Global Prevalence and Modifiers of Human Papillomavirus Positivity in Oral Cavity Cancer: A Systematic Review and Meta-Analysis of Prevalence (1995–2024)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Literature Search
2.2. Selection Strategy
- Population: patients with histologically confirmed oral cavity cancer.
- Exposure: none.
- Comparison: none.
- Outcome: HPV-positive rate.
- Study Design: epidemiological studies and cross-sectional studies. Case-control studies were only considered if they investigated the rate of HPV positivity in cancer and healthy individuals.
- Non-original research.
- Abstract-only publications.
- Experimental and investigation studies (clinical trials).
- Case reports and case series.
- Case-control studies including HPV-positive and HPV-negative controls.
- Duplicated records or studies with overlapping datasets (similar samples and baseline characteristics even if author lists differed).
- Non-oral cavity cancer (like oropharyngeal cancer—OPC).
- Studies including patients with oral cavity cancer and OPC without stratifying HPV data based on cancer location.
- Studies not reporting HPV-positivity rate.
- Animal studies plus in vivo or in vitro studies.
2.3. Data Collection and Outcomes
2.4. Risk of Bias Assessment
2.5. Statistical Analysis
3. Results
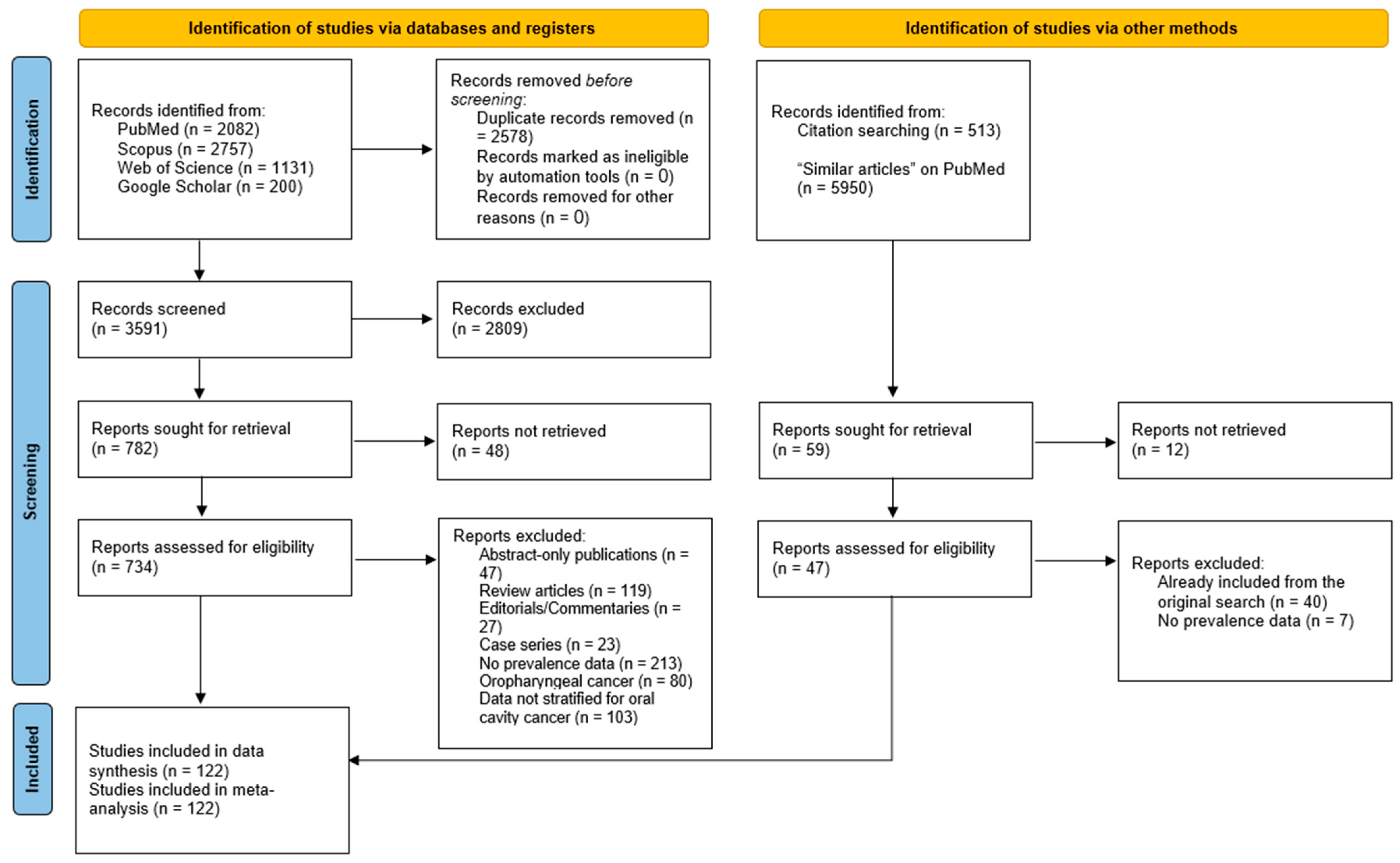
3.1. Literature Search Results
3.2. Baseline Characteristics
3.3. Methodological Quality
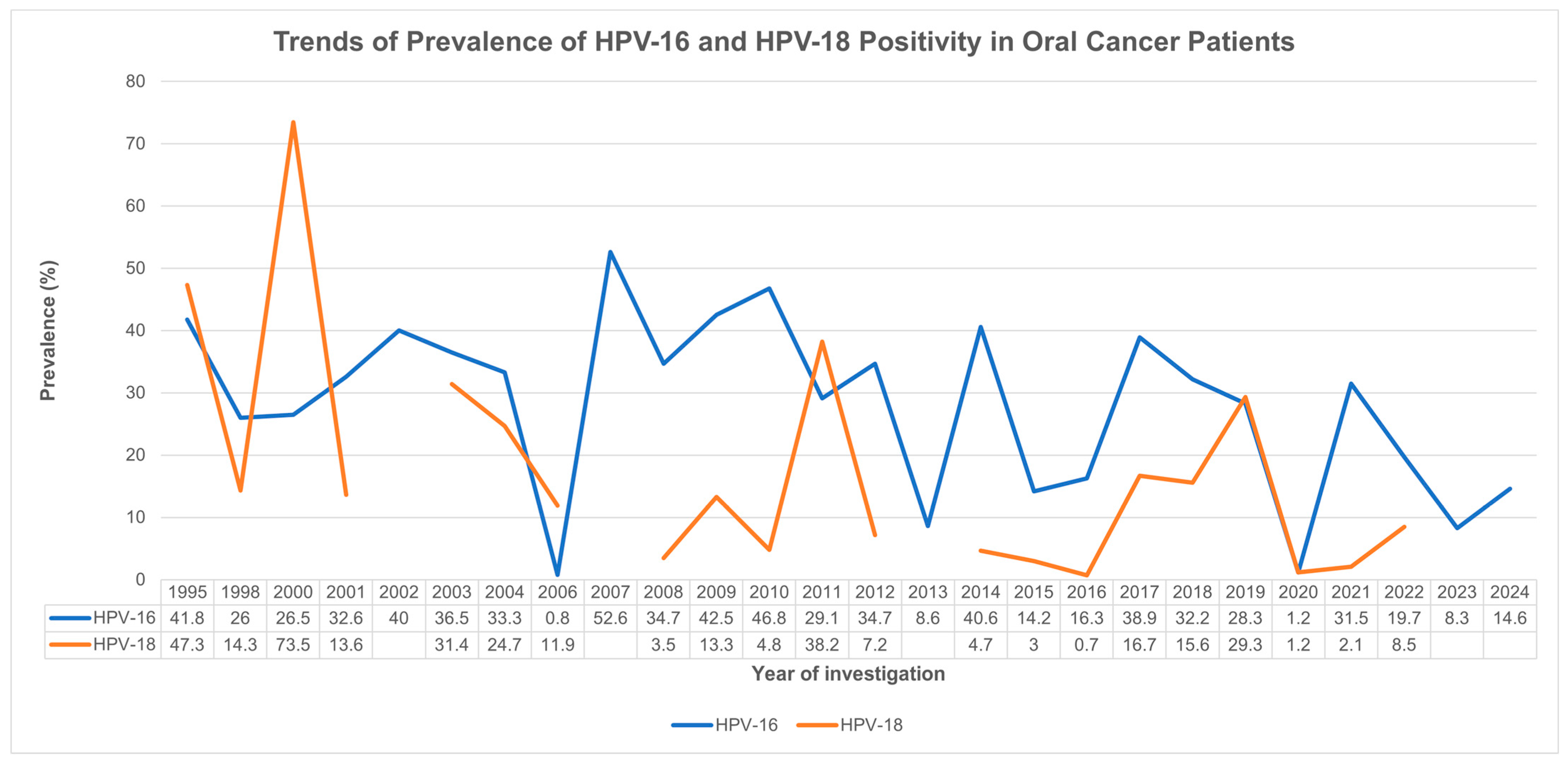
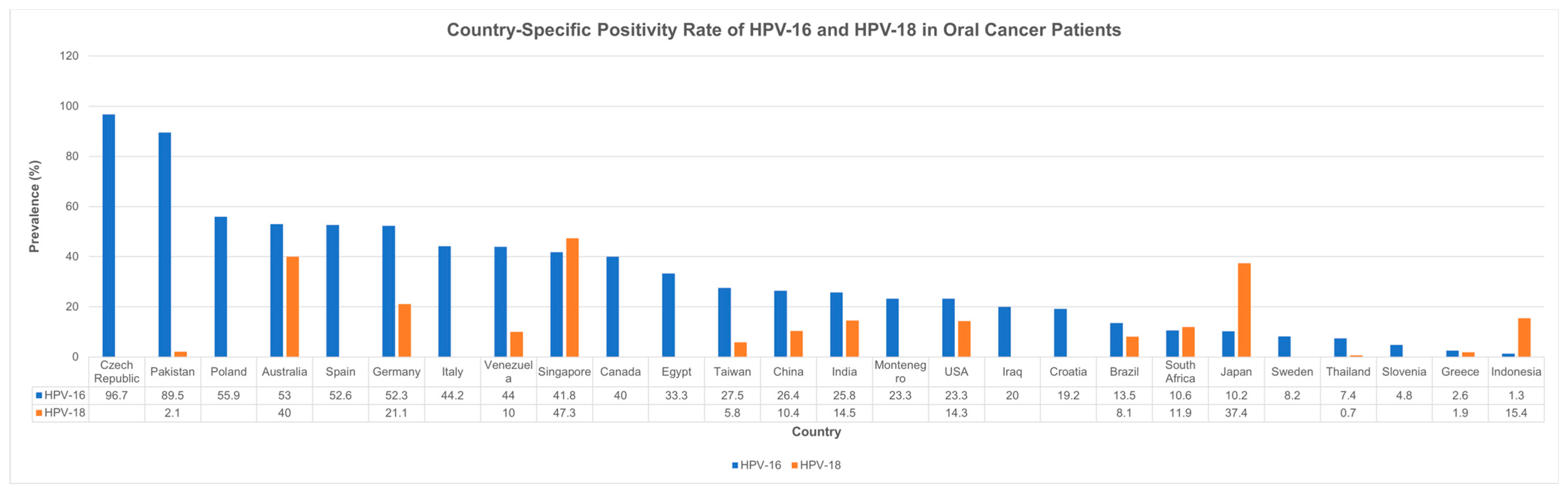
3.4. Country- and Year-Specific Prevalence Rates
3.5. Age- and Gender-Specific Prevalence
3.6. Smoking- and Alcohol-Specific Prevalence
3.7. Cancer Site-Specific Prevalence
3.8. Cancer Stage- and Grade-Specific Prevalence
3.9. Tumor Size (T Staging) and Nodal Involvement-Based Prevalence
3.10. Treatment-Specific Prevalence
3.11. P16-Specific Prevalence
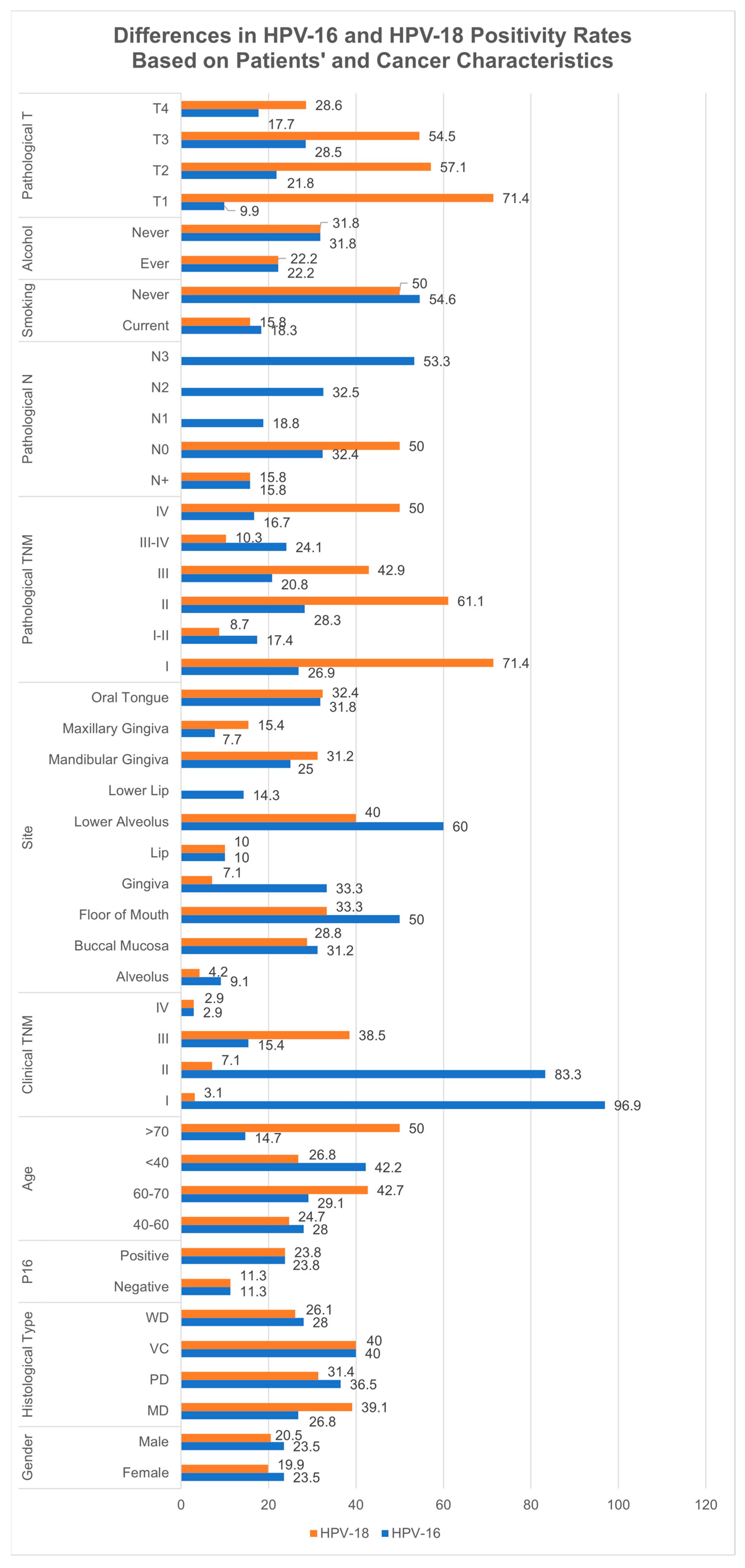
3.12. Subgroup Analyses Based on HPV-16 and HPV-18 Strains
4. Discussion
4.1. Overview of Findings
4.2. HPV Prevalence in Oral Cavity Cancer: A Global and Temporal Perspective
4.3. Age and Gender Differences in HPV Positivity
4.4. HPV Subtype-Specific Prevalence: HPV-16 and HPV-18
4.5. Cancer Site and Stage-Specific Differences
4.6. Methodological and Detection Challenges
4.7. Public Health and Clinical Implications
4.8. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| CI | Confidence interval |
| DNA | Deoxyribonucleic acid |
| E6/E7 | Early genes 6 and 7 of HPV |
| FISH | Fluorescence in Situ Hybridization |
| HPV | Human papillomavirus |
| IHC | Immunohistochemistry |
| I2 | I-squared |
| LD | Linear dichroism |
| MD | Moderately differentiated |
| OPC | Oropharyngeal cancer |
| OPSCC | Oropharyngeal squamous cell carcinoma |
| OR | Odds ratio |
| OS | Overall survival |
| P16 | Cyclin-dependent kinase inhibitor 2A |
| PD | Poorly differentiated |
| PECO | Population, exposure, comparison, and outcomes |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| REML | Restricted maximum likelihood |
| RNA | Ribonucleic acid |
| ROC | Receiver operating characteristics |
| SCC | Squamous cell carcinoma |
| TNM | Tumor, node, and metastasis |
| VC | Verrucous carcinoma |
| WD | Well differentiated |
References
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.T.; Grønhøj, C.; Zamani, M.; Brask, J.; Kjær, E.K.R.; Lajer, H.; von Buchwald, C. Association between oropharyngeal cancers with known HPV and p16 status and cervical intraepithelial neoplasia: A Danish population-based study. Acta Oncol. 2019, 58, 267–272. [Google Scholar] [CrossRef]
- Isayeva, T.; Li, Y.; Maswahu, D.; Brandwein-Gensler, M. Human papillomavirus in non-oropharyngeal head and neck cancers: A systematic literature review. Head Neck Pathol. 2012, 6, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Zokirovna, O.A. The incidence of cancer of the oral cavity and pharynx in The Bukhara Region. Int. J. Integr. Mod. Med. 2023, 1, 86–89. [Google Scholar]
- Lu, Y.; Sobue, T.; Kitamura, T.; Matsuse, R.; Kitamura, Y.; Matsuo, K.; Ito, H.; Oze, I.; Shimazu, T.; Yamaji, T. Cigarette smoking, alcohol drinking, and oral cavity and pharyngeal cancer in the Japanese: A population-based cohort study in Japan. Eur. J. Cancer Prev. 2018, 27, 171–179. [Google Scholar] [CrossRef]
- Lai, K.; Killingsworth, M.; Matthews, S.; Caixeiro, N.; Evangelista, C.; Wu, X.; Wykes, J.; Samakeh, A.; Forstner, D.; Niles, N. Differences in survival outcome between oropharyngeal and oral cavity squamous cell carcinoma in relation to HPV status. J. Oral Pathol. Med. 2017, 46, 574–582. [Google Scholar] [CrossRef]
- Ibieta, B.R.; Lizano, M.; Fras-Mendivil, M.; Barrera, J.L.; Carrillo, A.; Ma Ruz-Godoy, L.; Mohar, A. Human papilloma virus in oral squamous cell carcinoma in a Mexican population. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 311–315. [Google Scholar] [CrossRef]
- Kansky, A.A.; Poljak, M.; Seme, K.; Kocjan, B.J.; Gale, N.; Luzar, B.; Golouh, R. Human papillomavirus DNA in oral squamous cell carcinomas and normal oral mucosa. Acta Virol. 2003, 47, 11–16. [Google Scholar]
- Komolmalai, N.; Pongsiriwet, S.; Lertprasertsuke, N.; Lekwanavijit, S.; Kintarak, S.; Phattarataratip, E.; Subarnbhesaj, A.; Dhanuthai, K.; Chaisuparat, R.; Iamaroon, A. Human Papillomavirus 16 and 18 Infection in Oral Cancer in Thailand: A Multicenter Study. Asian Pac. J. Cancer Prev. 2020, 21, 3349–3355. [Google Scholar] [CrossRef]
- Machado, J.; Reis, P.P.; Zhang, T.; Simpson, C.; Xu, W.; Perez-Ordonez, B.; Goldstein, D.P.; Brown, D.H.; Gilbert, R.W.; Gullane, P.J.; et al. Low prevalence of human papillomavirus in oral cavity carcinomas. Head Neck Oncol. 2010, 2, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Osazuwa-Peters, N.; Adjei Boakye, E.; Rohde, R.L.; Ganesh, R.N.; Moiyadi, A.S.; Hussaini, A.S.; Varvares, M.A. Understanding of risk factors for the human papillomavirus (HPV) infection based on gender and race. Sci. Rep. 2019, 9, 297. [Google Scholar] [CrossRef]
- Petrović, A.; Čanković, M.; Avramov, M.; Popović, Ž.D.; Janković, S.; Mojsilović, S. High-Risk Human Papillomavirus in Patients with Oral Carcinoma and Oral Potentially Malignant Disorders in Serbia-A Pilot Study. Medicina 2023, 59, 1843. [Google Scholar] [CrossRef] [PubMed]
- Romanitan, M.; Näsman, A.; Ramqvist, T.; Dahlstrand, H.; Polykretis, L.; Vogiatzis, P.; Vamvakas, P.; Tasopoulos, G.; Valavanis, C.; Arapantoni-Dadioti, P.; et al. Human papillomavirus frequency in oral and oropharyngeal cancer in Greece. Anticancer Res. 2008, 28, 2077–2080. [Google Scholar] [PubMed]
- Makvandi, M.; Jalilian, S.; Faghihloo, E.; Khanizadeh, S.; Ramezani, A.; Bagheri, S.; Mirzaei, H. Prevalence of Human Papillomavirus and Co-Infection with Epstein-Barr Virus in Oral and Oropharyngeal Squamous Cell Carcinomas. Asian Pac. J. Cancer Prev. 2022, 23, 3931–3937. [Google Scholar] [CrossRef]
- Nekić, I.; Medić, A.; Puntarić, D.; Nemeth Blažić, T.; Šikić, N.L.; Konjevoda, S.; Nonković, D.; Sambunjak, J.; Dželalija, B. Prevalence of Human Papillomavirus in Laryngeal, Oropharyngeal and Oral Cavity Carcinomas in Zadar County, Croatia. Infektološki Glas. 2022, 42, 51–56. [Google Scholar] [CrossRef]
- Pongsapich, W.; Jotikaprasardhna, P.; Lianbanchong, C.; Phumchan, A.; Siritantikorn, S.; Chongkolwatana, C. Human Papillomavirus Infection in Oral Cavity and Oropharyngeal Cancers: Are They the Same Story? J. Med. Assoc. Thail. Chotmaihet Thangphaet 2016, 99, 684–690. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Shea, B.J.; Hamel, C.; Wells, G.A.; Bouter, L.M.; Kristjansson, E.; Grimshaw, J.; Henry, D.A.; Boers, M. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J. Clin. Epidemiol. 2009, 62, 1013–1020. [Google Scholar] [CrossRef]
- Muka, T.; Glisic, M.; Milic, J.; Verhoog, S.; Bohlius, J.; Bramer, W.; Chowdhury, R.; Franco, O.H. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur. J. Epidemiol. 2020, 35, 49–60. [Google Scholar] [CrossRef]
- Abdelaal, A.; Eltaras, M.M.; Katamesh, B.E.; Serhan, H.A.; Farahat, R.A.; Badr, H.; Abdelazeem, B. The prevalence and presentation patterns of microcystic macular oedema: A systematic review and meta-analysis of 2128 glaucomatous eyes. Eye 2023, 37, 3322–3333. [Google Scholar] [CrossRef]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef]
