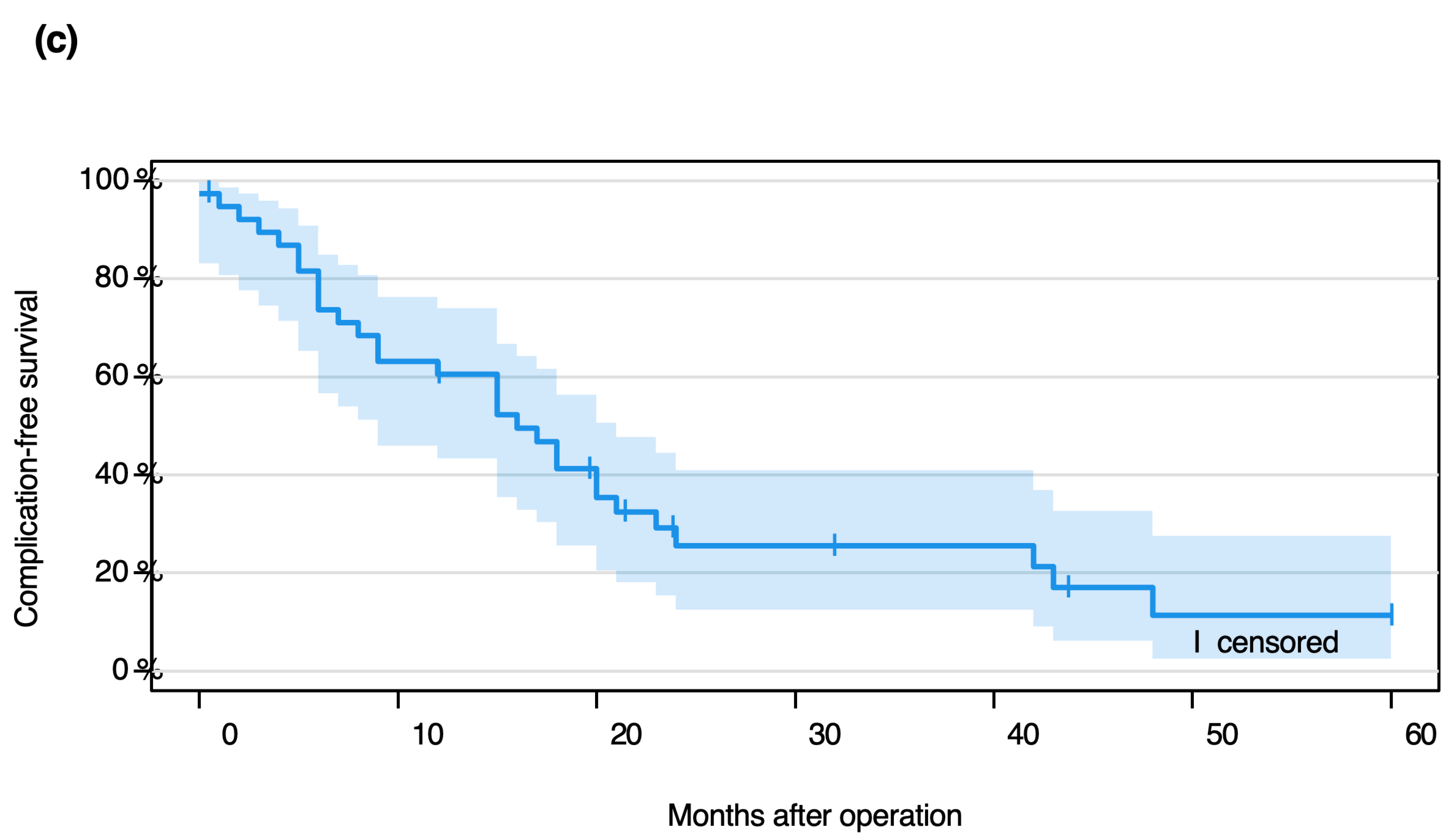
3.2. Event-Free Probability
- 61% (95% CI 43–74.0%) at 12 months,
- 26% (95% CI 13–41%) at 24 months,
- 11% (95% CI 3–28%) at 60 months.
