Clinicopathological Features and Prognosis of Lung Adenocarcinoma Presenting as Ground-Glass Opacity: A Single-Center Experience
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Pre-Operative Evaluation
2.2. Surgery
2.3. Histopathological Examination
2.4. Post-Operative Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Univariate and Multivariate Analysis
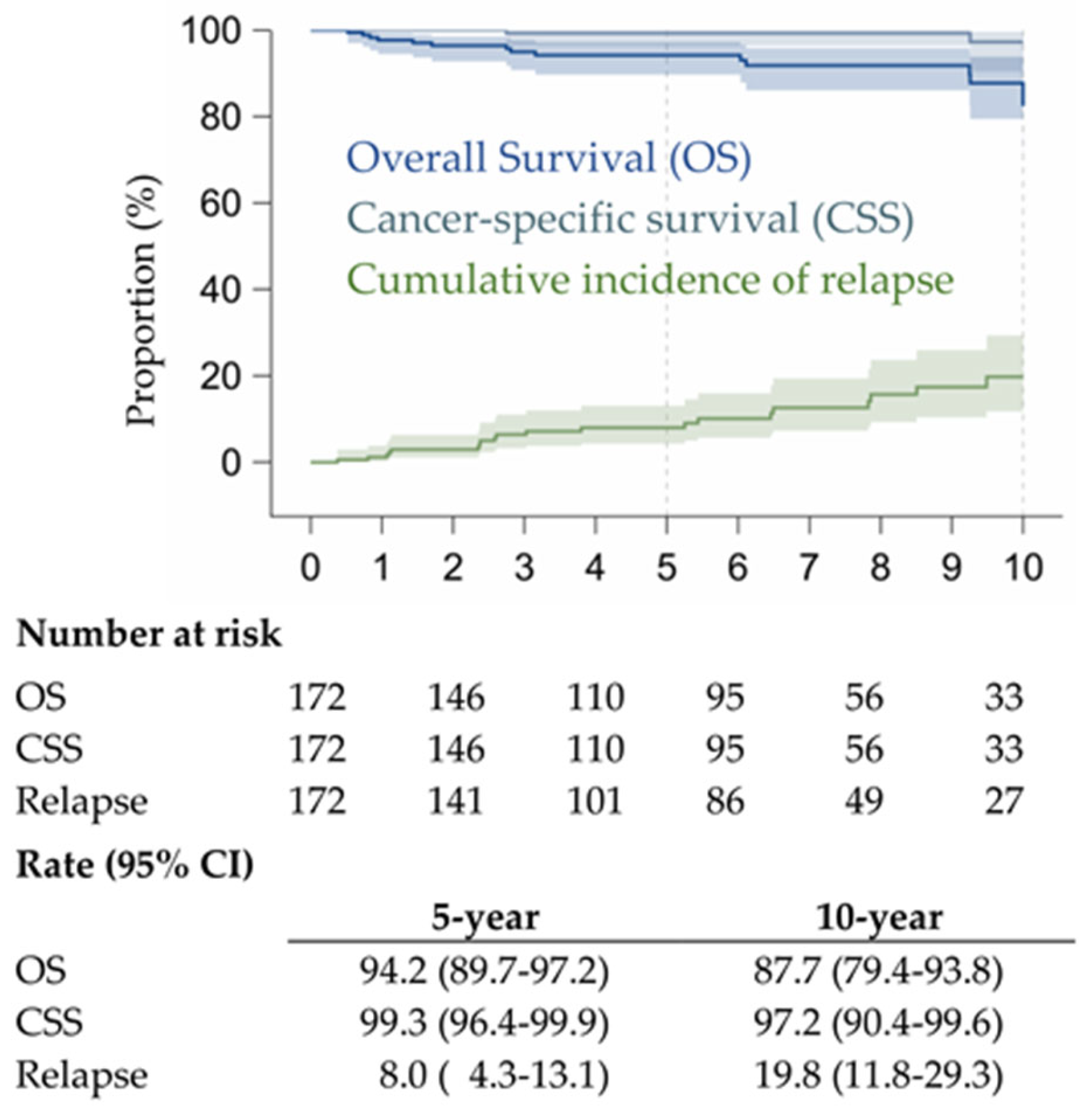
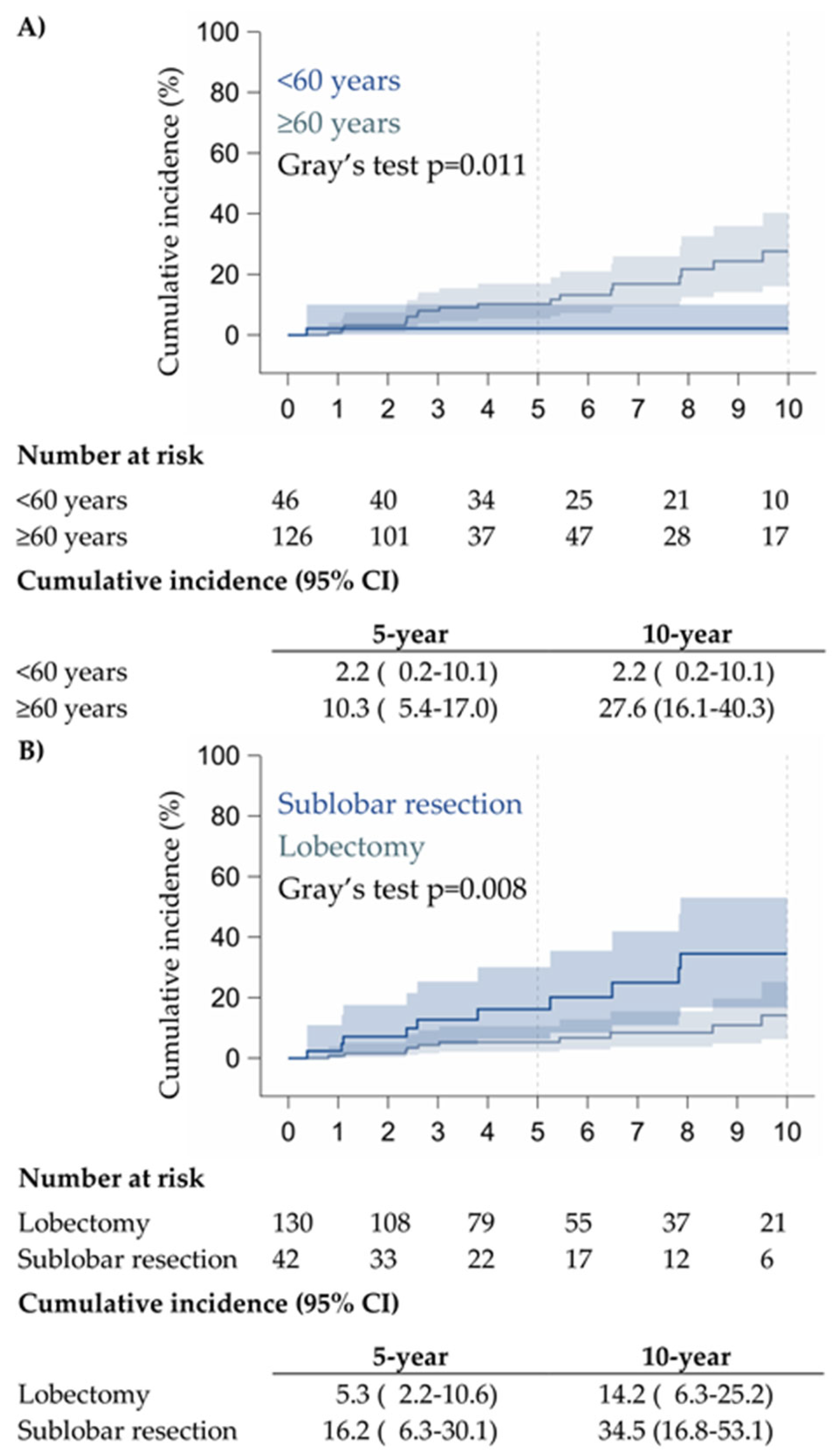
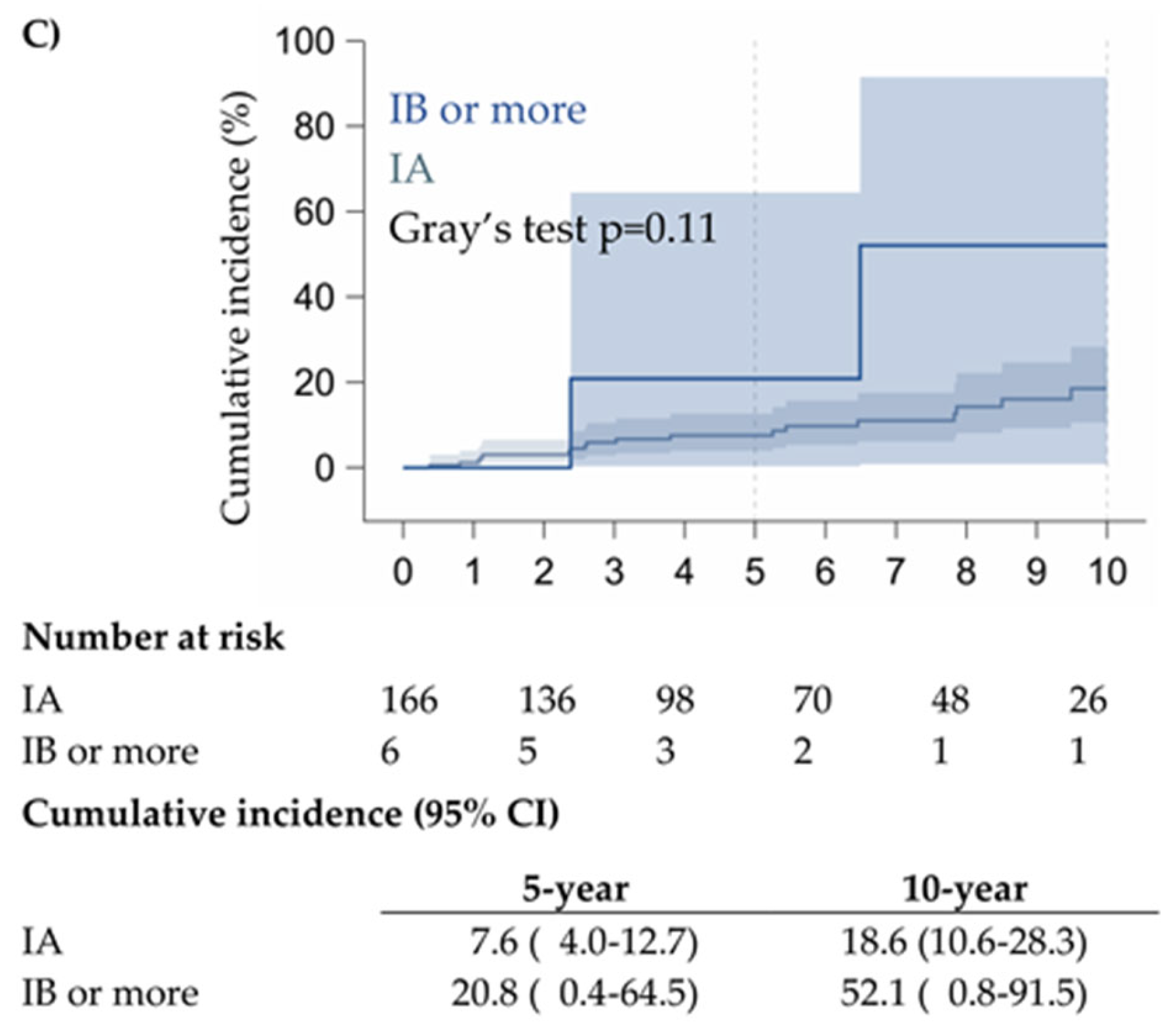
3.2. Prognostic Factors and Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GGO | ground-glass opacity; |
| CT | computed tomography; |
| CTR | consolidation-to-tumor ratio; |
| MIA | minimally invasive adenocarcinoma; |
| IA | invasive adenocarcinoma; |
| AIS | adenocarcinoma in situ; |
| AAH | atypical adenomatous hyperplasia; |
| PET-FDG | positron emission tomography with fluorodeoxyglucose; |
| EBUS-TBNA | endobronchial ultrasound-guided transbronchial needle aspiration; |
| WHO | World Health Organization; |
| RATS | robotic-assisted thoracic surgery; |
| VATS | video-assisted thoracic surgery; |
| OS | overall survival; |
| CSS | cancer-specific survival; |
References
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef]
- Gao, J.W.; Rizzo, S.; Ma, L.H.; Qiu, X.Y.; Warth, A.; Seki, N.; Hasegawa, M.; Zou, J.W.; Li, Q. Pulmonary ground-glass opacity: Computed tomography features, histopathology, and molecular pathology. Transl. Lung Cancer Res. 2017, 6, 68–75. [Google Scholar] [CrossRef]
- Aherne, E.A.; Plodkowski, A.J.; Montecalvo, J.; Hayan, S.; Zheng, J.; Capanu, M.; Adusumilli, P.S.; Travis, W.D.; Ginsberg, M.S. What CT characteristics of lepidic predominant pattern lung adenocarcinomas correlate with invasiveness on pathology? Lung Cancer 2018, 118, 83–89. [Google Scholar] [CrossRef]
- Aokage, K.; Miyoshi, T.; Ishii, G.; Kusumoto, M.; Nomura, S.; Katsumata, S.; Sekihara, K.; Hishida, T.; Tsuboi, M. Clinical and Pathological Staging Validation in the Eighth Edition of the TNM Classification for Lung Cancer: Correlation between Solid Size on Thin-Section Computed Tomography and Invasive Size in Pathological Findings in the New T Classification. J. Thorac. Oncol. 2017, 12, 1403–1412. [Google Scholar] [CrossRef]