- Mavridis, D.; Salanti, G.; Furukawa, T.A.; Cipriani, A.; Chaimani, A.; White, I.R. Allowing for uncertainty due to missing and LOCF imputed outcomes in meta-analysis. Stat. Med. 2019, 38, 720–737. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, P. Meta-analyses: Heterogeneity and subgroup analysis. BMJ 2013, 346, f4040. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Abreu, P.M.; Valle, I.B.; Damasceno, T.C.D.; Có, A.C.G.; Pansini, P.F.; Podestá, J.R.V.; Souza, E.D.; Rocha, R.M.; Curado, M.P.; Mehanna, H.; et al. Human Papillomavirus E6/E7 mRNA detection by in situ hybridization in oral cavity squamous cell carcinoma. Arch. Oral Biol. 2020, 116, 104746. [Google Scholar] [CrossRef]
- Adamopoulou, M.; Vairaktaris, E.; Panis, V.; Nkenke, E.; Neukam, F.W.; Yapijakis, C. HPV detection rate in saliva may depend on the immune system efficiency. In Vivo 2008, 22, 599–602. [Google Scholar]
- Adilbay, D.; Adilbayev, G.; Kidirbayeva, G.; Shipilova, V.; Sadyk, Z.; Koyanbekova, G.; Sokolenko, E.; Klozar, J. HPV infection and P16 expression in oral and oropharyngeal cancer in Kazakhstan. Infect. Agents Cancer 2018, 13, 2. [Google Scholar] [CrossRef]
- Afzal, A.; Liaqat, R.; Shafqat, F.; Kalsoom, F.; Loya, A. The Frequency of Human Papilloma Virus Related Oral Squamous Cell Carcinomas by P16 Immuno Histochemical Stain. Med. Forum Mon. 2019, 30, 117–121. [Google Scholar]
- Ahmed, S.M.; Jabar, S.K. Prevalence of human papillomavirus in oral and laryngeal squamous cell carcinoma: A comparative study by polymerase chain reaction. Med. Sci. 2019, 23, 42–47. [Google Scholar]
- Ajila, V.; Babu, S.; Shetty, V.; Shetty, P.; Devegowda, D.; Ramesh, P.; Natarajan, S. Human papillomavirus in oral squamous cell carcinoma: An institutional study. Clin. Cancer Investig. J. 2021, 10, 102–107. [Google Scholar] [CrossRef]
- Akhondnezhad, M.; Haghshenas, M.R.; Ghasemi, M.; Mousavi, T. The prevalence and genotyping of human papillomavirus in patients with oral tumors in health centers and clinics of Mazandaran in Iran. Virusdisease 2018, 29, 297–302. [Google Scholar] [CrossRef]
- Ali, S.; Awan, M.; Ghaffar, S.; Salahuddin, I.; Khan, S.; Mehraj, V.; Pervez, S. Human papillomavirus infection in oral squamous cell carcinomas: Correlation with histologic variables and survival outcome in a high risk population. Oral Surg. 2008, 1, 96–105. [Google Scholar] [CrossRef]
- Alsharif, U.; Hofmann, M.; Gebhard, M.; Tharun, L.; Rades, D.; Sieg, P.; Hakim, S.G. Double Positivity for HPV DNA/P16(INK4a) Does Not Influence Survival of Patients with Oral Squamous Cell Carcinoma. Anticancer Res. 2021, 41, 5557–5568. [Google Scholar] [CrossRef] [PubMed]
- Antunović, M.; Lopičić, M.; Vučković, L.; Raonić, J.; Mugoša, S. Prevalence and clinical implications of the HPV16 infection in oral cancer in Montenegro—Evidence to support the immunization program. Acta Microbiol. Immunol. Hung. 2022, 69, 241–246. [Google Scholar] [CrossRef]
- Anwar, N.; Chundriger, Q.; Awan, S.; Moatter, T.; Ali, T.S.; Abdul Rasheed, M.; Pervez, S. Prevalence of high-risk human papillomavirus in oral squamous cell carcinoma with or without chewing habits. PLoS ONE 2024, 19, e0300354. [Google Scholar] [CrossRef]
- Ashraf, M.J.; Hosseini, S.; Monabati, A.; Valibeigi, B.; Khademi, B.; Abedi, E.; Azarpira, N. The Prevalence of Human Papilloma Virus in Squamous Cell Carcinoma of Oral Tongue. Iran. J. Pathol. 2017, 12, 144–149. [Google Scholar] [CrossRef]
- Balaram, P.; Nalinakumari, K.R.; Abraham, E.; Balan, A.; Hareendran, N.K.; Bernard, H.U.; Chan, S.Y. Human papillomaviruses in 91 oral cancers from Indian betel quid chewers--high prevalence and multiplicity of infections. Int. J. Cancer 1995, 61, 450–454. [Google Scholar] [CrossRef]
- Belobrov, S.; Cornall, A.M.; Young, R.J.; Koo, K.; Angel, C.; Wiesenfeld, D.; Rischin, D.; Garland, S.M.; McCullough, M. The role of human papillomavirus in p16-positive oral cancers. J. Oral Pathol. Med. 2018, 47, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Bhawal, U.K.; Sugiyama, M.; Nomura, Y.; Sawajiri, M.; Tsukinoki, K.; Ikeda, M.A.; Kuniyasu, H. High-risk human papillomavirus type 16 E7 oncogene associates with Cdc25A over-expression in oral squamous cell carcinoma. Virchows Arch. Int. J. Pathol. 2007, 450, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Bijina, B.R.; Ahmed, J.; Shenoy, N.; Ongole, R.; Shenoy, S.; Baliga, S. Detection of human papilloma virus in potentially malignant and malignant lesions of the oral cavity and a study of associated risk factors. South Asian J. Cancer 2016, 5, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Boy, S.; Van Rensburg, E.J.; Engelbrecht, S.; Dreyer, L.; van Heerden, M.; van Heerden, W. HPV detection in primary intra-oral squamous cell carcinomas--commensal, aetiological agent or contamination? J. Oral Pathol. Med. 2006, 35, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Božinović, K.; Sabol, I.; Rakušić, Z.; Jakovčević, A.; Šekerija, M.; Lukinović, J.; Prgomet, D.; Grce, M. HPV-driven oropharyngeal squamous cell cancer in Croatia—Demography and survival. PLoS ONE 2019, 14, e0211577. [Google Scholar] [CrossRef]
- Campisi, G.; Giovannelli, L.; Calvino, F.; Matranga, D.; Colella, G.; Di Liberto, C.; Capra, G.; Leao, J.C.; Lo Muzio, L.; Capogreco, M.; et al. HPV infection in relation to OSCC histological grading and TNM stage. Evaluation by traditional statistics and fuzzy logic model. Oral Oncol. 2006, 42, 638–645. [Google Scholar] [CrossRef]
- Chakrobarty, B.; Roy, J.G.; Majumdar, S.; Uppala, D. Relationship among tobacco habits, human papilloma virus (HPV) infection, p53 polymorphism/mutation and the risk of oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2014, 18, 211–216. [Google Scholar] [CrossRef]
- Chen, S.F.; Yu, F.S.; Chang, Y.C.; Fu, E.; Nieh, S.; Lin, Y.S. Role of human papillomavirus infection in carcinogenesis of oral squamous cell carcinoma with evidences of prognostic association. J. Oral Pathol. Med. 2012, 41, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Sun, K.; Jiang, W.W. Absence of high-risk HPV 16 and 18 in Chinese patients with oral squamous cell carcinoma and oral potentially malignant disorders. Virol. J. 2016, 13, 81. [Google Scholar] [CrossRef]
- Chotipanich, A.; Siriarechakul, S.; Mungkung, O.O. Role of high-risk human papillomavirus in the etiology of oral and oropharyngeal cancers in Thailand: A case-control study. SAGE Open Med. 2018, 6, 2050312118765604. [Google Scholar] [CrossRef]
- Chowdary, S.D.; Sekhar, P.C.; Kattapagari, K.K.; Mani Deepthi, C.H.; Neelima, D.; Reddy, B.V.R. A study to assess expression of human papillomavirus types 16 and 18 in oral squamous cell carcinoma using polymerase chain reaction. J. Oral Maxillofac. Pathol. 2018, 22, 347–352. [Google Scholar] [CrossRef]
- Cutilli, T.; Leocata, P.; Dolo, V.; Altobelli, E. Association between p53 status, human papillomavirus infection, and overall survival in advanced oral cancer after resection and combination systemic treatment. Br. J. Oral Maxillofac. Surg. 2016, 54, 198–202. [Google Scholar] [CrossRef]
- Dahlgren, L.; Dahlstrand, H.M.; Lindquist, D.; Högmo, A.; Björnestål, L.; Lindholm, J.; Lundberg, B.; Dalianis, T.; Munck-Wikland, E. Human papillomavirus is more common in base of tongue than in mobile tongue cancer and is a favorable prognostic factor in base of tongue cancer patients. Int. J. Cancer 2004, 112, 1015–1019. [Google Scholar] [CrossRef]
- Dalla Torre, D.; Burtscher, D.; Soelder, E.; Offermanns, V.; Rasse, M.; Puelacher, W. Human papillomavirus prevalence in a Mid-European oral squamous cell cancer population: A cohort study. Oral Dis. 2018, 24, 948–956. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, J.; Saranath, D.; Dedhia, P.; Sanghvi, V.; Mehta, A.R. Detection of HPV-16 genome in human oral cancers and potentially malignant lesions from India. Oral Oncol. 1998, 34, 413–420. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, P.M.; Có, A.C.G.; Azevedo, P.L.; do Valle, I.B.; de Oliveira, K.G.; Gouvea, S.A.; Cordeiro-Silva, M.F.; Louro, I.D.; de Podestá, J.R.V.; Lenzi, J.; et al. Frequency of HPV in oral cavity squamous cell carcinoma. BMC Cancer 2018, 18, 324. [Google Scholar] [CrossRef]
