Multiplex Imaging Mass Cytometry Reveals Prognostic Immunosuppressive Subpopulations and Macrophage-Driven Metastasis in Osteosarcoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. TMA and Patient Characteristics
2.2. Tissue Antibody Staining and IMC Acquisition
2.3. Image Processing, Cell Segmentation, and Quality Control
2.4. Cell Phenotyping and Spatial Analysis
2.5. Mice and OS Cell Lines
2.6. Macrophage Polarization
2.7. Mouse Studies
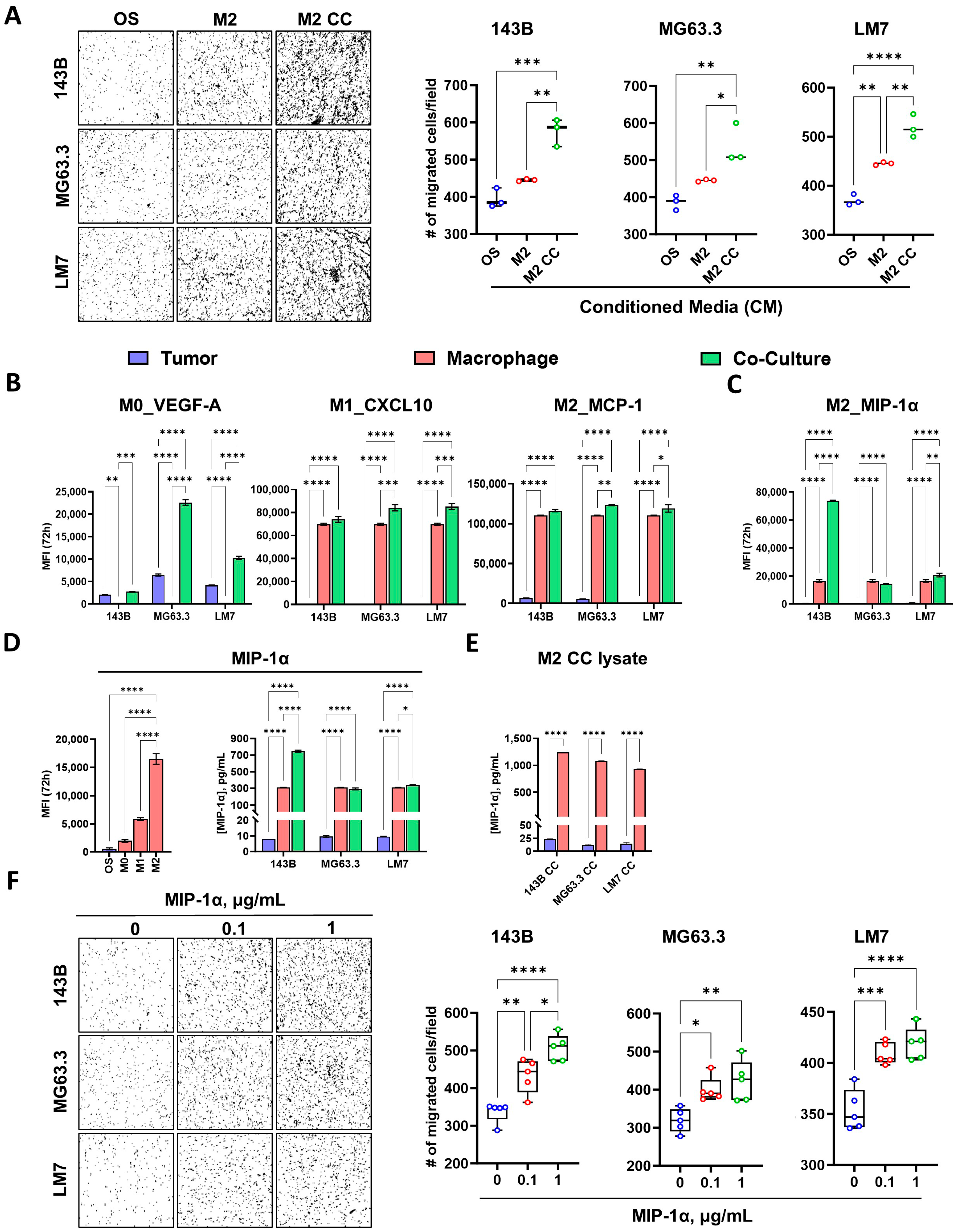
2.8. OS–Macrophage Co-Culture and Cytokine Analysis
2.9. Luminex Data Validation and Protein Source Verification
2.10. Cell Migration Assay
2.11. Statistical Analyses
3. Results
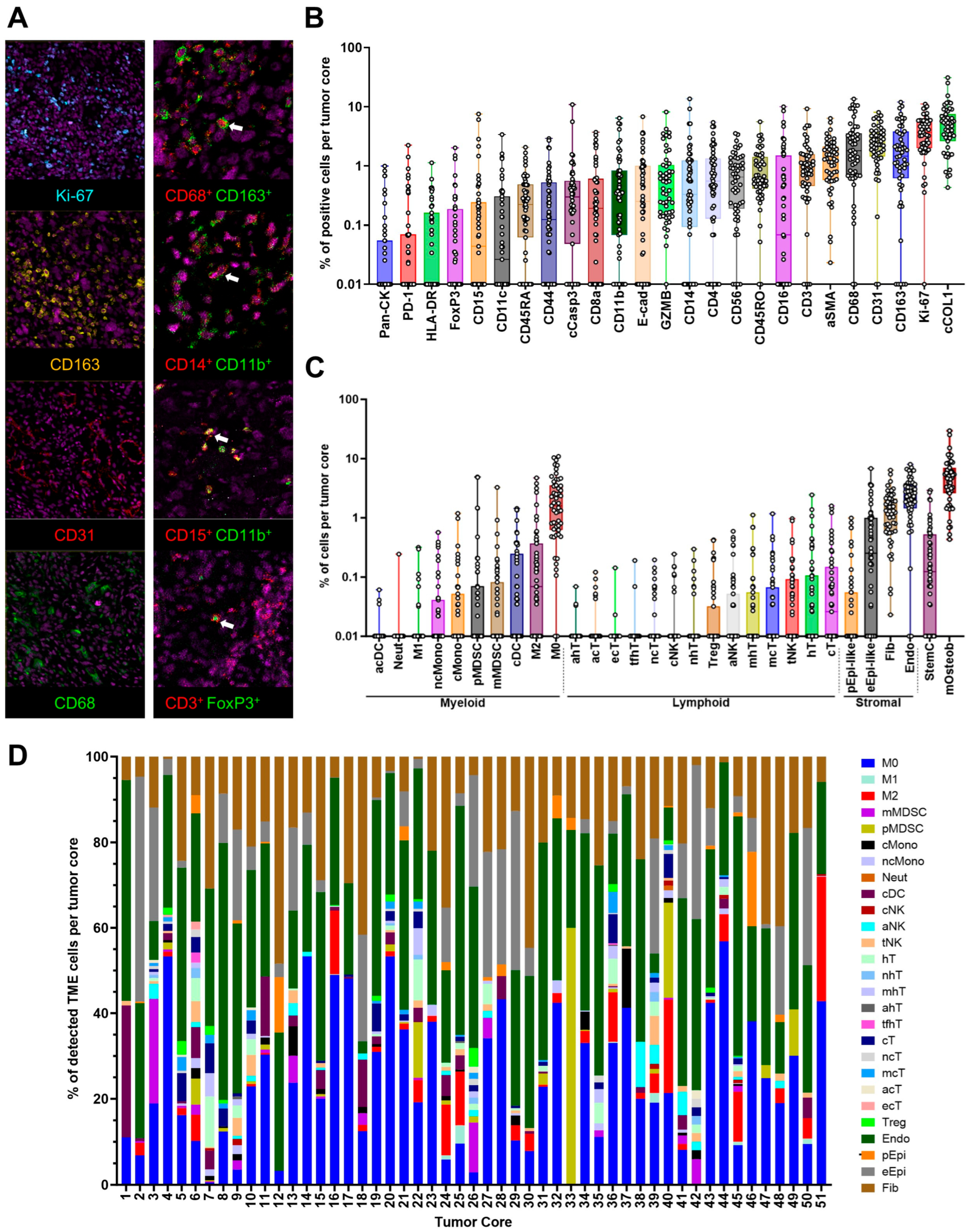
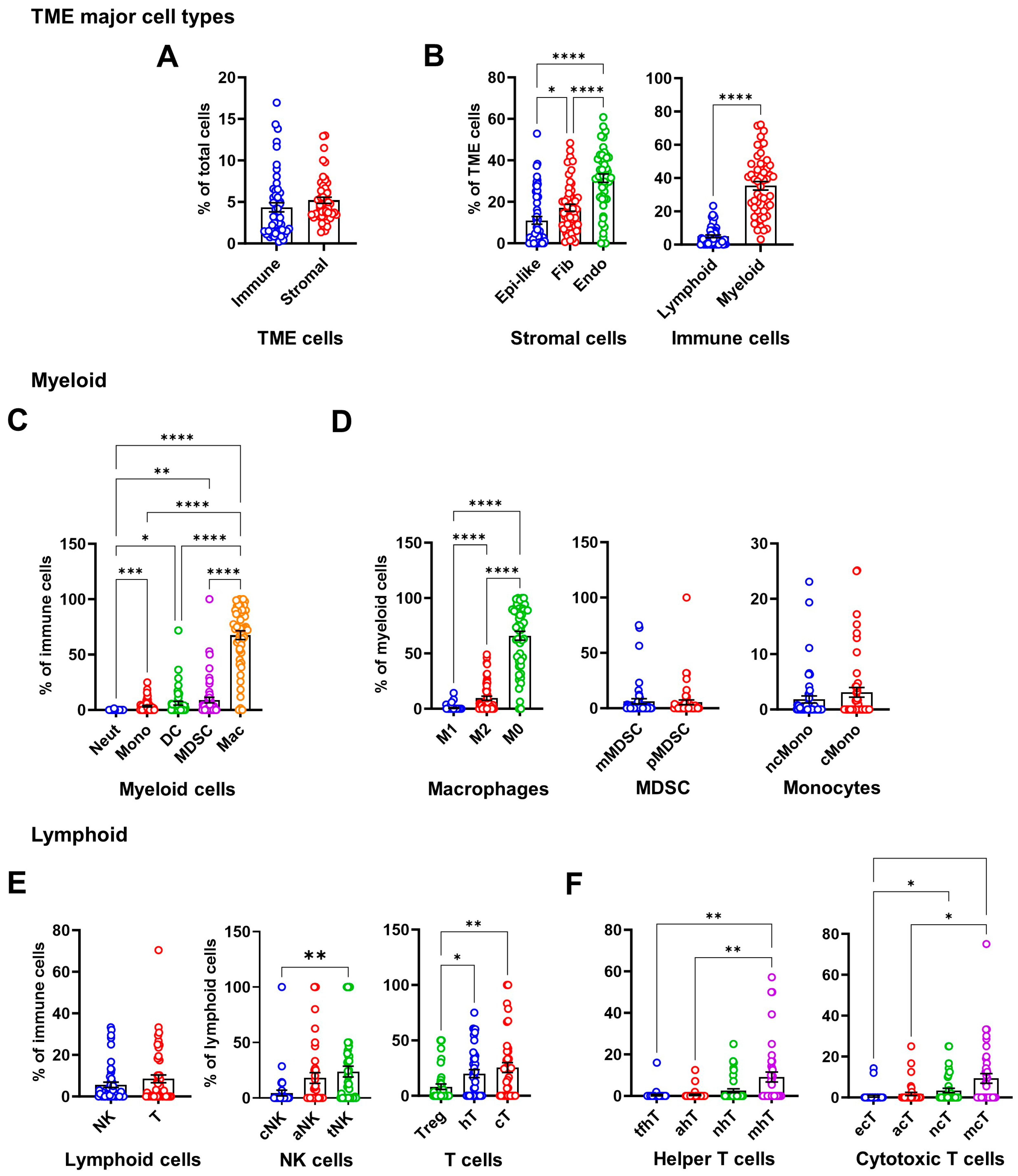
3.1. Cell Phenotyping Analysis of the Osteosarcoma Tumor Microenvironment
3.2. Prognostic Impact of Immunosuppressive Cell Enrichment in Osteosarcoma
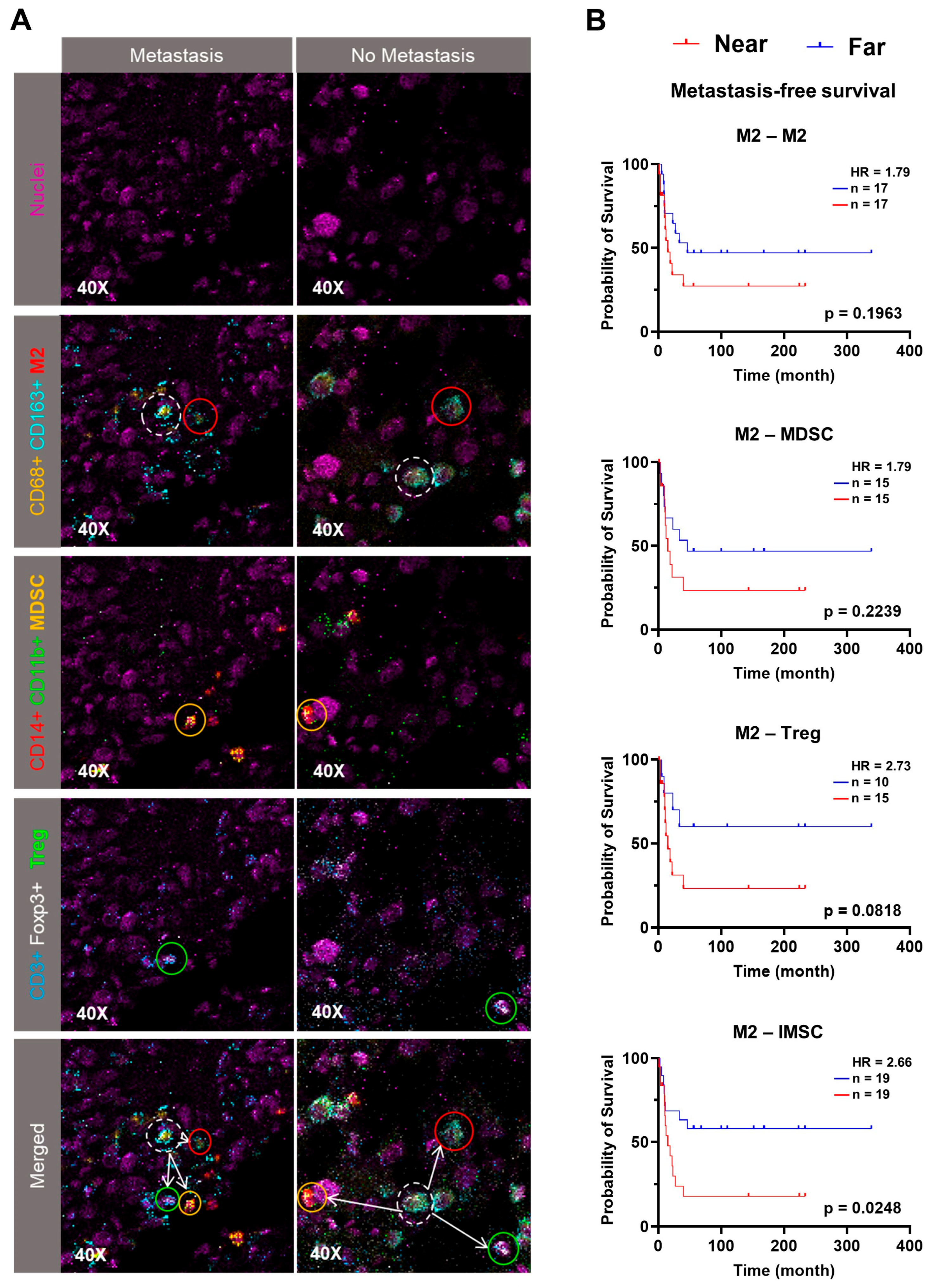
3.3. Spatial Proximity of IMSC with M2 Predicts Poorer Outcomes
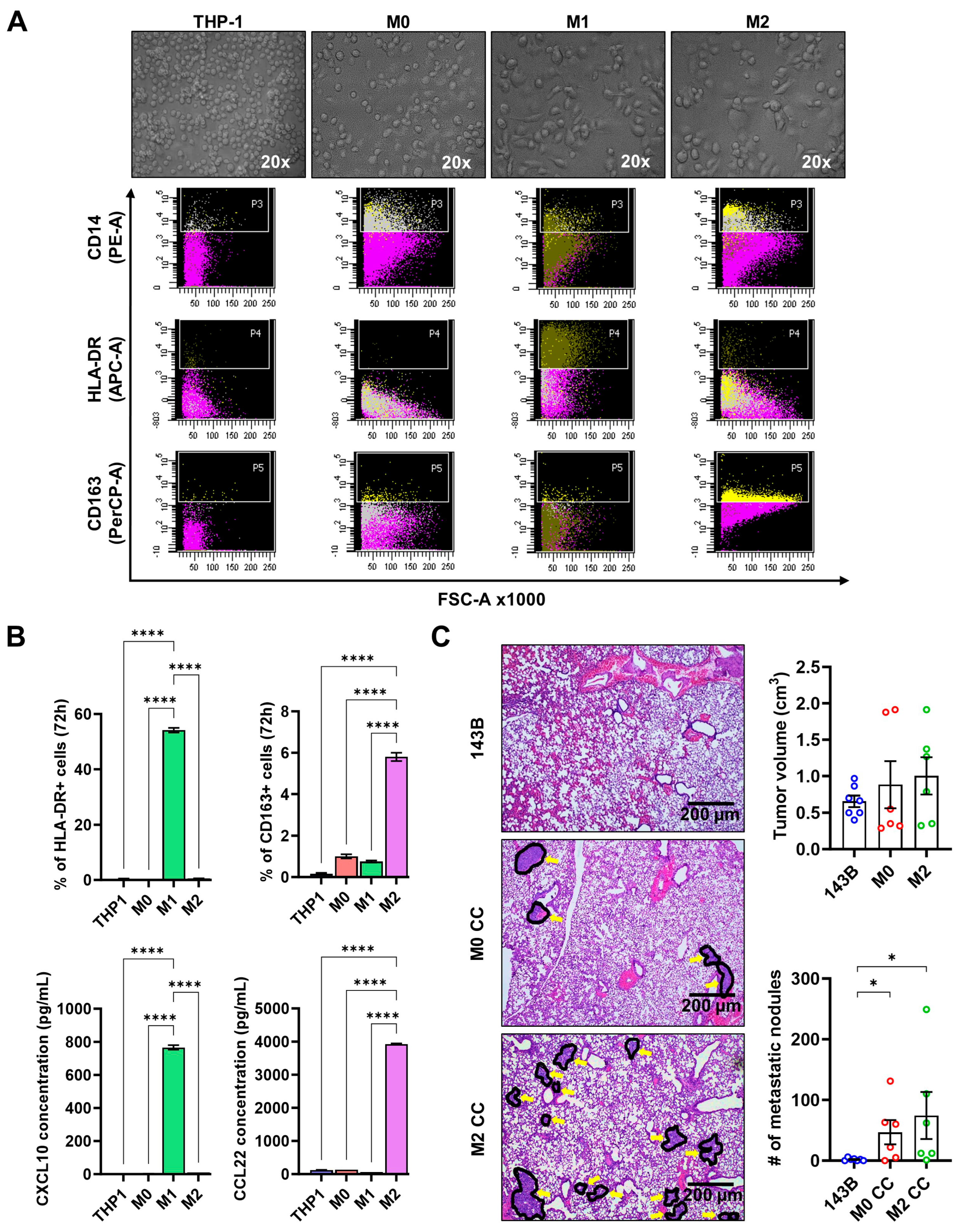
