Teledermatology vs. Face-to-Face Dermatology for the Diagnosis of Melanoma: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Registration
2.2. Eligibility Criteria
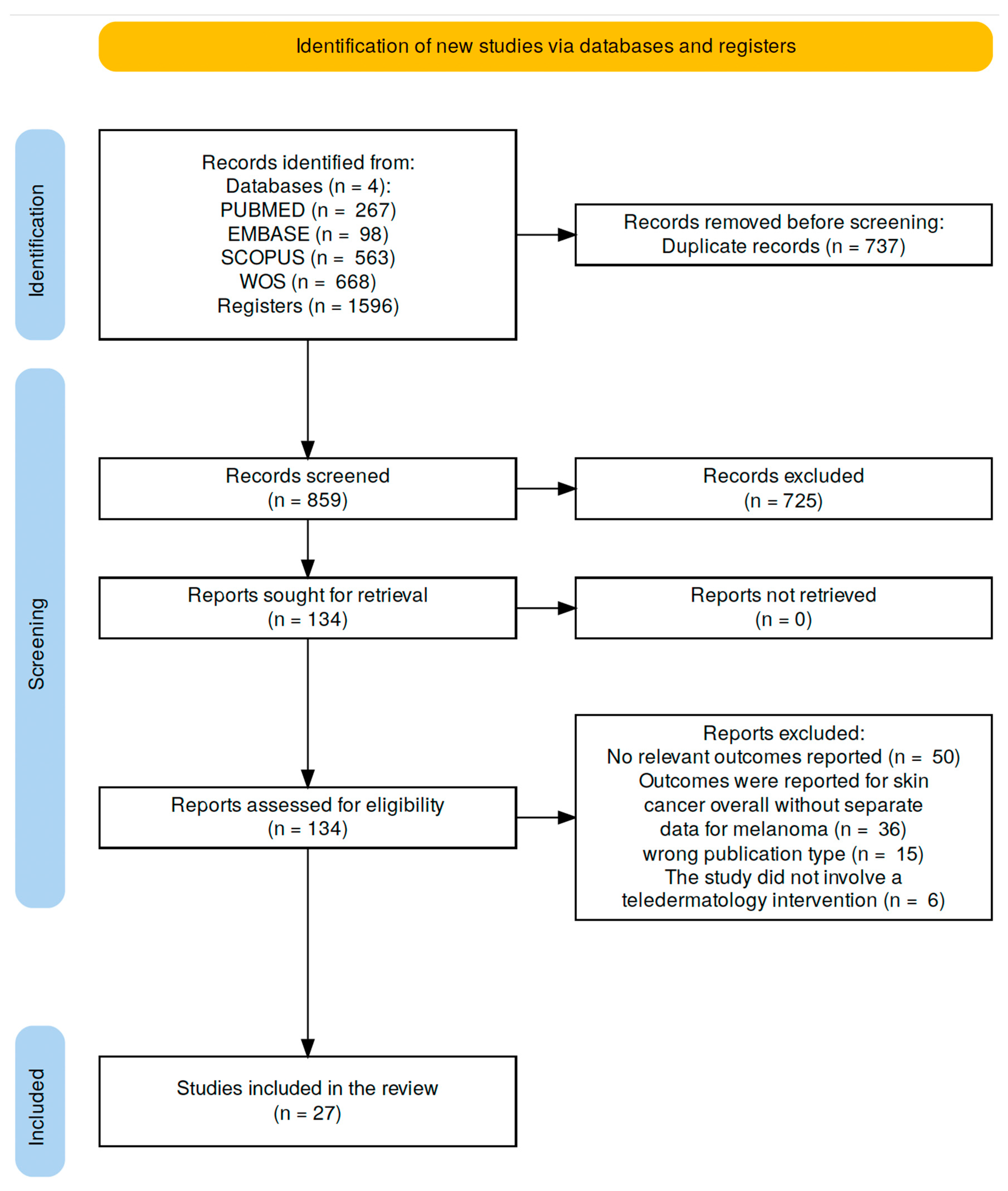
2.3. Information Sources and Search Strategy
2.4. Study Selection
2.5. Data Extraction and Processing
2.6. Risk of Bias Assessment
3. Results
3.1. Diagnostic Accuracy (Sensitivity and Specificity)
3.2. Prognostic Impact (Breslow Thickness)
3.3. Waiting-Time Outcomes
3.4. Satisfaction
3.5. Economic Outcomes
4. Discussion
4.1. Clinical Implications and Future Directions
4.2. Limitations of the Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Swetter, S.M.; Tsao, H.; Bichakjian, C.K.; Curiel-Lewandrowski, C.; Elder, D.E.; Gershenwald, J.E.; Guild, V.; Grant-Kels, J.M.; Halpern, A.C.; Johnson, T.M.; et al. Guidelines of Care for the Management of Primary Cutaneous Melanoma. J. Am. Acad. Dermatol. 2019, 80, 208–250. [Google Scholar] [CrossRef]
- Ahmed, B.; Qadir, M.I.; Ghafoor, S. Malignant Melanoma: Skin Cancer-Diagnosis, Prevention, and Treatment. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 291–297. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2022; Available online: https://gco.iarc.who.int/today (accessed on 13 June 2025).