- Gardiner, N.; Jogai, S.; Wallis, A. The revised lung adenocarcinoma classification-an imaging guide. J. Thorac. Dis. 2014, 6 (Suppl. 5), S537–S546. [Google Scholar] [CrossRef]
- Matsunaga, T.; Suzuki, K.; Takamochi, K.; Oh, S. What is the radiological definition of part-solid tumour in lung cancer? Eur. J. Cardiothor. Surg. 2017, 51, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, H.; Tanahashi, M.; Suzuki, E.; Yoshii, N.; Watanabe, T.; Yobita, S.; Uchiyama, S.; Iguchi, K.; Nakamura, M.; Endo, T. Impact of ground glass opacity in a three-dimensional analysis for pathological findings and prognosis in stage IA pure solid lung cancer. J. Thorac. Dis. 2023, 15, 3829–3839. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, T.; Aokage, K.; Katsumata, S.; Tane, K.; Ishii, G.; Tsuboi, M. Ground-Glass Opacity Is a Strong Prognosticator for Pathologic Stage IA Lung Adenocarcinoma. Ann. Thorac. Surg. 2019, 108, 249–255. [Google Scholar] [CrossRef]
- Suzuki, K.; Koike, T.; Asakawa, T.; Kusumoto, M.; Asamura, H.; Nagai, K.; Tada, H.; Mitsudomi, T.; Tsuboi, M.; Shibata, T.; et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J. Thorac. Oncol. 2011, 6, 751–756. [Google Scholar] [CrossRef]
- Asamura, H.; Hishida, T.; Suzuki, K.; Koike, T.; Nakamura, K.; Kusumoto, M.; Nagai, K.; Tada, H.; Mitsudomi, T.; Tsuboi, M.; et al. Radiographically determined noninvasive adenocarcinoma of the lung: Survival outcomes of Japan Clinical Oncology Group 0201. J. Thorac. Cardiovasc. Surg. 2013, 146, 24–30. [Google Scholar] [CrossRef]
- Aokage, K.; Miyoshi, T.; Ishii, G.; Kusumoto, M.; Nomura, S.; Katsumata, S.; Sekihara, K.; Tane, K.; Tsuboi, M. Influence of Ground Glass Opacity and the Corresponding Pathological Findings on Survival in Patients with Clinical Stage I Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 533–542. [Google Scholar] [CrossRef]