3.3. Implant Complications: Types and Timing
3.4. Risk Factors for Implant Complications
- •
- 35 were cemented and screwed;
- •
- 29 were cemented without screws;
- •
- 5 were screwed but not cemented;
- •
- 7 were neither cemented nor screwed;
- •
- 1 case lacked fixation documentation.
- •
- 7 of 64 cemented stems (11%);
- •
- 1 of 12 uncemented stems (8%);
- •
- 3 of 40 screwed stems (8%);
- •
- 5 of 36 non-screwed stems (14%).
- •
- 14 (18%) were custom-made;
- •
- 29 (37%) were off-the-shelf.
- •
- Femur: 16.4 cm (SD = 4.6);
- •
- Tibia: 14.8 cm (SD = 3.1);
- •
- Humerus: 8.6 cm (SD = 1.0).
3.5. Functional Outcome
- •
- Cemented: median 14.5 (IQR: 8.0–25.0);
- •
- Uncemented: median 27.0 (IQR: 17.0–29.0);
- •
- Screwed: median 15.5 (IQR: 10.3–25.3);
- •
- Non-screwed: median 14.5 (IQR: 10.3–25.3).
- •
- Femoral: median 15.5 (IQR: 8.8–25.8);
- •
- Tibial: median 17.5 (IQR: 9.0–25.3);
- •
- Humeral: 14.0.
- •
- Primary tumor: median 20.5 (IQR: 12.5–26.8);
- •
- Metastasis: median 8.0 (IQR: 6.5–20.5).
- •
- Without comorbidities: median 25.0 (IQR: 16.0–28.0);
- •
- With comorbidities: median 13.0 (IQR: 8.0–16.5).
4. Discussion
4.1. Diaphyseal Implants Have Higher Complication Rates Than Biological Reconstructions
4.2. Cement and Screw Fixation Reduces Aseptic Loosening Risk
4.3. Prosthesis Failure Is More Likely After Primary Tumor Resection, but Not Significantly
4.4. Internal Comorbidities Affect Function More than Survival
4.5. Overweight Patients Have Worse Function but No Increased Failure Risk
4.6. Functional Outcomes in Diaphyseal Implants
4.7. Tibial Implants Have Higher Failure Rates than Femoral Ones
4.8. Custom-Made Stems Are Linked to Worse Outcomes
4.9. Study Limitations
5. Conclusions
- -
- The fixation method significantly influences the outcome of the implants.
- -
- The risk of aseptic loosening tends to be lower for cemented and screwin stems than for cemented unscrewed stems. However, this result is not statistically significant.
- -
- Risk factors that can negatively influence the functional outcome in a statistically significant manner include increased body weight, advanced patient age, and preexisting internal medical conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henshaw, R.M. Surgical Advances in Bone and Soft Tissue Sarcoma: 50 Years of Progress. In American Society of Clinical Oncology Educational Book; American Society of Clinical Oncology: Alexandria, VA, USA, 2014; pp. 252–258. [Google Scholar] [CrossRef]
- Zekry, K.M.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Higuchi, T.; Abe, K.; Taniguchi, Y.; Alkhooly, A.Z.A.A.; Abd-Elfattah, A.S.; Fouly, E.H.; et al. Intercalary frozen autograft for reconstruction of malignant bone and soft tissue tumours. Int. Orthop. 2017, 41, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Zekry, K.M.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Alkhooly, A.Z.A.; Abd-Elfattah, A.S.; Elsaid, A.N.S.; Ahmed, A.R.; Tsuchiya, H. Reconstruction of intercalary bone defect after resection of malignant bone tumor. J. Orthop. Surg. 2019, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hilven, P.H.; Bayliss, L.; Cosker, T.; Dijkstra, P.D.S.; Jutte, P.C.; Lahoda, L.U.; Schaap, G.R.; Bramer, J.A.M.; van Drunen, G.K.; Strackee, S.D.; et al. The vascularized fibular graft for limb salvage after bone tumour surgery: A multicentre study. Bone Jt. J. 2015, 97, 853–861. [Google Scholar] [CrossRef]