- Brochez, L.; Volkmer, B.; Hoorens, I.; Garbe, C.; Röcken, M.; Schüz, J.; Whiteman, D.C.; Autier, P.; Greinert, R.; Boonen, B. Skin Cancer in Europe Today and Challenges for Tomorrow. J. Eur. Acad. Dermatol. Venereol. 2025, 39, 272–277. [Google Scholar] [CrossRef]
- Blank, C.U.; Lucas, M.W.; Scolyer, R.A.; van de Wiel, B.A.; Menzies, A.M.; Lopez-Yurda, M.; Hoeijmakers, L.L.; Saw, R.P.M.; Lijnsvelt, J.M.; Maher, N.G.; et al. Neoadjuvant Nivolumab and Ipilimumab in Resectable Stage III Melanoma. N. Engl. J. Med. 2024, 391, 1696–1708. [Google Scholar] [CrossRef]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-Year Survival Outcomes for Patients with Advanced Melanoma Treated with Pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Leiter, U.; Keim, U.; Garbe, C. Epidemiology of Skin Cancer: Update 2019. Adv. Exp. Med. Biol. 2020, 1268, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological Trends in Skin Cancer. Dermatol. Pract. Concept. 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Brodersen, J.; Gøtzsche, P.C.; Jørgensen, K.J. Screening for Reducing Morbidity and Mortality in Malignant Melanoma. Cochrane Database Syst. Rev. 2019, CD012352. [Google Scholar] [CrossRef]
- Pitre, L.D.; Linford, G.; Pond, G.R.; McWhirter, E.; Seow, H. Is Access to Care Associated with Stage at Presentation and Survival for Melanoma Patients? J. Cutan. Med. Surg. 2019, 23, 586–594. [Google Scholar] [CrossRef]
- Bashshur, R.L.; Shannon, G.W.; Tejasvi, T.; Kvedar, J.C.; Gates, M. The Empirical Foundations of Teledermatology: A Review of the Research Evidence. Telemed. E-Health 2015, 21, 953–979. [Google Scholar] [CrossRef]
- Loh, C.H.; Chong Tam, S.Y.; Oh, C.C. Teledermatology in the COVID-19 Pandemic: A Systematic Review. JAAD Int. 2021, 5, 54–64. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic Reviews of Etiology and Risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: North Adelaide, Australia, 2020. [Google Scholar]
- Tan, E.; Yung, A.; Jameson, M.; Oakley, A.; Rademaker, M. Successful Triage of Patients Referred to a Skin Lesion Clinic Using Teledermoscopy (IMAGE IT Trial). Br. J. Dermatol. 2010, 162, 803–811. [Google Scholar] [CrossRef]
- Wolf, J.A.; Moreau, J.F.; Akilov, O.; Patton, T.; English, J.C.; Ho, J.; Ferris, L.K. Diagnostic Inaccuracy of Smartphone Applications for Melanoma Detection. JAMA Dermatol. 2013, 149, 422–426. [Google Scholar] [CrossRef]
- Congalton, A.T.; Oakley, A.M.; Rademaker, M.; Bramley, D.; Martin, R.C.W. Successful Melanoma Triage by a Virtual Lesion Clinic (Teledermatoscopy). J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2423–2428. [Google Scholar] [CrossRef]
- Cazzaniga, S.; Castelli, E.; Di Landro, A.; Di Mercurio, M.; Imberti, G.; Locatelli, G.A.; Raponi, F.; Vezzoli, P.; Gambini, D.; Damiani, G.; et al. Mobile Teledermatology for Melanoma Detection: Assessment of the Validity in the Framework of a Population-Based Skin Cancer Awareness Campaign in Northern Italy. J. Am. Acad. Dermatol. 2019, 81, 257–260. [Google Scholar] [CrossRef]
- Jahn, A.S.; Navarini, A.A.; Cerminara, S.E.; Kostner, L.; Huber, S.M.; Kunz, M.; Maul, J.T.; Dummer, R.; Sommer, S.; Neuner, A.D.; et al. Over-Detection of Melanoma-Suspect Lesions by a CE-Certified Smartphone App: Performance in Comparison to Dermatologists, 2D and 3D Convolutional Neural Networks in a Prospective Data Set of 1204 Pigmented Skin Lesions Involving Patients’ Perception. Cancers 2022, 14, 3829. [Google Scholar] [CrossRef] [PubMed]
- Jobbágy, A.; Kiss, N.; Meznerics, F.A.; Farkas, K.; Plázár, D.; Bozsányi, S.; Fésűs, L.; Bartha, Á.; Szabó, E.; Lőrincz, K.; et al. Emergency Use and Efficacy of an Asynchronous Teledermatology System as a Novel Tool for Early Diagnosis of Skin Cancer during the First Wave of COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 2699. [Google Scholar] [CrossRef]
- Fazil Jaber, N.; Jerkovic Gulin, S.; Seifert, O. Analysis of Teledermoscopy and Face-to-Face Examination of Atypical Pigmented Lesions: A Cross-Sectional, Retrospective Study. Dermatol. Pract. Concept. 2023, 13, e2023212. [Google Scholar] [CrossRef]
- Koop, C.; Kruus, P.; Hallik, R.; Lehemets, H.; Vettus, E.; Niin, M.; Ross, P.; Kingo, K. A Country-Wide Teledermatoscopy Service in Estonia Shows Results Comparable to Those in Experimental Settings in Management Plan Development and Diagnostic Accuracy: A Retrospective Database Study. JAAD Int. 2023, 12, 81–89. [Google Scholar] [CrossRef]
- Gafoor, S.M.A.; Nelson, T.; Woodcock, E.; Adityani, B. Patient-Led Teledermatology for Skin Lesion Triage: A Service Evaluation of the DyplensTM Dermoscope. Clin. Exp. Dermatol. 2024, 49, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Zazo, V.; Boman, A.; Andersson, N. Diagnostic Accuracy and Safety of Teledermoscopy for Cutaneous Melanoma Triage in Northern Sweden. Acta Derm.-Venereol. 2024, 104, 15302. [Google Scholar] [CrossRef] [PubMed]
- Ferrámdiz, L.; Ruiz-De-Casas, A.; Martin-Gutierrez, F.J.; Peral-Rubio, F.; Mendez-Abad, C.; Rios-Martin, J.J.; Moreno-Ramirez, D. Effect of Teledermatology on the Prognosis of Patients with Cutaneous Melanoma. Arch. Dermatol. 2012, 148, 1025–1028. [Google Scholar] [CrossRef]