- de Menezes, S.A.F.; Miranda, Y.M.S.; da Silva, Y.M.; Carvalho, T.R.B.; Alves, F.R.S.; Silvestre, R.V.D.; Oliveira-Filho, A.B.; de Alencar Menezes, T.O.; de Souza Fonseca, R.R.; Laurentino, R.V.; et al. Prevalence and Genotyping of HPV in Oral Squamous Cell Carcinoma in Northern Brazil. Pathogens 2022, 11, 1106. [Google Scholar] [CrossRef]
- Ramos, G.M.D.; Cotter, T.G.; Ramos, L.F.; Floril, V.T.; Martinez, G.A.R.; Ruiz-Cabezas, J.C. A pilot study on the identification of human papillomavirus genotypes in tongue cancer samples from a single institution in Ecuador. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas Biol. 2018, 51, e7810. [Google Scholar] [CrossRef]
- Dhanapal, R.; Ranganathan, K.; Kondaiah, P.; Devi, R.U.; Joshua, E.; Saraswathi, T.R. High-risk human papilloma virus in archival tissues of oral pathosis and normal oral mucosa. Contemp. Clin. Dent. 2015, 6, 148–152. [Google Scholar] [CrossRef]
- Dirasantchu, S.; Marthala, M.; Shaik, S.; Jayam, R.; Venkata, S.S.; Bokkasam, V. Detection of human papilloma virus (HPV) and human immunodeficiency virus (HIV) in oral squamous cell carcinoma: A polymerized chain reaction (PCR) study. J. Indian Acad. Oral Med. Radiol. 2015, 27, 377–381. [Google Scholar] [CrossRef]
- Duncan, L.D.; Winkler, M.; Carlson, E.R.; Heidel, R.E.; Kang, E.; Webb, D. p16 immunohistochemistry can be used to detect human papillomavirus in oral cavity squamous cell carcinoma. J. Oral Maxillofac. Surg. 2013, 71, 1367–1375. [Google Scholar] [CrossRef]
- Elango, K.J.; Suresh, A.; Erode, E.M.; Subhadradevi, L.; Ravindran, H.K.; Iyer, S.K.; Iyer, S.K.; Kuriakose, M.A. Role of human papilloma virus in oral tongue squamous cell carcinoma. Asian Pac. J. Cancer Prev. 2011, 12, 889–896. [Google Scholar]
- Emmett, S.; Boros, S.; Whiteman, D.C.; Porceddu, S.V.; Panizza, B.J.; Antonsson, A. Sexual behaviour, HPV status and p16(INK4a) expression in oropharyngeal and oral cavity squamous cell carcinomas: A case-case comparison study. J. Gen. Virol. 2018, 99, 783–789. [Google Scholar] [CrossRef]
- Emmett, S.; Jenkins, G.; Boros, S.; Whiteman, D.C.; Panizza, B.; Antonsson, A. Low prevalence of human papillomavirus in oral cavity squamous cell carcinoma in Queensland, Australia. ANZ J. Surg. 2017, 87, 714–719. [Google Scholar] [CrossRef]
- Gan, L.L.; Zhang, H.; Guo, J.H.; Fan, M.W. Prevalence of human papillomavirus infection in oral squamous cell carcinoma: A case-control study in Wuhan, China. Asian Pac. J. Cancer Prev. 2014, 15, 5861–5865. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, L.; Campisi, G.; Colella, G.; Capra, G.; Di Liberto, C.; Caleca, M.P.; Matranga, D.; D’Angelo, M.; Lo Muzio, L.; Ammatuna, P. Brushing of oral mucosa for diagnosis of HPV infection in patients with potentially malignant and malignant oral lesions. Mol. Diagn. Ther. 2006, 10, 49–55. [Google Scholar] [CrossRef] [PubMed]
- González-Ramírez, I.; Irigoyen-Camacho, M.E.; Ramírez-Amador, V.; Lizano-Soberón, M.; Carrillo-García, A.; García-Carrancá, A.; Sánchez-Pérez, Y.; Méndez-Martínez, R.; Granados-García, M.; Ruíz-Godoy, L.; et al. Association between age and high-risk human papilloma virus in Mexican oral cancer patients. Oral Dis. 2013, 19, 796–804. [Google Scholar] [CrossRef]
- Goto, M.; Hanai, N.; Nishikawa, D.; Nagao, T.; Hasegawa, Y. Prognosis of HPV-Positive Oral Squamous Carcinoma: A Cohort Study from Japan. J. Hard Tissue Biol. 2023, 32, 77–82. [Google Scholar] [CrossRef]
- Götz, C.; Drecoll, E.; Straub, M.; Bissinger, O.; Wolff, K.D.; Kolk, A. Impact of HPV infection on oral squamous cell carcinoma. Oncotarget 2016, 7, 76704–76712. [Google Scholar] [CrossRef][Green Version]
- Grewal, R.K.; Sircar, K.; Bhat, K.G.; Grewal, D.S.; Tyagi, K.K.; David, S. Detection of human papilloma virus-E6/E7 proteins of high-risk human papilloma virus in saliva and lesional tissue of oral squamous cell carcinoma patients using nested multiplex polymerase chain reaction: A comparative study. J. Oral Maxillofac. Pathol. 2018, 22, 318–324. [Google Scholar] [CrossRef]
- Ha, P.K.; Pai, S.I.; Westra, W.H.; Gillison, M.L.; Tong, B.C.; Sidransky, D.; Califano, J.A. Real-time quantitative PCR demonstrates low prevalence of human papillomavirus type 16 in premalignant and malignant lesions of the oral cavity. Clin. Cancer Res. 2002, 8, 1203–1209. [Google Scholar]
- Harbor, S.N.; Schneider, J.W.; Solomons, N.; Sanderson, M.; Afrogheh, A.H. An Evaluation of High-Risk HPV in Squamous Cell Carcinomas of the Lip in a South African Cohort. Head Neck Pathol. 2024, 18, 36. [Google Scholar] [CrossRef]
- Huang, C.G.; Lee, L.A.; Liao, C.T.; Yen, T.C.; Yang, S.L.; Liu, Y.C.; Li, J.C.; Gong, Y.N.; Kang, C.J.; Huang, S.F.; et al. Molecular and serologic markers of HPV 16 infection are associated with local recurrence in patients with oral cavity squamous cell carcinoma. Oncotarget 2017, 8, 34820–34835. [Google Scholar] [CrossRef]
- Huang, S.F.; Li, H.F.; Liao, C.T.; Wang, H.M.; Chen, I.H.; Chang, J.T.; Chen, Y.J.; Cheng, A.J. Association of HPV infections with second primary tumors in early-staged oral cavity cancer. Oral Dis. 2012, 18, 809–815. [Google Scholar] [CrossRef]
- Ishibashi, M.; Kishino, M.; Sato, S.; Morii, E.; Ogawa, Y.; Aozasa, K.; Kogo, M.; Toyosawa, S. The prevalence of human papillomavirus in oral premalignant lesions and squamous cell carcinoma in comparison to cervical lesions used as a positive control. Int. J. Clin. Oncol. 2011, 16, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Jaber, L.; Fatani, H.; Aldhahri, S.F. Absence of human papillomavirus in oral cavity squamous cell carcinomas among Saudi patients. Clin. Exp. Dent. Res. 2019, 5, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Jalouli, J.; Ibrahim, S.O.; Mehrotra, R.; Jalouli, M.M.; Sapkota, D.; Larsson, P.A.; Hirsch, J.M. Prevalence of viral (HPV, EBV, HSV) infections in oral submucous fibrosis and oral cancer from India. Acta Oto-Laryngol. 2010, 130, 1306–1311. [Google Scholar] [CrossRef]
- Jalouli, J.; Jalouli, M.M.; Sapkota, D.; Ibrahim, S.O.; Larsson, P.A.; Sand, L. Human papilloma virus, herpes simplex virus and epstein barr virus in oral squamous cell carcinoma from eight different countries. Anticancer Res. 2012, 32, 571–580. [Google Scholar]
- Jitani, A.K.; Raphael, V.; Mishra, J.; Shunyu, N.B.; Khonglah, Y.; Medhi, J. Analysis of Human Papilloma Virus 16/18 DNA and its Correlation with p16 Expression in Oral Cavity Squamous Cell Carcinoma in North-Eastern India: A Chromogenic in-situ Hybridization Based Study. J. Clin. Diagn. Res. 2015, 9, Ec04–Ec07. [Google Scholar] [CrossRef] [PubMed]
- Kaminagakura, E.; Villa, L.L.; Andreoli, M.A.; Sobrinho, J.S.; Vartanian, J.G.; Soares, F.A.; Nishimoto, I.N.; Rocha, R.; Kowalski, L.P. High-risk human papillomavirus in oral squamous cell carcinoma of young patients. Int. J. Cancer 2012, 130, 1726–1732. [Google Scholar] [CrossRef]
- Khanna, R.; Rao, G.R.; Tiwary, S.K.; Rai, A.; Khanna, S.; Khanna, A.K. Detection of human papilloma virus 16 and 18 DNA sequences by southern blot hybridization in oral leukoplakia and squamous cell carcinoma. Indian J. Surg. 2009, 71, 69–72. [Google Scholar] [CrossRef][Green Version]
- Khovidhunkit, S.O.; Buajeeb, W.; Sanguansin, S.; Poomsawat, S.; Weerapradist, W. Detection of human papillomavirus in oral squamous cell carcinoma, leukoplakia and lichen planus in Thai patients. Asian Pac. J. Cancer Prev. 2008, 9, 771–775. [Google Scholar]
- Kim, S.M.; Kwon, I.J.; Myoung, H.; Lee, J.H.; Lee, S.K. Identification of human papillomavirus (HPV) subtype in oral cancer patients through microarray technology. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 535–543. [Google Scholar] [CrossRef]
- Klozar, J.; Kratochvil, V.; Salakova, M.; Smahelova, J.; Vesela, E.; Hamsikova, E.; Betka, J.; Tachezy, R. HPV status and regional metastasis in the prognosis of oral and oropharyngeal cancer. Eur. Arch. Oto-Rhino-Laryngol. 2008, 265 (Suppl. S1), S75–S82. [Google Scholar] [CrossRef]
