Targeting Oncogenic Activity and Signalling of Mutant Receptor Tyrosine Kinase FLT3
Simple Summary
Abstract
1. Introduction
2. Specific Targeting of FLT3 Activity with Tyrosine Kinase Inhibitors (TKIs)
3. Mediating FLT3 Activity by Controlling Its Maturation and Degradation
3.1. Targeting Glycosylation and Plasma Membrane Localisation
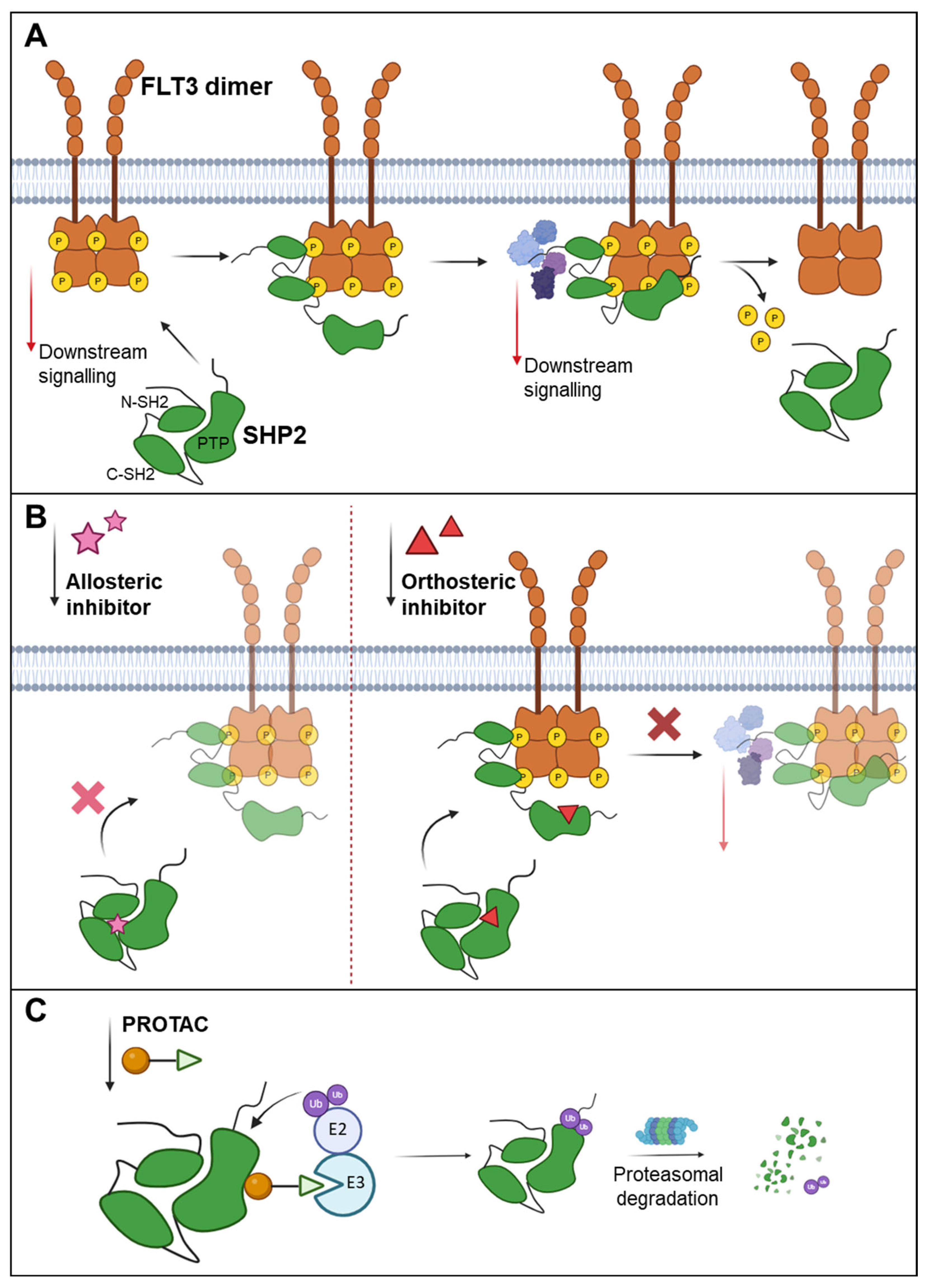
3.2. Targeting Degradation Through the Ubiquitin Pathway
3.3. Targeting Degradation by PROTACs
4. Controlling FLT3 Trans-Autophosphorylation by Complex Formation
5. Regulation of RTK Activity by Reactive Oxygen Species (ROS)
6. Controlling FLT3 Activity by Regulating the Activity of Protein Tyrosine Phosphatases (PTPs)
6.1. Targeting PTPs by PROTACs
6.2. Orthosteric Inhibition of PTPs
6.3. Allosteric Inhibition of PTPs
6.4. Dimerisation of PTPs
6.5. Redox Regulation of PTPs
6.6. Bispecific Antibody–Aptamer Chimeras—Immunologic Approaches to Enforce RTK-PTP Interaction
7. Targeting RTK Activity and AML by Modulating Genome-Wide Regulation of Gene Expression—Controlling Protein Acetylation and DNA Methylation
7.1. Zinc-Dependent Histone Deacetylases
7.2. NAD-Dependent Histone Deacetylases
7.3. DNA Methyltransferases
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dohner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Nakao, M.; Yokota, S.; Iwai, T.; Kaneko, H.; Horiike, S.; Kashima, K.; Sonoda, Y.; Fujimoto, T.; Misawa, S. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia 1996, 10, 1911–1918. [Google Scholar]
- Yokota, S.; Kiyoi, H.; Nakao, M.; Iwai, T.; Misawa, S.; Okuda, T.; Sonoda, Y.; Abe, T.; Kahsima, K.; Matsuo, Y.; et al. Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and myelodysplastic syndrome among various hematological malignancies. A study on a large series of patients and cell lines. Leukemia 1997, 11, 1605–1609. [Google Scholar] [CrossRef]
- Kiyoi, H.; Ohno, R.; Ueda, R.; Saito, H.; Naoe, T. Mechanism of constitutive activation of FLT3 with internal tandem duplication in the juxtamembrane domain. Oncogene 2002, 21, 2555–2563. [Google Scholar] [CrossRef]
- Griffith, J.; Black, J.; Faerman, C.; Swenson, L.; Wynn, M.; Lu, F.; Lippke, J.; Saxena, K. The structural basis for autoinhibition of FLT3 by the juxtamembrane domain. Mol. Cell 2004, 13, 169–178. [Google Scholar] [CrossRef]
- Ruglioni, M.; Crucitta, S.; Luculli, G.I.; Tancredi, G.; Del Giudice, M.L.; Mechelli, S.; Galimberti, S.; Danesi, R.; Del Re, M. Understanding mechanisms of resistance to FLT3 inhibitors in adult FLT3-mutated acute myeloid leukemia to guide treatment strategy. Crit. Rev. Oncol. Hematol. 2024, 201, 104424. [Google Scholar] [CrossRef] [PubMed]
- Levis, M.; Perl, A.E. Gilteritinib: Potent targeting of FLT3 mutations in AML. Blood Adv. 2020, 4, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Abu-Duhier, F.M.; Goodeve, A.C.; Wilson, G.A.; Care, R.S.; Peake, I.R.; Reilly, J.T. Identification of novel FLT-3 Asp835 mutations in adult acute myeloid leukaemia. Br. J. Haematol. 2001, 113, 983–988. [Google Scholar] [CrossRef]
- Kindler, T.; Breitenbuecher, F.; Kasper, S.; Estey, E.; Giles, F.; Feldman, E.; Ehninger, G.; Schiller, G.; Klimek, V.; Nimer, S.D.; et al. Identification of a novel activating mutation (Y842C) within the activation loop of FLT3 in patients with acute myeloid leukemia (AML). Blood 2005, 105, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Bacher, U.; Haferlach, C.; Kern, W.; Haferlach, T.; Schnittger, S. Prognostic relevance of FLT3-TKD mutations in AML: The combination matters—an analysis of 3082 patients. Blood 2008, 111, 2527–2537. [Google Scholar] [CrossRef]
- Zhong, Y.; Qiu, R.Z.; Sun, S.L.; Zhao, C.; Fan, T.Y.; Chen, M.; Li, N.G.; Shi, Z.H. Small-Molecule Fms-like Tyrosine Kinase 3 Inhibitors: An Attractive and Efficient Method for the Treatment of Acute Myeloid Leukemia. J. Med. Chem. 2020, 63, 12403–12428. [Google Scholar] [CrossRef]
- Rataj, J.; Gorecki, L.; Muthna, D.; Sorf, A.; Krystof, V.; Klener, P.; Ceckova, M.; Rezacova, M.; Korabecny, J. Targeting FMS-like tyrosine kinase 3 (FLT3) in acute myeloid leukemia: Novel molecular approaches and therapeutic challenges. Biomed. Pharmacother. 2025, 182, 117788. [Google Scholar] [CrossRef]
- Santoni, M.; Iacovelli, R.; Colonna, V.; Klinz, S.; Mauri, G.; Nuti, M. Antitumor effects of the multi-target tyrosine kinase inhibitor cabozantinib: A comprehensive review of the preclinical evidence. Expert. Rev. Anticancer. Ther. 2021, 21, 1029–1054. [Google Scholar] [CrossRef]
- Bohmer, A.; Barz, S.; Schwab, K.; Kolbe, U.; Gabel, A.; Kirkpatrick, J.; Ohlenschlager, O.; Gorlach, M.; Bohmer, F.D. Modulation of FLT3 signal transduction through cytoplasmic cysteine residues indicates the potential for redox regulation. Redox Biol. 2020, 28, 101325. [Google Scholar] [CrossRef]
- Williams, A.B.; Li, L.; Nguyen, B.; Brown, P.; Levis, M.; Small, D. Fluvastatin inhibits FLT3 glycosylation in human and murine cells and prolongs survival of mice with FLT3/ITD leukemia. Blood 2012, 120, 3069–3079. [Google Scholar] [CrossRef]
- Larrue, C.; Saland, E.; Vergez, F.; Serhan, N.; Delabesse, E.; Mansat-De Mas, V.; Hospital, M.A.; Tamburini, J.; Manenti, S.; Sarry, J.E.; et al. Antileukemic Activity of 2-Deoxy-d-Glucose through Inhibition of N-Linked Glycosylation in Acute Myeloid Leukemia with FLT3 ITD or c-KIT Mutations. Mol. Cancer Ther. 2015, 14, 2364–2373. [Google Scholar] [CrossRef] [PubMed]
- Tsitsipatis, D.; Jayavelu, A.K.; Muller, J.P.; Bauer, R.; Schmidt-Arras, D.; Mahboobi, S.; Schnoder, T.M.; Heidel, F.; Bohmer, F.D. Synergistic killing of FLT3ITD-positive AML cells by combined inhibition of tyrosine-kinase activity and N-glycosylation. Oncotarget 2017, 8, 26613–26624. [Google Scholar] [CrossRef]
- Marcotegui, N.; Romero-Murillo, S.; Marco-Sanz, J.; Peris, I.; Berrozpe, B.S.; Vicente, C.; Odero, M.D.; Arriazu, E. Set Protein Is Involved in FLT3 Membrane Trafficking. Cancers 2023, 15, 2233. [Google Scholar] [CrossRef] [PubMed]
- Reiter, K.; Polzer, H.; Krupka, C.; Maiser, A.; Vick, B.; Rothenberg-Thurley, M.; Metzeler, K.H.; Dorfel, D.; Salih, H.R.; Jung, G.; et al. Tyrosine kinase inhibition increases the cell surface localization of FLT3 ITD and enhances FLT3-directed immunotherapy of acute myeloid leukemia. Leukemia 2018, 32, 313–322. [Google Scholar] [CrossRef]
- Fleischmann, M.; Fischer, M.; Schnetzke, U.; Fortner, C.; Kirkpatrick, J.; Heidel, F.H.; Hochhaus, A.; Scholl, S. Modulation of FLT3 ITD Localization and Targeting of Distinct Downstream Signaling Pathways as Potential Strategies to Overcome FLT3-Inhibitor Resistance. Cells 2021, 10, 2992. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Bai, Y.; Zhou, L.; Jin, J.; Zhang, M.; Wang, Y.; Lin, R.; Huang, W.; Ren, X.; Ma, N.; et al. Discovery of LWY713 as a potent and selective FLT3 PROTAC degrader with in vivo activity against acute myeloid leukemia. Eur. J. Med. Chem. 2024, 264, 115974. [Google Scholar] [CrossRef]