3.4. M2 Drives Pulmonary Metastasis in an Orthotopic Osteosarcoma Xenograft Model
3.5. M2 Enhances Osteosarcoma Cell Migration via MIP-1α Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OS | Osteosarcoma |
| TME | Tumor Microenvironment |
| M2 | M2 Macrophage |
| MDSC | Myeloid-Derived Suppressor Cell |
| Treg | Regulatory T Cell |
| IMSC | Immunosuppressive Cell |
| MIP-1α/CCL3 | Macrophage Inflammatory Protein-1α |
| R/M | Relapsed or Metastatic |
| IMC | Imaging Mass Cytometry |
| TOF | Time-of-Flight |
| CM | Conditioned Media |
| MFI | Median Fluorescence Intensities |
| RFS | Recurrence-Free Survival |
| MFS | Metastasis-Free Survival |
| HR | Hazard Ratio |
| cCOL1 | Collagen Type 1 (Cellular) |
| eCOL1 | Collagen Type 1 (Non-cellular) |
| cNK | Classical NK Cell |
| aNK | Activated NK Cell |
| tNK | T-like NK Cell |
| Mono | Monocyte |
| DC | Dendritic Cell |
| hT | Helper T Cell |
| cT | Cytotoxic T Cell |
| mhT | Memory T Helper Cell |
| mcT | Memory Cytotoxic T Cell |
| tfhT | T Follicular Helper T Cell |
| ahT | Activated Helper T Cell |
| ecT | Effector Cytotoxic T Cell |