- Veronesi, G.; Travaini, L.L.; Maisonneuve, P.; Rampinelli, C.; Bertolotti, R.; Spaggiari, L.; Bellomi, M.; Paganelli, G. Positron emission tomography in the diagnostic work-up of screening-detected lung nodules. Eur. Respir. J. 2015, 45, 501–510. [Google Scholar] [CrossRef]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for management of incidental pulmonary nodules detected on CT images: From the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef]
- Fu, F.; Zhang, Y.; Wen, Z.; Zheng, D.; Gao, Z.; Han, H.; Deng, L.; Wang, S.; Liu, Q.; Li, Y.; et al. Distinct Prognostic Factors in Patients with Stage I Non–Small Cell Lung Cancer with Radiologic Part-Solid or Solid Lesions. J. Thorac. Oncol. 2019, 14, 2133–2142. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, S.; Aokage, K.; Ishii, G.; Hoshino, H.; Suzuki, J.; Miyoshi, T.; Tane, K.; Samejima, J.; Tsuboi, M. Pathological features and prognostic implications of ground-glass opacity components on computed tomography for clinical stage I lung adenocarcinoma. Surg. Today 2021, 51, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Matsunaga, T.; Takamochi, K.; Oh, S.; Suzuki, K. Neither Maximum Tumor Size nor Solid Component Size Is Prognostic in Part-Solid Lung Cancer: Impact of Tumor Size Should Be Applied Exclusively to Solid Lung Cancer. Ann. Thorac. Surg. 2016, 102, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Matsunaga, T.; Takamochi, K.; Oh, S.; Suzuki, K. Prognostic impact of a ground glass opacity component in the clinical T classification of non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2017, 154, 2102–2110.e1. [Google Scholar] [CrossRef]
- Suzuki, S.; Sakurai, H.; Yotsukura, M.; Masai, K.; Asakura, K.; Nakagawa, K.; Motoi, N.; Watanabe, S.-I. Clinical Features of Ground Glass Opacity–Dominant Lung Cancer Exceeding 3.0 cm in the Whole Tumor Size. Ann. Thorac. Surg. 2018, 105, 1499–1506. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, L.; Wang, G. Risk factors for lymph node metastasis and surgical methods in patients with early-stage peripheral lung adenocarcinoma presenting as ground glass opacity. J. Cardiothorac. Surg. 2020, 15, 121. [Google Scholar] [CrossRef]