- Weisberg, E.L.; Schauer, N.J.; Yang, J.; Lamberto, I.; Doherty, L.; Bhatt, S.; Nonami, A.; Meng, C.; Letai, A.; Wright, R.; et al. Inhibition of USP10 induces degradation of oncogenic FLT3. Nat. Chem. Biol. 2017, 13, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Y.; Liao, W.; Mou, Y.; Zhan, X.; Hu, Q.; Zhao, Z.; Xiong, D. Decursin induces FLT3 ITD acute myeloid leukemia cell apoptosis by increasing the expression of the ubiquitin-conjugase UBE2L6. Cell Commun. Signal. 2025, 23, 162. [Google Scholar] [CrossRef] [PubMed]
- Oshikawa, G.; Nagao, T.; Wu, N.; Kurosu, T.; Miura, O. c-Cbl and Cbl-b ligases mediate 17-allylaminodemethoxygeldanamycin-induced degradation of autophosphorylated Flt3 kinase with internal tandem duplication through the ubiquitin proteasome pathway. J. Biol. Chem. 2011, 286, 30263–30273. [Google Scholar] [CrossRef]
- Burslem, G.M.; Song, J.; Chen, X.; Hines, J.; Crews, C.M. Enhancing Antiproliferative Activity and Selectivity of a FLT-3 Inhibitor by Proteolysis Targeting Chimera Conversion. J. Am. Chem. Soc. 2018, 140, 16428–16432. [Google Scholar] [CrossRef]
- Ferrari, G.; Greene, L.A. Prevention of neuronal apoptotic death by neurotrophic agents and ganglioside GM1: Insights and speculations regarding a common mechanism. Perspect. Dev. Neurobiol. 1996, 3, 93–100. [Google Scholar]
- Sorby, M.; Sandstrom, J.; Ostman, A. An extracellular ligand increases the specific activity of the receptor-like protein tyrosine phosphatase DEP-1. Oncogene 2001, 20, 5219–5224. [Google Scholar] [CrossRef] [PubMed]
- Jayavelu, A.K.; Moloney, J.N.; Bohmer, F.D.; Cotter, T.G. NOX-driven ROS formation in cell transformation of FLT3 ITD-positive AML. Exp. Hematol. 2016, 44, 1113–1122. [Google Scholar] [CrossRef]
- Jayavelu, A.K.; Muller, J.P.; Bauer, R.; Bohmer, S.A.; Lassig, J.; Cerny-Reiterer, S.; Sperr, W.R.; Valent, P.; Maurer, B.; Moriggl, R.; et al. NOX4-driven ROS formation mediates PTP inactivation and cell transformation in FLT3ITD-positive AML cells. Leukemia 2016, 30, 473–483. [Google Scholar] [CrossRef]
- Li, W.; Lu, W.; Liu, Z. A phosphatase-recruiting bispecific antibody-aptamer chimera for enhanced suppression of tumor growth. Chem. Commun. 2023, 59, 6572–6575. [Google Scholar] [CrossRef]
- Wang, J.; Rong, Q.; Ye, L.; Fang, B.; Zhao, Y.; Sun, Y.; Zhou, H.; Wang, D.; He, J.; Cui, Z.; et al. Discovery of a Novel Orally Bioavailable FLT3-PROTAC Degrader for Efficient Treatment of Acute Myeloid Leukemia and Overcoming Resistance of FLT3 Inhibitors. J. Med. Chem. 2024, 67, 7197–7223. [Google Scholar] [CrossRef]
- Nabinger, S.C.; Li, X.J.; Ramdas, B.; He, Y.; Zhang, X.; Zeng, L.; Richine, B.; Bowling, J.D.; Fukuda, S.; Goenka, S.; et al. The protein tyrosine phosphatase, Shp2, positively contributes to FLT3 ITD-induced hematopoietic progenitor hyperproliferation and malignant disease in vivo. Leukemia 2013, 27, 398–408. [Google Scholar] [CrossRef][Green Version]
- Hellmuth, K.; Grosskopf, S.; Lum, C.T.; Wurtele, M.; Roder, N.; von Kries, J.P.; Rosario, M.; Rademann, J.; Birchmeier, W. Specific inhibitors of the protein tyrosine phosphatase Shp2 identified by high-throughput docking. Proc. Natl. Acad. Sci. USA 2008, 105, 7275–7280. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Zhan, Z.; Gan, L.; Bai, O. Mechanisms of HDACs in cancer development. Front. Immunol. 2025, 16, 1529239. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, Y.; Madlambayan, G.; Edwards, H.; Polin, L.; Kushner, J.; Dzinic, S.H.; White, K.; Ma, J.; Knight, T.; et al. Antileukemic activity and mechanism of action of the novel PI3K and histone deacetylase dual inhibitor CUDC-907 in acute myeloid leukemia. Haematologica 2019, 104, 2225–2240. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; He, Z.; Jin, G.; Jin, S.; Du, Y.; Yuan, S.; Zhang, J. Targeting DNA methyltransferases for cancer therapy. Bioorg. Chem. 2024, 151, 107652. [Google Scholar] [CrossRef]
- Dhillon, S. Gilteritinib: First Global Approval. Drugs 2019, 79, 331–339. [Google Scholar] [CrossRef]
- Kim, E.S. Midostaurin: First Global Approval. Drugs 2017, 77, 1251–1259. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2024 update. Pharmacol. Res. 2024, 200, 107059. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.; Hernandez, D.; Rajkhowa, T.; Smith, S.C.; Raman, J.R.; Nguyen, B.; Small, D.; Levis, M. Preclinical studies of gilteritinib, a next-generation FLT3 inhibitor. Blood 2017, 129, 257–260. [Google Scholar] [CrossRef]
- Perl, A.E.; Altman, J.K.; Cortes, J.; Smith, C.; Litzow, M.; Baer, M.R.; Claxton, D.; Erba, H.P.; Gill, S.; Goldberg, S.; et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: A multicentre, first-in-human, open-label, phase 1–2 study. Lancet Oncol. 2017, 18, 1061–1075. [Google Scholar] [CrossRef]
- Aikawa, T.; Togashi, N.; Iwanaga, K.; Okada, H.; Nishiya, Y.; Inoue, S.; Levis, M.J.; Isoyama, T. Quizartinib, a selective FLT3 inhibitor, maintains antileukemic activity in preclinical models of RAS-mediated midostaurin-resistant acute myeloid leukemia cells. Oncotarget 2020, 11, 943–955. [Google Scholar] [CrossRef]
- Lewis, N.L.; Lewis, L.D.; Eder, J.P.; Reddy, N.J.; Guo, F.; Pierce, K.J.; Olszanski, A.J.; Cohen, R.B. Phase I study of the safety, tolerability, and pharmacokinetics of oral CP-868,596, a highly specific platelet-derived growth factor receptor tyrosine kinase inhibitor in patients with advanced cancers. J. Clin. Oncol. 2009, 27, 5262–5269. [Google Scholar] [CrossRef]
- Chang, Y.T.; Hernandez, D.; Alonso, S.; Gao, M.; Su, M.; Ghiaur, G.; Levis, M.J.; Jones, R.J. Role of CYP3A4 in bone marrow microenvironment-mediated protection of FLT3/ITD AML from tyrosine kinase inhibitors. Blood Adv. 2019, 3, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.P.; Schmidt-Arras, D. Novel Approaches to Target Mutant FLT3 Leukaemia. Cancers 2020, 12, 2806. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Ding, X.; Wang, Y.; Li, Z.; Zhao, R.; Cheng, H.E.; Sun, Y. Efficacy and safety of FLT3 inhibitors in monotherapy of hematological and solid malignancies: A systemic analysis of clinical trials. Front. Pharmacol. 2024, 15, 1294668. [Google Scholar] [CrossRef] [PubMed]
- Park, I.K.; Trotta, R.; Yu, J.; Caligiuri, M.A. Axl/Gas6 pathway positively regulates FLT3 activation in human natural killer cell development. Eur. J. Immunol. 2013, 43, 2750–2755. [Google Scholar] [CrossRef]
- Park, I.K.; Mundy-Bosse, B.; Whitman, S.P.; Zhang, X.; Warner, S.L.; Bearss, D.J.; Blum, W.; Marcucci, G.; Caligiuri, M.A. Receptor tyrosine kinase Axl is required for resistance of leukemic cells to FLT3-targeted therapy in acute myeloid leukemia. Leukemia 2015, 29, 2382–2389. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, J.; Liu, J.; Ma, W.; Duan, Y.; Liu, D. Novel AXL-targeted agents overcome FLT3 inhibitor resistance in FLT3 ITD(+) acute myeloid leukemia cells. Oncol. Lett. 2021, 21, 397. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.Y.; Buelow, D.R.; Garrison, D.A.; Niu, M.; Eisenmann, E.D.; Huang, K.M.; Zavorka Thomas, M.E.; Weber, R.H.; Whatcott, C.J.; Warner, S.L.; et al. TP-0903 is active in models of drug-resistant acute myeloid leukemia. JCI Insight. 2020, 5, e140169. [Google Scholar] [CrossRef]
