The RNA-Binding Protein RBMX Mediates the Immunosuppressive Microenvironment of Osteosarcoma by Regulating CD8+T Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Single-Cell Transcriptome Sequencing and Bulk Sequencing Analysis of Human Osteosarcoma
2.2. Single-Cell Transcriptome Analysis of Mouse Samples
2.3. Transcriptome Data Analysis of Mouse Osteosarcoma Cells
2.4. Multiplex Immunofluorescence Staining of Human Osteosarcoma Tissues and Subcutaneous Tumor Tissues of Mouse Osteosarcoma
2.5. Cell Culture
2.6. RBMX Knockout Cells
2.7. Western Blot
2.8. Real-Time Quantitative PCR
2.9. 5-Ethynyl-2′-Deoxyuridine (EdU) Cell Proliferation Assay
2.10. Mouse Subcutaneous Tumor Model
2.11. Cell Coculture
2.12. Tumor Cell Dissociation and Staining
2.13. Statistical Analysis
3. Results
3.1. RBMX Could Serve as an Indicator of Poor Prognosis in Osteosarcoma Patients
3.2. Downregulation of RBMX Inhibits the Proliferation of Osteosarcoma Cells
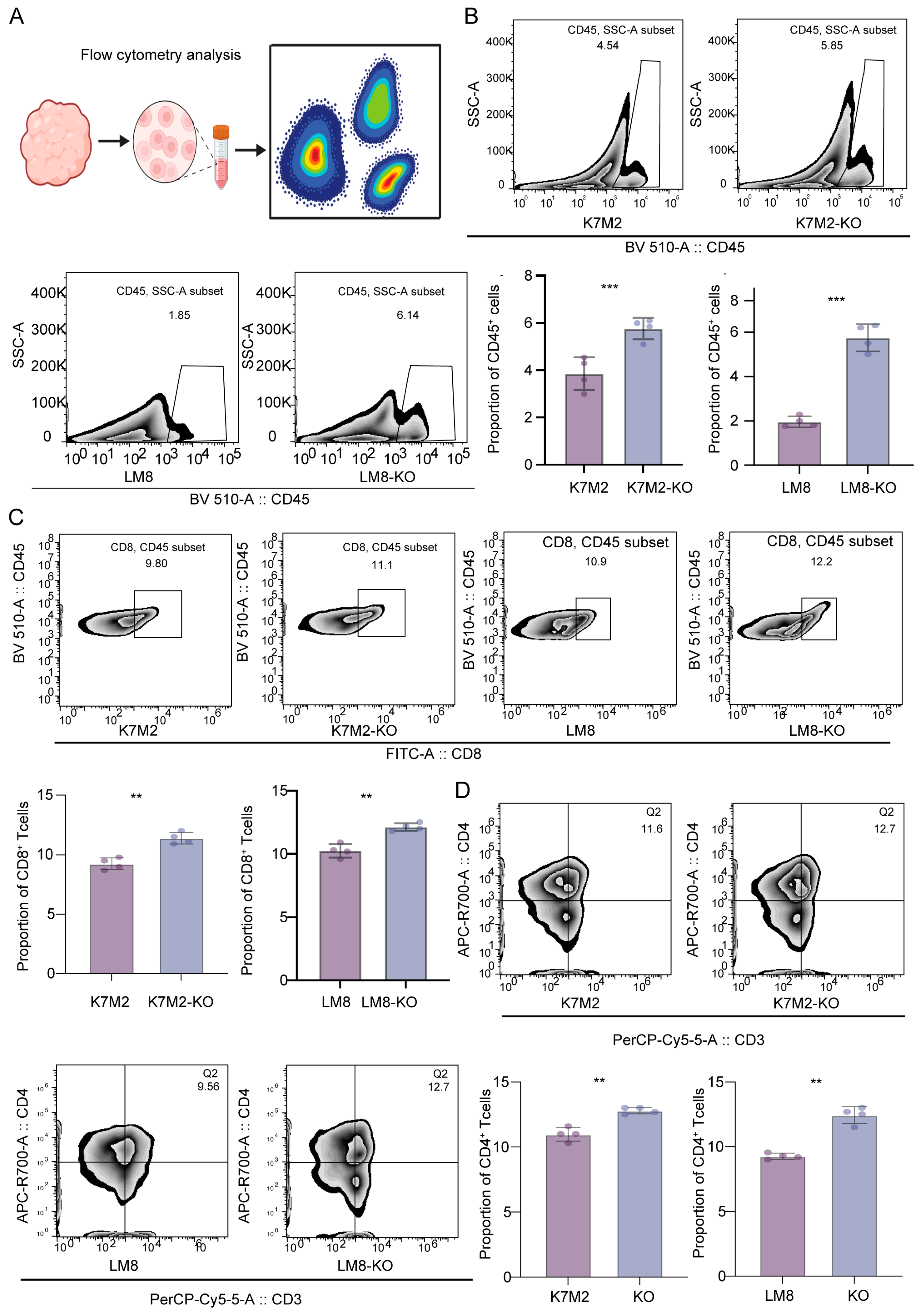
3.3. Knockout of RBMX Significantly Activates the Immune Microenvironment of Osteosarcoma
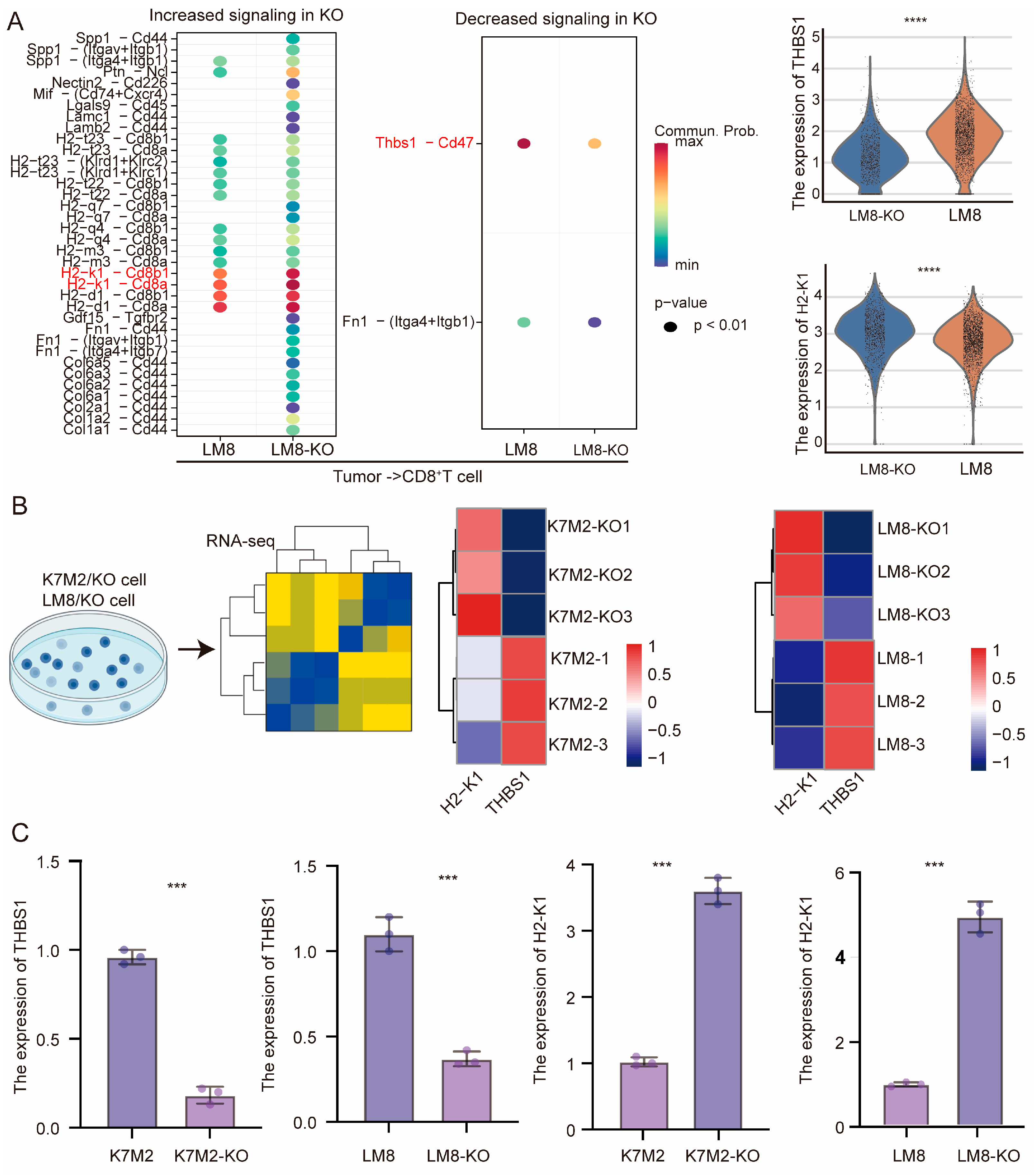
3.4. CD8+T Cells Might Mediate the Suppressive Capacity of RBMX Knockout in the Osteosarcoma Microenvironment
3.5. RBMX Knockout Promoted CD8+T Cell Infiltration in the Osteosarcoma Microenvironment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Behjati, S.; Gilbertson, R.J.; Pfister, S.M. Maturation Block in Childhood Cancer. Cancer Discov. 2021, 11, 542–544. [Google Scholar] [CrossRef]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int. J. Cancer 2009, 125, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Bull, E.C.; Singh, A.; Harden, A.M.; Soanes, K.; Habash, H.; Toracchio, L.; Carrabotta, M.; Schreck, C.; Shah, K.M.; Riestra, P.V.; et al. Targeting metastasis in paediatric bone sarcomas. Mol. Cancer 2025, 24, 153. [Google Scholar] [CrossRef]
- Filbin, M.; Monje, M. Developmental origins and emerging therapeutic opportunities for childhood cancer. Nat. Med. 2019, 25, 367–376. [Google Scholar] [CrossRef]
- Yu, S.; Yao, X. Advances on immunotherapy for osteosarcoma. Mol. Cancer 2024, 23, 192. [Google Scholar] [CrossRef]
- Tian, H.; Cao, J.; Li, B.; Nice, E.C.; Mao, H.; Zhang, Y.; Huang, C. Managing the immune microenvironment of osteosarcoma: The outlook for osteosarcoma treatment. Bone Res. 2023, 11, 11. [Google Scholar] [CrossRef]