- Casiraghi, M.; Travaini, L.L.; Maisonneuve, P.; Tessitore, A.; Brambilla, D.; Agoglia, B.G.; Guarize, J.; Spaggiari, L. Lymph node involvement in T1 non-small-cell lung cancer: Could glucose uptake and maximal diameter be predictive criteria? Eur. J. Cardiothorac. Surg. 2011, 39, e38–e43. [Google Scholar] [CrossRef]
- Hattori, A.; Hirayama, S.; Matsunaga, T.; Hayashi, T.; Takamochi, K.; Oh, S.; Suzuki, K. Distinct Clinicopathologic Characteristics and Prognosis Based on the Presence of Ground Glass Opacity Component in Clinical Stage IA Lung Adenocarcinoma. J. Thorac. Oncol. 2019, 14, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, F.; Chen, H. Management of Ground-Glass Opacities in the Lung Cancer Spectrum. Ann. Thorac. Surg. 2020, 110, 1796–1804. [Google Scholar] [CrossRef]
- Ye, T.; Deng, L.; Wang, S.; Xiang, J.; Zhang, Y.; Hu, H.; Sun, Y.; Li, Y.; Shen, L.; Xie, L.; et al. Lung Adenocarcinomas Manifesting as Radiological Part-Solid Nodules Define a Special Clinical Subtype. J. Thorac. Oncol. 2019, 14, 617–627. [Google Scholar] [CrossRef]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L):a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Altorki, N.; Wang, X.; Kozono, D.; Watt, C.; Landrenau, R.; Wigle, D.; Port, J.; Jones, D.R.; Conti, M.; Ashrafi, A.S.; et al. Lobar or Sublobar Resection for Peripheral Stage IA Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 388, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Choi, Y.S.; Kim, J.; Kim, H.K.; Zo, J.I.; Shim, Y.M. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann. Thorac. Surg. 2015, 99, 218–222. [Google Scholar] [CrossRef]
- Zini Radaelli, L.F.; Fabbri, E.; Costantini, M.; Gaudio, M.; Dubini, A.; Giampalma, E.; Stella, F.; Aramini, B. Prognostic role of subsolid ground-glass opacity, pure ground-glass opacity, and solid nodules of the lung: A retrospective observational study. J. Thorac. Dis. 2025, 17, 2239–2247. [Google Scholar] [CrossRef]