- Patel, A.B.; Pomicter, A.D.; Yan, D.; Eiring, A.M.; Antelope, O.; Schumacher, J.A.; Kelley, T.W.; Tantravahi, S.K.; Kovacsovics, T.J.; Shami, P.J.; et al. Dasatinib overcomes stroma-based resistance to the FLT3 inhibitor quizartinib using multiple mechanisms. Leukemia 2020, 34, 2981–2991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, G.; Zhang, H.; Basyal, M.; Ly, C.; Yuan, B.; Ruvolo, V.; Piya, S.; Bhattacharya, S.; Zhang, Q.; et al. Concomitant targeting of FLT3 and BTK overcomes FLT3 inhibitor resistance in acute myeloid leukemia through the inhibition of autophagy. Haematologica 2023, 108, 1500–1514. [Google Scholar] [CrossRef]
- Pratz, K.W.; Kaplan, J.; Levy, M.; Bixby, D.; Burke, P.W.; Erba, H.; Wise-Draper, T.M.; Roboz, G.J.; Papadantonakis, N.; Rajkhowa, T.; et al. A phase Ib trial of mivavotinib (TAK-659), a dual SYK/FLT3 inhibitor, in patients with relapsed/refractory acute myeloid leukemia. Haematologica 2023, 108, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Popescu, B.; Stahlhut, C.; Tarver, T.C.; Wishner, S.; Lee, B.J.; Peretz, C.A.C.; Luck, C.; Phojanakong, P.; Camara Serrano, J.A.; Hongo, H.; et al. Allosteric SHP2 inhibition increases apoptotic dependency on BCL2 and synergizes with venetoclax in FLT3- and KIT-mutant AML. Cell Rep. Med. 2023, 4, 101290. [Google Scholar] [CrossRef]
- Lancet, J.E.; Uy, G.L.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; Bixby, D.L.; et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021, 8, e481–e491. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Lin, T.L.; Asubonteng, K.; Faderl, S.; Lancet, J.E.; Prebet, T. Efficacy and safety of CPX-351 versus 7 + 3 chemotherapy by European LeukemiaNet 2017 risk subgroups in older adults with newly diagnosed, high-risk/secondary AML: Post hoc analysis of a randomized, phase 3 trial. J. Hematol. Oncol. 2022, 15, 155. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; DiNardo, C.D.; Kadia, T.M.; Daver, N.G.; Altman, J.K.; Stein, E.M.; Jabbour, E.; Schiffer, C.A.; Lang, A.; Ravandi, F. Acute myeloid leukemia management and research in 2025. CA Cancer J. Clin. 2025, 75, 46–67. [Google Scholar] [CrossRef]
- Long, J.; Jia, M.Y.; Fang, W.Y.; Chen, X.J.; Mu, L.L.; Wang, Z.Y.; Shen, Y.; Xiang, R.F.; Wang, L.N.; Wang, L.; et al. FLT3 inhibition upregulates HDAC8 via FOXO to inactivate p53 and promote maintenance of FLT3 ITD+ acute myeloid leukemia. Blood 2020, 135, 1472–1483. [Google Scholar] [CrossRef]
- Li, J.; Fu, S.; Ye, C.; Li, J. Combination therapy involving azacitidine for acute myeloid leukemia patients ineligible for intensive chemotherapy. Leuk. Res. 2025, 148, 107638. [Google Scholar] [CrossRef]
- Tan, Y.; Xin, L.; Wang, Q.; Xu, R.; Tong, X.; Chen, G.; Ma, L.; Yang, F.; Jiang, H.; Zhang, N.; et al. FLT3-selective PROTAC: Enhanced safety and increased synergy with Venetoclax in FLT3 ITD mutated acute myeloid leukemia. Cancer Lett. 2024, 592, 216933. [Google Scholar] [CrossRef]
- Schmidt-Arras, D.E.; Böhmer, A.; Markova, B.; Choudhary, C.; Serve, H.; Böhmer, F.D. Tyrosine phosphorylation regulates maturation of receptor tyrosine kinases. Mol. Cell. Biol. 2005, 25, 3690–3703. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Jacobi, A.; Ryser, M.; Ehninger, G.; Thiede, C. Abnormal localization and accumulation of FLT3 ITD, a mutant receptor tyrosine kinase involved in leukemogenesis. Cells Tissues Organs 2008, 188, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Kazi, J.U.; Ronnstrand, L. FMS-like Tyrosine Kinase 3/FLT3: From Basic Science to Clinical Implications. Physiol. Rev. 2019, 99, 1433–1466. [Google Scholar] [CrossRef]
- Yamawaki, K.; Shiina, I.; Murata, T.; Tateyama, S.; Maekawa, Y.; Niwa, M.; Shimonaka, M.; Okamoto, K.; Suzuki, T.; Nishida, T.; et al. FLT3 ITD transduces autonomous growth signals during its biosynthetic trafficking in acute myelogenous leukemia cells. Sci. Rep. 2021, 11, 22678. [Google Scholar] [CrossRef]
- Natarajan, K.; Xie, Y.; Burcu, M.; Linn, D.E.; Qiu, Y.; Baer, M.R. Pim-1 Kinase Phosphorylates and Stabilizes 130 kDa FLT3 and Promotes Aberrant STAT5 Signaling in Acute Myeloid Leukemia with FLT3 Internal Tandem Duplication. PLoS ONE 2013, 8, e74653. [Google Scholar] [CrossRef]
- Schmidt-Arras, D.; Böhmer, S.A.; Koch, S.; Müller, J.P.; Blei, L.; Cornils, H.; Bauer, R.; Korasikha, S.; Thiede, C.; Böhmer, F.D. Anchoring of FLT3 in the endoplasmic reticulum alters signaling quality. Blood 2009, 113, 3568–3576. [Google Scholar] [CrossRef]
- Choudhary, C.; Olsen, J.V.; Brandts, C.; Cox, J.; Reddy, P.N.; Böhmer, F.D.; Gerke, V.; Schmidt-Arras, D.E.; Berdel, W.E.; Müller-Tidow, C.; et al. Mislocalized activation of oncogenic RTKs switches downstream signaling outcomes. Mol. Cell 2009, 36, 326–339. [Google Scholar] [CrossRef]
- Caldarelli, A.; Muller, J.P.; Paskowski-Rogacz, M.; Herrmann, K.; Bauer, R.; Koch, S.; Heninger, A.K.; Krastev, D.; Ding, L.; Kasper, S.; et al. A genome-wide RNAi screen identifies proteins modulating aberrant FLT3 ITD signaling. Leukemia 2013, 27, 2301–2310. [Google Scholar] [CrossRef][Green Version]
- Corsini, A.; Maggi, F.M.; Catapano, A.L. Pharmacology of competitive inhibitors of HMG-CoA reductase. Pharmacol. Res. 1995, 31, 9–27. [Google Scholar] [CrossRef]
- Mo, H.; Elson, C.E. Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Exp. Biol. Med. 2004, 229, 567–585. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, H.; Martin, D.S.; Xu, R.H.; Huang, P. Glycolysis inhibition for anticancer treatment. Oncogene 2006, 25, 4633–4646. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Kurtoglu, M.; Liu, H.; Wangpaichitr, M.; You, M.; Liu, X.; Savaraj, N.; Lampidis, T.J. 2-Deoxy-D-glucose activates autophagy via endoplasmic reticulum stress rather than ATP depletion. Cancer Chemother. Pharmacol. 2011, 67, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Andresen, L.; Skovbakke, S.L.; Persson, G.; Hagemann-Jensen, M.; Hansen, K.A.; Jensen, H.; Skov, S. 2-deoxy D-glucose prevents cell surface expression of NKG2D ligands through inhibition of N-linked glycosylation. J. Immunol. 2012, 188, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Kurtoglu, M.; Gao, N.; Shang, J.; Maher, J.C.; Lehrman, M.A.; Wangpaichitr, M.; Savaraj, N.; Lane, A.N.; Lampidis, T.J. Under normoxia, 2-deoxy-D-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation. Mol. Cancer Ther. 2007, 6, 3049–3058. [Google Scholar] [CrossRef]
- Weisberg, E.; Boulton, C.; Kelly, L.M.; Manley, P.; Fabbro, D.; Meyer, T.; Gilliland, D.G.; Griffin, J.D. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell 2002, 1, 433–443. [Google Scholar] [CrossRef]