- Karavan, M.; Compton, N.; Knezevich, S.; Raugi, G.; Kodama, S.; Taylor, L.; Reiber, G.E. Teledermatology in the Diagnosis of Melanoma. J. Telemed. Telecare 2013, 20, 18–23. [Google Scholar] [CrossRef]
- Börve, A.; Dahlén Gyllencreutz, J.; Terstappen, K.; Johansson Backman, E.; Alden-Bratt, A.; Danielsson, M.; Gillstedt, M.; Sandberg, C.; Paoli, J. Smartphone Teledermoscopy Referrals: A Novel Process for Improved Triage of Skin Cancer Patients. Acta Derm.-Venereol. 2015, 95, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Teague, R.; Wang, M.; Wen, D.; Sunderland, M.; Rolfe, G.; Oakley, A.M.M.; Rademaker, M.; Martin, R. Virtual Lesion Clinic—Evaluation of a Teledermatology Triage System for Referrals for Suspected Melanoma. Australas. J. Dermatol. 2022, 63, e33–e40. [Google Scholar] [CrossRef]
- Jaklitsch, E.; Shah, V.K.; Smith, B.; Agarwal, A.; Chen, J.; Sweeney, A.; English, J.C.; Ferris, L.K. Melanoma Detected Through Teledermatology Versus In-Person Visits. Telemed. E-Health 2024, 30, E1192–E1196. [Google Scholar] [CrossRef]
- May, C.; Giles, L.; Gupta, G. Prospective Observational Comparative Study Assessing the Role of Store and Forward Teledermatology Triage in Skin Cancer. Clin. Exp. Dermatol. 2008, 33, 736–739. [Google Scholar] [CrossRef]
- Dahlén Gyllencreutz, J.; Paoli, J.; Bjellerup, M.; Bucharbajeva, Z.; Gonzalez, H.; Nielsen, K.; Sandberg, C.; Synnerstad, I.; Terstappen, K.; Wennberg Larkö, A.M. Diagnostic Agreement and Interobserver Concordance with Teledermoscopy Referrals. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 898–903. [Google Scholar] [CrossRef]
- Teoh, N.S.C.; Oakley, A. A 9-Year Teledermoscopy Service in New Zealand: Retrospective Service Review. JMIR Dermatol. 2022, 5, e36351. [Google Scholar] [CrossRef]
- Bouton, C.; Schmeltz, H.; Lévèque, C.; Gaultier, A.; Quereux, G.; Dreno, B.; Nguyen, J.M.; Rat, C. Early Diagnosis of Melanoma: A Randomized Trial Assessing the Impact of the Transmission of Photographs Taken with a Smartphone from the General Practitioner to the Dermatologist on the Time to Dermatological Consultation. BMC Health Serv. Res. 2024, 24, 660. [Google Scholar] [CrossRef] [PubMed]
- Sahin, C.; Carlsson, M.; Munir Ehrlington, F.; Micu, E.; Falk, M. Increased Use of Dermoscopy in Primary Healthcare Following the Implementation of Teledermatology in Southeast Sweden: A Retrospective Cohort Study of 2,137 Patients. Acta Derm.-Venereol. 2024, 104, adv40890. [Google Scholar] [CrossRef]
- Spinks, J.; Janda, M.; Soyer, H.P.; Whitty, J.A. Consumer Preferences for Teledermoscopy Screening to Detect Melanoma Early. J. Telemed. Telecare 2015, 22, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Horsham, C.; Loescher, L.J.; Whiteman, D.C.; Soyer, H.P.; Janda, M. Consumer Acceptance of Patient-Performed Mobile Teledermoscopy for the Early Detection of Melanoma. Br. J. Dermatol. 2016, 175, 1301–1310. [Google Scholar] [CrossRef]
- Kirtava, Z.; Shulaia, T.; Kiladze, N.; Korsantia, N.; Gogitidze, T.; Jorjoliani, D. 2016 IEEE 18th International Conference on e-Health Networking, Applications and Services (Healthcom 2016), Munich, Germany, 14–16 September 2016; IEEE: Piscataway, NJ, USA, 2016; ISBN 9781509033706. [Google Scholar]
- Koh, U.; Horsham, C.; Soyer, H.P.; Loescher, L.J.; Gillespie, N.; Vagenas, D.; Janda, M. Consumer Acceptance and Expectations of a Mobile Health Application to Photograph Skin Lesions for Early Detection of Melanoma. Dermatology 2018, 235, 4–10. [Google Scholar] [CrossRef]
- Chin, Y.P.; Huang, I.H.; Hou, Z.Y.; Chen, P.Y.; Bassir, F.; Wang, H.H.; Lin, Y.T.; Li, Y.C. User Satisfaction with a Smartphone-Compatible, Artificial Intelligence-Based Cutaneous Pigmented Lesion Evaluator. Comput. Methods Programs Biomed. 2020, 195, 105649. [Google Scholar] [CrossRef]
- Damsin, T.; Canivet, G.; Jacquemin, P.; Seidel, L.; Gillet, P.; Giet, D.; Nikkels, A.F. Value of Teledermoscopy in Primary Healthcare Centers: Preliminary Results of the TELESPOT Project in Belgium. Dermatol. Ther. 2020, 10, 1405–1413. [Google Scholar] [CrossRef]
- Horsham, C.; Snoswell, C.; Vagenas, D.; Loescher, L.J.; Gillespie, N.; Peter Soyer, H.; Janda, M. Is Teledermoscopy Ready to Replace Face-to-Face Examinations for the Early Detection of Skin Cancer? Consumer Views, Technology Acceptance, and Satisfaction with Care. Dermatology 2020, 236, 90–96. [Google Scholar] [CrossRef]
- Deda, L.C.; Goldberg, R.H.; Jamerson, T.A.; Lee, I.; Tejasvi, T. Dermoscopy Practice Guidelines for Use in Telemedicine. NPJ Digit Med 2022, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Sangers, T.E.; Kittler, H.; Blum, A.; Braun, R.P.; Barata, C.; Cartocci, A.; Combalia, M.; Esdaile, B.; Guitera, P.; Haenssle, H.A.; et al. Position Statement of the EADV Artificial Intelligence (AI) Task Force on AI-Assisted Smartphone Apps and Web-Based Services for Skin Disease. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 22–30. [Google Scholar] [CrossRef]