- Bai, J.; Fu, F.; Sun, W.; Deng, C.; Ma, Z.; Wang, S.; Deng, L.; Zhang, Y.; Chen, H. Prognostic effect of ground-glass opacity in subcentimeter invasive lung adenocarcinoma. J. Thorac. Dis. 2023, 15, 1559–1571. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Chen, Y.; Zhong, C.; Fang, W. Ground glass opacity resection extent assessment trial (GREAT): A study protocol of multi-institutional, prospective, open-label, randomized phase III trial of minimally invasive segmentectomy versus lobectomy for ground glass opacity (GGO)-containing early-stage invasive lung adenocarcinoma. Front. Oncol. 2023, 13, 1052796. [Google Scholar] [CrossRef]
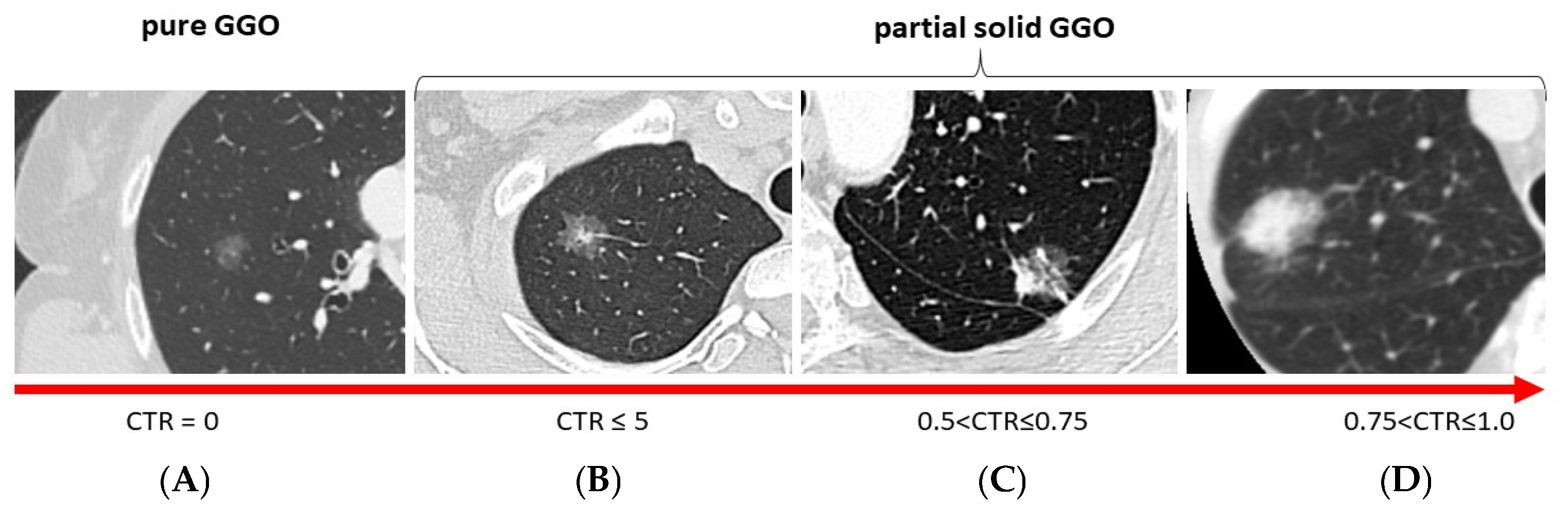
| Patients | Patients | ||
|---|---|---|---|
| N (%) | N (%) | ||
| All patients | 172 (100.) | Histological subtype | |
| Acinar | 32 (18.6) | ||
| Sex | Papillary | 7 (4.1) | |
| Male | 74 (43.0) | Lepidic predominant | 41 (23.8) |
| Female | 98 (57.0) | Mixed Acinar + papillary | 6 (3.5) |
| Age | Acinar + lepidic predominant | 70 (40.7) | |
| Median [range] | 65 [38–81] | Papillary + lepidic predominant | 16 (9.3) |
| <60 | 46 (26.7) | CTR | |
| 60–69 | 69 (40.1) | Pure GGO | 90 (52.3) |
| 70+ | 57 (33.1) | ≤0.50 | 56 (32.6) |
| Clinical stage | 0.50–0.75 | 18 (10.5) | |
| IA | 166 (96.5) | 0.75–1.00 | 8 (4.7) |
| IB/II/III | 6 (3.5) | Invasiveness | |
| Site | AIS | 5 (2.9) | |
| RUL | 74 (43.0) | MIA | 31 (18.0) |
| ML | 11 (6.4) | IA | 136 (79.1) |
| RLL | 28 (16.3) | Size | |
| LUL | 38 (22.1) | Median [range] | 13 [3–90] |
| LLL | 21 (12.2) | ≤10 | 61 (35.5) |
| Access | 11–20 | 87 (50.6) | |
| Open | 38 (22.1) | >20 | 24 (14.0) |
| Robot | 86 (51.7) | pT | |
| VATS | 48 (27.9) | 1a | 69 (40.1) |
| Surgery | 1b | 80 (46.5) | |
| Lobectomy | 130 (75.6) | 1c | 16 (9.3) |
| Segmentectomy | 10 (5.8) | 2a | 4 (2.3) |
| Wedge | 32 (18.6) | 2b | 2 (1.2) |
| Lymphadenectomy | 3–4 * | 1 (0.6) | |
| No | 27 (15.7) | pN | |
| Yes | 45 (26.2) | pN− | 143 (83.1) |
| Radicality | pN+ | 2 (1.2) | |