- Zong, Z.; Zhang, Z.; Wu, L.; Zhang, L.; Zhou, F. The Functional Deubiquitinating Enzymes in Control of Innate Antiviral Immunity. Adv. Sci. 2021, 8, 2002484. [Google Scholar] [CrossRef]
- Buchwald, M.; Pietschmann, K.; Muller, J.P.; Bohmer, F.D.; Heinzel, T.; Kramer, O.H. Ubiquitin conjugase UBCH8 targets active FMS-like tyrosine kinase 3 for proteasomal degradation. Leukemia 2010, 24, 1412–1421. [Google Scholar] [CrossRef]
- Kazi, J.U.; Sun, J.; Phung, B.; Zadjali, F.; Flores-Morales, A.; Ronnstrand, L. Suppressor of cytokine signaling 6 (SOCS6) negatively regulates Flt3 signal transduction through direct binding to phosphorylated tyrosines 591 and 919 of Flt3. J. Biol. Chem. 2012, 287, 36509–36517. [Google Scholar] [CrossRef]
- Liu, J.; Gu, J. Importance of PTM of FLT3 in acute myeloid leukemia. Acta Biochim. Biophys. Sin. 2024, 56, 1199–1207. [Google Scholar] [CrossRef]
- Kazi, J.U.; Ronnstrand, L. Suppressor of cytokine signaling 2 (SOCS2) associates with FLT3 and negatively regulates downstream signaling. Mol. Oncol. 2013, 7, 693–703. [Google Scholar] [CrossRef]
- Lu, Y.C.; Wang, X.F.; Zhu, W.B.; Cheng, X.; Cui, Z.J. Degradation of cyanide and maturity in cassava processing wastes composting. Huan Jing Ke Xue 2009, 30, 1556–1560. [Google Scholar]
- Reindl, C.; Quentmeier, H.; Petropoulos, K.; Greif, P.A.; Benthaus, T.; Argiropoulos, B.; Mellert, G.; Vempati, S.; Duyster, J.; Buske, C.; et al. CBL exon 8/9 mutants activate the FLT3 pathway and cluster in core binding factor/11q deletion acute myeloid leukemia/myelodysplastic syndrome subtypes. Clin. Cancer Res. 2009, 15, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Umezawa, Y.; Ishida, S.; Okada, K.; Nogami, A.; Miura, O. Inhibition of USP9X induces apoptosis in FLT3 ITD-positive AML cells cooperatively by inhibiting the mutant kinase through aggresomal translocation and inducing oxidative stress. Cancer Lett. 2019, 453, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Isaji, T.; Komatsu, S.; Sun, Y.; Xu, X.; Fukuda, T.; Fujimura, T.; Takahashi, S.; Gu, J. BRCC36 associates with FLT3 ITD to regulate its protein stability and intracellular signaling in acute myeloid leukemia. Cancer Sci. 2024, 115, 1196–1208. [Google Scholar] [CrossRef]
- Minami, Y.; Kiyoi, H.; Yamamoto, Y.; Yamamoto, K.; Ueda, R.; Saito, H.; Naoe, T. Selective apoptosis of tandemly duplicated FLT3-transformed leukemia cells by Hsp90 inhibitors. Leukemia 2002, 16, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, Y. Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy. J. Hematol. Oncol. 2020, 13, 50. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, Q.; Song, D.; Tang, K.; Tang, Z.; Hu, M.; Luo, Y.; Xie, Y. Discovery of degrader for FLT3, GSPT1 and IKZF1/3 proteins merging PROTAC and molecular glue targeting FLT3 ITD mutant acute myeloid leukemia. Eur. J. Med. Chem. 2025, 296, 117893. [Google Scholar] [CrossRef]
- Cao, S.; Ma, L.; Liu, Y.; Wei, M.; Yao, Y.; Li, C.; Wang, R.; Liu, N.; Dong, Z.; Li, X.; et al. Proteolysis-Targeting Chimera (PROTAC) Modification of Dovitinib Enhances the Antiproliferative Effect against FLT3 ITD-Positive Acute Myeloid Leukemia Cells. J. Med. Chem. 2021, 64, 16497–16511. [Google Scholar] [CrossRef]
- Reznickova, E.; Krajcovicova, S.; Perina, M.; Kovalova, M.; Soural, M.; Krystof, V. Modulation of FLT3 ITD and CDK9 in acute myeloid leukaemia cells by novel proteolysis targeting chimera (PROTAC). Eur. J. Med. Chem. 2022, 243, 114792. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, X.; Liu, C.; Huang, F.; Lu, T.; Chen, Y.; Liu, L.; Lu, S. Discovery of FLT3-targeting PROTACs with potent antiproliferative activity against acute myeloid leukemia cells harboring FLT3 mutations. Eur. J. Med. Chem. 2024, 268, 116237. [Google Scholar] [CrossRef]
- Ye, L.; Cui, Z.; Sun, Y.; Zhou, H.; Rong, Q.; Wang, D.; Jin, J.; Zhang, Q.; Kang, D.; Hu, L.; et al. Discovery of a potent Gilteritinib-based FLT3-PROTAC degrader for the treatment of Acute myeloid leukemia. Bioorg. Chem. 2024, 149, 107477. [Google Scholar] [CrossRef]
- Moharram, S.A.; Chougule, R.A.; Su, X.; Li, T.; Sun, J.; Zhao, H.; Ronnstrand, L.; Kazi, J.U. Src-like adaptor protein 2 (SLAP2) binds to and inhibits FLT3 signaling. Oncotarget 2016, 7, 57770–57782. [Google Scholar] [CrossRef]
- Masson, K.; Liu, T.; Khan, R.; Sun, J.; Rönnstrand, L. A role of Gab2 association in Flt3 ITD mediated Stat5 phosphorylation and cell survival. Br. J. Haematol. 2009, 146, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Sonnino, S.; Mauri, L.; Chigorno, V.; Prinetti, A. Gangliosides as components of lipid membrane domains. Glycobiology 2007, 17, 1R–13R. [Google Scholar] [CrossRef] [PubMed]
- Ruzzi, F.; Cappello, C.; Semprini, M.S.; Scalambra, L.; Angelicola, S.; Pittino, O.M.; Landuzzi, L.; Palladini, A.; Nanni, P.; Lollini, P.L. Lipid rafts, caveolae, and epidermal growth factor receptor family: Friends or foes? Cell Commun. Signal. 2024, 22, 489. [Google Scholar] [CrossRef] [PubMed]
- Mutoh, T.; Tokuda, A.; Miyadai, T.; Hamaguchi, M.; Fujiki, N. Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc. Natl. Acad. Sci. USA 1995, 92, 5087–5091. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Fukui, T.; Hikichi, C.; Ishikawa, T.; Murate, K.; Adachi, T.; Imai, H.; Fukuhara, K.; Ueda, A.; Kaplan, A.P.; et al. Neurotropin promotes NGF signaling through interaction of GM1 ganglioside with Trk neurotrophin receptor in PC12 cells. Brain Res. 2015, 1596, 13–21. [Google Scholar] [CrossRef]
- Zakharova, I.O.; Sokolova, T.V.; Vlasova, Y.A.; Furaev, V.V.; Rychkova, M.P.; Avrova, N.F. GM1 ganglioside activates ERK1/2 and Akt downstream of Trk tyrosine kinase and protects PC12 cells against hydrogen peroxide toxicity. Neurochem. Res. 2014, 39, 2262–2275. [Google Scholar] [CrossRef]
- Zurita, A.R.; Maccioni, H.J.; Daniotti, J.L. Modulation of epidermal growth factor receptor phosphorylation by endogenously expressed gangliosides. Biochem. J. 2001, 355 Pt 2, 465–472. [Google Scholar] [CrossRef]
- Sasaki, N.; Toyoda, M.; Ishiwata, T. Gangliosides as Signaling Regulators in Cancer. Int. J. Mol. Sci. 2021, 22, 5076. [Google Scholar] [CrossRef]
- Peckys, D.B.; Gaa, D.; de Jonge, N. Quantification of EGFR-HER2 Heterodimers in HER2-Overexpressing Breast Cancer Cells Using Liquid-Phase Electron Microscopy. Cells 2021, 10, 3244. [Google Scholar] [CrossRef]
- Van Brocklyn, J.; Bremer, E.G.; Yates, A.J. Gangliosides inhibit platelet-derived growth factor-stimulated receptor dimerization in human glioma U-1242MG and Swiss 3T3 cells. J. Neurochem. 1993, 61, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Holmstrom, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Sundaresan, M.; Yu, Z.X.; Ferrans, V.J.; Irani, K.; Finkel, T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 1995, 270, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.M.; Kalantar-Zadeh, K.; Streja, E.; Carrero, J.J.; Ma, J.Z.; Lu, J.L.; Kovesdy, C.P. The relationship between thyroid function and estimated glomerular filtration rate in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2015, 30, 282–287. [Google Scholar] [CrossRef]