- Fu, J.; Li, T.; Yang, Y.; Jiang, L.; Wang, W.; Fu, L.; Zhu, Y.; Hao, Y. Activatable nanomedicine for overcoming hypoxia-induced resistance to chemotherapy and inhibiting tumor growth by inducing collaborative apoptosis and ferroptosis in solid tumors. Biomaterials 2021, 268, 120537. [Google Scholar] [CrossRef]
- Zhong, L.; Liao, D.; Li, J.; Liu, W.; Wang, J.; Zeng, C.; Wang, X.; Cao, Z.; Zhang, R.; Li, M.; et al. Rab22a-NeoF1 fusion protein promotes osteosarcoma lung metastasis through its secretion into exosomes. Signal Transduct. Target. Ther. 2021, 6, 59. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, C.; Chen, H.; Song, Y.; Xu, H.; Song, G.; Wang, X.; Luo, T.; Chen, W.; Ma, J.; et al. Osteosarcoma Cells Secrete CXCL14 That Activates Integrin α11β1 on Fibroblasts to Form a Lung Metastatic Niche. Cancer Res. 2024, 84, 994–1012. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, E.; Meazza, C.; Tamburini, A.; Márquez-Vega, C.; Bisogno, G.; Fagioli, F.; Ferraresi, V.; Milano, G.M.; Coccoli, L.; Rubio-San-Simón, A.; et al. Is There a Role for Mifamurtide in Nonmetastatic High-Grade Osteosarcoma? Results From the Italian Sarcoma Group (ISG/OS-2) and Spanish Sarcoma Group (GEIS-33) Trials. J. Clin. Oncol. 2025, Jco2500210. [Google Scholar] [CrossRef] [PubMed]
- Pahl, J.H.; Kwappenberg, K.M.; Varypataki, E.M.; Santos, S.J.; Kuijjer, M.L.; Mohamed, S.; Wijnen, J.T.; van Tol, M.J.; Cleton-Jansen, A.M.; Egeler, R.M.; et al. Macrophages inhibit human osteosarcoma cell growth after activation with the bacterial cell wall derivative liposomal muramyl tripeptide in combination with interferon-γ. J. Exp. Clin. Cancer Res. 2014, 33, 27. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Reiss, K.A.; Angelos, M.G.; Dees, E.C.; Yuan, Y.; Ueno, N.T.; Pohlmann, P.R.; Johnson, M.L.; Chao, J.; Shestova, O.; Serody, J.S.; et al. CAR-macrophage therapy for HER2-overexpressing advanced solid tumors: A phase 1 trial. Nat. Med. 2025, 31, 1171–1182. [Google Scholar] [CrossRef]
- Shi, J.X.; Zhang, Z.C.; Yin, H.Z.; Piao, X.J.; Liu, C.H.; Liu, Q.J.; Zhang, J.C.; Zhou, W.X.; Liu, F.C.; Yang, F.; et al. RNA m6A modification in ferroptosis: Implications for advancing tumor immunotherapy. Mol. Cancer 2024, 23, 213. [Google Scholar] [CrossRef]