- Nabil, R.; Bergman, W.; Kukutsch, N.A. Conflicting Results between the Analysis of Skin Lesions Using a Mobile-Phone Application and a Dermatologist’s Clinical Diagnosis: A Pilot Study. Br. J. Dermatol. 2017, 177, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; van der Sande, A.A.J.; de Roos, K.P.; Bekkenk, M.W.; de Haas, E.R.M.; Kelleners-Smeets, N.W.J.; Kukutsch, N.A. Poor Agreement between the Automated Risk Assessment of a Smartphone Application for Skin Cancer Detection and the Rating by Dermatologists. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Thissen, M.; Udrea, A.; Hacking, M.; Von Braunmuehl, T.; Ruzicka, T. MHealth App for Risk Assessment of Pigmented and Nonpigmented Skin Lesions-A Study on Sensitivity and Specificity in Detecting Malignancy. Telemed. e-Health 2017, 23, 948–954. [Google Scholar] [CrossRef]
- Tejasvi, T.; Plaska, A.; Scharnitz, T.; Hesseler, M.; Ellis, C. Incidental Melanoma Detection Following Teledermatology Consultation. J. Investig. Dermatol. 2019, 139, S44. [Google Scholar] [CrossRef]
- Soyer, H.P.; Jayasinghe, D.; Rodriguez-Acevedo, A.J.; Collins, L.G.; Caffery, L.J.; Whiteman, D.C.; Betz-Stablein, B.; Osborne, S.R.; Finnane, A.; Horsham, C.; et al. 3D Total-Body Photography in Patients at High Risk for Melanoma: A Randomized Clinical Trial. JAMA Dermatol. 2025, 161, 472–481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smak Gregoor, A.M.; Sangers, T.E.; Bakker, L.J.; Hollestein, L.; Uyl-de Groot, C.A.; Nijsten, T.; Wakkee, M. An artificial intelligence based app for skin cancer detection evaluated in a population based setting. NPJ Digit. Med. 2023, 6, 90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lindsay, D.; Soyer, H.P.; Janda, M.; Whiteman, D.C.; Osborne, S.; Finnane, A.; Caffery, L.J.; Collins, L.G. Cost-Effectiveness Analysis of 3D Total-Body Photography for People at High Risk of Melanoma. JAMA Dermatol. 2025, 161, 482–489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vestergaard, T.; Prasad, S.C.; Schuster, A.; Laurinaviciene, R.; Andersen, M.K.; Bygum, A. Diagnostic accuracy and interobserver concordance: Teledermoscopy of 600 suspicious skin lesions in Southern Denmark. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
| Author & Year | Country/Setting | Study Design | Population | TD Intervention | Comparator | Melanoma Results | Risk of Bias |
|---|---|---|---|---|---|---|---|
| Tan et al., 2010 [18] | New Zealand/Waikato Hospital lesion clinic | Prospective diagnostic accuracy study | 200 patients; 491 lesions; 2 dermatologists | Store-and-forward TD with macroscopic + dermoscopic images reviewed remotely | FTF consultation and histopathology | Sensitivity for melanoma: Derm A: 100% (vs. FTF & histology) Derm B: 93.3% (vs. FTF), 100% (vs. histology) Specificity for melanoma: Derm A: 98.7% (vs. FTF), 98.4% (vs. histology) Derm B: 100% (vs. FTF), 99.5% (vs. histology) | Risk of bias discussed; measures to minimise recall and selection bias described, although no formal tool used. |
| Wolf et al., 2013 [19] | USA/University dermatology department | Case-control diagnostic accuracy study | 188 digital images (60 melanoma, 128 benign); histopathologically confirmed | Four smartphone apps: mobile app 1: AI analysis with user-confirmed lesion border mobile app 2: Fully automated AI (“melanoma” vs. “looks good”) mobile app 3: AI with risk score (low/medium/high); “medium” counted as positive mobile app 4: Store-and-forward TD image to a dermatologist | Histopathology | Sensitivity/Specificity: mobile app 1: 70.0%/39.3% mobile app 2: 69.0%/37.0% mobile app 3: 6.8%/93.7% mobile app 4: 98.1%/30.4%. mobile app 4 (teledermatologist) was significantly more sensitive than AI apps (p < 0.001 vs. mobile app 1 & 3; p = 0.02 vs. mobile app 2). | Not formally assessed; descriptive study without discussion of potential bias or methodological limitations. |
| Congalton et al., 2015 [20] | New Zealand/Waitematā District Health Board | Retrospective observational study | 310 patients; 613 lesions assessed via VLC; 129 lesions excised; 48 melanomas confirmed (23 in situ, 24 invasive, 1 metastatic) | Store-and-forward TD with macroscopic + dermoscopic images evaluated remotely | FTF consultation and histopathology | Sensitivity of 96% (95% CI: 86–99%) Specificity of 62% (95% CI: 50–73%) | Not formally assessed; no discussion of potential sources of bias or methodological limitations. |
| Cazzaniga et al., 2019 [21] | Italy/General population in Bergamo province | Prospective validity study within public awareness campaign | 232 adults who submitted lesion photos via mobile app; followed by clinical exam | Mobile app allowing users to send images of suspicious lesions to dermatologists for triage | FTF whole-body clinical examination | sensitivity was 92.9% (95% CI 66.1–99.8%), specificity 80.3% (95% CI 74.4–85.3) | Not formally assessed; self-selection bias likely due to participant profile and lack of data on non-users. |
| Jahn et al., 2022 [22] | Switzerland/University Hospital of Basel | Prospective, single-centre, observational cohort | 114 patients (55 high-risk, 59 with melanoma); 1204 lesions; 61 lesions with histology available | mobile app SkinVision® (macroscopic image-based AI risk stratification) | Dermatologists; 2D FotoFinder ATBM®; 3D Vectra; histopathology | Sensitivity/Specificity: SkinVision® 83%/60.0%; 2D FotoFinder ATBM® 83%/40.0%; 3D Vectra 83%/63.6%. Dermatologists 83%/92.7% Dermatologists + AI scores 83%/88% | Not formally assessed; single-centre design acknowledged as limitation. |