| acT | Activated Cytotoxic T Cell |
| nhT | Naïve Helper T Cell |
| ncT | Naïve Cytotoxic T Cell |
| Endo | Endothelial Cell |
| Epi-like | Epithelial-like Cell |
| Fib | Fibroblast |
| StemC | Stem Cell |
| TAM | Tumor-Associated Macrophage |
References
- Jenney, M.E.M. Principles and Practice of Pediatric Oncology. Arch. Dis. Child. 1994, 70, 73. [Google Scholar] [CrossRef]
- Bacci, G.; Rocca, M.; Salone, M.; Balladelli, A.; Ferrari, S.; Palmerini, E.; Forni, C.; Briccoli, A. High grade osteosarcoma of the extremities with lung metastases at presentation: Treatment with neoadjuvant chemotherapy and simultaneous resection of primary and metastatic lesions. J. Surg. Oncol. 2008, 98, 415–420. [Google Scholar] [CrossRef]
- Smeland, S.; Bielack, S.S.; Whelan, J.; Bernstein, M.; Hogendoorn, P.; Krailo, M.D.; Gorlick, R.; Janeway, K.A.; Ingleby, F.C.; Anninga, J.; et al. Survival and prognosis with osteosarcoma: Outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur. J. Cancer 2019, 109, 36–50. [Google Scholar] [CrossRef]
