ESFT13: A Phase II Study Evaluating the Addition of Window and Maintenance Therapy to a Standard Chemotherapy Backbone for the Treatment of High-Risk Ewing Sarcoma
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Eligibility
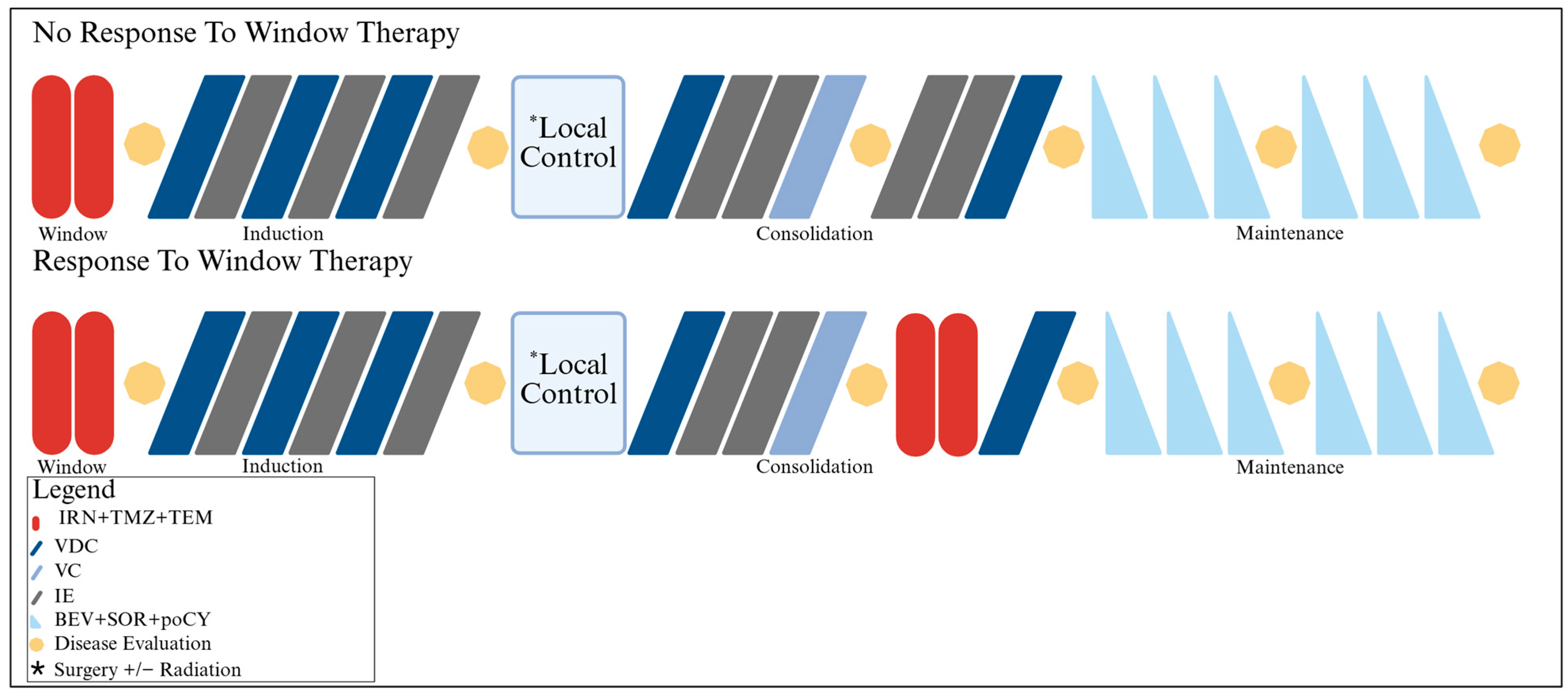
2.2. Protocol Therapy
2.2.1. Chemotherapy
2.2.2. Local Control
2.2.3. Toxicity
2.2.4. Response
2.2.5. Quality of Life
2.2.6. Functional Assessments
2.3. Objectives and Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Disease Characteristics
3.3. Treatment Completed
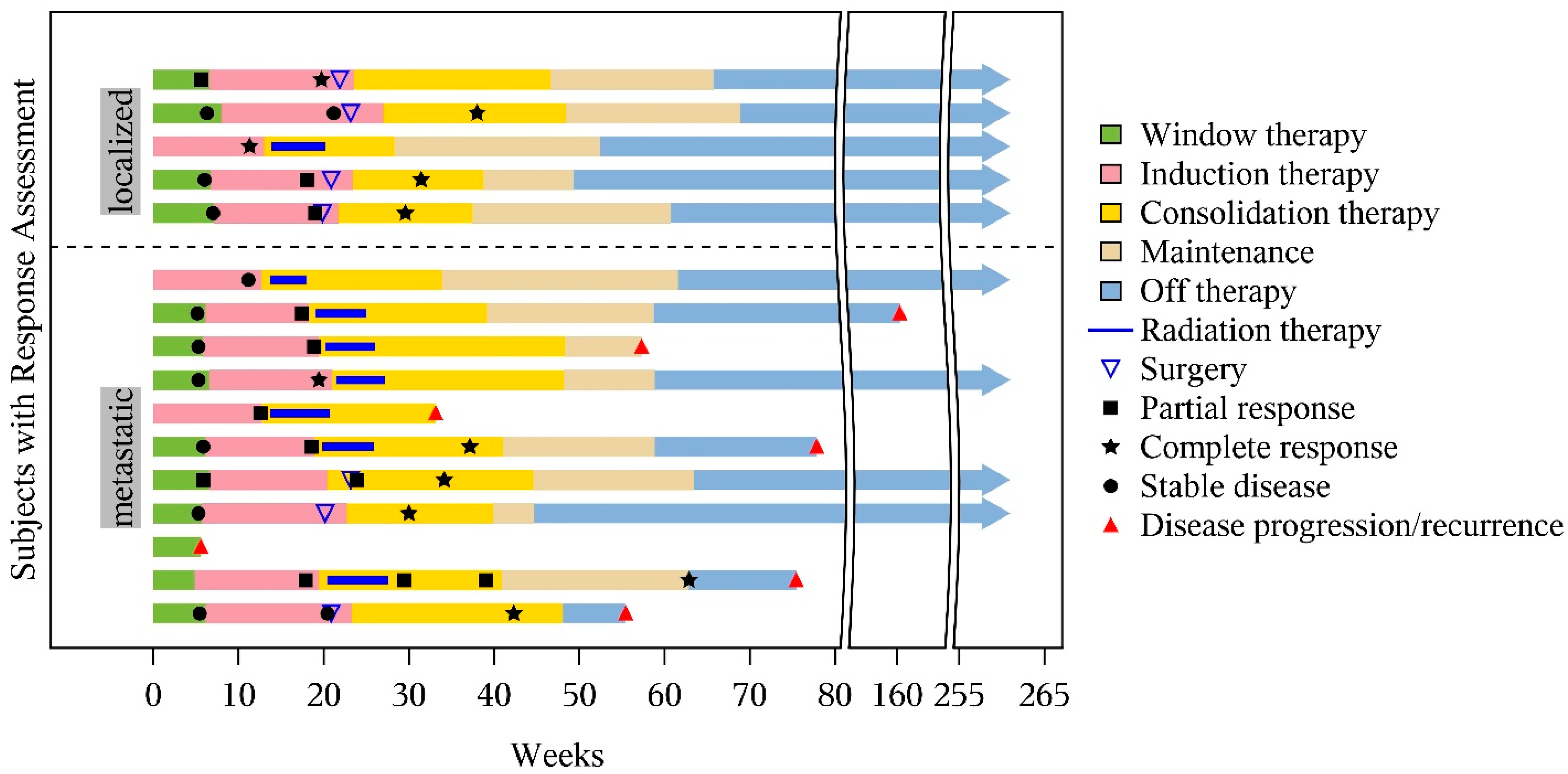
3.4. Tumor Response
3.5. Survival Outcomes
3.6. Primary-Site Local Control and Treatment Failures
3.7. Toxicity
3.8. Functional Outcomes
3.9. Quality of Life Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Womer, R.B.; West, D.C.; Krailo, M.D.; Dickman, P.S.; Pawel, B.R.; Grier, H.E.; Marcus, K.; Sailer, S.; Healey, J.H.; Dormans, J.P.; et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: A report from the Children’s Oncology Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 4148–4154. [Google Scholar] [CrossRef]
- Brennan, B.; Kirton, L.; Marec-Bérard, P.; Gaspar, N.; Laurence, V.; Martín-Broto, J.; Sastre, A.; Gelderblom, H.; Owens, C.; Fenwick, N.; et al. Comparison of two chemotherapy regimens in patients with newly diagnosed Ewing sarcoma (EE2012): An open-label, randomised, phase 3 trial. Lancet 2022, 400, 1513–1521. [Google Scholar] [CrossRef]
- Rodriguez-Galindo, C.; Billups, C.A.; Kun, L.E.; Rao, B.N.; Pratt, C.B.; Merchant, T.E.; Santana, V.M.; Pappo, A.S. Survival after recurrence of Ewing tumors: The St Jude Children’s Research Hospital experience, 1979–999. Cancer 2002, 94, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Ranft, A.; Paulussen, M.; Bölling, T.; Vieth, V.; Bielack, S.; Görtitz, I.; Braun-Munzinger, G.; Hardes, J.; Jürgens, H.; et al. Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatr. Blood Cancer 2011, 57, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.G.; Ashley, S.; Craft, A.W.; Pinkerton, C.R. Outcome after relapse in an unselected cohort of children and adolescents with Ewing sarcoma. Med. Pediatr. Oncol. 2003, 40, 141–147. [Google Scholar] [CrossRef]
- Rodríguez-Galindo, C.; Navid, F.; Liu, T.; Billups, C.A.; Rao, B.N.; Krasin, M.J. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann. Oncol. 2008, 19, 814–820. [Google Scholar] [CrossRef]
- Rodríguez-Galindo, C.; Liu, T.; Krasin, M.J.; Wu, J.; Billups, C.A.; Daw, N.C.; Spunt, S.L.; Rao, B.N.; Santana, V.M.; Navid, F. Analysis of prognostic factors in ewing sarcoma family of tumors: Review of St. Jude Children’s Research Hospital studies. Cancer 2007, 110, 375–384. [Google Scholar] [CrossRef]