| Jobbágy et al., 2022 [23] | Hungary/Semmelweis University, Budapest | Retrospective single-centre case-control study | Subset of 100 lesions (30 malignant, 70 benign) retrospectively selected from a larger dataset of 779 cases (during first COVID-19 wave) | Store-and-forward TD using mobile app (photos and questionnaire submitted) | FTF consultation and histopathology | Sensitivity (melanoma): Primary diagnosis: 66.7% (95% CI: 41.7–84.8) Aggregated diagnosis (including any differencial diagnosis during teledermatology): 93.3% (95% CI: 70.2–99.7) Specificity (melanoma): Primary: 97.1% (95% CI: 95.7–98.1) Aggregated: 96.3% (95% CI: 94.8–97.5) | Not formally assessed; retrospective design with acknowledged limitations including lack of dermoscopy and variable image quality. |
| Fazil Jaber et al., 2023 [24] | Sweden/Ryhov County Hospital, Jönköping | Cross-sectional, retrospective study | 112 TD patients and 134 FTF patients with suspected melanoma or atypical melanocytic lesions | Store-and-forward TD with clinical + dermoscopic images captured by GPs and reviewed by dermatologists | FTF consultation and histopathology | TD showed 75% sensitivity and 83.9% specificity vs. 47.8% and 81.2% for FTF | Not formally assessed; retrospective design, high-quality images only, real-world setting |
| Koop et al., 2023 [25] | Estonia/National primary care network | Retrospective database study | 4748 teledermatoscopy cases from 3403 patients, 50 melanomas (October 2017–August 2019) | Store-and-forward TD mobile teledermatoscopy (smartphone + dermatoscope, dermatologist-reviewed) | Histopathology | Sensitivity: 90.5% (95% CI: 69.6–98.8); Specificity: 92.6% (95% CI: 91.8–93.3). | Formally assessed using STARD and ISPOR standards; limitations of retrospective design discussed. |
| Gafoor et al., 2024 [26] | UK/Dermatology clinics (2 sites) | Service evaluation (prospective) | 78 patients (390 image assessments) | Patient-led Mobile TD with Dyplens™ dermoscope and smartphone; images reviewed by 5 dermatologists | FTF consultation and histopathology | Sensitivity: 100% for melanoma Specificity: 89% for melanoma | Not formally assessed. |
| Zazo et al., 2024 [27] | Sweden/Västerbotten County healthcare system | Retrospective cross-sectional study | 135 patients diagnosed with melanoma, 95 via TD | Store-and-forward TD (macro + dermoscopic images) from primary care to dermatology | FTF consultation and histopathology | TD showed sensitivity of 98.9%. | Not formally assessed; retrospective design with good coverage and real-life data |
| Author & Year | Country/Setting | Study Design | Population | TD Intervention | Comparator | Melanoma Results | Risk of Bias |
|---|---|---|---|---|---|---|---|
| Ferrándiz et al., 2012 [28] | Spain/Hospital Universitario Virgen Macarena, Seville | Descriptive and longitudinal observational study | 201 patients with primary cutaneous melanoma | Store-and-forward TD TD (TD)system using standard digital photographs transmitted via intranet or email | Conventional FTF referral system | Mean Breslow thickness significantly lower in TD group (1.06 mm vs. 1.64 mm, p = 0.03); higher rate of early-stage tumours (Tis + T1a: 70.1% vs. 56.9%, OR = 1.96, p = 0.04) | Not formally assessed; potential selection bias and loss to follow-up acknowledged as limitations. |
| Karavan et al., 2013 [29] | USA/Veterans Affairs network (Pacific NW) | Retrospective cohort study | 529 Veterans with 567 pathology-confirmed melanomas (112 TD, 455 non-TD) | Store-and-forward TD TD with digital photography by trained technicians | Conventional FTF referral system | Mean Breslow: 1.91 mm (TD) vs. 1.14 mm (non-TD) (p = 0.03). Higher % of thick melanomas (>4 mm) in TD group (14% vs. 4%, p < 0.01). Lower proportion of thin melanomas (<1 mm) in TD (59% vs. 74%, p = 0.06). In situ melanoma: 44% (TD) vs. 42% (non-TD). | Not formally assessed; descriptive study without discussion of potential bias or methodological limitations. |
| Börve et al., 2015 [30] | Sweden/20 PHCs and 2 hospitals | Open, multicentre, prospective observational study | 772 patients with skin lesions of concern referred via smartphone TDS | Smartphone TD using iDoc24 PRO mobile app and dermoscope | Conventional FTF referral system | TDS: 46% of MM were in situ vs. 35% with paper. Median Breslow: 1.0 mm (TDS) vs. 2.2 mm (paper). | Not formally assessed; controlled design with robust sample. |
| Congalton et al., 2015 [20] | New Zealand/Waitematā District Health Board | Retrospective observational study | 310 patients; 613 lesions assessed via VLC; 129 lesions excised; 48 melanomas confirmed (23 in situ, 24 invasive, 1 metastatic) | Store-and-forward TD TD with macroscopic + dermoscopic images evaluated remotely | Conventional FTF referral system | The median Breslow thickness of the 24 invasive primary lesions was 0.69 mm (range 0.3–5.0 mm). Sixty-two percent were <1.0 mm in thickness without ulceration or mitoses. | Not formally assessed; small sample size, good clinical relevance |