- Kager, L.; Zoubek, A.; Pötschger, U.; Kastner, U.; Flege, S.; Kempf-Bielack, B.; Branscheid, D.; Kotz, R.; Salzer-Kuntschik, M.; Winkelmann, W.; et al. Primary metastatic osteosarcoma: Presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J. Clin. Oncol. 2003, 21, 2011–2018. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Mahoney, M.R.; Van Tine, B.A.; Atkins, J.; Milhem, M.M.; Jahagirdar, B.N.; Antonescu, C.R.; Horvath, E.; Tap, W.D.; Schwartz, G.K.; et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018, 19, 416–426. [Google Scholar] [CrossRef]
- Anderson, P.M.; Bielack, S.S.; Gorlick, R.G.; Skubitz, K.; Daw, N.C.; Herzog, C.E.; Monge, O.R.; Lassaletta, A.; Boldrini, E.; Pápai, Z.; et al. A phase II study of clinical activity of SCH 717454 (robatumumab) in patients with relapsed osteosarcoma and Ewing sarcoma. Pediatr. Blood Cancer 2016, 63, 1761–1770. [Google Scholar] [CrossRef]
- Rickel, K.; Fang, F.; Tao, J. Molecular genetics of osteosarcoma. Bone 2017, 102, 69–79. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Yang, Q.; Lv, X.; Huang, W.; Zhou, Z.; Wang, Y.; Zhang, Z.; Yuan, T.; Ding, X.; et al. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat. Commun. 2020, 11, 6322. [Google Scholar] [CrossRef]