- Raciborska, A.; Bilska, K.; Drabko, K.; Chaber, R.; Pogorzala, M.; Wyrobek, E.; Polczyńska, K.; Rogowska, E.; Rodriguez-Galindo, C.; Wozniak, W. Vincristine, irinotecan, and temozolomide in patients with relapsed and refractory Ewing sarcoma. Pediatr. Blood Cancer 2013, 60, 1621–1625. [Google Scholar] [CrossRef]
- Bisogno, G.; Riccardi, R.; Ruggiero, A.; Arcamone, G.; Prete, A.; Surico, G.; Provenzi, M.; Bertolini, P.; Paolucci, P.; Carli, M. Phase II study of a protracted irinotecan schedule in children with refractory or recurrent soft tissue sarcoma. Cancer 2006, 106, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.M.; Crews, K.R.; Iacono, L.C.; Houghton, P.J.; Fuller, C.E.; McCarville, M.B.; Goldsby, R.E.; Albritton, K.; Stewart, C.F.; Santana, V.M. Phase I trial of temozolomide and protracted irinotecan in pediatric patients with refractory solid tumors. Clin. Cancer Res. 2004, 10, 840–848. [Google Scholar] [CrossRef]
- Casey, D.A.; Wexler, L.H.; Merchant, M.S.; Chou, A.J.; Merola, P.R.; Price, A.P.; Meyers, P.A. Irinotecan and temozolomide for Ewing sarcoma: The Memorial Sloan-Kettering experience. Pediatr. Blood Cancer 2009, 53, 1029–1034. [Google Scholar] [CrossRef]
- Zenali, M.J.; Zhang, P.L.; Bendel, A.E.; Brown, R.E. Morphoproteomic confirmation of constitutively activated mTOR, ERK, and NF-kappaB pathways in Ewing family of tumors. Ann. Clin. Lab. Sci. 2009, 39, 160–166. [Google Scholar]
- Subbiah, V.; Brown, R.E.; Jiang, Y.; Buryanek, J.; Hayes-Jordan, A.; Kurzrock, R.; Anderson, P.M. Morphoproteomic profiling of the mammalian target of rapamycin (mTOR) signaling pathway in desmoplastic small round cell tumor (EWS/WT1), Ewing’s sarcoma (EWS/FLI1) and Wilms’ tumor(WT1). PLoS ONE 2013, 8, e68985. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Morton, C.L.; Gorlick, R.; Lock, R.B.; Carol, H.; Reynolds, C.P.; Kang, M.H.; Maris, J.M.; Keir, S.T.; Kolb, E.A.; et al. Stage 2 combination testing of rapamycin with cytotoxic agents by the Pediatric Preclinical Testing Program. Mol. Cancer Ther. 2010, 9, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Thornton, K.A.; Chen, A.R.; Trucco, M.M.; Shah, P.; Wilky, B.A.; Gul, N.; Carrera-Haro, M.A.; Ferreira, M.F.; Shafique, U.; Powell, J.D.; et al. A dose-finding study of temsirolimus and liposomal doxorubicin for patients with recurrent and refractory bone and soft tissue sarcoma. Int. J. Cancer 2013, 133, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Bagatell, R.; Norris, R.; Ingle, A.M.; Ahern, C.; Voss, S.; Fox, E.; Little, A.R.; Weigel, B.J.; Adamson, P.C.; Blaney, S. Phase 1 trial of temsirolimus in combination with irinotecan and temozolomide in children, adolescents and young adults with relapsed or refractory solid tumors: A Children’s Oncology Group Study. Pediatr. Blood Cancer 2014, 61, 833–839. [Google Scholar] [CrossRef]