- Kouketsu, A.; Sato, I.; Abe, S.; Oikawa, M.; Shimizu, Y.; Takahashi, T.; Kumamoto, H. Detection of human papillomavirus infection in oral squamous cell carcinoma: A cohort study of Japanese patients. J. Oral Pathol. Med. 2016, 45, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.S.; Kulkarni, S.S.; Vastrad, P.P.; Kulkarni, B.B.; Markande, A.R.; Kadakol, G.S.; Hiremath, S.V.; Kaliwal, S.; Patil, B.R.; Gai, P.B. Prevalence and distribution of high risk human papillomavirus (HPV) Types 16 and 18 in Carcinoma of cervix, saliva of patients with oral squamous cell carcinoma and in the general population in Karnataka, India. Asian Pac. J. Cancer Prev. 2011, 12, 645–648. [Google Scholar]
- Lee, L.A.; Huang, C.G.; Liao, C.T.; Lee, L.Y.; Hsueh, C.; Chen, T.C.; Lin, C.Y.; Fan, K.H.; Wang, H.M.; Huang, S.F.; et al. Human papillomavirus-16 infection in advanced oral cavity cancer patients is related to an increased risk of distant metastases and poor survival. PLoS ONE 2012, 7, e40767. [Google Scholar] [CrossRef]
- Lee, L.A.; Huang, C.G.; Tsao, K.C.; Liao, C.T.; Kang, C.J.; Chang, K.P.; Huang, S.F.; Chen, I.H.; Fang, T.J.; Li, H.Y.; et al. Human Papillomavirus Infections are Common and Predict Mortality in a Retrospective Cohort Study of Taiwanese Patients with Oral Cavity Cancer. Medicine 2015, 94, e2069. [Google Scholar] [CrossRef]
- Liang, X.H.; Lewis, J.; Foote, R.; Smith, D.; Kademani, D. Prevalence and significance of human papillomavirus in oral tongue cancer: The Mayo Clinic experience. J. Oral Maxillofac. Surg. 2008, 66, 1875–1880. [Google Scholar] [CrossRef]
- Lukesova, E.; Boucek, J.; Rotnaglova, E.; Salakova, M.; Koslabova, E.; Grega, M.; Eckschlager, T.; Rihova, B.; Prochazka, B.; Klozar, J.; et al. High level of Tregs is a positive prognostic marker in patients with HPV-positive oral and oropharyngeal squamous cell carcinomas. BioMed Res. Int. 2014, 2014, 303929. [Google Scholar] [CrossRef] [PubMed]
- Matzow, T.; Boysen, M.; Kalantari, M.; Johansson, B.; Hagmar, B. Low detection rate of HPV in oral and laryngeal carcinomas. Acta Oncol. 1998, 37, 73–76. [Google Scholar] [CrossRef]
- Montaldo, C.; Mastinu, A.; Zorco, S.; Santini, N.; Pisano, E.; Piras, V.; Denotti, G.; Peluffo, C.; Erriu, M.; Garau, V.; et al. Distribution of human papillomavirus genotypes in sardinian patients with oral squamous cell carcinoma. Open Virol. J. 2010, 4, 163–168. [Google Scholar] [CrossRef]
- More, P.; Kheur, S.; Patekar, D.; Kheur, M.; Gupta, A.A.; Raj, A.T.; Patil, S. Assessing the nature of the association of human papillomavirus in oral cancer with and without known risk factors. Transl. Cancer Res. 2020, 9, 3119–3125. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, J.K.; Patnaik, S.; Das, B.R. Prevalence of high-risk human papilloma virus types and its association with P53 codon 72 polymorphism in tobacco addicted oral squamous cell carcinoma (OSCC) patients of Eastern India. Int. J. Cancer 2002, 97, 649–653. [Google Scholar] [CrossRef]
- Naqvi, S.U.; Khan, S.; Ahmed, A.; Lail, A.; Gul, S.; Ahmed, S. Prevalence of EBV, CMV, and HPV in oral squamous cell carcinoma patients in the Pakistani population. J. Med. Virol. 2020, 92, 3880–3883. [Google Scholar] [CrossRef]
- Nauta, I.H.; Heideman, D.A.M.; Brink, A.; van der Steen, B.; Bloemena, E.; Koljenović, S.; Baatenburg de Jong, R.J.; Leemans, C.R.; Brakenhoff, R.H. The unveiled reality of human papillomavirus as risk factor for oral cavity squamous cell carcinoma. Int. J. Cancer 2021, 149, 420–430. [Google Scholar] [CrossRef]
- Nola-Fuchs, P.; Boras, V.V.; Plecko, V.; Plestina, S.; Milenović, A.; Susić, M.; Brailo, V. The prevalence of human papillomavirus 16 and Epstein-Barr virus in patients with oral squamous cell carcinoma. Acta Clin. Croat. 2012, 51, 609–614. [Google Scholar]
- Oliveira, L.R.; Ribeiro-Silva, A.; Ramalho, L.N.; Simões, A.L.; Zucoloto, S. HPV infection in Brazilian oral squamous cell carcinomapatients and its correlation with clinicopathological outcomes. Mol. Med. Rep. 2008, 1, 123–129. [Google Scholar] [CrossRef]
- Ostwald, C.; Rutsatz, K.; Schweder, J.; Schmidt, W.; Gundlach, K.; Barten, M. Human papillomavirus 6/11, 16 and 18 in oral carcinomas and benign oral lesions. Med. Microbiol. Immunol. 2003, 192, 145–148. [Google Scholar] [CrossRef]
- Palmieri, A.; Scapoli, L.; Martinelli, M.; Pezzetti, F.; Girardi, A.; Spinelli, G.; Lucchese, A.; Carinci, F. Incidence of low risk human papillomavirus in oral cancer: A real time PCR study on 278 patients. Int. J. Immunopathol. Pharmacol. 2011, 24, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, K.; Rameshkumar, A.; Rajkumar, K.; Ramadoss, R. Detection of human papillomavirus 16 and 18 in patients with oral squamous cell carcinoma and potentially malignant oral disorders in South Indian population: A pilot study. J. Cancer Res. Ther. 2019, 15, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Panzarella, V.; Campisi, G.; Giardina, Y.; Maniscalco, L.; Capra, G.; Rodolico, V.; Di Fede, O.; Mauceri, R. Low Frequency of Human Papillomavirus in Strictly Site-Coded Oral Squamous Cell Carcinomas, Using the Latest NHI/SEER-ICD Systems: A Pilot Observational Study and Critical Review. Cancers 2021, 13, 4595. [Google Scholar] [CrossRef] [PubMed]
- Parshad, S.; Nandi, S.; Marwah, N.; Mehta, P.; Tripathi, M.; Netrapal; Gogna, S.; Karwasra, R.K. Human papillomavirus 16 and 18 in squamous cell carcinoma of oral cavity and sexual practices: A pilot study at a Tertiary Care Hospital of North India. Natl. J. Maxillofac. Surg. 2015, 6, 185–189. [Google Scholar] [CrossRef]
- Patel, K.R.; Vajaria, B.N.; Begum, R.; Desai, A.; Patel, J.B.; Shah, F.D.; Shukla, S.N.; Patel, P.S. Prevalence of high-risk human papillomavirus type 16 and 18 in oral and cervical cancers in population from Gujarat, West India. J. Oral Pathol. Med. 2014, 43, 293–297. [Google Scholar] [CrossRef]
- Petito, G.; Carneiro, M.A.; Santos, S.H.; Silva, A.M.; Alencar, R.C.; Gontijo, A.P.; Saddi, V.A. Human papillomavirus in oral cavity and oropharynx carcinomas in the central region of Brazil. Braz. J. Otorhinolaryngol. 2017, 83, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Phusingha, P.; Ekalaksananan, T.; Vatanasapt, P.; Loyha, K.; Promthet, S.; Kongyingyoes, B.; Patarapadungkit, N.; Chuerduangphui, J.; Pientong, C. Human papillomavirus (HPV) infection in a case-control study of oral squamous cell carcinoma and its increasing trend in northeastern Thailand. J. Med. Virol. 2017, 89, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Pintos, V.L.J. Human Papillomavirus Infection and Oral Cancer: A Case-Control Study. Ph.D. Thesis, McGill University, Montréal, QC, Canada, 2002. [Google Scholar]
- Polz, D.; Polz-Dacewicz, M.; Morshed, K.; Jędrych, M. Prevalence of human papillomavirus in oral and oropharynx squamous cell carcinoma. Bull. Vet. Inst. Pulawy 2010, 54, 675–681. [Google Scholar] [CrossRef]
- Polz-Gruszka, D.; Morshed, K.; Stec, A.; Polz-Dacewicz, M. Prevalence of Human papillomavirus (HPV) and Epstein-Barr virus (EBV) in oral and oropharyngeal squamous cell carcinoma in south-eastern Poland. Infect. Agents Cancer 2015, 10, 37. [Google Scholar] [CrossRef]
- Premoli-De-Percoco, G.; Ramirez, J.L. High risk human papillomavirus in oral squamous carcinoma: Evidence of risk factors in a Venezuelan rural population. Preliminary report. J. Oral Pathol. Med. 2001, 30, 355–361. [Google Scholar] [CrossRef]
- Purwanto, D.J.; Soedarsono, N.; Reuwpassa, J.O.; Adisasmita, A.C.; Ramli, M.; Djuwita, R. The prevalence of oral high-risk HPV infection in Indonesian oral squamous cell carcinoma patients. Oral Dis. 2020, 26, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Rahbarnia, L.; Farajnia, S.; Bayazian, G.; Naderpour, M.; Feizi, H. Prevalence of human papillomavirus in patients with oral squamous cell carcinoma in Tabriz, Iran. Crescent J. Med. Biol. Sci. 2019, 6, 105–108. [Google Scholar]
- Prakash, S.M.R.; Jha, R.K.; Chawla, R.; Kupendra, S.; Kamarthi, N.; Jugade, S.C.; Tiwari, H.D. Assessment of the Impact of HPV Infection on the Incidence and Prognosis of Oral Cancers. J. Pharm. Bioallied Sci. 2024, 16, S2733–S2736. [Google Scholar] [CrossRef]
- Rivero, E.R.; Nunes, F.D. HPV in oral squamous cell carcinomas of a Brazilian population: Amplification by PCR. Braz. Oral Res. 2006, 20, 21–24. [Google Scholar] [CrossRef][Green Version]
- Rodríguez-Santamarta, T.; Rodrigo, J.P.; García-Pedrero, J.M.; Álvarez-Teijeiro, S.; Ángeles Villaronga, M.; Suárez-Fernández, L.; Alvarez-Argüelles, M.E.; Astudillo, A.; de Vicente, J.C. Prevalence of human papillomavirus in oral squamous cell carcinomas in northern Spain. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 4549–4559. [Google Scholar] [CrossRef] [PubMed]