- He, M.; Jiang, X.; Miao, J.; Feng, W.; Xie, T.; Liao, S.; Qin, Z.; Tang, H.; Lin, C.; Li, B.; et al. A new insight of immunosuppressive microenvironment in osteosarcoma lung metastasis. Exp. Biol. Med. 2023, 248, 1056–1073. [Google Scholar] [CrossRef]
- Ligon, J.A.; Choi, W.; Cojocaru, G.; Fu, W.; Hsiue, E.H.C.; Oke, T.F.; Siegel, N.; Fong, M.H.; Ladle, B.; Pratilas, C.A.; et al. Pathways of immune exclusion in metastatic osteosarcoma are associated with inferior patient outcomes. J. Immunother. Cancer 2021, 9, e001772. [Google Scholar] [CrossRef]
- Lacinski, R.A.; Dziadowicz, S.A.; Melemai, V.K.; Fitzpatrick, B.; Pisquiy, J.J.; Heim, T.; Lohse, I.; Schoedel, K.E.; Llosa, N.J.; Weiss, K.R.; et al. Spatial multiplexed immunofluorescence analysis reveals coordinated cellular networks associated with overall survival in metastatic osteosarcoma. Bone Res. 2024, 12, 55. [Google Scholar] [CrossRef]
- Najem, H.; Pacheco, S.; Kowal, J.; Winkowski, D.; Burks, J.K.; Heimberger, A.B. Protocol to quantify immune cell distribution from the vasculature to the glioma microenvironment on sequential immunofluorescence multiplex images. STAR Protoc. 2024, 5, 103079. [Google Scholar] [CrossRef]
- Scheurlen, K.M.; Snook, D.L.; Gardner, S.A.; Eichenberger, M.R.; Galandiuk, S. Macrophage Differentiation and Polarization into an M2-Like Phenotype using a Human Monocyte-Like THP-1 Leukemia Cell Line. J. Vis. Exp. 2021, 2021, e62652. [Google Scholar] [CrossRef]
- Kanta, J. Collagen matrix as a tool in studying fibroblastic cell behavior. Cell Adhes. Migr. 2015, 9, 308–316. [Google Scholar] [CrossRef]
- Shimizu, T.; Sagara, A.; Fukuchi, Y.; Muto, A. Single-agent nintedanib suppresses metastatic osteosarcoma growth by inhibiting tumor vascular formation. Oncol. Lett. 2024, 27, 123. [Google Scholar] [CrossRef]
- Francis, K.; Palsson, B.O. Effective intercellular communication distances are determined by the relative time constants for cyto/chemokine secretion and diffusion. Proc. Natl. Acad. Sci. USA 1997, 94, 12258. [Google Scholar] [CrossRef]