| Yes | 172 (100.) | pNx | 27 (15.7) |
| No | - | Stage | |
| PET | IA | 163 (94.8) | |
| Missing | 6 (3.5) | IB | 3 (1.7) |
| Negative | 93 (54.1) | IIA | 2 (1.2) |
| Positive | 73 (42.4) | IIB | 3 (1.7) |
| Radiologic GGO | IIIA | 1 (0.6) | |
| Pure GGO | 90 (52.3) | Grading | |
| Solid component | 82 (47.7) | G1 | 90 (52.3) |
| CTR | G2 | 72 (41.9) | |
| Pure GGO | 90 (52.3) | G3 | 10 (5.8) |
| ≤0.50 | 56 (32.6) | Post-operative treatment | |
| 0.50–0.75 | 18 (10.5) | No | 170 (98.8) |
| 0.75–1.00 | 8 (4.7) | Yes | 2 (1.2) |
| Patients (n = 172) | Relapses (n = 21) | Grays’ p-Value | Univariate HR (95% CI) | |
|---|---|---|---|---|
| Age | ||||
| <60 | 46 | 1 | 1.00 | |
| 60–69 | 69 | 11 | 0.025 | 8.35 (1.04–67.2) |
| 70+ | 57 | 9 | 10.2 (1.23–84.7) | |
| Clinical stage | ||||
| IA | 166 | 18 | 1.00 | |
| IB/II/III | 6 | 3 | 0.010 | 4.94 (2.07–11.8) |
| Surgery | ||||
| Lobectomy | 130 | 11 | 1.00 | |
| Segmentectomy | 10 | 4 | 0.035 | 3.99 (1.32–12.1) |
| Wedge | 32 | 6 | 2.48 (0.92–6.71) | |
| Lymphadenectomy | ||||
| No | 27 | 5 | 1.00 | |
| Yes | 145 | 16 | 0.20 | 0.52 (0.18–1.48) |
| PET | ||||
| Negative | 93 | 11 | 1.00 | |
| Positive | 73 | 8 | 0.17 | 1.18 (0.48–2.87) |
| Missing | 6 | 2 | 4.29 (0.87–21.1) | |
| GGO | ||||
| Pure GGO | 90 | 13 | 1.00 | |
| Solid component | 82 | 8 | 0.57 | 0.77 (0.32–1.83) |
| CTR | ||||
| Pure GGO | 90 | 13 | 1.00 | |
| ≤0.50 | 56 | 5 | 0.72 (0.26–1.98) | |
| 0.50–0.75 | 18 | 2 | 0.95 (0.21–4.38) | |
| 0.75–1.00 | 8 | 1 | 0.94 | 0.74 (0.10–5.82) |
| Histological subtype | ||||
| Acinar | 32 | 4 | 1.00 | |
| Papillary | 7 | 0 | - | |
| Lepidic predominant | 41 | 4 | 0.82 (0.21–3.26) | |
| Mix Acinar + papillary | 6 | 0 | - | |
| Acinar + lepidic predominant | 70 | 11 | 1.33 (0.42–4.21) | |
| Papillary + lepidic predominant | 16 | 2 | 0.73 | 0.86 (0.17–4.38) |
| Invasiveness | ||||
| AIS | 5 | 0 | - | |
| MIA | 31 | 5 | 1.15 (0.44–3.00) | |
| IA | 136 | 16 | 0.69 | 1.00 |
| Size | ||||
| ≤10 | 61 | 7 | 1.00 | |
| 11–20 | 87 | 11 | 1.43 (0.55–3.72) | |
| >20 | 24 | 3 | 0.67 | 1.55 (0.41–5.88) |