| Teague et al., 2022 [31] | New Zealand/Waitematā District Health Board | Retrospective audit (5 years) | 810 patients; 3546 lesions referred to VLC; 504 excised lesions analysed | VLC: TD triage of pigmented lesions via MoleMap imaging and remote dermatologist review | None (descriptive retrospective audit) | The mean Breslow thickness for invasive melanoma was 1.26 mm (95% CI: 0.90–1.61, median 0.60 mm), and the IM:MIS ratio was 0.76 (80 invasive melanoma: 105 melanoma in situ). | Not formally assessed; acknowledged limitations include incomplete follow-up of excision recommendations and potential selection bias. |
| Jaklitsch et al., 2024 [32] | USA/University of Pittsburgh Medical Center | Retrospective cohort (2020–2022) | 836 melanomas (35 TD, 801 FTF) | Asynchronous (eDerm) and synchronous video TD for triage | Conventional FTF referral system | TD vs. FTF: Median Breslow: 0.95 vs. 0.40 mm (p < 0.001) Invasive melanomas: 74.3% vs. 46.6% (p = 0.001) Ulcerated: 32.0% vs. 9.0% (p = 0.002) Aggressive subtype: 22.9% vs. 7.9% (p = 0.007) | Not formally assessed; retrospective design and single-centre setting may introduce selection bias and limit generalisability. |
| Zazo et al., 2024 [27] | Sweden/Västerbotten County healthcare system | Retrospective cross-sectional study | 135 patients diagnosed with melanoma, 95 via TD | Store-and-forward TD (macro + dermoscopic images) from primary care to dermatology | Conventional FTF referral system | The mean Breslow thicknes of invasive melanomas was 1.55 mm and the proportion of patients with Breslow thickness ≤ 1.0 mm was 60%. There were no significant differences in Breslow thickness (p = 0.4), between patients evaluated with or without TD consultations. | Not formally assessed; retrospective design with good coverage and real-life data |
| Author & Year | Country/Setting | Study Design | Population | TD Intervention | Comparator | Melanoma Results | Risk of Bias |
|---|---|---|---|---|---|---|---|
| May et al., 2008 [33] | UK/Lanarkshire dermatology clinics | Prospective observational comparative study | 76 patients with melanoma and SCC | Store-and-forward TD TD with digital images and dermoscopy reviewed for triage | Conventional referral without images | Median time to clinic (melanoma): With photos: 14 days Without photos: 24 days (urgent), 44 days (‘soon’), 130 days (routine) Median time to treatment (melanoma): With photos: median 21.5 days Without photos: 41 days (urgent), 51 days (‘soon’), 136 days (routine). No routine-priority patients without photos were treated within 62 days; all but one with photos were. | Not formally assessed; observational design without discussion of potential sources of bias. |
| Börve et al., 2015 [30] | Sweden/20 PHCs and 2 hospitals | Open, multicentre, prospective observational study | 772 patients with skin lesions of concern referred via smartphone TDS | Smartphone TD using iDoc24 PRO mobile app and dermoscope | Conventional referral without images | TD: first visit in 9/10 days and surgery in 9/12 days for MM/MMIS vs. 14/17 and 35/62 days with paper (p < 0.0001 and p = 0.028). Dermatologist response in <24 h (median 1.8 h; TD) vs. 4-day delay (paper). | Not formally assessed; controlled design with robust sample. |
| Congalton et al., 2015 [20] | New Zealand/Waitematā District Health Board | Retrospective observational study | 310 patients; 613 lesions assessed via VLC; 129 lesions excised; 48 melanomas confirmed (23 in situ, 24 invasive, 1 metastatic) | Store-and-forward TD TD with macroscopic + dermoscopic images evaluated remotely | Conventional referral without images | Reduced wait time to first assessment (9 vs. 26.5 days). The median wait time for diagnostic excision from VLC assessment report was 40 days for lesions suspicious of melanoma (range 14–210 days) | Not formally assessed; no discussion of potential sources of bias or methodological limitations. |
| Dahlén Gyllencreutz et al., 2017 [34] | Sweden/Primary care to dermatology services | Comparative study (TDS vs. paper-based referrals) | 157 cases (80 TDS, 77 paper); evaluated by 6 dermatologists | Mobile TD: macroscopic + dermoscopic images assessed remotely | Conventional referral without images | TDS significantly improved correct prioritisation of invasive melanoma (98% vs. 62%; p = 0.012) and allowed more patients to be booked directly for surgery (91% vs. 36%) | Not formally assessed; methodological limitations discussed, including interobserver variability and potential misclassification in triage decisions. |
| Teoh & Oakley, 2022. [35] | New Zealand/Te Whatu Ora Waikato | Retrospective service review (9 years, 5 months) | 6479 patients; 11,005 lesions imaged; 330 histologically confirmed melanomas | Nurse-led imaging clinics using digital and dermoscopic photography for remote diagnosis by dermatologists | None (descriptive study) | median waiting time of 44.5 (mean 57.9; range 8–218) days for imaging and a median waiting time of 63 (mean 63.2; range 28–94) for the first treatment received | Not formally assessed; retrospective design with limited detail on comparator groups or bias control methods. |
| Koop et al., 2023 [25] | Estonia/National primary care network | Retrospective database study | 4748 teledermatoscopy cases from 3403 patients (October 2017–August 2019) | Store-and-forward TD mobile teledermatoscopy (smartphone + dermatoscope, dermatologist-reviewed) | None | Mean time to excision: 45.5 days; to histology: 67.4 days. | Formally assessed using STARD and ISPOR standards; limitations of retrospective design discussed. |