- Rout, T.; Panda, S.K.; Shankar, K.V.; Kar, D.; Mohanty, D.P.; Agrawala, S. Prevalence of HPV in Oral Squamous Cell Carcinoma Through p16 IHC: A Hospital-Based Study in Eastern India. Niger. J. Basic Clin. Sci. 2024, 21, 132–136. [Google Scholar] [CrossRef]
- Rungraungrayabkul, D.; Panpradit, N.; Lapthanasupkul, P.; Kitkumthorn, N.; Klanrit, P.; Subarnbhesaj, A.; Sresumatchai, V.; Klongnoi, B.; Khovidhunkit, S.P. Detection of Human Papillomavirus and p16(INK4a) Expression in Thai Patients with Oral Squamous Cell Carcinoma. Head Neck Pathol. 2022, 16, 444–452. [Google Scholar] [CrossRef]
- Saini, R.; Tang, T.H.; Zain, R.B.; Cheong, S.C.; Musa, K.I.; Saini, D.; Ismail, A.R.; Abraham, M.T.; Mustafa, W.M.; Santhanam, J. Significant association of high-risk human papillomavirus (HPV) but not of p53 polymorphisms with oral squamous cell carcinomas in Malaysia. J. Cancer Res. Clin. Oncol. 2011, 137, 311–320. [Google Scholar] [CrossRef]
- Schwartz, S.R.; Yueh, B.; McDougall, J.K.; Daling, J.R.; Schwartz, S.M. Human papillomavirus infection and survival in oral squamous cell cancer: A population-based study. Otolaryngol.—Head Neck Surg. 2001, 125, 1–9. [Google Scholar] [CrossRef]
- Shima, K.; Kobayashi, I.; Saito, I.; Kiyoshima, T.; Matsuo, K.; Ozeki, S.; Ohishi, M.; Sakai, H. Incidence of human papillomavirus 16 and 18 infection and p53 mutation in patients with oral squamous cell carcinoma in Japan. Br. J. Oral Maxillofac. Surg. 2000, 38, 445–450. [Google Scholar] [CrossRef]
- Sichero, L.; Gonçalves, M.G.; Bettoni, F.; Coser, E.M.; Mota, G.; Nunes, R.A.L.; Mercante, A.; Natalino, R.; Uno, M.; Ferreira Alves, M.J.; et al. Detection of serum biomarkers of HPV-16 driven oropharynx and oral cavity cancer in Brazil. Oral Oncol. 2024, 149, 106676. [Google Scholar] [CrossRef]
- Simonato, L.E.; Garcia, J.F.; Sundefeld, M.L.; Mattar, N.J.; Veronese, L.A.; Miyahara, G.I. Detection of HPV in mouth floor squamous cell carcinoma and its correlation with clinicopathologic variables, risk factors and survival. J. Oral Pathol. Med. 2008, 37, 593–598. [Google Scholar] [CrossRef]
- Singh, A.K.; Kushwaha, J.K.; Anand, A.; Sonkar, A.A.; Husain, N.; Srivastava, K.; Singh, S. Human Papilloma Virus in Oral Cavity Cancer and Relation to Change in Quality of Life Following Treatment-a Pilot Study from Northern India. Indian J. Surg. Oncol. 2016, 7, 386–391. [Google Scholar] [CrossRef]
- Singh, V.; Husain, N.; Akhtar, N.; Kumar, V.; Tewari, S.; Mishra, S.; Misra, S.; Khan, M.Y. Do Human Papilloma Viruses Play Any Role in Oral Squamous Cell Carcinoma in North Indians? Asian Pac. J. Cancer Prev. 2015, 16, 7077–7084. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Hoffman, H.T.; Summersgill, K.S.; Kirchner, H.L.; Turek, L.P.; Haugen, T.H. Human papillomavirus and risk of oral cancer. Laryngoscope 1998, 108, 1098–1103. [Google Scholar] [CrossRef]
- Soares, R.C.; Oliveira, M.C.; Souza, L.B.; Costa, A.L.; Medeiros, S.R.; Pinto, L.P. Human papillomavirus in oral squamous cells carcinoma in a population of 75 Brazilian patients. Am. J. Otolaryngol. 2007, 28, 397–400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sri, S.; Ramani, P.; Premkumar, P.; Ramshankar, V.; Ramasubramanian, A.; Krishnan, R.P. Prevalence of Human Papillomavirus (HPV) 16 and 18 in Oral Malignant and Potentially Malignant Disorders: A Polymerase Chain Reaction Analysis—A Comparative Study. Ann. Maxillofac. Surg. 2021, 11, 6–11. [Google Scholar] [CrossRef]
- Taberna, M.; Inglehart, R.C.; Pickard, R.K.; Fakhry, C.; Agrawal, A.; Katz, M.L.; Gillison, M.L. Significant changes in sexual behavior after a diagnosis of human papillomavirus-positive and human papillomavirus-negative oral cancer. Cancer 2017, 123, 1156–1165. [Google Scholar] [CrossRef]
- Tachezy, R.; Klozar, J.; Saláková, M.; Smith, E.; Turek, L.; Betka, J.; Kodet, R.; Hamsíková, E. HPV and other risk factors of oral cavity/oropharyngeal cancer in the Czech Republic. Oral Dis. 2005, 11, 181–185. [Google Scholar] [CrossRef]
- Tang, K.D.; Menezes, L.; Baeten, K.; Walsh, L.J.; Whitfield, B.C.S.; Batstone, M.D.; Kenny, L.; Frazer, I.H.; Scheper, G.C.; Punyadeera, C. Oral HPV16 Prevalence in Oral Potentially Malignant Disorders and Oral Cavity Cancers. Biomolecules 2020, 10, 223. [Google Scholar] [CrossRef]
- Tangthongkum, M.; Phisalmongkhon, S.; Leelasawatsuk, P.; Supanimitjaroenporn, P.; Kirtsreesakul, V.; Tantipisit, J. Impact of human papillomavirus status on survival in patients with oral cancer. Laryngoscope Investig. Otolaryngol. 2024, 9, e1294. [Google Scholar] [CrossRef]
- Tealab, S.H.; Sedhom, N.F.H.; Hassouna, A.; Gouda, I.; Ismail, H. Prevalence of human papilloma virus in oropharyngeal, tongue and lip squamous cell carcinoma: An experience from the Egyptian National Cancer Institute. J. Investig. Med. 2019, 67, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Tokuzen, N.; Nakashiro, K.I.; Tojo, S.; Goda, H.; Kuribayashi, N.; Uchida, D. Human papillomavirus-16 infection and p16 expression in oral squamous cell carcinoma. Oncol. Lett. 2021, 22, 528. [Google Scholar] [CrossRef] [PubMed]
- Tsimplaki, E.; Argyri, E.; Xesfyngi, D.; Daskalopoulou, D.; Stravopodis, D.J.; Panotopoulou, E. Prevalence and expression of human papillomavirus in 53 patients with oral tongue squamous cell carcinoma. Anticancer Res. 2014, 34, 1021–1025. [Google Scholar]
- Valls-Ontañón, A.; Hernández-Losa, J.; Somoza Lopez de Haro, R.; Bellosillo-Paricio, B.; Ramón, Y.C.S.; Bescós-Atín, C.; Munill-Ferrer, M.; Alberola-Ferranti, M. Impact of human papilloma virus in patients with oral and oropharyngeal squamous cell carcinomas. Med. Clin. 2019, 152, 174–180. [Google Scholar] [CrossRef]
- Vanshika, S.; Preeti, A.; Sumaira, Q.; Vijay, K.; Shikha, T.; Shivanjali, R.; Shankar, S.U.; Mati, G.M. Incidence OF HPV and EBV in oral cancer and their clinico-pathological correlation- a pilot study of 108 cases. J. Oral Biol. Craniofacial Res. 2021, 11, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Verma, H.; Singh, S.K.; Phulambrikar, T.; Gupta, A. Evaluation of human papillomavirus as an independent risk factor in known patients of oral squamous cell carcinoma using immunohistochemistry. J. Indian Acad. Oral Med. Radiol. 2018, 30, 367–371. [Google Scholar] [CrossRef]
- Loustau, A.C.V.; Dulguerov, N.; Curvoisier, D.; McKee, T.; Lombardi, T. Low prevalence of HPV-induced oral squamous cell carcinoma in Geneva, Switzerland. Oral Dis. 2019, 25, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Q.; Xiao, X.; Li, C.X.; Wu, W.Y.; Shen, X.M.; Zhou, Z.T.; Fan, Y.; Shi, L.J. Human papillomavirus genotypes and p16 expression in oral leukoplakia and squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2019, 12, 1022–1028. [Google Scholar]
- Zhang, Z.Y.; Sdek, P.; Cao, J.; Chen, W.T. Human papillomavirus type 16 and 18 DNA in oral squamous cell carcinoma and normal mucosa. Int. J. Oral Maxillofac. Surg. 2004, 33, 71–74. [Google Scholar] [CrossRef]
- Christianto, S.; Li, K.Y.; Huang, T.H.; Su, Y.X. The Prognostic Value of Human Papilloma Virus Infection in Oral Cavity Squamous Cell Carcinoma: A Meta-Analysis. Laryngoscope 2022, 132, 1760–1770. [Google Scholar] [CrossRef]
- Lima, M.A.P.d.; Silva, C.G.L.d.; Rabenhorst, S.H.B. Association between human papillomavirus (HPV) and the oral squamous cell carcinoma: A systematic review. J. Bras. Patol. Med. Lab. 2014, 50, 75–84. [Google Scholar] [CrossRef][Green Version]
- Shigeishi, H.; Sugiyama, M. Risk factors for oral human papillomavirus infection in healthy individuals: A systematic review and meta-analysis. J. Clin. Med. Res. 2016, 8, 721. [Google Scholar] [CrossRef]
- Kaur, G.; Yap, T.; Ramani, R.; McCullough, M.; Singh, A. Assessing bias in the causal role of HPV in oral cancer: A systematic review and meta-analysis. Oral Dis. 2024, 30, 5379–5387. [Google Scholar] [CrossRef]
- Sathish, N.; Wang, X.; Yuan, Y. Human papillomavirus (HPV)-associated oral cancers and treatment strategies. J. Dent. Res. 2014, 93, 29S–36S. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.H.; McMillan, N.A.; Johnson, N.W. HPV-associated head and neck cancers in the Asia Pacific: A critical literature review & meta-analysis. Cancer Epidemiol. 2015, 39, 923–938. [Google Scholar] [PubMed]