- Bus, M.P.; Dijkstra, P.D.; Van De Sande, M.A.; Taminiau, A.H.; Schreuder, H.W.; Jutte, P.C.; van der Geest, I.C.M.; Schaap, G.R.; Bramer, J.A.M. Intercalary allograft reconstructions following resection of primary bone tumors: A nationwide multicenter study. J. Bone Jt. Surg. Am. 2014, 96, e26. [Google Scholar] [CrossRef]
- Panagopoulos, G.N.; Mavrogenis, A.F.; Mauffrey, C.; Lesenský, J.; Angelini, A.; Megaloikonomos, P.D.; Igoumenou, V.G.; Papanastassiou, J.; Savvidou, O.; Ruggieri, P.; et al. Intercalary reconstructions after bone tumor resections: A review of treatments. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 737–746. [Google Scholar] [CrossRef]
- Damron, T.A.; Malhotra, A. Intercalary Diaphyseal Endoprosthetic Reconstruction. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2025, 9, e24.00201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shao, X.; Dou, M.; Yang, Q.; Li, J.; Zhang, A.; Yao, Y.; Chu, Q.; Li, K.; Li, Z. Reconstruction of massive bone defects after femoral tumor resection using two new-designed 3D-printed intercalary prostheses: A clinical analytic study with the cooperative utilization of multiple technologies. BMC Musculoskelet. Disord. 2023, 24, 67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goebel, L.; Kohn, D.; Orth, P. Endoprosthetic replacement following intercalary resection. Orthopade 2019, 48, 572–581. [Google Scholar] [CrossRef]
- Hanna, S.A.; Sewell, M.D.; Aston, W.J.S.; Pollock, R.C.; Skinner, J.A.; Cannon, S.R.; Briggs, T.W.R. Femoral diaphyseal endoprosthetic reconstruction after segmental resection of primary bone tumours. J. Bone Jt. Surg.—Ser. B 2010, 92, 867–874. [Google Scholar] [CrossRef]
- Huang, H.C.; Hu, Y.C.; Lun, D.X.; Miao, J.; Wang, F.; Yang, X.G.; Ma, X.L. Outcomes of intercalary prosthetic reconstruction for pathological diaphyseal femoral fractures secondary to metastatic tumors. Orthop. Surg. 2017, 9, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, D.; De Man, F.H.R.; De Jong, P.T.; Van Stralen, R.A.; Marti, R.K. Is the long-term outcome of cemented THA jeopardized by patients being overweight? Clin. Orthop. Relat. Res. 2008, 466, 1162–1168. [Google Scholar] [CrossRef]
- Henderson, E.R.; Groundland, J.S.; Pala, E.; Dennis, J.A.; Wooten, R.; Cheong, D.; Windhager, R.; Kotz, R.I.; Mercuri, M.; Funovics, P.T.; et al. Failure mode classification for tumor endoprostheses: Retrospective review of five institutions and a literature review. J. Bone Jt. Surg.—Ser. A 2011, 93, 418–429. [Google Scholar] [CrossRef]