- Truong, T.H.; Ung, P.M.; Palde, P.B.; Paulsen, C.E.; Schlessinger, A.; Carroll, K.S. Molecular Basis for Redox Activation of Epidermal Growth Factor Receptor Kinase. Cell Chem. Biol. 2016, 23, 837–848. [Google Scholar] [CrossRef]
- Tonks, N.K. Protein tyrosine phosphatases: From genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 2006, 7, 833–846. [Google Scholar] [CrossRef]
- Stoker, A.W. Protein tyrosine phosphatases and signalling. J. Endocrinol. 2005, 185, 19–33. [Google Scholar] [CrossRef]
- Tartaglia, M.; Mehler, E.L.; Goldberg, R.; Zampino, G.; Brunner, H.G.; Kremer, H.; van der Burgt, I.; Crosby, A.H.; Ion, A.; Jeffery, S.; et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat. Genet. 2001, 29, 465–468. [Google Scholar] [CrossRef]
- Yamamoto, T.; Isomura, M.; Xu, Y.; Liang, J.; Yagasaki, H.; Kamachi, Y.; Kudo, K.; Kiyoi, H.; Naoe, T.; Kojma, S. PTPN11, RAS and FLT3 mutations in childhood acute lymphoblastic leukemia. Leuk. Res. 2006, 30, 1085–1089. [Google Scholar] [CrossRef]
- Loh, M.L.; Martinelli, S.; Cordeddu, V.; Reynolds, M.G.; Vattikuti, S.; Lee, C.M.; Wulfert, M.; Germing, U.; Haas, P.; Niemeyer, C.; et al. Acquired PTPN11 mutations occur rarely in adult patients with myelodysplastic syndromes and chronic myelomonocytic leukemia. Leuk. Res. 2005, 29, 459–462. [Google Scholar] [CrossRef]
- Loh, M.L.; Vattikuti, S.; Schubbert, S.; Reynolds, M.G.; Carlson, E.; Lieuw, K.H.; Cheng, J.W.; Lee, C.M.; Stokoe, D.; Bonifas, J.M.; et al. Mutations in PTPN11 implicate the SHP-2 phosphatase in leukemogenesis. Blood 2004, 103, 2325–2331. [Google Scholar] [CrossRef]
- Müller, J.P.; Schönherr, C.; Markova, B.; Bauer, R.; Stocking, C.; Böhmer, F.D. Role of SHP2 for FLT3-dependent proliferation and transformation in 32D cells. Leukemia 2008, 22, 1945–1948. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arora, D.; Stopp, S.; Bohmer, S.A.; Schons, J.; Godfrey, R.; Masson, K.; Razumovskaya, E.; Ronnstrand, L.; Tanzer, S.; Bauer, R.; et al. Protein-tyrosine phosphatase DEP-1 controls receptor tyrosine kinase FLT3 signaling. J. Biol. Chem. 2011, 286, 10918–10929. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Rizzo, S.; Paz, W.; Kresinsky, A.; Thevenin, D.; Müller, J. Disrupting PTPRJ Transmembrane-Mediated Oligomerization Counteracts Oncogenic Receptor Tyrosine Kinase FLT3 ITD. Front. Oncol. Submitt. 2022, 12, 1017947. [Google Scholar] [CrossRef]
- Kresinsky, A.; Bauer, R.; Schnoder, T.M.; Berg, T.; Meyer, D.; Ast, V.; Konig, R.; Serve, H.; Heidel, F.H.; Bohmer, F.D.; et al. Loss of DEP-1 (Ptprj) promotes myeloproliferative disease in FLT3 ITD acute myeloid leukemia. Haematologica 2018, 103, e505–e509. [Google Scholar] [CrossRef]
- Kresinsky, A.; Schnoder, T.M.; Jacobsen, I.D.; Rauner, M.; Hofbauer, L.C.; Ast, V.; Konig, R.; Hoffmann, B.; Svensson, C.M.; Figge, M.T.; et al. Lack of CD45 in FLT3 ITD mice results in a myeloproliferative phenotype, cortical porosity, and ectopic bone formation. Oncogene 2019, 38, 4773–4787. [Google Scholar] [CrossRef]
- Lossius-Cott, C.; Annoh, A.; Bens, M.; Nietzsche, S.; Hoffmann, B.; Figge, M.T.; Rauner, M.; Hofbauer, L.C.; Muller, J.P. Oncogenic FLT3 internal tandem duplications (ITD) and CD45/PTPRC control osteoclast functions and bone microarchitecture. JBMR Plus. 2025, 9, ziae173. [Google Scholar] [CrossRef]
- Barrios, A.M. PTPs: Degrading the Undruggable. J. Med. Chem. 2020, 63, 7508–7509. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Miao, J.; Miao, Y.; Qu, Z.; Zhang, S.; Zhu, P.; Wiede, F.; Jassim, B.A.; Bai, Y.; Nguyen, Q.; et al. Small Molecule Degraders of Protein Tyrosine Phosphatase 1B and T-Cell Protein Tyrosine Phosphatase for Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2023, 62, e202303818. [Google Scholar] [CrossRef]
- Miao, J.; Dong, J.; Miao, Y.; Bai, Y.; Qu, Z.; Jassim, B.A.; Huang, B.; Nguyen, Q.; Ma, Y.; Murray, A.A.; et al. Discovery of a selective TC-PTP degrader for cancer immunotherapy. Chem. Sci. 2023, 14, 12606–12614. [Google Scholar] [CrossRef]
- Coronell-Tovar, A.; Pardo, J.P.; Rodriguez-Romero, A.; Sosa-Peinado, A.; Vasquez-Bochm, L.; Cano-Sanchez, P.; Alvarez-Anorve, L.I.; Gonzalez-Andrade, M. Protein tyrosine phosphatase 1B (PTP1B) function, structure, and inhibition strategies to develop antidiabetic drugs. FEBS Lett. 2024, 598, 1811–1838. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Dong, J.; Zhang, Z.Y. Protein tyrosine phosphatases as emerging targets for cancer immunotherapy. Br. J. Pharmacol. 2023. [Google Scholar] [CrossRef]
- Combs, A.P. Recent advances in the discovery of competitive protein tyrosine phosphatase 1B inhibitors for the treatment of diabetes, obesity, and cancer. J. Med. Chem. 2010, 53, 2333–2344. [Google Scholar] [CrossRef]
- Liu, R.; Mathieu, C.; Berthelet, J.; Zhang, W.; Dupret, J.M.; Rodrigues Lima, F. Human Protein Tyrosine Phosphatase 1B (PTP1B): From Structure to Clinical Inhibitor Perspectives. Int. J. Mol. Sci. 2022, 23, 7027. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Jiang, B.; Wu, N.; Wang, S.Y.; Shi, D.Y. Small molecules as potent protein tyrosine phosphatase 1B (PTP1B) inhibitors documented in patents from 2009 to 2013. Mini. Rev. Med. Chem. 2015, 15, 104–122. [Google Scholar] [CrossRef]
- Krishnan, N.; Koveal, D.; Miller, D.H.; Xue, B.; Akshinthala, S.D.; Kragelj, J.; Jensen, M.R.; Gauss, C.M.; Page, R.; Blackledge, M.; et al. Targeting the disordered C terminus of PTP1B with an allosteric inhibitor. Nat. Chem. Biol. 2014, 10, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, C.; Barr, K.J.; Kung, J.; Zhu, J.; Erlanson, D.A.; Shen, W.; Fahr, B.J.; Zhong, M.; Taylor, L.; Randal, M.; et al. Allosteric inhibition of protein tyrosine phosphatase 1B. Nat. Struct. Mol. Biol. 2004, 11, 730–737. [Google Scholar] [CrossRef]
- Friedman, A.J.; Liechty, E.T.; Kramer, L.; Sarkar, A.; Fox, J.M.; Shirts, M.R. Allosteric Inhibition of PTP1B by a Nonpolar Terpenoid. J. Phys. Chem. B 2022, 126, 8427–8438. [Google Scholar] [CrossRef]
- Barford, D.; Neel, B.G. Revealing mechanisms for SH2 domain mediated regulation of the protein tyrosine phosphatase SHP-2. Structure 1998, 6, 249–254. [Google Scholar] [CrossRef]
- Chen, Y.N.; LaMarche, M.J.; Chan, H.M.; Fekkes, P.; Garcia-Fortanet, J.; Acker, M.G.; Antonakos, B.; Chen, C.H.; Chen, Z.; Cooke, V.G.; et al. Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature 2016, 535, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Ramdas, B.; Wan, C.; Sandusky, G.; Mohseni, M.; Zhang, C.; Kapur, R. SHP2 inhibition reduces leukemogenesis in models of combined genetic and epigenetic mutations. J. Clin. Investig. 2019, 129, 5468–5473. [Google Scholar] [CrossRef]