- Shavers, V.L.; Harlan, L.C.; Winn, D.; Davis, W.W. Racial/ethnic patterns of care for cancers of the oral cavity, pharynx, larynx, sinuses, and salivary glands. Cancer Metastasis Rev. 2003, 22, 25. [Google Scholar] [CrossRef] [PubMed]


| Author (YOP) | Country | Year of Investigation | Design | Sample Size | Cancer Location (Number) | Diagnostic Method (HPV) | Age | Gender | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Male | Female | |||||||
| De Abreu (2018) [54] | Brazil | 2012–2015 | Cross-sectional | 90 | Tongue (49), FOM (22), Other (19) | Nested PCR using MY09/MY11 and GP5+/GP6+ primers | 57.9 | 12.73 | 68 | 22 |
| Abreu (2020) [26] | UK | 2011–2015 | Prospective cohort | 99 | Tongue (72), FOM (9) | ISH | 60.5 | 13.3 | 77 | 22 |
| ADAMOPOULOU (2008) [27] | Germany | 2008 | Cross-sectional | 102 | Oral cavity cancer (68) | PCR protocol | 52.1 | 10.3 | 51 | 51 |
| Adilbay (2018) [28] | Kazakhistan | 2015–2017 | Prospective cohort | 76 | Oral cavity cancer (42) | PCR protocol | 57.2 | 11.45 | 50 | 26 |
| Afzal (2019) [29] | Pakistan | 2018–2019 | Cross-sectional | 140 | Oral cavity cancer (140) | PCR protocol | 48.86 | 9.37 | 114 | 26 |
| Ahmed (2019) [30] | Iraq | 2019 | Cross-sectional | 80 | Oral cavity cancer (40) | PCR protocol | - | - | 24 | 16 |
| Ajila (2021) [31] | India | 2021 | Case-control | 60 | Oral cavity cancer (30) | PCR protocol | 58 | 8.86 | 25 | 5 |
| Akhondnezhad (2018) [32] | Iran | 2006–2016 | Cross-sectional | 83 | Oral cavity cancer (83) | PCR protocol | 46.2 | 15.5 | 43 | 40 |
| Ali (2008) [33] | Pakistan | 1991–2004 | Retrospective cohort | 140 | Oral cavity (86), tongue (54) | PCR protocol/primers GP5/6 | 50 | 13 | 82 | 58 |
| Alsharif (2021) [34] | Germany | 2002–2011 | Cross-sectional | 280 | Not specified | ISH | 62.8 | 12 | 188 | 92 |
| Vidal Loustau (2019) [136] | Switzerland | 2001–2011 | Retrospective cohort | 155 | Mobile tongue (61) | PCR protocol | 66.5 | 13.63 | 107 | 48 |
| Antuncov (2022) [35] | Montenegro | 2012–2018 | Cross-sectional | 60 | Tonge (22), FOM (10), lower lip (28) | PCR protocol | 62 | 10.5 | 47 | 13 |
| Anwar (2024) [36] | Pakistan | 2017 | Cross-sectional | 186 | Not specified | PCR protocol | - | - | - | - |
| Ashraf (2017) [37] | Iran | 2017 | Case-control | 100 | Oral tongue SCC (50) | nested PCR | 53.54 | 11.19 | 41 | 59 |
| Balaram (1995) [38] | Singapore | 1995 | Cross-sectional | 91 | Oral cavity (91) | PCR protocol | - | - | - | - |
| Belobrov (2017) [39] | Australia | 2007–2011 | Prospective cohort | 46 | Tongue (20), FOM (5), check mucosa (5), Mandibular Alveolus (2) | Laser capture microdissection | - | - | 26 | 20 |
| Bijina (2020) [41] | India | 2020 | Case-control | 90 | Oral cavity (47) | PCR protocol, gel electrophoresis | 55 | 14.96 | 70 | 20 |
| Boy (2006) [42] | South Africa | 1998–2003 | Cross-sectional | 59 | Oral cavity (59) | ISH/signal enhancement (Genpoint)/PCR | 57.58 | 8.41 | 41 | 18 |
| Božinović (2020) [43] | Serbia | 2005–2006 | Cross-sectional | 63 | Tonsil (13), Tongue (9) | ISH | 54.7 | 4.6 | 39 | 24 |
| Campisi (2006) [44] | Italy | 2006 | Cross-sectional | 63 | Not specified | PCR protocol | 68.89 | 11.78 | 28 | 35 |
| Chakrobarty (2014) [45] | India | 2006–2008 | Case-control | 183 | Oral cancer (83) | PCR protocol | 50.81 | 10.56 | 136 | 47 |
| Chen (2012) [46] | Taiwan | 2003–2004 | Cross-sectional | 65 | Tongue (35), buccal mucosa (20), gingiva (2), hard palate (1), FOM (7) | ISH | 54.3 | 10.88 | 52 | 13 |
| Chen (2016) [47] | China | 2016 | Cross-sectional | 99 | Oral cavity cancer (40) | PCR protocol | 56.7 | - | 35 | 5 |
| Chotipanich (2018) [48] | Thailand | 2018 | Case-control | 208 | Oral cavity (52) | PCR protocol | 60 | 11.7 | 154 | 54 |
| Chowdary (2018) [49] | India | 2018 | Case-control | 40 | Oral cavity (20) | PCR protocol | - | - | 24 | 16 |
| Cutilli (2016) [50] | Italy | 1992–2012 | Retrospective cohort | 75 | Not specified | PCR protocol | 67 | 3.15 | 57 | 18 |
| DAHLGREN (2004) [51] | Sweden | 1970–2002 | Cross-sectional | 110 | Mobile tongue (85), base of tongue (25) | PCR protocol/primers GP5/6 | 62.46 | 12.72 | 69 | 41 |
| D’Costa (1998) [53] | India | 1998 | Cross-sectional | 100 | Buccal (57), tongue (14), FOM (2) | PCR protocol | 51.3 | 12.2 | 72 | 28 |
| Dhanapal (2015) [57] | India | 2015 | Cross-sectional | 23 | Buccal mucosa (8), FOM (2), tongue (1) | PCR protocol | 61.5 | 6.5 | 7 | 7 |
| Duncan (2013) [59] | USA | 2002–2007 | Cross-sectional | 81 | Tongue (36), FOM (11), buccal mucosa (4), lip (2) | PCR protocol/IHC | 63.9 | 12.57 | 44 | 37 |
| Elango (2011) [60] | India | 2004–2007 | Case-control | 106 | Oral tongue cancer (60) | PCR protocol, IHC, ISH | 53.87 | 13.32 | 76 | 30 |
| Emmett (2017) [62] | Australia | 2006–2012 | Cross-sectional | 63 | Tongue (48), FOM (14), Oral cavity (1) | PCR protocol | 60.7 | 13 | 47 | 16 |
| Emmett (2018) [61] | Australia | 2018 | Cross-sectional | 136 | Oral cavity (40) | PCR protocol | - | - | 113 | 23 |
| Nola-Fuchs (2012) [95] | Croatia | 2012 | Case-control | 54 | Not specified | Swab | 53.9 | 10.1 | 45 | 9 |
| Gan (2014) [63] | China | 2009–2013 | Case-control | 268 | Not specified | PCR protocol | - | - | - | - |
| Giovannelli (2006) [64] | Italy | 2004 | Cross-sectional | 116 | Oral cavity (17) | PCR protocol | 58.9 | 12.75 | 49 | 67 |
| Goto (2023) [66] | Japan | 2009–2013 | Cross-sectional | 67 | Tongue (34), FOM (5) | PCR protocol | - | - | 54 | 13 |
| Götz (2016) [67] | Germany | 2009–2011 | Cross-sectional | 202 | Not specified | IHC | 57.58 | 10.23 | 145 | 57 |
| Ha (2022) [69] | Maryland | 1982–2000 | Cross-sectional | 102 | Oral cavity (34) | PCR protocol | 59 | 15.5 | 85 | 17 |
| Harbor (2024) [70] | South Africa | 2009–2019 | Cross-sectional | 50 | Lip (50) | HybriSpot HPV Direct Flow Chip kit | 61 | 14 | 38 | 12 |
| Huang (2012) [72] | Taiwan | 1997–2003 | Cross-sectional | 103 | Tongue (60), lip (1), mouth floor (6) | PCR protocol | 94.4 | 10.9 | 96 | 7 |
| Huang (2017) [71] | Taiwan | 2017 | Cross-sectional | 85 | Not specified | PCR protocol | - | - | 78 | 7 |
| Ibieta (2005) [8] | Mexico | 1999–2001 | Cross-sectional | 50 | Tongue (13), mouth of floor (4) | PCR protocol | - | - | 36 | 14 |
| Ishibashi (2011) [73] | Japan | 2011 | Cross-sectional | 107 | Oral cavity (50) | PCR protocol/using consensus primers (My09/My11, GP5?/GP6?) | 59.2 | 13.72 | 57 | 50 |
| Jaber (2019) [74] | Saudi Arabia | 2010–2014 | Retrospective cohort | 45 | Not specified | ISH | 60.25 | - | 24 | 21 |
| JALOULI (2010) [75] | India | 2010 | Cross-sectional | 74 | Tongue (18), buccal (12), lip (6) | PCR protocol | 55.3 | 10.7 | 59 | 15 |
| Jalouli (2012) [76] | Sweden | 2012 | Cross-sectional | 155 | Tongue (41), FOM (23) | PCR protocol | 63.3 | - | - | - |
| JitAni (2015) [77] | India | 2010–2013 | Cross-sectional | 31 | Not specified | PCR protocol/ISH | - | - | 16 | 15 |
| Kaminagakura (2012) [78] | Brazil | 1970 to 2006 | Case-control | 114 | Tongue (23), buccal (1) | PCR protocol/IHC | 34 | 5.4 | 83 | 33 |
| KANSKY (2003) [9] | Slovenia | 1994–1998 | Case-control | 124 | Oral cavity (62) | PCR protocol | 58.2 | 7.3 | 55 | 7 |
| Grewal (2018) [68] | India | 2011–2014 | Cross-sectional | 47 | Tongue (23), lip (4), buccal (9) | nested PCR | - | - | 36 | 11 |
| Khanna (2009) [79] | India | 2007–2009 | Case-control | 120 | Not specified | PCR protocol | 50.6 | - | 90 | 30 |
| Khovidhunkit (2008) [80] | Thailand | 2008 | Cross-sectional | 65 | Buccal mucosa (11) | PCR protocol | 58.22 | 13.06 | 15 | 50 |
| Kim (2018) [81] | South Korea | 2010–2015 | Retrospective cohort | 187 | Tongue (54), gum (80) | DNA chip kit | 64 | 11.9 | 116 | 71 |
| Klozar (2008) [82] | Czech Republic | 2001–2005 | Cross-sectional | 81 | Tonsil (51), oral (10), tongue (4), base of tongue (10) | PCR protocol | - | - | 51 | 30 |
| Komolmala (2020) [10] | Thailand | 1999–2019 | Cross-sectional | 403 | Tongue (46), FOM (8) | PCR protocol | 66 | - | 78 | 94 |
| Kouketsu (2015) [83] | Japan | 2012–2013 | Cross-sectional | 174 | Tongue (90), gingiva (43), buccal (22), FOM (7), lip (11) | PCR protocol | 67.6 | 12.7 | 76 | 98 |