- Lun, D.X.; Hu, Y.C.; Yang, X.G.; Wang, F.; Xu, Z.W. Short-term outcomes of reconstruction subsequent to intercalary resection of femoral diaphyseal metastatic tumor with pathological fracture: Comparison between segmental allograft and intercalary prosthesis. Oncol. Lett. 2018, 15, 3508–3517. [Google Scholar] [CrossRef]
- Gerrand, C.H.; Rankin, K. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. In Classic Papers in Orthopaedics; Springer: London, UK, 1993; Volume 2014, pp. 489–490. [Google Scholar] [CrossRef]
- Benevenia, J.; Kirchner, R.; Patterson, F.; Beebe, K.; Wirtz, D.C.; Rivero, S.; Palma, M.; Friedrich, M.J. Outcomes of a Modular Intercalary Endoprosthesis as Treatment for Segmental Defects of the Femur, Tibia, and Humerus. Clin. Orthop. Relat. Res. 2016, 474, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Errani, C.; Ceruso, M.; Donati, D.M.; Manfrini, M. Microsurgical reconstruction with vascularized fibula and massive bone allograft for bone tumors. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Streitbürger, A.; Hardes, J.; Nottrott, M.; Guder, W.K. Reconstruction survival of segmental megaendoprostheses: A retrospective analysis of 28 patients treated for intercalary bone defects after musculoskeletal tumor resections. Arch. Orthop. Trauma Surg. 2022, 142, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Houdek, M.T.; Wagner, E.R.; Stans, A.A.; Shin, A.Y.; Bishop, A.T.; Sim, F.H.; Moran, S.L. What Is the Outcome of Allograft and Intramedullary Free Fibula (Capanna Technique) in Pediatric and Adolescent Patients With Bone Tumors? Clin. Orthop. Relat. Res. 2016, 474, 660–668. [Google Scholar] [CrossRef]
- Yao, W.; Cai, Q.; Wang, J.; Zhang, P.; Wang, X.; Du, X.; Niu, X. Biological reconstruction in the treatment of extremity sarcoma in femur, tibia, and humerus. Medicine 2020, 99, e20715. [Google Scholar] [CrossRef]
- Bellan, D.G.; Filho, R.J.-G.; Garcia, J.G.; de Toledo Petrilli, M.; Maia Viola, D.C.; Schoedl, M.F.; Petrilli, A.S. Ewing’S Sarcoma: Epidemiology and Prognosis for Patients Treated At the Pediatric Oncology Institute, Iop-Graacc-Unifesp. Rev. Bras. Ortop. (Engl. Ed.) 2012, 47, 446–450. [Google Scholar] [CrossRef]
- Pu, F.; Zhang, Z.; Wang, B.; Liu, J.; Shao, Z. En bloc resection and intercalary prosthesis implantation for the treatment of humeral diaphyseal bone metastases. Int. Orthop. 2021, 45, 281–288. [Google Scholar] [CrossRef]
- Stelzl, A.; Aziz, F.; Riedl, J.M.; Posch, F.; Smolle, M.A.; Stojakovic, T.; Terbuch, A.; Pichler, M.; Bergovec, M.; Leithner, A.; et al. Diabetes mellitus is independently associated with adverse clinical outcome in soft tissue sarcoma patients. Sci. Rep. 2020, 10, 12438. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Guo, W.; Yang, R.; Li, D.; Tang, S.; Yang, Y.; Dong, S.; Zang, J. Reconstruction of segmental bone defect of long bones after tumor resection by devitalized tumor-bearing bone. World J. Surg. Oncol. 2015, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Organization WH. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 7 July 2025).