- Geoerger, B.; Kieran, M.W.; Grupp, S.; Perek, D.; Clancy, J.; Krygowski, M.; Ananthakrishnan, R.; Boni, J.P.; Berkenblit, A.; Spunt, S.L. Phase II trial of temsirolimus in children with high-grade glioma, neuroblastoma and rhabdomyosarcoma. Eur. J. Cancer 2012, 48, 253–262. [Google Scholar] [CrossRef]
- Bisogno, G.; Minard-Colin, V.; Jenney, M.; Ferrari, A.; Chisholm, J.; Di Carlo, D.; Hjalgrim, L.L.; Orbach, D.; Merks, J.H.M.; Casanova, M. Maintenance Chemotherapy for Patients with Rhabdomyosarcoma. Cancers 2023, 15, 4012. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Erber, R.; Thurnher, A.; Katsen, A.D.; Groth, G.; Kerger, H.; Hammes, H.P.; Menger, M.D.; Ullrich, A.; Vajkoczy, P. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004, 18, 338–340. [Google Scholar] [CrossRef]
- Ning, S.; Laird, D.; Cherrington, J.M.; Knox, S.J. The antiangiogenic agents SU5416 and SU6668 increase the antitumor effects of fractionated irradiation. Radiat. Res. 2002, 157, 45–51. [Google Scholar] [CrossRef]
- Pietras, K.; Hanahan, D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, R.M.; Tseng, W.W.; Vellagas, R.; Liu, W.; Ahmad, S.A.; Jung, Y.D.; Reinmuth, N.; Drazan, K.E.; Bucana, C.D.; Hicklin, D.J.; et al. Effects of an antibody to vascular endothelial growth factor receptor-2 on survival, tumor vascularity, and apoptosis in a murine model of colon carcinomatosis. Int. J. Oncol. 2001, 18, 221–226. [Google Scholar] [CrossRef]
- Federico, S.M.; Caldwell, K.J.; McCarville, M.B.; Daryani, V.M.; Stewart, C.F.; Mao, S.; Wu, J.; Davidoff, A.M.; Santana, V.M.; Furman, W.L.; et al. Phase I expansion cohort to evaluate the combination of bevacizumab, sorafenib and low-dose cyclophosphamide in children and young adults with refractory or recurrent solid tumours. Eur. J. Cancer 2020, 132, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Navid, F.; Baker, S.D.; McCarville, M.B.; Stewart, C.F.; Billups, C.A.; Wu, J.; Davidoff, A.M.; Spunt, S.L.; Furman, W.L.; McGregor, L.M.; et al. Phase I and clinical pharmacology study of bevacizumab, sorafenib, and low-dose cyclophosphamide in children and young adults with refractory/recurrent solid tumors. Clin. Cancer Res. 2013, 19, 236–246. [Google Scholar] [CrossRef][Green Version]