| Kulkarni (2011) [84] | India | 2009–2010 | Cross-sectional | 490 | Oral cavity (34) | PCR protocol | - | - | - | - |
| Bhawal (2007) [40] | Japan | 2007 | Cross-sectional | 22 | Oral cavity (22) | PCR protocol/PT-PCR | 66.6 | 12.6 | 13 | 9 |
| Lee (2012) [85] | Taiwan | 2004–2006 | Prospective cohort | 333 | Not specified | PCR protocol | - | - | 316 | 17 |
| Lee (2015) [86] | Taiwan | 2004–2011 | Retrospective cohort | 1002 | Tongue (322), lip (35), FOM (31) | PCR protocol | - | - | 938 | 64 |
| Liang (2008) [87] | China | 2004–2006 | Cross-sectional | 51 | Oral tongue (51) | PCR protocol | 59.5 | 12.4 | 31 | 20 |
| Lukesova (2014) [88] | Czech Republic | 2014 | Cross-sectional | 60 | Oral cavity (5) | PCR protocol | 56.5 | - | 54 | 6 |
| Machado (2010) [11] | Canada | 1995–2007 | Retrospective cohort | 92 | Oral cavity, tongue, FOM, palate, buccal mucosa and gingiva (53) | PCR protocol | - | - | 64 | 28 |
| Makvandi (2022) [15] | Iran | 2013–2019 | Cross-sectional | 166 | Oral tongue (140), base of tongue (22), tonsils (4) | Nested PCR | 53.23 | 15.9 | 144 | 22 |
| Matzow (2009) [89] | Sweden | 2009 | Cross-sectional | 54 | Tongue (11), FOM (7), gingiva (10), buccal (2) | PCR protocol | - | - | - | - |
| De Menezes (2022) [55] | Brazil | 2019 | Cross-sectional | 101 | Tongue (19), lip (16), gingiva (46) | PCR/”Inno-Lipa Genotyping Extra II System | - | - | 46 | 55 |
| Montaldo (2010) [90] | Italy | 2007–2008 | Case-control | 120 | Not specified | PCR protocol | 61.7 | 13.3 | 72 | 48 |
| More (2020) [91] | Saudi Arabia | 2020 | Cross-sectional | 45 | Oral cavity (30) | PCR protocol | - | - | 31 | 14 |
| NAGPAL (2001) [92] | India | 2001 | Case-control | 110 | Tongue (6), lip (4) | PCR assay | - | - | 68 | 42 |
| Naqvi (2020) [93] | Pakistan | 2015–2017 | Cross-sectional | 58 | Tongue (17), lip (11), buccal mucosa (24) | PCR protocol | 42 | 12 | 48 | 10 |
| Nauta (2021) [94] | The Netherlands | 2008–2014 | Retrospective cohort | 940 | Tongue (451), FOM (268) | PCR protocol | 64.86 | 12 | 551 | 389 |
| Nekić (2022) [16] | Croatia | 2022 | Retrospective cohort | 99 | Oral cavity (26) | PCR protocol | - | - | 89 | 10 |
| OLIVEIRA (2003) [96] | Brazil | 2008 | Retrospective cohort | 87 | Tongue (22), lip (13) | PCR protocol | - | - | 73 | 14 |
| Ostwald (2003) [97] | Germany | 2003 | Cross-sectional | 267 | Intraorally (93), lips (21) | PCR protocol | 58.57 | - | 186 | 81 |
| PALMIER (2011) [98] | Italy | 1990–2007 | Case-control | 278 | Oral cavity | RT-PCR | - | - | - | - |
| Panneerselvam (2019) [99] | India | 2019 | Cross-sectional | 30 | Not specified | PCR protocol | 46.7 | - | 27 | 3 |
| Panzarella (2021) [100] | Italy | 2021 | Cross-sectional | 40 | Not specified | PCR protocol | 66.5 | 14.1 | 17 | 23 |
| Parshad (2015) [101] | India | 2015 | Prospective cohort | 50 | Tonsil (15), base of tongue (16) | PCR protocol | 55.32 | 10.2 | 44 | 6 |
| Patel (2015) [102] | India | 2015 | Cross-sectional | 149 | Tongue (21), buccal (39) | PCR protocol | 48.3 | 10.8 | 84 | 65 |
| Premoli-De-Percoco (2001) [108] | Venezuela | 2001 | Cross-sectional | 50 | Tongue (18), buccal mucosa (7), FOM (7) | PCR protocol | 56.3 | - | 0 | 50 |
| Petito (2017) [103] | Brazil | 2005–2007 | Cross-sectional | 82 | Oral cavity (39) | PCR protocol | - | - | 64 | 18 |
| Petrovic (2023) [13] | Serbia | 2018–2022 | Cross-sectional | 90 | Tongue (19), lip (4), buccal (4) | PCR protocol | 62.95 | - | 48 | 42 |
| Phusingha (2016) [104] | Thailand | 2005–2010 | Case-control | 191 | Tongue (20), lip (16), FOM (16) | Reverse line blot hybridization (RLBH) | - | - | 115 | 76 |
| POLZ (2010) [106] | Poland | 1998–2004 | Cross-sectional | 60 | Oral cavity (21) | PCR protocol | 57.5 | - | 54 | 6 |
| Polz-Gruszka (2015) [107] | Poland | 2006–2009 | Retrospective cohort | 154 | Oral cavity (92) | PCR protocol | 56.8 | 8.8 | 131 | 23 |
| Pongsapich (2016) [17] | Thailand | 2010–2012 | Cross-sectional | 46 | Not specified | PCR protocol | 59.6 | 15.16 | 29 | 17 |
| Ravi Prakash (2024) [111] | India | 2020–2022 | Retrospective cohort | 100 | Not specified | ISH or PCR. | 58.75 | 8.1 | 74 | 26 |
| Purwanto (2019) [109] | Indonesia | 2003–2013 | Cross-sectional | 78 | Tongue (58), lip (6), buccal (2) | PCR protocol | 47.08 | 14.15 | 47 | 31 |
| Rahbarnia (2019) [110] | Iran | 2012–2014 | Case-control | 60 | Tongue (30) | PCR protocol | 61.3 | 13.7 | 26 | 34 |
| González-Ramírez (2013) [65] | Mexico | 2007–2011 | Case-control | 400 | Tongue (47), palate (11), buccal (1), Gingival (21) | PCR protocol | - | - | - | - |
| Delgado Ramos (2018) [56] | Ecuador | 2006–2011 | Cross-sectional | 53 | Tongue (100%) | PCR protocol | 61.8 | 17.3 | 29 | 24 |
| Rivero (2006) [112] | Brazil | 2006 | Cross-sectional | 40 | Lip (20), Tongue (14), gingiva (3), FOM (2) and palate (1) | PCR protocol | 57 | 13.6 | 32 | 8 |
| Rodríguez-Santamarta (2016) [113] | Spain | 1996–2007 | Retrospective cohort | 125 | Tongue (51), FOM (37), buccal (7) | PCR protocol/ ISH | 58.6 | 14.4 | 82 | 43 |
| ROMANITAN (2008) [14] | Greece | 1986–2007 | Cross-sectional | 115 | Tonsil (31), tongue (38) | PCR protocol | 62 | 7.9 | - | - |
| Rout (2024) [114] | India | 2024 | Cross-sectional | 140 | Not specified | PCR protocol | 54.5 | - | 117 | 23 |
| Rungraungrayabkul (2022) [115] | Thailand | 2013–2019 | Retrospective cohort | 81 | Tongue (24) buccal mucosa (11) lip (5) | PCR protocol | - | - | 32 | 49 |
| Saini (2010) [116] | Malaysia | 2010 | Case-control | 210 | Tongue (29), lip (1) | GP5+/GP6+ in a nested PCR | 49.12 | 13.4 | 109 | 101 |
| Schwartz (2001) [117] | USA | 1988–1995 | Cross-sectional | 254 | Tongue (81), tonsil (44) | PCR protocol | 54.2 | - | 163 | 91 |
| Shima (2000) [118] | Japan | 1991–1996 | Cross-sectional | 46 | Tongue (27), buccal (3), FOM (3) | PCR protocol | 50 | 14 | 32 | 14 |
| Sichero (2024) [119] | brazil | 2015–2019 | Cross-sectional | 146 | Oral cavity (89) | PCR protocol | - | - | 118 | 28 |
| Simonato (2008) [120] | Brazil | 1991–2005 | Cross-sectional | 29 | Not specified | PCR protocol/GP5+⁄GP6+ (35) | - | - | 27 | 2 |
| Singh (2015) [122] | India | 2013–2015 | Prospective cohort | 250 | Buccal mucosa (127), FOM (4) | Real-Time PCR, Conventional PCR/IHC | - | - | 200 | 50 |
| Singh (2016) [121] | India | 2013–2014 | Prospective cohort | 43 | Not specified | PCR protocol | 45.56 | 10.04 | 37 | 6 |
| Smith (1998) [123] | USA | 1994–1996 | Case-control | 298 | Not specified | PCR protocol | - | - | 198 | 100 |
| Soares (2007) [124] | Brazil | 2000–2003 | Cross-sectional | 75 | Tongue (20), FOM (17), lips (14) | PCR protocol | 65.45 | 13.2 | 49 | 26 |
| Sri (2021) [125] | India | 2010–2012 | Cross-sectional | 40 | Not specified | Qiagen QIAamp DNA tissue Kit (Qiagen Inc., USA). | - | - | - | - |
| Dirasantchu (2015) [58] | India | 2015 | Case-control | 35 | Buccal mucosa (10), tongue (5), alveolus (4), retromolar (3), buccal sulcus (1) | PCR protocol | - | - | 24 | 11 |
| Taberna (2017) [126] | USA | July 1905 | Prospective cohort | 262 | Oral cavity (90) | ISH | - | - | 213 | 49 |
| Tachezy (2005) [127] | Czech Republic | 2000–2003 | Cross-sectional | 68 | Tongue (5), tonsil (8) | PCR protocol | 57 | - | 54 | 14 |
| Tang (2020) [128] | The Netherlands | 2020 | Cross-sectional | 183 | Not specified | nested PCR | - | - | 118 | 65 |
| Tangthongkum (2024) [129] | Thailand | 2012–2021 | Retrospective cohort | 381 | Not specified | PCR protocol | - | - | 232 | 149 |
| Tealab (2009) [130] | Egypt | 2008–2015 | Retrospective cohort | 99 | tongue (48), lip (45) | PCR protocol/ISH | 57.2 | 13 | 55 | 44 |
| Tokuzen (2021) [131] | Japan | 2004–2013 | Cross-sectional | 100 | Tongue (36), mandibular gingiva (31), maxillary gingiva (13), FOM (9), buccal mucosa (9), or lower lip (2) | RT-qPCR | 68.2 | 10.08 | 54 | 46 |
| Dalla Torre (2018) [52] | Australia | 2008–2012 | Retrospective cohort | 106 | Not specified | PCR protocol | 58.9 | 7.9 | 71 | 35 |
| TSIMPLAKI (2014) [132] | Greece | 2012–2013 | Cross-sectional | 53 | Not specified | PCR protocol | 51 | 12.4 | 39 | 14 |
| Valls-Ontanón (2007) [133] | Spain | 2010–2011 | Retrospective cohort | 155 | Tongue (47), buccal (11), lip (8) | PCR protocol | 72.7 | 13.4 | 107 | 48 |