| Bouton et al., 2024 [36] | France/Primary care practices (Nantes region) | Cluster-randomised controlled trial | 250 patients referred for suspected melanoma (125 per group) | Email transmission of smartphone photos by GPs to dermatologists | Conventional referral without images | No significant difference in consultation delay for lesions requiring resection (56.5 vs. 63.7 days, p = 0.53). | Not formally assessed; strengths include randomised design, but limitations in statistical power and lack of standardised bias assessment tool. |
| Jaklitsch et al., 2024 [32] | USA/University of Pittsburgh Medical Center | Retrospective cohort (2020–2022) | 836 melanomas (35 TD, 801 FTF) | Asynchronous (eDerm) and synchronous video TD for triage | Conventional referral without images | TD vs. FTF: Median time to first visit: 0 vs. 42 days (p < 0.001) Median time to biopsy: 6 vs. 47 days (p < 0.001) | Not formally assessed; retrospective design and single-centre setting may introduce selection bias and limit generalisability. |
| Sahin et al., 2024 [37] | Sweden/Östergötland County, 3 PHCs | Retrospective cohort study (Pre vs. Post implementation) | 2137 patients with skin tumours, including 44 melanomas | Store-and-forward TD TD with macroscopic and dermoscopic images | Conventional referral without images | No significant difference in melanoma management times (PreT: 56.7 vs. PostT: 49.7 days until excision of a melanoma; p = 0.705)). | Not formally assessed; naturalistic design, representative sample |
| Author & Year | Country/Setting | Study Design | Population | TD Intervention | Comparator | Melanoma/PL Results | Risk of Bias |
|---|---|---|---|---|---|---|---|
| Spinks et al., 2015 [38] | Australia/Queensland | Discrete choice experiment with prior TD users | 35 participants aged 50–64 at moderate/high melanoma risk | Store-and-forward TD Mobile TD with dermatologist image review | Skin self-exam, GP or skin clinic visit | Participants strongly preferred TD with dermatologist review. Key drivers: higher detection rate, fewer unnecessary removals, shorter time away from activities. | Not formally assessed; small sample and selection bias acknowledged as study limitations. |
| Horsham et al., 2016 [39] | Australia/Brisbane, community | Mixed-methods study: survey + home use trial | 228 survey participants (mean age 57); 49 home users (mean age 59) | Patient-performed Mobile TD using dermatoscope + iPhone + Handyscope mobile app; Store-and-forward TD to dermatologist | None (descriptive study) | 94% found the device easy to use; 86% said it motivated regular skin self-exam; 78% would use it again. However, 35% needed help submitting photos, 18% couldn’t image hard-to-see areas, and 12% felt anxious. 84% found the AC rule helpful. Trust in telediagnosis was moderate (46–56% agreement). Overall satisfaction was high, but confidence varied. | Not formally assessed; self-reported outcomes, participant self-selection and lack of clinical outcome verification may introduce bias. |
| Kirtava et al., 2016 [40] | Georgia/Outpatient dermatology clinics in Tbilisi and Adjara | Prospective observational study | 584 patients screened for skin cancer, mainly pigmented lesions: 13 melanomas or Spitz tumours | Store-and-forward TD Mobile TD using DermLite DL3/DL1 attached to smartphones, with e-registry and remote review | Clinical diagnosis and follow-up; histopathology confirmation. | Approximately 60% of patients agreed or strongly agreed that TD is beneficial, reliable, and time- and cost-saving. Moreover, 90% of physicians agreed or strongly agreed that TD is beneficial and reliable, and 70% reported it saves time and money. | Not formally assessed; technical and operational limitations discussed, but no mention of methodological sources of bias. |
| Koh et al., 2019 [41] | Australia/Community (Brisbane) | Mixed methods: online survey + focus groups | 88 online participants (mean age 38); 28 focus group participants (mean age 46) | Consumer-directed Mobile TD mobile app for photographing skin lesions and sending to a medical practitioner | None (descriptive study) | 95% would use the mobile app to send images. Main perceived benefits: convenience (73%), ease of use (32%), cost reduction (13%). Barriers: privacy (25%), confidence in identifying lesions, system overload, and anxiety while awaiting results. Overall high acceptance. | Not formally assessed; limitations include single-centre design and potential diagnostic verification bias. |
| Chin et al., 2020 [42] | Taiwan/General public using mobile app | Cross-sectional satisfaction survey | 1231 users of MoleMe mobile app for pigmented lesion evaluation | AI-based mobile app (MoleMe) analysing mole photos + clinical info; outputs risk and referral recommendation | None (descriptive study) | Over 90% satisfied (score 4–5); >75% strongly satisfied (score 5). High satisfaction in usability (95%), interaction (94%), impact on daily life (92%), and overall performance (94%). No significant differences by gender, age, or risk category. | Not formally assessed; self-reported, no diagnostic validation, limited to Asian population |