- Costelloe, C.M.; Chuang, H.H.; Madewell, J.E.; Ueno, N.T. Cancer Response Criteria and Bone Metastases: RECIST 1.1, MDA and PERCIST. J. Cancer 2010, 1, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Schmidkonz, C.; Krumbholz, M.; Atzinger, A.; Cordes, M.; Goetz, T.I.; Prante, O.; Ritt, P.; Schaefer, C.; Agaimy, A.; Hartmann, W.; et al. Correction to: Assessment of treatment responses in children and adolescents with Ewing sarcoma with metabolic tumor parameters derived from (18)F-FDG-PET/CT and circulating tumor DNA. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1613. [Google Scholar] [CrossRef]
- Annovazzi, A.; Ferraresi, V.; Anelli, V.; Covello, R.; Vari, S.; Zoccali, C.; Biagini, R.; Sciuto, R. [(18)F]FDG PET/CT quantitative parameters for the prediction of histological response to induction chemotherapy and clinical outcome in patients with localised bone and soft-tissue Ewing sarcoma. Eur. Radiol. 2021, 31, 7012–7021. [Google Scholar] [CrossRef]
- El-Hennawy, G.; Moustafa, H.; Omar, W.; Elkinaai, N.; Kamel, A.; Zaki, I.; Farid, N.; El-Kholy, E. Different (18) F-FDG PET parameters for the prediction of histological response to neoadjuvant chemotherapy in pediatric Ewing sarcoma family of tumors. Pediatr. Blood Cancer 2020, 67, e28605. [Google Scholar] [CrossRef]
- Orsatti, G.; Beltrame, V.; Crimì, F.; Frigo, A.C.; Bisogno, G.; Stramare, R. Radiologic Response Assessment in Pediatric Soft Tissue Sarcoma: Computed-Assisted Volume Evaluation. J. Pediatr. 2017, 182, 327–334.e322. [Google Scholar] [CrossRef]
- Orsatti, G.; Zucchetta, P.; Varotto, A.; Crimì, F.; Weber, M.; Cecchin, D.; Bisogno, G.; Spimpolo, A.; Giraudo, C.; Stramare, R. Volumetric histograms-based analysis of apparent diffusion coefficients and standard uptake values for the assessment of pediatric sarcoma at staging: Preliminary results of a PET/MRI study. Radiol. Medica 2021, 126, 878–885. [Google Scholar] [CrossRef]
- Haveman, L.M.; Ranft, A.; Vd Berg, H.; Smets, A.; Kruseova, J.; Ladenstein, R.; Brichard, B.; Paulussen, M.; Kuehne, T.; Juergens, H.; et al. The relation of radiological tumor volume response to histological response and outcome in patients with localized Ewing Sarcoma. Cancer Med. 2019, 8, 1086–1094. [Google Scholar] [CrossRef]
- Park, J.R.; Bagatell, R.; Cohn, S.L.; Pearson, A.D.; Villablanca, J.G.; Berthold, F.; Burchill, S.; Boubaker, A.; McHugh, K.; Nuchtern, J.G.; et al. Revisions to the International Neuroblastoma Response Criteria: A Consensus Statement From the National Cancer Institute Clinical Trials Planning Meeting. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 2580–2587. [Google Scholar] [CrossRef]