| Vanshika (2021) [134] | India | 2018–2019 | Cross-sectional | 216 | Not specified | (RT-PCR) | 45.6 | - | 172 | 44 |
| Pintos Vega (2002) [105] | Canada | 1997–2001 | Case-control | 201 | Tongue except base (21), FOM (12), lips (1) | PCR protocol/DNA sequencing | 62.7 | - | 143 | 58 |
| Verma (2018) [135] | India | 2018 | Case-control | 100 | Tongue (16), buccal (12). lip (3) | PCR | 47.69 | 6.73 | - | - |
| Yang (2019) [137] | China | 2016–2017 | Case-control | 163 | Tongue (70), buccal (40), FOM (3) | IHC | 81.5 | 12 | 76 | 87 |
| Zhang (2004) [138] | China | 1997–1999 | Case-control | 113 | Tongue (35), buccal (14), FOM (10) | PCR protocol | - | - | 72 | 41 |
| Group | Prevalence (%) | 95% CI | Studies | Q | p-Value | Tau2 | I2 (%) | H2 |
|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||
| Female | 24.6 | 19.3–29.8 | 65 | 1022.39 | 0.000 | 0.038 | 96.39 | 27.71 |
| Male | 23.5 | 18.8–28.2 | 66 | 1201.37 | 0.000 | 0.035 | 96.84 | 31.63 |
| Age | ||||||||
| <40 | 29.7 | 20.3–39 | 20 | 75.32 | 0.000 | 0.026 | 81.45 | 5.39 |
| 40–60 | 25.4 | 19.8–30.9 | 61 | 812.97 | 0.000 | 0.043 | 95.15 | 20.61 |
| 60–70 | 24.3 | 17.4–31.3 | 26 | 242.16 | 0.000 | 0.027 | 91.43 | 11.67 |
| >70 | 23.8 | 8.6–39.1 | 8 | 42.02 | 0.000 | 0.037 | 90.78 | 10.85 |
| Smoking | ||||||||
| Current | 27.2 | 18.4–36 | 31 | 584.64 | 0.000 | 0.058 | 97.58 | 41.28 |
| Ever | 23.3 | 4.1–42.4 | 7 | 126.02 | 0.000 | 0.064 | 97.5 | 39.95 |
| Former | 9.4 | 0–19 | 4 | 1.87 | 0.599 | 0.000 | 0 | 1 |
| Never | 25.4 | 18.1–32.8 | 33 | 297.04 | 0.000 | 0.039 | 93.98 | 16.62 |
| Alcohol | ||||||||
| Ever | 22.7 | 14.9–30.4 | 24 | 207.95 | 0.000 | 0.032 | 94.81 | 19.26 |
| Excessive | 12.2 | 7.2–17.2 | 1 | 0.00 | 0.000 | |||
| Never | 21.8 | 13.9–29.8 | 26 | 254.81 | 0.000 | 0.036 | 95.68 | 23.15 |
| Histological Type | ||||||||
| MD | 23.4 | 16.8–30 | 38 | 542.57 | 0.000 | 0.037 | 96.61 | 29.48 |
| PD | 26.7 | 18.7–34.7 | 37 | 257.32 | 0.000 | 0.039 | 88.89 | 9 |
| VC | 34.1 | 3.9–64.4 | 4 | 22.09 | 0.000 | 0.080 | 90.88 | 10.97 |
| WD | 26.8 | 19.6–34 | 36 | 659.14 | 0.000 | 0.043 | 97.12 | 34.75 |
| AJCC | ||||||||
| I | 7.8 | 0–15.8 | 2 | 0.17 | 0.676 | 0.000 | 0 | 1 |
| II | 3.3 | 0–8.2 | 3 | 0.95 | 0.622 | 0.000 | 0 | 1 |
| III | 3.9 | 0–9.2 | 3 | 0.47 | 0.791 | 0.000 | 0.01 | 1 |
| IV | 10.3 | 5.5–15.1 | 3 | 0.31 | 0.859 | 0.000 | 0.01 | 1 |
| Site | ||||||||
| Lip | 25 | 14.7–35.3 | 19 | 79.63 | 0.000 | 0.034 | 78.07 | 4.56 |
| Lower Lip | 14.8 | 6–23.6 | 5 | 1.43 | 0.838 | 0.000 | 0 | 1 |
| Upper Lip | 16.7 | 0–58.8 | 1 | 0.00 | 0.000 | |||
| Gingiva | 18.2 | 10.9–25.5 | 20 | 64.75 | 0.000 | 0.018 | 83.7 | 6.14 |
| Lower Gingiva | 18.8 | 2.3–35.3 | 2 | 2.1 | 0.147 | 0.007 | 52.49 | 2.1 |
| Upper Gingiva | 3.9 | 0–10.5 | 3 | 0.81 | 0.665 | 0.000 | 0 | 1 |
| Mandibular Gingiva | 24.6 | 3.3–45.9 | 5 | 12.93 | 0.012 | 0.035 | 67.33 | 3.06 |
| Maxillary Gingiva | 23.1 | 8.9–37.2 | 4 | 4.05 | 0.256 | 0.000 | 0 | 1 |
| Alveolus | 24.5 | 0–56.1 | 4 | 26.89 | 0.000 | 0.094 | 91.53 | 11.81 |
| Lower Alveolus | 29.5 | 0–76.5 | 3 | 32.68 | 0.000 | 0.166 | 97.98 | 49.6 |
| Upper Alveolus | 7.1 | 0–26.2 | 1 | 0.00 | 0.000 | |||
| Oral Tongue | 22.7 | 16.7–28.7 | 51 | 504.77 | 0.000 | 0.042 | 95.38 | 21.67 |
| Mobile Tongue | 11.2 | 0–24.1 | 5 | 21.66 | 0.000 | 0.018 | 97.43 | 38.92 |
| Tongue Border | 17.1 | 0–34.9 | 2 | 0.16 | 0.687 | 0.000 | 0 | 1 |
| Buccal Mucosa | 20.9 | 14.2–27.6 | 39 | 423.85 | 0.000 | 0.036 | 93.46 | 15.3 |
| Floor of Mouth | 14.8 | 10.7–19 | 38 | 91.97 | 0.000 | 0.007 | 66.44 | 2.98 |
| Gingivobuccal sulcus | 4.7 | 0–13 | 2 | 0.45 | 0.504 | 0.000 | 0 | 1 |
| Hard Palate | 18.9 | 10.8–26.9 | 24 | 54.4 | 0.000 | 0.019 | 61.98 | 2.63 |
| Retromolar Trigone | 10.5 | 5–16 | 17 | 31.82 | 0.011 | 0.004 | 45.64 | 1.84 |
| Vestibulum of Mouth | 0.4 | 0–1.5 | 1 | 0.00 | 0.000 | |||
| Waldeyer ring | 25.8 | 10.4–41.2 | 1 | 0.00 | 0.000 | |||
| Pathological TNM | ||||||||
| I | 31.9 | 19.7–44.2 | 18 | 254.33 | 0.000 | 0.060 | 95.98 | 24.9 |
| II | 36.4 | 24.3–48.6 | 18 | 194.61 | 0.000 | 0.059 | 92.81 | 13.92 |
| III | 32.3 | 20.1–44.5 | 17 | 156.03 | 0.000 | 0.056 | 90.88 | 10.97 |
| IV | 29.1 | 17.3–40.8 | 15 | 129.22 | 0.000 | 0.045 | 93.4 | 15.15 |
| I–II | 28.8 | 19.1–38.4 | 25 | 556.59 | 0.000 | 0.055 | 97.47 | 39.6 |
| III–IV | 27.7 | 19.3–36.1 | 23 | 282.89 | 0.000 | 0.038 | 95.14 | 20.59 |
| Pathological T | ||||||||
| T1 | 25.4 | 13.5–37.2 | 18 | 224.66 | 0.000 | 0.056 | 97.82 | 45.9 |
| T2 | 25.9 | 15–36.8 | 19 | 415.85 | 0.000 | 0.053 | 98.56 | 69.44 |
| T3 | 28.4 | 16.5–40.3 | 20 | 241.49 | 0.000 | 0.066 | 96.11 | 25.71 |
| T4 | 25.9 | 14.9–37 | 19 | 312.68 | 0.000 | 0.052 | 96.05 | 25.31 |
| T1–T2 | 25 | 15.8–34.1 | 25 | 621.71 | 0.000 | 0.050 | 98.74 | 79.1 |
| T3–T4 | 25.9 | 16.8–35.1 | 25 | 582.91 | 0.000 | 0.051 | 97.54 | 40.6 |
| Pathological N | ||||||||
| N0 | 17.9 | 9.5–26.4 | 18 | 291.31 | 0.000 | 0.029 | 97.03 | 33.68 |
| N+ | 16.8 | 10.8–22.8 | 18 | 153.19 | 0.000 | 0.013 | 90.42 | 10.44 |
| N1 | 18.3 | 5.5–31.1 | 10 | 86.29 | 0.000 | 0.036 | 93.42 | 15.2 |
| N2 | 24 | 15.4–32.5 | 9 | 14.09 | 0.079 | 0.007 | 48.27 | 1.93 |
| N2a | 11.7 | 0–40.6 | 2 | 2.67 | 0.102 | 0.031 | 62.52 | 2.67 |
| N2b | 4.4 | 0–9.5 | 2 | 1.8 | 0.180 | 0.001 | 44.33 | 1.8 |
| N2c | 9 | 2.6–15.4 | 2 | 0.18 | 0.672 | 0.000 | 0 | 1 |
| N3 | 28.7 | 11.1–46.3 | 6 | 8.46 | 0.132 | 0.018 | 38.27 | 1.62 |
| N3a | 16.7 | 0–58.8 | 1 | 0.00 | 0.000 | |||
| N3b | 2.6 | 0–5.7 | 2 | 0.39 | 0.534 | 0.000 | 0 | 1 |
| N4 | 16.7 | 0–46.5 | 1 | 0.00 | 0.000 | |||
| Clinical TNM | ||||||||
| I | 41.8 | 3.3–80.4 | 4 | 193.14 | 0.000 | 0.146 | 97.55 | 40.81 |
| I–II | 24 | 5.6–42.5 | 10 | 328.29 | 0.000 | 0.085 | 97.48 | 39.61 |
| II | 27.7 | 3.7–51.8 | 5 | 29.47 | 0.000 | 0.067 | 94.05 | 16.82 |
| III | 12.4 | 1.7–23.1 | 4 | 6.94 | 0.074 | 0.006 | 56.97 | 2.32 |
| III–IV | 12.7 | 8.5–16.8 | 10 | 20.66 | 0.014 | 0.002 | 58.14 | 2.39 |
| IV | 10.4 | 1.7–19.2 | 4 | 9.75 | 0.021 | 0.006 | 74.24 | 3.88 |
| Clinical N | ||||||||
| N0 | 12 | 3.4–20.5 | 8 | 144.06 | 0.000 | 0.013 | 97.72 | 43.94 |
| N+ | 16.2 | 2.4–29.9 | 8 | 158.24 | 0.000 | 0.038 | 98.24 | 56.93 |
| N1 | 8 | 1.4–14.7 | 6 | 22.91 | 0.000 | 0.005 | 80.84 | 5.22 |
| N2 | 9.8 | 2.9–16.7 | 6 | 50.75 | 0.000 | 0.006 | 88.6 | 8.77 |
| N3 | 7 | 0–23.4 | 3 | 1.73 | 0.422 | 0.007 | 13.79 | 1.16 |
| Management | ||||||||
| Chemoradiation | 10.5 | 6–15 | 5 | 2.15 | 0.709 | 0.000 | 0.02 | 1 |
| Chemotherapy | 12.6 | 6.2–19 | 2 | 0.00 | 0.980 | 0.000 | 0 | 1 |
| Radiotherapy | 12 | 1.2–22.9 | 4 | 16.22 | 0.001 | 0.007 | 75.15 | 4.02 |
| Surgery alone | 7.1 | 2.1–12.1 | 6 | 52.12 | 0.000 | 0.003 | 93.99 | 16.63 |
| Surgery plus chemoradiation | 9.2 | 2.4–16 | 5 | 19.63 | 0.001 | 0.004 | 79.2 | 4.81 |
| Surgery plus radiotherapy | 12 | 4.1–19.9 | 5 | 24.7 | 0.000 | 0.006 | 86.21 | 7.25 |
| Treatment-naïve | 3 | 0–8.1 | 2 | 0.34 | 0.559 | 0.000 | 0 | 1 |
| P16 Positivity | ||||||||
| Negative | 7.2 | 3.1–11.4 | 13 | 55.19 | 0.000 | 0.004 | 82.66 | 5.77 |
| Positive | 26.7 | 13.3–40 | 13 | 154.28 | 0.000 | 0.050 | 95.15 | 20.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iraqui, A.; Safia, A.; Mahameed, M.; Abd Elhadi, U.; Merchavy, S. Global Prevalence and Modifiers of Human Papillomavirus Positivity in Oral Cavity Cancer: A Systematic Review and Meta-Analysis of Prevalence (1995–2024). Cancers 2025, 17, 2870. https://doi.org/10.3390/cancers17172870
Iraqui A, Safia A, Mahameed M, Abd Elhadi U, Merchavy S. Global Prevalence and Modifiers of Human Papillomavirus Positivity in Oral Cavity Cancer: A Systematic Review and Meta-Analysis of Prevalence (1995–2024). Cancers. 2025; 17(17):2870. https://doi.org/10.3390/cancers17172870
Chicago/Turabian StyleIraqui, Areeb, Alaa Safia, Mohamad Mahameed, Uday Abd Elhadi, and Shlomo Merchavy. 2025. "Global Prevalence and Modifiers of Human Papillomavirus Positivity in Oral Cavity Cancer: A Systematic Review and Meta-Analysis of Prevalence (1995–2024)" Cancers 17, no. 17: 2870. https://doi.org/10.3390/cancers17172870
APA StyleIraqui, A., Safia, A., Mahameed, M., Abd Elhadi, U., & Merchavy, S. (2025). Global Prevalence and Modifiers of Human Papillomavirus Positivity in Oral Cavity Cancer: A Systematic Review and Meta-Analysis of Prevalence (1995–2024). Cancers, 17(17), 2870. https://doi.org/10.3390/cancers17172870