- Röder, C.; Eggli, S.; Münger, P.; Melloh, M.; Busato, A. Patient characteristics differently affect early cup and stem loosening in THA: A case-control study on 7535 patients. Int. Orthop. 2008, 32, 33–38. [Google Scholar] [CrossRef]
- Werner, F.W.; Ayers, D.C.; Maletsky, L.P.; Rullkoetter, P.J. The effect of valgus/varus malalignment on load distribution in total knee replacements. J. Biomech. 2005, 38, 349–355. [Google Scholar] [CrossRef]
- McLaughlin, J.R.; Lee, K.R. The outcome of total hip replacement in obese and non-obese patients at 2008, 10- to 18-years. J. Bone Jt. Surg.—Ser. B 2006, 88, 1286–1292. [Google Scholar] [CrossRef]
- Yeung, E.; Jackson, M.; Sexton, S.; Walter, W.; Zicat, B. The effect of obesity on the outcome of hip and knee arthroplasty. Int. Orthop. 2011, 35, 929–934. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, M.; Zheng, K.; Yu, X. Limb salvage surgery with joint preservation for malignant humeral bone tumors: Operative procedures and clinical application. BMC Surg. 2019, 19, 57. [Google Scholar] [CrossRef]
- Mahdal, M.; Pazourek, L.; Apostolopoulos, V.; Krákorová, D.A.; Zambo, I.S.; Tomáš, T. Outcomes of Intercalary Endoprostheses as a Treatment for Metastases in the Femoral and Humeral Diaphysis. Curr. Oncol. 2022, 29, 3519–3530. [Google Scholar] [CrossRef]
- Biau, D.; Faure, F.; Katsahian, S.; Jeanrot, C.; Tomeno, B.; Anract, P. Survival of total knee replacement with a megaprosthesis after bone tumor resection. J. Bone Jt. Surg.—Ser. A 2006, 88, 1285–1293. [Google Scholar] [CrossRef][Green Version]
- Hennen, J.; Sabo, D.; Martini, A.K.; Bernd, L. Das Manteltransplantat zur Defektrekonstruktion nach Resektion maligner Knochentumoren an der unteren Extremität. Unfallchirurg 2002, 105, 120–127. [Google Scholar] [CrossRef]
- Saaiq, M.; Zimri, F.U.K. Clinical Applications and Outcome of Proximally Based Medial Gastrocnemius Muscle Flap. World J. Plast. Surg. 2020, 9, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Trieb, K.; Göggel, M.; Dürr, H.R. Proximaler Tibiaersatz mit Transfer des M. Gastrocnemius. Oper. Orthop. Traumatol. 2012, 24, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Aponte-Tinao, L.A.; Ayerza, M.A.; Albergo, J.I.; Farfalli, G.L. Do Massive Allograft Reconstructions for Tumors of the Femur and Tibia Survive 10 or More Years after Implantation? Clin. Orthop. Relat. Res. 2020, 478, 517–524. [Google Scholar] [CrossRef]
- Grzebień, A.; Chabowski, M.; Malinowski, M.; Uchmanowicz, I.; Milan, M.; Janczak, D. Analysis of selected factors determining quality of life in patients after lower limb amputation—A review article. Pol. J. Surg. 2017, 89, 57–61. [Google Scholar] [CrossRef]
- Lesensky, J.; Prince, D.E. Distraction osteogenesis reconstruction of large segmental bone defects after primary tumor resection: Pitfalls and benefits. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 715–727. [Google Scholar] [CrossRef]
- Moran, S.L.; Shin, A.Y.; Bishop, A.T. The use of massive bone allograft with intramedullary free fibular flap for limb salvage in a pediatric and adolescent population. Plast. Reconstr. Surg. 2006, 118, 413–419. [Google Scholar] [CrossRef]