- Gitto, S.; Cuocolo, R.; Huisman, M.; Messina, C.; Albano, D.; Omoumi, P.; Kotter, E.; Maas, M.; Van Ooijen, P.; Sconfienza, L.M. CT and MRI radiomics of bone and soft-tissue sarcomas: An updated systematic review of reproducibility and validation strategies. Insights Imaging 2024, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Hu, Y.; Si, L.; Jia, G.; Xing, Y.; Zhang, H.; Yao, W. A systematic review of radiomics in osteosarcoma: Utilizing radiomics quality score as a tool promoting clinical translation. Eur. Radiol. 2021, 31, 1526–1535. [Google Scholar] [CrossRef]
- Gitto, S.; Corino, V.D.A.; Annovazzi, A.; Milazzo Machado, E.; Bologna, M.; Marzorati, L.; Albano, D.; Messina, C.; Serpi, F.; Anelli, V.; et al. 3D vs. 2D MRI radiomics in skeletal Ewing sarcoma: Feature reproducibility and preliminary machine learning analysis on neoadjuvant chemotherapy response prediction. Front. Oncol. 2022, 12, 1016123. [Google Scholar] [CrossRef]
- DuBois, S.G.; Krailo, M.D.; Glade-Bender, J.; Buxton, A.; Laack, N.; Randall, R.L.; Chen, H.X.; Seibel, N.L.; Boron, M.; Terezakis, S.; et al. Randomized Phase III Trial of Ganitumab With Interval-Compressed Chemotherapy for Patients With Newly Diagnosed Metastatic Ewing Sarcoma: A Report From the Children’s Oncology Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 2098–2107. [Google Scholar] [CrossRef]
- Raciborska, A.; Bilska, K.; Rodriguez-Galindo, C. Maintenance treatment with trofosfamide in patients with primary bone ewing sarcoma—single center experience. Dev. Period. Med. 2019, 23, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Puma, N.; Sebastian, A.D.; Paioli, A.; Bisogno, G.; Rabusin, M.; Coccoli, L.; Tamburini, A.; Milano, G.M.; Mascarin, M.; Bertulli, R.; et al. Maintenance Therapy with Oral Cyclophosphamide Plus Celecoxib in Patients with Metastatic Ewingn Sarcoma: Results of the Italian Sarcoma Group/AIEPOP EW-2 Study. JCO 1994, 16, 10517. [Google Scholar] [CrossRef]
- Moromizato, K.; Kimura, R.; Fukase, H.; Yamaguchi, K.; Ishida, H. Whole-body patterns of the range of joint motion in young adults: Masculine type and feminine type. J. Physiol. Anthropol. 2016, 35, 23. [Google Scholar] [CrossRef] [PubMed]
- Soucie, J.M.; Wang, C.; Forsyth, A.; Funk, S.; Denny, M.; Roach, K.E.; Boone, D. Range of motion measurements: Reference values and a database for comparison studies. Haemophilia 2011, 17, 500–507. [Google Scholar] [CrossRef]
- Troke, M.; Moore, A.P.; Maillardet, F.J.; Hough, A.; Cheek, E. A new, comprehensive normative database of lumbar spine ranges of motion. Clin. Rehabil. 2001, 15, 371–379. [Google Scholar] [CrossRef]