| Damsin et al., 2020 [43] | Belgium/PHCs near Liège | Pilot observational study (6 months) | 80 patients, 105 lesions; PHCs with GP/nurse staff | Smartphone-based TD (TELESPOT) with macroscopic and dermoscopic imaging; remote review by dermatologists | None (descriptive study) | Patient satisfaction: 8.8/10 Comfort: 9.4 Tech confidence: 8.6 Trust in advice: 8.2 Physician satisfaction: 9.4/10 Integration: 8.6 Time efficiency: 9.4 Report usefulness: 9.6 Diagnostic support: 8.8 Value to PHC: 9.2 Skill improvement: 6.8 | Not formally assessed; observational design and lack of control group may introduce selection and performance bias. |
| Horsham et al., 2020 [44] | Australia/Community-based, Brisbane | Randomised controlled trial | 98 adults at high risk of skin cancer, aged 19–73, performing self-exams with mobile dermatoscope | Home-based Mobile TD using smartphone + dermatoscope for self-selected lesion imaging | Baseline self-report; no traditional FTF comparator | No change in melanoma worry; high baseline acceptance, but slight decline post-intervention; 92% found device easy to use, 71% would use again | Not formally assessed; self-reported outcomes, participant self-selection and lack of clinical outcome verification may introduce bias. |
| Jahn et al., 2022 [22] | Switzerland/University Hospital of Basel | Prospective, single-centre, observational cohort | 114 patients (55 high-risk, 59 with melanoma); 1204 lesions; 61 lesions with histology available | mobile app SkinVision® (macroscopic image-based AI risk stratification) | Dermatologists; 2D FotoFinder ATBM®; 3D Vectra; histopathology | Most patients expressed full trust in dermatologists (100%) and high confidence in 2D/3D total body imaging devices (>88%). In contrast, trust in the mobile app was notably lower (36% in high-risk patients, 49% in melanoma patients). While dermatologist assessments significantly reduced anxiety (81–89%), the mobile app alone achieved this in only about one-third of cases. The preferred screening method was dermatologist combined with 3D imaging (chosen by 51–64%), whereas no patient preferred the mobile app alone. Nearly all participants (95–98%) believed AI could support clinical decision-making. Dermatologists, however, reported low confidence in the mobile app (8.8%). | Not formally assessed; single-centre design acknowledged as limitation. |
| Author & Year | Country/Setting | Study Design | Population | TD Intervention | Comparator | Melanoma Results | Risk of Bias |
|---|---|---|---|---|---|---|---|
| Congalton et al., 2015 [20] | New Zealand/Waitematā District Health Board | Retrospective observational study | 310 patients; 613 lesions assessed via VLC; 129 lesions excised; 48 melanomas confirmed (23 in situ, 24 invasive, 1 metastatic) | Store-and-forward TD TD with macroscopic + dermoscopic images evaluated remotely | Conventional outpatient referral system | The VLC triage service resulted in an estimated cost reduction in NZ$364 330, or NZ$1174/patient seen (exchange rate Dec 2014: NZ$1.00 = US$ 0.77, Euro 0.62, UK£ 0.49). | Not formally assessed; no discussion of potential sources of bias or methodological limitations. |
| Kirtava et al., 2016 [40] | Georgia/Outpatient dermatology clinics in Tbilisi and Adjara (rural region) | Prospective observational study | 584 patients screened for skin cancer, mainly pigmented lesions: 13 melanomas or Spitz tumours | Store-and-forward TD Mobile TD using DermLite DL3/DL1 attached to smartphones, with e-registry and remote review | Clinical diagnosis and follow-up; histopathology confirmation. | Traditional FTF care in the study region was at least 3.65 times more expensive than TD. | Not formally assessed; technical and operational limitations discussed, but no mention of methodological sources of bias. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Pardo Rico, M.; Ginarte Val, M.; Sánchez-Aguilar Rojas, M.D.; Martínez Leboráns, L.; Rodríguez Otero, C.; Flórez, Á. Teledermatology vs. Face-to-Face Dermatology for the Diagnosis of Melanoma: A Systematic Review. Cancers 2025, 17, 2836. https://doi.org/10.3390/cancers17172836
López-Pardo Rico M, Ginarte Val M, Sánchez-Aguilar Rojas MD, Martínez Leboráns L, Rodríguez Otero C, Flórez Á. Teledermatology vs. Face-to-Face Dermatology for the Diagnosis of Melanoma: A Systematic Review. Cancers. 2025; 17(17):2836. https://doi.org/10.3390/cancers17172836
Chicago/Turabian StyleLópez-Pardo Rico, María, Manuel Ginarte Val, María Dolores Sánchez-Aguilar Rojas, Lorena Martínez Leboráns, Carmen Rodríguez Otero, and Ángeles Flórez. 2025. "Teledermatology vs. Face-to-Face Dermatology for the Diagnosis of Melanoma: A Systematic Review" Cancers 17, no. 17: 2836. https://doi.org/10.3390/cancers17172836
APA StyleLópez-Pardo Rico, M., Ginarte Val, M., Sánchez-Aguilar Rojas, M. D., Martínez Leboráns, L., Rodríguez Otero, C., & Flórez, Á. (2025). Teledermatology vs. Face-to-Face Dermatology for the Diagnosis of Melanoma: A Systematic Review. Cancers, 17(17), 2836. https://doi.org/10.3390/cancers17172836