- Refaat, Y.; Gunnoe, J.; Hornicek, F.J.; Mankin, H.J. Comparison of quality of life after amputation or limb salvage. Clin. Orthop. Relat. Res. 2002, 298–305. [Google Scholar] [CrossRef]
- Guder, W.K.; Hardes, J.; Gosheger, G.; Nottrott, M.; Streitbürger, A. Ultra-short stem anchorage in the proximal tibial epiphysis after intercalary tumor resections: Analysis of reconstruction survival in four patients at a mean follow-up of 56 months. Arch. Orthop. Trauma Surg. 2017, 137, 481–488. [Google Scholar] [CrossRef]
- Fuchs, B.; Ossendorf, C.; Leerapun, T.; Sim, F.H. Intercalary segmental reconstruction after bone tumor resection. Eur. J. Surg. Oncol. 2008, 34, 1271–1276. [Google Scholar] [CrossRef]
- Zhao, D.; Tang, F.; Min, L.; Lu, M.; Wang, J.; Zhang, Y.; Zhao, K.; Zhou, Y.; Luo, Y.; Tu, C. Intercalary reconstruction of the “ultra-critical sized bone defect” by 3D-printed porous prosthesis after resection of tibial malignant tumor. Cancer Manag. Res. 2020, 12, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Bischel, O.E.; Nadorf, J.; Klein, S.B.; Gantz, S.; Jakubowitz, E.; Kretzer, J.P.; Arnholdt, J.; Seeger, J.B. Modular tumor prostheses: Are current stem designs suitable for distal femoral reconstruction? A biomechanical implant stability analysis in Sawbones. Arch. Orthop. Trauma Surg. 2019, 139, 843–849. [Google Scholar] [CrossRef] [PubMed]
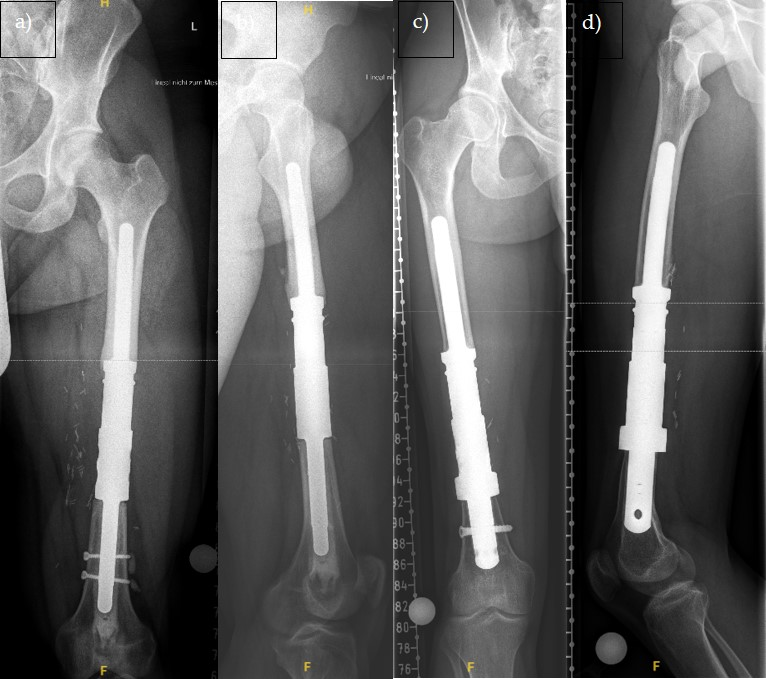
| No | Age | Diagnosis | Localiza tion | Bone/Soft Tissue | Seize | Enneking Classification | Prior Operation | Chemo | Response | Radiation | FU |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 26 | Extraskelettal | Femur | ST | 5–10 | IIa | - | Y | 2 | N | NED 42 |
| OS | |||||||||||
| 2 | 63 | OS | Femur | Bone | >10 | IIb | - | Y | 2 | N | DOUC |
| 76 | |||||||||||
| 3 | 16 | EWS | Femur | Bone | 5–10 | IIa | - | Y | 1 | N | NED 54 |
| 4 | 32 | Extraskelettal EWS | Femur | ST | 5–10 | na | - | Y | 1 | N | NED 24 |
| 5 | 35 | Soft Tissue Sarcoma | Femur | ST | 5–10 | IIb | - | Y | Na | N | NED 76 |
| 6 | 59 | Soft Tissue Sarcoma | Femur | ST | 5–10 | IIb | - | Y | Na | Y | AWD 32 |
| 7 | 59 | Soft Tissue Sarcoma | Femur | ST | 5–10 | IIa | - | Y | Na | Y | AWD 91 |
| 8 | 80 | Soft Tissue Sarcoma | Femur | ST | 5–10 | IIb | - | N | - | Y | DOD 4 |
| 9 | 55 | MFH | Femur | ST | >10 | IIa | Intralesional. Resection | N | - | N | DOD 17 |
| 10 | 48 | Soft Tissue Sarcoma | Femur | ST | 5–10 | IIa | Intralesional Resection | N | - | Y | DOD 10 |
| 11 | 59 | Soft Tissue Sarcoma | Femur | ST | 5–10 | IIb | Intralesional Resection | Y | Na | Y | DOUC 6 |
| 12 | 53 | Soft Tissue Sarcoma | Femur | ST | >10 | IIa | Marginal Resection | N | - | Y | NED 97 |
| 13 | 41 | Soft Tissue Sarcoma | Femur | ST | >10 | IIa | Intralesional Resection | Y | Na | Y | AWD 104 |
| 14 | 52 | Soft Tissue Sarcoma | Femur | ST | >10 | IIb | - | Y | Na | Y | DOD 20 |
| 15 | 54 | Soft Tissue Sarcoma | Femur | ST | >10 | IIa | - | Y | Na | Y | DOUC 42 |