- Bilwes, A.M.; den Hertog, J.; Hunter, T.; Noel, J.P. Structural basis for inhibition of receptor protein-tyrosine phosphatase-alpha by dimerization. Nature 1996, 382, 555–559. [Google Scholar] [CrossRef]
- Bohmer, S.A.; Weibrecht, I.; Soderberg, O.; Bohmer, F.D. Association of the protein-tyrosine phosphatase DEP-1 with its substrate FLT3 visualized by in situ proximity ligation assay. PLoS ONE 2013, 8, e62871. [Google Scholar] [CrossRef]
- Noordman, Y.E.; Augustus, E.D.; Schepens, J.T.; Chirivi, R.G.; Rios, P.; Pulido, R.; Hendriks, W.J. Multimerisation of receptor-type protein tyrosine phosphatases PTPBR7 and PTP-SL attenuates enzymatic activity. Biochim. Biophys. Acta 2008, 1783, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Hower, A.E.; Beltran, P.J.; Bixby, J.L. Dimerization of tyrosine phosphatase PTPRO decreases its activity and ability to inactivate TrkC. J. Neurochem. 2009, 110, 1635–1647. [Google Scholar] [CrossRef]
- Walchli, S.; Espanel, X.; Hooft van Huijsduijnen, R. Sap-1/PTPRH activity is regulated by reversible dimerization. Biochem. Biophys. Res. Commun. 2005, 331, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Bloch, E.; Sikorski, E.L.; Pontoriero, D.; Day, E.K.; Berger, B.W.; Lazzara, M.J.; Thevenin, D. Disrupting the transmembrane domain-mediated oligomerization of protein tyrosine phosphatase receptor J inhibits EGFR-driven cancer cell phenotypes. J. Biol. Chem. 2019, 294, 18796–18806. [Google Scholar] [CrossRef]
- Arora, D.; Kothe, S.; van den Eijnden, M.; Hooft van Huijsduijnen, R.; Heidel, F.; Fischer, T.; Scholl, S.; Tolle, B.; Bohmer, S.A.; Lennartsson, J.; et al. Expression of protein-tyrosine phosphatases in Acute Myeloid Leukemia cells: FLT3 ITD sustains high levels of DUSP6 expression. Cell Commun. Signal. 2012, 10, 19. [Google Scholar] [CrossRef]
- Sternberg, M.J.; Gullick, W.J. A sequence motif in the transmembrane region of growth factor receptors with tyrosine kinase activity mediates dimerization. Protein. Eng. 1990, 3, 245–248. [Google Scholar] [CrossRef]
- Kim, S.; Jeon, T.J.; Oberai, A.; Yang, D.; Schmidt, J.J.; Bowie, J.U. Transmembrane glycine zippers: Physiological and pathological roles in membrane proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 14278–14283. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Takahashi, K.; Mernaugh, R.L.; Tsuboi, N.; Liu, H.; Daniel, T.O. A monoclonal antibody against CD148, a receptor-like tyrosine phosphatase, inhibits endothelial-cell growth and angiogenesis. Blood 2006, 108, 1234–1242. [Google Scholar] [CrossRef][Green Version]
- Groen, A.; Overvoorde, J.; van der Wijk, T.; den Hertog, J. Redox regulation of dimerization of the receptor protein-tyrosine phosphatases RPTPalpha, LAR, RPTPmu and CD45. FEBS J. 2008, 275, 2597–2604. [Google Scholar] [CrossRef]
- Tabernero, L.; Aricescu, A.R.; Jones, E.Y.; Szedlacsek, S.E. Protein tyrosine phosphatases: Structure-function relationships. FEBS J. 2008, 275, 867–882. [Google Scholar] [CrossRef]
- Blanchetot, C.; den Hertog, J. Multiple interactions between receptor protein-tyrosine phosphatase (RPTP) alpha and membrane-distal protein-tyrosine phosphatase domains of various RPTPs. J. Biol. Chem. 2000, 275, 12446–12452. [Google Scholar] [CrossRef]
- van der Wijk, T.; Overvoorde, J.; den Hertog, J. H2O2-induced intermolecular disulfide bond formation between receptor protein-tyrosine phosphatases. J. Biol. Chem. 2004, 279, 44355–44361. [Google Scholar] [CrossRef]
- Chin, C.N.; Sachs, J.N.; Engelman, D.M. Transmembrane homodimerization of receptor-like protein tyrosine phosphatases. FEBS Lett. 2005, 579, 3855–3858. [Google Scholar] [CrossRef]
- Rizzo, S.; Sikorski, E.; Park, S.; Im, W.; Vasquez-Montes, V.; Ladokhin, A.S.; Thevenin, D. Promoting the activity of a receptor tyrosine phosphatase with a novel pH-responsive transmembrane agonist inhibits cancer-associated phenotypes. Protein. Sci. 2023, 32, e4742. [Google Scholar] [CrossRef] [PubMed]
- Paduano, F.; Ortuso, F.; Campiglia, P.; Raso, C.; Iaccino, E.; Gaspari, M.; Gaudio, E.; Mangone, G.; Carotenuto, A.; Bilotta, A.; et al. Isolation and functional characterization of peptide agonists of PTPRJ, a tyrosine phosphatase receptor endowed with tumor suppressor activity. ACS Chem. Biol. 2012, 7, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Weiss, A. Negative regulation of CD45 by differential homodimerization of the alternatively spliced isoforms. Nat. Immunol. 2002, 3, 764–771. [Google Scholar] [CrossRef]
- Rhee, S.G.; Chang, T.S.; Bae, Y.S.; Lee, S.R.; Kang, S.W. Cellular regulation by hydrogen peroxide. J. Am. Soc. Nephrol. 2003, 14 (Suppl. S3), S211–S215. [Google Scholar] [CrossRef]
- Dagnell, M.; Pace, P.E.; Cheng, Q.; Frijhoff, J.; Ostman, A.; Arner, E.S.J.; Hampton, M.B.; Winterbourn, C.C. Thioredoxin reductase 1 and NADPH directly protect protein tyrosine phosphatase 1B from inactivation during H(2)O(2) exposure. J. Biol. Chem. 2017, 292, 14371–14380. [Google Scholar] [CrossRef]
- Barrett, W.C.; DeGnore, J.P.; Keng, Y.F.; Zhang, Z.Y.; Yim, M.B.; Chock, P.B. Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase 1B. J. Biol. Chem. 1999, 274, 34543–34546. [Google Scholar] [CrossRef]
- Tonks, N.K. Redox redux: Revisiting PTPs and the control of cell signaling. Cell 2005, 121, 667–670. [Google Scholar] [CrossRef]
- Ostman, A.; Frijhoff, J.; Sandin, A.; Bohmer, F.D. Regulation of protein tyrosine phosphatases by reversible oxidation. J. Biochem. 2011, 150, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Sallmyr, A.; Fan, J.; Datta, K.; Kim, K.T.; Grosu, D.; Shapiro, P.; Small, D.; Rassool, F. Internal tandem duplication of FLT3 (FLT3/ITD) induces increased ROS production, DNA damage, and misrepair: Implications for poor prognosis in AML. Blood 2008, 111, 3173–3182. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, R.; Arora, D.; Bauer, R.; Stopp, S.; Muller, J.P.; Heinrich, T.; Bohmer, S.A.; Dagnell, M.; Schnetzke, U.; Scholl, S.; et al. Cell transformation by FLT3 ITD in acute myeloid leukemia involves oxidative inactivation of the tumor suppressor protein-tyrosine phosphatase DEP-1/ PTPRJ. Blood 2012, 119, 4499–4511. [Google Scholar] [CrossRef] [PubMed]
- den Hertog, J.; Ostman, A.; Bohmer, F.D. Protein tyrosine phosphatases: Regulatory mechanisms. FEBS J. 2008, 275, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yi, M.; Zhu, S.; Wang, H.; Wu, K. Recent advances and challenges of bispecific antibodies in solid tumors. Exp. Hematol. Oncol. 2021, 10, 56. [Google Scholar] [CrossRef]
- Wei, J.; Yang, Y.; Wang, G.; Liu, M. Current landscape and future directions of bispecific antibodies in cancer immunotherapy. Front. Immunol. 2022, 13, 1035276. [Google Scholar] [CrossRef]
- Weidanz, J. Targeting cancer with bispecific antibodies. Science 2021, 371, 996–997. [Google Scholar] [CrossRef]