| pT | ||||
| pT1 | 165 | 19 | 1.00 | |
| pT2 | 6 | 1 | 1.61 (0.41–6.34) | |
| pT3–4 * | 1 | 1 | 0.0001 | 22.6 (10.7–47.8) |
| pN | ||||
| pN− | 143 | 16 | 1.00 | |
| pN+ | 2 | 0 | - | |
| pNx | 27 | 5 | 0.38 | 1.89 (0.66–5.41) |
| Stage | ||||
| IA/IB | 166 | 18 | 1.00 | |
| IIA/B | 5 | 2 | 2.58 (0.86–7.75) | |
| IIIA | 1 | 1 | <0.0001 | 23.3 (10.9–49.6) |
| Grading | ||||
| G1 | 90 | 11 | 1.00 | |
| G2 | 72 | 7 | 1.00 (0.40–2.51) | |
| G3 | 10 | 3 | 0.38 | 2.46 (0.64–9.44) |
| HR (95% CI) * | p-Value | Time-Dependent Area Under the Curve (95% CI) | |
|---|---|---|---|
| Age | 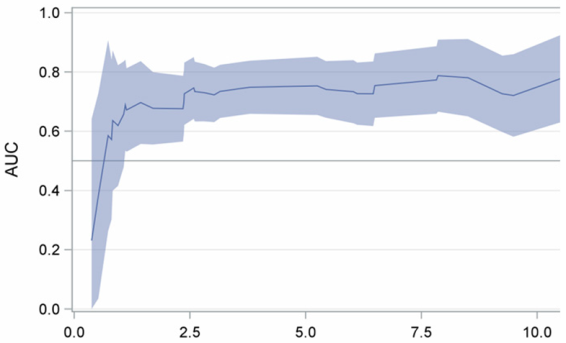 | ||
| <60 years | 1.00 | ||
| ≥60 years | 8.92 (1.14–69.9) | 0.037 | |
| Type of surgery | |||
| Lobectomy | 1.00 | ||
| Sublobar resection | 3.17 (1.42–7.10) | 0.005 | |
| Clinical stage | |||
| IA | 1.00 | ||
| IB or more | 5.06 (1.80–14.2) | 0.002 |
| All | Consolidation-to-Tumor Ratio (CTR) | |||||
|---|---|---|---|---|---|---|
| Patients | Pure GGO | ≤0.50 | 0.50–0.75 | 0.75–1.00 | ||
| N (%) | N (%) | N (%) | N (%) | N (%) | p-Value * | |
| All patients | 172 (100.) | 90 (100.) | 56 (100.) | 18 (100.) | 8 (100.) | |
| Invasiveness | ||||||
| AIS | 5 (2.9) | 5 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| MIA | 31 (18.0) | 24 (26.7) | 6 (10.7) | 0 (0.0) | 1 (12.5) | |
| IA | 136 (79.1) | 61 (67.8) | 50 (89.3) | 18 (100.) | 7 (87.5) | 0.0006 |
| Size | ||||||
| ≤10 | 61 (35.5) | 39 (43.3) | 18 (32.1) | 1 (5.6) | 3 (37.5) | |
| 11–20 | 87 (50.6) | 43 (47.8) | 30 (53.6) | 10 (55.6) | 4 (50.0) | |
| >20 | 24 (14.0) | 8 (8.9) | 8 (14.3) | 7 (38.9) | 1 (12.5) | 0.004 |
| pT | ||||||
| 1a | 69 (40.1) | 44 (48.9) | 20 (35.7) | 1 (5.6) | 4 (50.0) | |
| 1b | 80 (46.5) | 39 (43.3) | 28 (50.0) | 10 (55.6) | 3 (37.5) | |
| 1c | 16 (9.3) | 4 (4.4) | 5 (8.9) | 6 (33.3) | 1 (12.5) | |
| 2a | 4 (2.3) | 2 (2.2) | 1 (1.8) | 1 (5.6) | 0 (0.0) | |
| 2b | 2 (1.2) | 1 (1.1) | 1 (1.8) | 0 (0.0) | 0 (0.0) | |
| 3–4 * | 1 (0.6) | 0 (0.0) | 1 (1.8) | 0 (0.0) | 0 (0.0) | 0.020 |
| Stage | ||||||