- Xiao, S.; Duan, S.; Caligiuri, M.A.; Ma, S.; Yu, J. YTHDF2: A key RNA reader and antitumor target. Trends Immunol. 2025, 46, 485–498. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, Y.; Shen, H.; Xie, W. m(6)A-binding proteins: The emerging crucial performers in epigenetics. J. Hematol. Oncol. 2020, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Sun, H.; Zhang, Z.; Zheng, Y.; Zheng, S.; Bin, J.; Liao, Y.; Shi, M.; Zhou, R.; Liao, W. High baseline tumor burden-associated macrophages promote an immunosuppressive microenvironment and reduce the efficacy of immune checkpoint inhibitors through the IGFBP2-STAT3-PD-L1 pathway. Cancer Commun. 2023, 43, 562–581. [Google Scholar] [CrossRef]
- Bao, Y.; Zhai, J.; Chen, H.; Wong, C.C.; Liang, C.; Ding, Y.; Huang, D.; Gou, H.; Chen, D.; Pan, Y.; et al. Targeting m(6)A reader YTHDF1 augments antitumour immunity and boosts anti-PD-1 efficacy in colorectal cancer. Gut 2023, 72, 1497–1509. [Google Scholar] [CrossRef]
- Ma, F.; Liu, X.; Zhou, S.; Li, W.; Liu, C.; Chadwick, M.; Qian, C. Long non-coding RNA FGF13-AS1 inhibits glycolysis and stemness properties of breast cancer cells through FGF13-AS1/IGF2BPs/Myc feedback loop. Cancer Lett. 2019, 450, 63–75. [Google Scholar] [CrossRef]
- Elliott, D.J.; Dalgliesh, C.; Hysenaj, G.; Ehrmann, I. RBMX family proteins connect the fields of nuclear RNA processing, disease and sex chromosome biology. Int. J. Biochem. Cell Biol. 2019, 108, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Guo, T.; Wu, T.; Zhang, M.; Luo, D.; Dou, K.; Yang, Y.; Jin, C.; Zhang, B.; et al. SOCS5-RBMX stimulates SREBP1-mediated lipogenesis to promote metastasis in steatotic HCC with HBV-related cirrhosis. NPJ Precis. Oncol. 2024, 8, 58. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, K.; Zhi, Y.; Wu, Y.; Chen, B.; Bai, J.; Wang, X. Tumor-derived exosomal miR-19b-3p facilitates M2 macrophage polarization and exosomal LINC00273 secretion to promote lung adenocarcinoma metastasis via Hippo pathway. Clin. Transl. Med. 2021, 11, e478. [Google Scholar] [CrossRef]
- Li, A.; Hong, J.; Ma, X.; Huang, Y.; Jiang, Q.; Zhang, C.; Wang, Y.; Huang, Y. Cancer-Derived Exosomal LINC01615 Induces M2 Polarization of Tumor-Associated Macrophages via RBMX-EZH2 Axis to Promote Colorectal Cancer Progression. Int. J. Nanomed. 2025, 20, 7343–7358. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, X.; Zhang, X.; Zhao, Z.; Wu, W.; Yu, S. A single-cell and spatially resolved atlas of human osteosarcomas. J. Hematol. Oncol. 2024, 17, 71. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Yang, Q.; Lv, X.; Huang, W.; Zhou, Z.; Wang, Y.; Zhang, Z.; Yuan, T.; Ding, X.; et al. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat. Commun. 2020, 11, 6322. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Skrzypczynska, K.M.; Fang, Q.; Zhang, W.; O’Brien, S.A.; He, Y.; Wang, L.; Zhang, Q.; Kim, A.; et al. Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell 2020, 181, 442–459 e429. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Plikus, M.V.; Nie, Q. CellChat for systematic analysis of cell-cell communication from single-cell transcriptomics. Nat. Protoc. 2025, 20, 180–219. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Yin, R.X.; Pan, S.L.; Yang, S.; Yang, D.Z.; Lin, W.X. Circulating miR-3659 may be a potential biomarker of dyslipidemia in patients with obesity. J. Transl. Med. 2019, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Jing, X.; Chen, Z.; Pan, X.; Xu, D.; Yu, X.; Zhong, F.; Zhao, L.; Yang, C.; Wang, B.; et al. Histone deacetylase-mediated tumor microenvironment characteristics and synergistic immunotherapy in gastric cancer. Theranostics 2023, 13, 4574–4600. [Google Scholar] [CrossRef]
- Ji, X.; Qian, X.; Luo, G.; Yang, W.; Huang, W.; Lei, Z.; Zhou, J.; Zhong, G.; Zhou, J.; Liu, N.; et al. Engineered macrophage nanoparticles enhance microwave ablation efficacy in osteosarcoma via targeting the CD47-SIRPα Axis: A novel Biomimetic immunotherapeutic approach. Bioact. Mater. 2025, 47, 248–265. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Bai, X.; Zhang, M.; Lv, W.; Ma, Y.; Sun, Y.; Zhang, H.; Jiang, Q.; Yao, Q.; et al. Osteosarcoma Cell-Derived Migrasomes Promote Macrophage M2 Polarization to Aggravate Osteosarcoma Proliferation and Metastasis. Adv. Sci. 2025, 12, e2409870. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Wang, Y.; Cheng, J.; Pan, B.; Zang, X.; Liu, R.; Deng, Y. Single-cell RNA-seq reveals T cell exhaustion and immune response landscape in osteosarcoma. Front. Immunol. 2024, 15, 1362970. [Google Scholar] [CrossRef]
- Lussier, D.M.; O’Neill, L.; Nieves, L.M.; McAfee, M.S.; Holechek, S.A.; Collins, A.W.; Dickman, P.; Jacobsen, J.; Hingorani, P.; Blattman, J.N. Enhanced T-cell immunity to osteosarcoma through antibody blockade of PD-1/PD-L1 interactions. J. Immunother. 2015, 38, 96–106. [Google Scholar] [CrossRef]
- Kumar, S.; Sinha, M. Targeting intracellular mRNA m(6)A-modifiers in advancing immunotherapeutics. J. Adv. Res. 2025; in press. [Google Scholar] [CrossRef]