- Wachholz, V.; Mustafa, A.M.; Zeyn, Y.; Henninger, S.J.; Beyer, M.; Dzulko, M.; Piee-Staffa, A.; Brachetti, C.; Haehnel, P.S.; Sellmer, A.; et al. Inhibitors of class I HDACs and of FLT3 combine synergistically against leukemia cells with mutant FLT3. Arch. Toxicol. 2022, 96, 177–193. [Google Scholar] [CrossRef]
- Buchwald, M.; Pietschmann, K.; Brand, P.; Gunther, A.; Mahajan, N.P.; Heinzel, T.; Kramer, O.H. SIAH ubiquitin ligases target the nonreceptor tyrosine kinase ACK1 for ubiquitinylation and proteasomal degradation. Oncogene 2013, 32, 4913–4920. [Google Scholar] [CrossRef]
- Li, Y.; Seto, E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 2016, 6, a026831. [Google Scholar] [CrossRef]
- Nishioka, C.; Ikezoe, T.; Yang, J.; Takeuchi, S.; Koeffler, H.P.; Yokoyama, A. MS-275, a novel histone deacetylase inhibitor with selectivity against HDAC1, induces degradation of FLT3 via inhibition of chaperone function of heat shock protein 90 in AML cells. Leuk Res. 2008, 32, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Bali, P.; Pranpat, M.; Bradner, J.; Balasis, M.; Fiskus, W.; Guo, F.; Rocha, K.; Kumaraswamy, S.; Boyapalle, S.; Atadja, P.; et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: A novel basis for antileukemia activity of histone deacetylase inhibitors. J. Biol. Chem. 2005, 280, 26729–26734. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.K.; Qi, J.; Cai, Q.; Carnahan, E.; Ayala Ramirez, M.; Li, L.; Marcucci, G.; Kuo, Y.H. HDAC8 regulates long-term hematopoietic stem-cell maintenance under stress by modulating p53 activity. Blood 2017, 130, 2619–2630. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Singh, S.; Hua, W.K.; Cai, Q.; Chao, S.W.; Li, L.; Liu, H.; Ho, Y.; McDonald, T.; Lin, A.; et al. HDAC8 Inhibition Specifically Targets Inv(16) Acute Myeloid Leukemic Stem Cells by Restoring p53 Acetylation. Cell Stem. Cell 2015, 17, 597–610. [Google Scholar] [CrossRef]
- Yao, M.; Yan, W.; Wang, Y.; Zhao, Y.; Xu, X.; Chen, Y.; Yu, C.; Li, Y.; Jiang, H.; Shen, J.; et al. IHCH9033, a novel class I HDAC inhibitor, synergizes with FLT3 inhibitor and rescues quizartinib resistance in FLT3 ITD AML via enhancing DNA damage response. Exp. Hematol. Oncol. 2025, 14, 15. [Google Scholar] [CrossRef]
- George, P.; Bali, P.; Cohen, P.; Tao, J.; Guo, F.; Sigua, C.; Vishvanath, A.; Fiskus, W.; Scuto, A.; Annavarapu, S.; et al. Cotreatment with 17-allylamino-demethoxygeldanamycin and FLT-3 kinase inhibitor PKC412 is highly effective against human acute myelogenous leukemia cells with mutant FLT-3. Cancer Res. 2004, 64, 3645–3652. [Google Scholar] [CrossRef]
- Pietschmann, K.; Bolck, H.A.; Buchwald, M.; Spielberg, S.; Polzer, H.; Spiekermann, K.; Bug, G.; Heinzel, T.; Bohmer, F.D.; Kramer, O.H. Breakdown of the FLT3 ITD/STAT5 axis and synergistic apoptosis induction by the histone deacetylase inhibitor panobinostat and FLT3-specific inhibitors. Mol. Cancer Ther. 2012, 11, 2373–2383. [Google Scholar] [CrossRef]
- Strzalka, P.; Krawiec, K.; Wisnik, A.; Jarych, D.; Czemerska, M.; Zawlik, I.; Pluta, A.; Wierzbowska, A. The Role of the Sirtuin Family Histone Deacetylases in Acute Myeloid Leukemia-A Promising Road Ahead. Cancers 2025, 17, 1009. [Google Scholar] [CrossRef]
- Ianni, A.; Kumari, P.; Tarighi, S.; Braun, T.; Vaquero, A. SIRT7: A novel molecular target for personalized cancer treatment? Oncogene 2024, 43, 993–1006. [Google Scholar] [CrossRef]
- Vaziri, H.; Dessain, S.K.; Ng Eaton, E.; Imai, S.I.; Frye, R.A.; Pandita, T.K.; Guarente, L.; Weinberg, R.A. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001, 107, 149–159. [Google Scholar] [CrossRef]
- Sasca, D.; Hahnel, P.S.; Szybinski, J.; Khawaja, K.; Kriege, O.; Pante, S.V.; Bullinger, L.; Strand, S.; Strand, D.; Theobald, M.; et al. SIRT1 prevents genotoxic stress-induced p53 activation in acute myeloid leukemia. Blood 2014, 124, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, B.; Yu, D.; Zuo, Y.; Cai, R.; Yang, J.; Cheng, J. SIRT3 deacetylase activity confers chemoresistance in AML via regulation of mitochondrial oxidative phosphorylation. Br. J. Haematol. 2019, 187, 49–64. [Google Scholar] [CrossRef]
- Zhao, K.; Zhou, Y.; Qiao, C.; Ni, T.; Li, Z.; Wang, X.; Guo, Q.; Lu, N.; Wei, L. Oroxylin A promotes PTEN-mediated negative regulation of MDM2 transcription via SIRT3-mediated deacetylation to stabilize p53 and inhibit glycolysis in wt-p53 cancer cells. J. Hematol. Oncol. 2015, 8, 41. [Google Scholar] [CrossRef]
- Barber, M.F.; Michishita-Kioi, E.; Xi, Y.; Tasselli, L.; Kioi, M.; Moqtaderi, Z.; Tennen, R.I.; Paredes, S.; Young, N.L.; Chen, K.; et al. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature 2012, 487, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bhatia, R. The controversial role of Sirtuins in tumorigenesis-SIRT7 joins the debate. Cell Res. 2013, 23, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.; Voit, R.; Liszt, G.; Magin, C.; Grummt, I.; Guarente, L. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 2006, 20, 1075–1080. [Google Scholar] [CrossRef]
- Raza, U.; Tang, X.; Liu, Z.; Liu, B. SIRT7: The seventh key to unlocking the mystery of aging. Physiol. Rev. 2024, 104, 253–280. [Google Scholar] [CrossRef]
- Metzeler, K.H.; Hummel, M.; Bloomfield, C.D.; Spiekermann, K.; Braess, J.; Sauerland, M.C.; Heinecke, A.; Radmacher, M.; Marcucci, G.; Whitman, S.P.; et al. An 86-probe-set gene-expression signature predicts survival in cytogenetically normal acute myeloid leukemia. Blood 2008, 112, 4193–4201. [Google Scholar] [CrossRef]
- Kaiser, A.; Schmidt, M.; Huber, O.; Frietsch, J.J.; Scholl, S.; Heidel, F.H.; Hochhaus, A.; Muller, J.P.; Ernst, T. SIRT7: An influence factor in healthy aging and the development of age-dependent myeloid stem-cell disorders. Leukemia 2020, 36, 2206–2216. [Google Scholar] [CrossRef]
- Nowicki, M.; Wierzbowska, A.; Stec-Martyna, E.; Kulczycka-Wojdala, D.; Nowicki, G.; Szmigielska-Kaplon, A. SIRT1-SIRT7 Expression in Patients with Lymphoproliferative Disorders Undergoing Hematopoietic Stem Cell Mobilization. Cancers 2022, 14, 1213. [Google Scholar] [CrossRef]
- Jenke, R.; Ressing, N.; Hansen, F.K.; Aigner, A.; Buch, T. Anticancer Therapy with HDAC Inhibitors: Mechanism-Based Combination Strategies and Future Perspectives. Cancers 2021, 13, 634. [Google Scholar] [CrossRef]
- Hao, B.B.; Ma, K.; Xu, J.Y.; Fan, R.F.; Zhao, W.S.; Jia, X.L.; Zhai, L.H.; Lee, S.; Xie, D.; Tan, M.J. Proteomics analysis of histone deacetylase inhibitor-resistant solid tumors reveals resistant signatures and potential drug combinations. Acta Pharmacol. Sin. 2024, 45, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Contieri, B.; Duarte, B.K.L.; Lazarini, M. Updates on DNA methylation modifiers in acute myeloid leukemia. Ann. Hematol. 2020, 99, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Weeks, O.; Yang, F.C.; Xu, M. The TET2 interactors and their links to hematological malignancies. IUBMB Life 2015, 67, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Chang, Y.; Ruan, G.; Zhou, S.; Jiang, H.; Jiang, Q.; Huang, X.; Zhao, X.S. TET2 mutations contribute to adverse prognosis in acute myeloid leukemia (AML): Results from a comprehensive analysis of 502 AML cases and the Beat AML public database. Clin. Exp. Med. 2024, 24, 35. [Google Scholar] [CrossRef]