| 16 | 69 | Renal-CA | Femur | Bone | 5–10 | Met | - | N | - | N | AWD 28 |
| Metastasis | |||||||||||
| 17 | 62 | Renal-CA | Femur | Bone | <5 | Met | Intramed | N | - | Y | DOD 80 |
| Metastasis | Nailing | ||||||||||
| 18 | 62 | Renal-CA | Femur | Bone | 5–10 | Met | Intramed | N | - | N | DOD 9 |
| Metastasis | Nailing | ||||||||||
| 19 | 58 | Renal-CA Metastasis | Femur | Bone | 5–10 | Met | Plate-Osteosynthesis (ORIF) | N | - | N | DOD 43 |
| 20 | 60 | Renal-CA | Femur | Bone | <5 | Met | - | N | - | Y | DOD 98 |
| Metastasis | |||||||||||
| 21 | 79 | Renal-CA | Femur | Bone | <5 | Met | - | N | - | N | DOD 1 |
| Metastasis | |||||||||||
| 22 | 62 | CS | Femur | Bone | Na | IIa | Intralesio- | Y | Na | N | DOD 23 |
| nal Cur- | |||||||||||
| retage | |||||||||||
| 23 | 72 | NSCLC Meta- | Femur | Bone | <5 | Met | - | N | - | Y | DOD 6 |
| stasis | |||||||||||
| 24 | 85 | Breast-Ca | Femur | Bone | <5 | Met | - | Na | - | Y | DOD 7 |
| Metastasis | |||||||||||
| 25 | 82 | Breast-Ca | Femur | Bone | 5–10 | Met | - | N | - | Y | DOD 49 |
| Metastasis | |||||||||||
| 26 | 17 | OS | Femur | Bone | >10 | IIb | - | Y | 2 | N | NED 44 |
| 27 | 10 | EWS | Femur | Bone | 5–10 | IIb | - | Y | 2 | N | AWD |
| 144 | |||||||||||
| 28 | 60 | Soft Tissue Sarcoma | Femur | ST | >10 | IIa | - | N | - | Y | DOD 48 |
| 29 | 69 | Renal-CA | Femur | Bone | 5–10 | Met | Intramed | Y | Na | Y | DOD 17 |
| Metastasis | Nailing | ||||||||||
| 30 | 54 | Soft Tissue Sarcoma | Tibia | Bone | <5 | IIa | - | N | - | N | AWD 6 |
| 31 | 44 | NOS | Tibia | Bone | 5–10 | IIb | - | Y | 2 | N | DOD 32 |
| 32 | 47 | NOS | Tibia | Bone | >10 | IIb | - | Y | Na | N | DOD |
| 102 | |||||||||||
| 33 | 59 | Renal-CA | Tibia | Bone | >10 | Met | - | Y | Na | Y | DOD 73 |
| Metastasis | |||||||||||
| 34 | 44 | Adamantinoma | Tibia | Bone | 5–10 | IIb | - | N | - | N | AWD |
| 114 | |||||||||||
| 35 | 60 | Colon-CA Metastasis | Tibia | Bone | 5 | Met | - | Na | - | Y | DOD 54 |
| 36 | 76 | Soft Tissue Sarcoma | Humerus | ST | 5–10 | IIb | - | Y | Na | Y | NED 50 |
| 37 | 68 | Renal-CA | Humerus | Bone | Na | Met | - | Na | - | Na | DOD 81 |
| Metastasis | |||||||||||
| 38 | 64 | NSCLC Meta- | Humerus | Bone | <5 | Met | - | N | - | N | DOD 20 |
| stasis | |||||||||||
| 39 | 66 | Breast-Ca | Humerus | Bone | 5–10 | Met | - | N | - | Y | DOD 15 |
| Metastasis |
| 1-Year Survival | 2-Year Survival | 5-Year Survival | |
|---|---|---|---|
| Overall | 82% | 66% | 39% |
| Primary tumor | 87% | 74% | 47% |
| metastasis | 73% | 53% | 29% |
| Diagnosis | Number of Patients | 1-Year Survival | 2-Year Survival | 5-Year Survival |
|---|---|---|---|---|
| Osteosarcoma | 3 | 100% | 100% | 100% |
| Ewing sarcoma | 3 | 100% | 100% | 100% |
| Soft tissue sarcoma | 16 | 80% | 67% | 38% |
| Other primary tumor | 2 | 100% | 50% | 50% |
| Renal cancer metastasis | 9 | 77.8% | 66.7% | 50% |
| Pulmonary cancer metastasis | 2 | 50% | 0% | 0% |
| Breast cancer metastasis | 3 | 67% | 33% | 0% |
| Colrectal cancer metastasis Metastase | 1 | 100% | 100% | 0% |