- Ding, Y.P.; Liu, C.C.; Yu, K.D. RNA modifications in the tumor microenvironment: Insights into the cancer-immunity cycle and beyond. Exp. Hematol. Oncol. 2025, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, X.; Chen, D.; Li, Z.; Wu, X.; Wang, J.; Deng, Y. N6-Methyladenosine-Related LncRNAs Are Potential Remodeling Indicators in the Tumor Microenvironment and Prognostic Markers in Osteosarcoma. Front. Immunol. 2021, 12, 806189. [Google Scholar] [CrossRef]
- Yang, Y.; Ni, W.J.; Yang, Y.; Liao, J.; Yang, Y.; Li, J.; Zhu, X.; Guo, C.; Xie, F.; Leng, X.M. Research progress on N6-methyladenosine RNA modification in osteosarcoma: Functions, mechanisms, and potential clinical applications. Med. Oncol. 2025, 42, 55. [Google Scholar] [CrossRef]
- Matsunaga, S.; Takata, H.; Morimoto, A.; Hayashihara, K.; Higashi, T.; Akatsuchi, K.; Mizusawa, E.; Yamakawa, M.; Ashida, M.; Matsunaga, T.M.; et al. RBMX: A regulator for maintenance and centromeric protection of sister chromatid cohesion. Cell Rep. 2012, 1, 299–308. [Google Scholar] [CrossRef]
- Hao, C.; Zheng, Y.; Jönsson, J.; Cui, X.; Yu, H.; Wu, C.; Kajitani, N.; Schwartz, S. hnRNP G/RBMX enhances HPV16 E2 mRNA splicing through a novel splicing enhancer and inhibits production of spliced E7 oncogene mRNAs. Nucleic Acids Res. 2022, 50, 3867–3891. [Google Scholar] [CrossRef]
- Wu, Z.; Zuo, X.; Zhang, W.; Li, Y.; Gui, R.; Leng, J.; Shen, H.; Pan, B.; Fan, L.; Li, J.; et al. m6A-Modified circTET2 Interacting with HNRNPC Regulates Fatty Acid Oxidation to Promote the Proliferation of Chronic Lymphocytic Leukemia. Adv. Sci. 2023, 10, e2304895. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Zhou, H.; Li, X.; Peng, D.; Yang, Y.; Zeng, Y.; Liu, H.; Ren, J.; Zhao, Y. RBMX is required for activation of ATR on repetitive DNAs to maintain genome stability. Cell Death Differ. 2020, 27, 3162–3176. [Google Scholar] [CrossRef]
- Jaeger, A.M.; Stopfer, L.E.; Ahn, R.; Sanders, E.A.; Sandel, D.A.; Freed-Pastor, W.A.; Rideout, W.M., III; Naranjo, S.; Fessenden, T.; Nguyen, K.B.; et al. Deciphering the immunopeptidome in vivo reveals new tumour antigens. Nature 2022, 607, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Vitale, T.; Zhang, X.; Jackson, T.D.; Yu, D.; Jedrychowski, M.; Gygi, S.P.; Widlund, H.R.; Wucherpfennig, K.W.; Puigserver, P. Selective deficiency of mitochondrial respiratory complex I