- Li, Z.; Cai, X.; Cai, C.L.; Wang, J.; Zhang, W.; Petersen, B.E.; Yang, F.C.; Xu, M. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood 2011, 118, 4509–4518. [Google Scholar] [CrossRef]
- Abbas, S.; Lugthart, S.; Kavelaars, F.G.; Schelen, A.; Koenders, J.E.; Zeilemaker, A.; van Putten, W.J.; Rijneveld, A.W.; Lowenberg, B.; Valk, P.J. Acquired mutations in the genes encoding IDH1 and IDH2 both are recurrent aberrations in acute myeloid leukemia: Prevalence and prognostic value. Blood 2010, 116, 2122–2126. [Google Scholar] [CrossRef]
- Paschka, P.; Schlenk, R.F.; Gaidzik, V.I.; Habdank, M.; Kronke, J.; Bullinger, L.; Spath, D.; Kayser, S.; Zucknick, M.; Gotze, K.; et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J. Clin. Oncol. 2010, 28, 3636–3643. [Google Scholar] [CrossRef]
- Bill, M.; Jentzsch, M.; Bischof, L.; Kohlschmidt, J.; Grimm, J.; Schmalbrock, L.K.; Backhaus, D.; Brauer, D.; Goldmann, K.; Franke, G.N.; et al. Impact of IDH1 and IDH2 mutation detection at diagnosis and in remission in patients with AML receiving allogeneic transplantation. Blood Adv. 2023, 7, 436–444. [Google Scholar] [CrossRef]
- Calzaferri, F.; Daher, H.; Gilbert, J.; Yang, Y.; Tauziet, M.; Jallet, C.; Bessin, Y.; van der Lee, A.; Arimondo, P.B.; Krimm, I.; et al. Rational Design and Synthesis of Highly Stable Haloflavanone DNA Methyltransferase Inhibitors Inducing Tumor Suppressor Gene Re-expression in Cancer Cells. J. Med. Chem. 2025, 68, 10704–10721. [Google Scholar] [CrossRef]
- San Jose-Eneriz, E.; Agirre, X.; Rabal, O.; Vilas-Zornoza, A.; Sanchez-Arias, J.A.; Miranda, E.; Ugarte, A.; Roa, S.; Paiva, B.; Estella-Hermoso de Mendoza, A.; et al. Discovery of first-in-class reversible dual small molecule inhibitors against G9a and DNMTs in hematological malignancies. Nat. Commun. 2017, 8, 15424. [Google Scholar] [CrossRef] [PubMed]
- Chua, G.N.L.; Wassarman, K.L.; Sun, H.; Alp, J.A.; Jarczyk, E.I.; Kuzio, N.J.; Bennett, M.J.; Malachowsky, B.G.; Kruse, M.; Kennedy, A.J. Cytosine-Based TET Enzyme Inhibitors. ACS Med. Chem. Lett. 2019, 10, 180–185. [Google Scholar] [CrossRef]
- Guan, Y.; Tiwari, A.D.; Phillips, J.G.; Hasipek, M.; Grabowski, D.R.; Pagliuca, S.; Gopal, P.; Kerr, C.M.; Adema, V.; Radivoyevitch, T.; et al. A Therapeutic Strategy for Preferential Targeting of TET2 Mutant and TET-dioxygenase Deficient Cells in Myeloid Neoplasms. Blood Cancer Discov. 2021, 2, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Schlenk, R.F.; Russell, N.H.; Levis, M.J. Targeting FLT3 mutations in AML: Review of current knowledge and evidence. Leukemia 2019, 33, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.E. The role of targeted therapy in the management of patients with AML. Hematol. Am. Soc. Hematol. Educ. Program 2017, 2017, 54–65. [Google Scholar] [CrossRef]


| Target | Mode of Action | Agent | References |
|---|---|---|---|
| Kinase activity | Inhibition | FLT3-specific TKIs (midostaurin, gilteritinib, quizartinib, crenolanib) | reviewed in [12] |
| Multi-kinase-targeting TKIs (cabozantinib, dasatinib, mivavotinib) | [13] | ||
| ROS quenchers (diphenyleneiodonium (DPI), N-acetylcysteine) | [14] | ||
| Glycosylation/surface expression | Inhibition | Statins (fluvastatin) | [15] |
| 2-DG | [16] | ||
| Tunicamycin | [17] | ||
| Promotion | TKIs (midostaurin, sorafenib, quizartinib) | [18,19] | |
| Valproic acid | [20] | ||
| Degradation | Promotion | Inhibitors of deubiquitination enzymes (thiolutin, HBX19818, P22077, WP1130, EOAI3402143) | [21,22] |
| Stimulators of E2-conjugating enzymes/E3 ligases (decursin) | [23] | ||
| Inhibitor of HSP90 (17-AAG) | [24] | ||
| PROTACs (quizartinib-based, dovitinib-based, pomalidomide, A2, LWY713, FLT3 PROTAC molecule 35, A20, gilteritinib-based B3-2) | [25] | ||
| Dimerisation (of TRK, EGFR, PDGFR) * | Inhibition | Gangliosides (GM1, modification of endogenously expressed gangliosides GM2, GD1a, GD1b, GD3, GT1b) | [26] |
| Target | Mode of Action | Agents | References |
|---|---|---|---|
| Tumour-suppressive PTPs * | Promoting activation | Natural ligands (TSP1), peptide agonists (PTPRJ-pep5, PTPRJ-pep19, PTPRJ-pep23, PTPRJ-pep24) | [27] |
| Inhibition of deactivation | ROS quencher (schisandrin B) | [28,29] | |
| Promoting interaction | Bispecific antibody-aptamer chimeras (PTPRJ-MET) | [30] | |
| Pro-oncogenic PTPs | Promoting degradation | PROTACs (SHP2-D26) | [31] |
| Inhibition of activity | Allosteric inhibitors (SHP099) | [32] | |
| Orthosteric inhibitors (PHPS1) | [33] | ||
| Acetylation/methylation | Inhibition of histone deacetylases (HDACs) activity | HDAC inhibitors (multiple inhibitors, CUDC-907) | [34,35] |
| Inhibition of methyltransferases | Nucleoside DNMT inhibitors (azacytidine and decitabine) | [36] |
| No. | Target | Mode of Action | Agent | Reference |
|---|---|---|---|---|
| 1. | FLT3 kinase activity | Inhibition | TKI | [46] |
| BCL-2 | Inhibition | Venetoclax | ||
| 2. | FLT3 kinase activity | Inhibition | TKI | [17] |
| FLT3 glycosylation | Inhibition | Fluvastatin, tunicamycin | ||
| 3. | FLT3 kinase activity | Inhibition | TKI | [58] |
| HDAC activity | Inhibition | HDAC8 inhibitor 22d, HDACi HCH9033, 17-AAG, panobinostat | ||
| 4. | FLT3 kinase activity | Inhibition | TKI | [59] |
| Methyltransferase activity | Inhibition | Azacitidine | ||
| 5. | FLT3 degradation | Promotion | Decursin | [23] |
| BCL-2 | Inhibition | Venetoclax | ||
| 6. | FLT3 degradation | Promotion | PROTAC | [60] |
| BCL-2 | Inhibition | Venetoclax |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrevski, B.; Willems, H.; Lossius-Cott, C.; Müller, J.P. Targeting Oncogenic Activity and Signalling of Mutant Receptor Tyrosine Kinase FLT3. Cancers 2025, 17, 2931. https://doi.org/10.3390/cancers17172931
Dobrevski B, Willems H, Lossius-Cott C, Müller JP. Targeting Oncogenic Activity and Signalling of Mutant Receptor Tyrosine Kinase FLT3. Cancers. 2025; 17(17):2931. https://doi.org/10.3390/cancers17172931
Chicago/Turabian StyleDobrevski, Boban, Hannah Willems, Carolin Lossius-Cott, and Jörg P. Müller. 2025. "Targeting Oncogenic Activity and Signalling of Mutant Receptor Tyrosine Kinase FLT3" Cancers 17, no. 17: 2931. https://doi.org/10.3390/cancers17172931
APA StyleDobrevski, B., Willems, H., Lossius-Cott, C., & Müller, J. P. (2025). Targeting Oncogenic Activity and Signalling of Mutant Receptor Tyrosine Kinase FLT3. Cancers, 17(17), 2931. https://doi.org/10.3390/cancers17172931