| Complication According to Henderson | Total Number of Cases (n = 21) | Femoral (n = 13) | Tibial (n = 5) | Humeral (n = 3) | Time in Months After Implantation Mean (SD) | |
|---|---|---|---|---|---|---|
| Type 1 | 1 | 5% | 0 | 1 | 0 | 3 (-) |
| Type 2 | 8 | 38% | 5 | 2 | 1 | 10 (6) |
| Type 3 | 2 | 10% | 2 | 0 | 0 | 17 (2) |
| Type 4 | 4 | 19% | 2 | 2 | 0 | 8 (11) |
| Type 5 | 4 | 19% | 3 | 0 | 1 | 22 (18) |
| unclear | 2 | 10% | 1 | 0 | 1 | 6 (9) |
| Localization | Number of Protheses | Aseptic Loosening | Total Prosthesis Explantation | ||
|---|---|---|---|---|---|
| Femoral | 29 | 5 | 17% | 11 | 38% |
| Tibial | 6 | 2 | 33% | 5 | 83% |
| Humeral | 4 | 1 | 25% | 2 | 50% |
| Diagnosis | Number of Prostheses (n = 39) | Prosthesis Failure During Lifetime | Prosthesis Failure During Lifetime in % |
|---|---|---|---|
| Osteosarcoma | 3 | 1 | 33% |
| Ewing-Sarcoma | 3 | 2 | 67% |
| Soft Tissue Sarcoma | 16 | 9 | 56% |
| Renal Cell Carcinoma Metastasis | 9 | 3 | 33% |
| Other Primary Tumor | 2 | 1 | 50% |
| Lung Cancer Metastasis | 2 | 0 | 0.0% |
| Breast Cancer Metastasis | 3 | 1 | 33% |
| Colon Cancer Metastasis | 1 | 1 | 100% |
| Fixation Mechanism | Number of Stems | Number of Cases with Aseptic Loosening | Loosening Rate |
|---|---|---|---|
| cemented+ screwed | 35 | 2 | 6% |
| cemented+ unscrewed | 29 | 5 | 17% |
| uncemented + screwed | 5 | 1 | 20% |
| uncemented + unscrewed | 7 | 0 | 0% |
| No information | 2 | 0 | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budny, T.; Rachbauer, A.M.; Gosheger, G.; Lückel, F.; Vaal, M.D.; Klingebiel, S.; Theil, J.C.; Deventer, N. Long-Term Outcomes Following Reconstruction of Diaphyseal Defects of the Upper and Lower Extremities Using Diaphyseal Implants: A Retrospective Study with Focus on Fixation Technique. Cancers 2025, 17, 3059. https://doi.org/10.3390/cancers17183059
Budny T, Rachbauer AM, Gosheger G, Lückel F, Vaal MD, Klingebiel S, Theil JC, Deventer N. Long-Term Outcomes Following Reconstruction of Diaphyseal Defects of the Upper and Lower Extremities Using Diaphyseal Implants: A Retrospective Study with Focus on Fixation Technique. Cancers. 2025; 17(18):3059. https://doi.org/10.3390/cancers17183059
Chicago/Turabian StyleBudny, Tymoteusz, Anna Maria Rachbauer, Georg Gosheger, Felix Lückel, Marieke De Vaal, Sebastian Klingebiel, Jan Christoph Theil, and Niklas Deventer. 2025. "Long-Term Outcomes Following Reconstruction of Diaphyseal Defects of the Upper and Lower Extremities Using Diaphyseal Implants: A Retrospective Study with Focus on Fixation Technique" Cancers 17, no. 18: 3059. https://doi.org/10.3390/cancers17183059
APA StyleBudny, T., Rachbauer, A. M., Gosheger, G., Lückel, F., Vaal, M. D., Klingebiel, S., Theil, J. C., & Deventer, N. (2025). Long-Term Outcomes Following Reconstruction of Diaphyseal Defects of the Upper and Lower Extremities Using Diaphyseal Implants: A Retrospective Study with Focus on Fixation Technique. Cancers, 17(18), 3059. https://doi.org/10.3390/cancers17183059