subunits Ndufs4/6 causes tumor immunogenicity. Nat. Cancer 2025, 6, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Rodriguez-Zabala, M.; Dushime, G.T.; Reinbach, K.; Ramakrishnan, R.; Sitnicka, E.; Järås, M.; Lymphoid Development and Regulation (Ewa Sitnicka). H2-K1 protects murine MLL-AF9 leukemia stem cells from natural killer cell-mediated immune surveillance. Haematologica 2025, 110, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Rogers, N.M.; Ghimire, K. Thrombospondin-1 CD47 Signalling: From Mechanisms to Medicine. Int. J. Mol. Sci. 2021, 22, 4062. [Google Scholar] [CrossRef]
- Nejo, T.; Krishna, S.; Yamamichi, A.; Lakshmanachetty, S.; Jimenez, C.; Lee, K.Y.; Baker, D.L.; Young, J.S.; Chen, T.; Phyu, S.S.S.; et al. Glioma-neuronal circuit remodeling induces regional immunosuppression. Nat. Commun. 2025, 16, 4770. [Google Scholar] [CrossRef]
- Omatsu, M.; Nakanishi, Y.; Iwane, K.; Aoyama, N.; Duran, A.; Muta, Y.; Martinez-Ordoñez, A.; Han, Q.; Agatsuma, N.; Mizukoshi, K.; et al. THBS1-producing tumor-infiltrating monocyte-like cells contribute to immunosuppression and metastasis in colorectal cancer. Nat. Commun. 2023, 14, 5534. [Google Scholar] [CrossRef]
- Chi, J.J.; Xie, P.; Cheng, M.H.; Zhu, Y.; Cui, X.; Watson, J.; Zeng, L.; Uddin, A.; Nguyen, H.; Li, L.; et al. MGAT1-Guided complex N-Glycans on CD73 regulate immune evasion in triple-negative breast cancer. Nat. Commun. 2025, 16, 3552. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Pu, C.; Wang, C.; Quan, Z. The RNA-Binding Protein RBMX Mediates the Immunosuppressive Microenvironment of Osteosarcoma by Regulating CD8+T Cells. Cancers 2025, 17, 2928. https://doi.org/10.3390/cancers17172928
Qiu Y, Pu C, Wang C, Quan Z. The RNA-Binding Protein RBMX Mediates the Immunosuppressive Microenvironment of Osteosarcoma by Regulating CD8+T Cells. Cancers. 2025; 17(17):2928. https://doi.org/10.3390/cancers17172928
Chicago/Turabian StyleQiu, Yu, Chao Pu, Chengguang Wang, and Zhengxue Quan. 2025. "The RNA-Binding Protein RBMX Mediates the Immunosuppressive Microenvironment of Osteosarcoma by Regulating CD8+T Cells" Cancers 17, no. 17: 2928. https://doi.org/10.3390/cancers17172928
APA StyleQiu, Y., Pu, C., Wang, C., & Quan, Z. (2025). The RNA-Binding Protein RBMX Mediates the Immunosuppressive Microenvironment of Osteosarcoma by Regulating CD8+T Cells. Cancers, 17(17), 2928. https://doi.org/10.3390/cancers17172928