- Neeter, C.; Gustavsson, A.; Thomeé, P.; Augustsson, J.; Thomeé, R.; Karlsson, J. Development of a strength test battery for evaluating leg muscle power after anterior cruciate ligament injury and reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Harbo, T.; Brincks, J.; Andersen, H. Maximal isokinetic and isometric muscle strength of major muscle groups related to age, body mass, height, and sex in 178 healthy subjects. Eur. J. Appl. Physiol. 2012, 112, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Holm, I.; Fredriksen, P.; Fosdahl, M.; Vøllestad, N. A normative sample of isotonic and isokinetic muscle strength measurements in children 7 to 12 years of age. Acta Paediatr. 2008, 97, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, K.A.; Parnianpour, M. A normative database of isokinetic upper-extremity joint strengths: Towards the evaluation of dynamic human performance. Biomed. Eng. Appl. Basis Commun. 2001, 13, 79–92. [Google Scholar] [CrossRef]
- Günther, C.M.; Bürger, A.; Rickert, M.; Crispin, A.; Schulz, C.U. Grip strength in healthy caucasian adults: Reference values. J. Hand Surg. Am. 2008, 33, 558–565. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Wiemer, D.M.; Federman, S.M. Grip and pinch strength: Norms for 6- to 19-year-olds. Am. J. Occup. Ther. 1986, 40, 705–711. [Google Scholar] [CrossRef]
- Marchese, V.G.; Oriel, K.N.; Fry, J.A.; Kovacs, J.L.; Weaver, R.L.; Reilly, M.M.; Ginsberg, J.P. Development of reference values for the Functional Mobility Assessment. Pediatr. Phys. Ther. 2012, 24, 224–230. [Google Scholar] [CrossRef]
- Fleming, S.; Thompson, M.; Stevens, R.; Heneghan, C.; Plüddemann, A.; Maconochie, I.; Tarassenko, L.; Mant, D. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: A systematic review of observational studies. Lancet 2011, 377, 1011–1018. [Google Scholar] [CrossRef]
- James, F.W.; Kaplan, S.; Glueck, C.J.; Tsay, J.Y.; Knight, M.J.; Sarwar, C.J. Responses of normal children and young adults to controlled bicycle exercise. Circulation 1980, 61, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Kaminsky, L.A.; Lima, R.; Christle, J.W.; Ashley, E.; Arena, R. A Reference Equation for Normal Standards for VO(2) Max: Analysis from the Fitness Registry and the Importance of Exercise National Database (FRIEND Registry). Prog. Cardiovasc. Dis. 2017, 60, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Shuter, B.; Aslani, A. Body surface area: Du Bois and Du Bois revisited. Eur. J. Appl. Physiol. 2000, 82, 250–254. [Google Scholar] [CrossRef] [PubMed]