| IA | 163 (94.8) | 85 (94.4) | 53 (94.6) | 17 (94.4) | 8 (100.) | |
| IB | 3 (1.7) | 1 (1.1) | 1 (1.8) | 1 (5.6) | 0 (0.0) | |
| IIA | 2 (1.2) | 1 (1.1) | 1 (1.8) | 0 (0.0) | 0 (0.0) | |
| IIB | 3 (1.7) | 3 (3.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| IIIA | 1 (0.6) | 0 (0.0) | 1 (1.8) | 0 (0.0) | 0 (0.0) | 0.46 |
| Grading | ||||||
| G1 | 90 (52.3) | 61 (67.8) | 23 (41.1) | 3 (16.7) | 3 (37.5) | |
| G2 | 72 (41.9) | 24 (26.7) | 31 (55.4) | 13 (72.2) | 4 (50.0) | |
| G3 | 10 (5.8) | 5 (5.6) | 2 (3.6) | 2 (11.1) | 1 (12.5) | 0.0002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casiraghi, M.; Girelli, L.; Elettore, A.; Bertolaccini, L.; Mazzella, A.; Bardoni, C.; Lombardi, M.; Midolo, V.; Petralia, G.; Maisonneuve, P.; et al. Clinicopathological Features and Prognosis of Lung Adenocarcinoma Presenting as Ground-Glass Opacity: A Single-Center Experience. Cancers 2025, 17, 3016. https://doi.org/10.3390/cancers17183016
Casiraghi M, Girelli L, Elettore A, Bertolaccini L, Mazzella A, Bardoni C, Lombardi M, Midolo V, Petralia G, Maisonneuve P, et al. Clinicopathological Features and Prognosis of Lung Adenocarcinoma Presenting as Ground-Glass Opacity: A Single-Center Experience. Cancers. 2025; 17(18):3016. https://doi.org/10.3390/cancers17183016
Chicago/Turabian StyleCasiraghi, Monica, Lara Girelli, Attilio Elettore, Luca Bertolaccini, Antonio Mazzella, Claudia Bardoni, Mariano Lombardi, Valeria Midolo, Giuseppe Petralia, Patrick Maisonneuve, and et al. 2025. "Clinicopathological Features and Prognosis of Lung Adenocarcinoma Presenting as Ground-Glass Opacity: A Single-Center Experience" Cancers 17, no. 18: 3016. https://doi.org/10.3390/cancers17183016
APA StyleCasiraghi, M., Girelli, L., Elettore, A., Bertolaccini, L., Mazzella, A., Bardoni, C., Lombardi, M., Midolo, V., Petralia, G., Maisonneuve, P., Guarize, J., & Spaggiari, L. (2025). Clinicopathological Features and Prognosis of Lung Adenocarcinoma Presenting as Ground-Glass Opacity: A Single-Center Experience. Cancers, 17(18), 3016. https://doi.org/10.3390/cancers17183016