| Variables | Freq (%) (n = 16) |
|---|---|
| Age at Enrollment | |
| Median (Min, Max) | 12.2 (4.8, 23.6) |
| Age Group at Enrollment | |
| <14 years | 10 |
| ≥14 years | 6 |
| Gender | |
| Male | 7 (43.8) |
| Female | 9 (56.3) |
| Race | |
| White | 14 (87.5) |
| Multiple Race (NOS) | 1 (6.3) |
| Asian | 1 (6.3) |
| EWSR1 Translocation * | |
| Positive | 15 (93.8) |
| Negative | 1 (6.2) |
| Stage | |
| Metastatic | 11 (68.8) |
| Localized | 5 (31.3) |
| Primary Site | |
| Extremity | 6 (37.5) |
| Chest Wall | 2 (12.5) |
| Pelvis | 6 (37.5) |
| Spine | 1 (6.3) |
| Renal | 1 (6.3) |
| Metastatic Site | |
| Bone | 1 (9.1) |
| Pulmonary | 3 (27.3) |
| Bone + Bone Marrow | 1 (9.1) |
| Bone + Pulmonary | 3 (27.3) |
| Bone + Bone Marrow + Pulmonary | 3 (27.3) |
| Primary Tumor Volume | |
| n | 12 |
| Median (Min, Max) | 181.3 (12.5, 654.2) |
| Category | Grade | |||
|---|---|---|---|---|
| 2 * | 3 | 4 | 5 | |
| Blood and lymphatic system disorders | ||||
| Anemia | - | 3 (23.1%) | - | - |
| Lymphocyte count decreased | 3 (23.1%) | 2 (15.4%) | - | - |
| Neutrophil count decreased | 5 (38.5%) | 2 (15.4%) | - | - |
| White blood cell count decreased | 3 (23.1%) | - | ||
| Gastrointestinal disorders | ||||
| Abdominal pain | 4 (30.8%) | 1 (7.7%) | - | - |
| Alanine aminotransferase increased | - | 1 (7.7%) | - | - |
| Ascites | - | 1 (7.7%) | - | - |
| Colitis | - | 4 (30.8%) | - | - |
| Diarrhea | 4 (30.8%) | 4 (30.8%) | - | - |
| Mucositis oral | - | 2 (15.4%) | - | - |
| Nausea | 6 (46.2%) | 3 (23.1%) | - | - |
| Vomiting | 4 (80%) | 2 (15.4%) | - | - |
| General Disorder | ||||
| Fever | 2 (15.4%) | - | - | - |
| Immune system disorders | ||||
| Allergic reaction | 3 (23.1%) | - | - | - |
| Infections and infestations | ||||
| Catheter-related infection | - | 1 (7.7%) | - | - |
| Enterocolitis infectious | - | 2 (15.4%) | - | - |
| Esophageal infection | - | 1 (7.7%) | - | - |
| Febrile neutropenia | - | 1 (7.7%) | - | - |
| Mucosal infection | 3 (23.1%) | - | - | - |
| Skin infection | - | 1 (7.7%) | - | - |
| Metabolism and nutritional disorders | ||||
| Anorexia | 8 (61.5%) | 2 (15.4%) | - | - |
| Dehydration | 2 (15.4%) | 1 (7.7%) | - | - |
| Hypercalcemia | 2 (15.4%) | - | - | - |
| Hypoalbuminemia | 4 (30.8%) | 1 (7.7%) | - | - |
| Hypocalcemia | - | 2 (15.4%) | - | - |
| Hypokalemia | - | 2 (15.4%) | - | - |
| Hyponatremia | - | 5 (38.5%) | - | - |
| Hypophosphatemia | 2 (15.4%) | 3 (23.1%) | - | - |
| Weight loss | 4 (30.8%) | |||
| Respiratory, thoracic and mediastinal disorders | ||||
| Pneumothorax | - | 1 (7.7%) | - | - |
| Maximum Grade Any Event | 3 (23.1%) | 10 (76.9%) | - | - |
| Category | Grade | |||
|---|---|---|---|---|
| 2 * | 3 | 4 | 5 | |
| Blood and lymphatic system disorders | ||||
| Lymphocyte count decreased | - | 5 (38.5%) | 6 (46.2%) | - |
| Neutrophil count decreased | - | 3 (23.1%) | 4 (30.8%) | - |
| Platelet count decreased | - | 2 (15.4%) | - | - |
| White blood cell count decreased | - | 4 (30.8%) | 3 (23.1%) | - |
| Gastrointestinal disorders | ||||
| Diarrhea | - | 2 (15.4%) | - | - |
| Vomiting | - | 1 (7.7%) | - | - |
| Infections and infestations | ||||
| Bladder infection | - | 1 (7.7%) | - | - |
| Renal and urinary disorders | ||||
| Cystitis noninfective | - | 1 (7.7%) | - | - |
| Proteinuria | - | 1 (7.7%) | - | - |
| Skin and subcutaneous tissue disorders | ||||
| Palmar–plantar erythrodysesthesia syndrome | 6 (46.2%) | 2 (15.4%) | - | - |
| Maximum Grade Any Event | 2 (15.4%) | 3 (23.1%) | 8 (61.5%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gartrell, J.; Navid, F.; Yuan, X.; Ness, K.K.; Dubrovin, M.; Wang, F.; Pan, H.; McCarville, M.B.; Shulkin, B.L.; Helmig, S.; et al. ESFT13: A Phase II Study Evaluating the Addition of Window and Maintenance Therapy to a Standard Chemotherapy Backbone for the Treatment of High-Risk Ewing Sarcoma. Cancers 2025, 17, 2894. https://doi.org/10.3390/cancers17172894
Gartrell J, Navid F, Yuan X, Ness KK, Dubrovin M, Wang F, Pan H, McCarville MB, Shulkin BL, Helmig S, et al. ESFT13: A Phase II Study Evaluating the Addition of Window and Maintenance Therapy to a Standard Chemotherapy Backbone for the Treatment of High-Risk Ewing Sarcoma. Cancers. 2025; 17(17):2894. https://doi.org/10.3390/cancers17172894
Chicago/Turabian StyleGartrell, Jessica, Fariba Navid, Xiaomeng Yuan, Kirsten K. Ness, Mikhail Dubrovin, Fang Wang, Haitao Pan, Mary Beth McCarville, Barry L. Shulkin, Sara Helmig, and et al. 2025. "ESFT13: A Phase II Study Evaluating the Addition of Window and Maintenance Therapy to a Standard Chemotherapy Backbone for the Treatment of High-Risk Ewing Sarcoma" Cancers 17, no. 17: 2894. https://doi.org/10.3390/cancers17172894
APA StyleGartrell, J., Navid, F., Yuan, X., Ness, K. K., Dubrovin, M., Wang, F., Pan, H., McCarville, M. B., Shulkin, B. L., Helmig, S., Krasin, M. J., Neel, M. D., Davidoff, A. M., Mandrell, B. N., Levine, D. R., Cai, Z., Bishop, M. W., Pappo, A. S., & Federico, S. M. (2025). ESFT13: A Phase II Study Evaluating the Addition of Window and Maintenance Therapy to a Standard Chemotherapy Backbone for the Treatment of High-Risk Ewing Sarcoma. Cancers, 17(17), 2894. https://doi.org/10.3390/cancers17172894