- Júnior, L.C.L.; da Silveira, D.S.C.; Vulczak, A.; dos Santos, J.C.; Veronez, L.C.; Fisch, A.; Flória-Santos, M.; Lima, R.A.G.; Pereira-Da-Silva, G. Emerging cytokine networks in osteosarcoma. Cancer Cell Microenviron. 2017, 4, e1510. [Google Scholar] [CrossRef][Green Version]
- Fu, R.; Liu, H.; Zhao, S.; Wang, Y.; Li, L.; Gao, S.; Ruan, E.; Wang, G.; Wang, H.; Song, J.; et al. Osteoblast inhibition by chemokine cytokine ligand3 in myeloma-induced bone disease. Cancer Cell Int. 2014, 14, 132. [Google Scholar] [CrossRef]
- Eigenbrood, J.; Wong, N.; Mallory, P.; Pereira, J.S.; Williams, D.; Morris-II, D.W.; Beck, J.A.; Cronk, J.C.; Sayers, C.M.; Mendez, M.; et al. Spatial Profiling Identifies Regionally Distinct Microenvironments and Targetable Immunosuppressive Mechanisms in Pediatric Osteosarcoma Pulmonary Metastases. Cancer Res. 2025, 85, 2320–2337. [Google Scholar] [CrossRef]
- Rajan, S.; Franz, E.M.; McAloney, C.A.; Vetter, T.A.; Cam, M.; Gross, A.C.; Taslim, C.; Wang, M.; Cannon, M.V.; Oles, A.; et al. Osteosarcoma tumors maintain intra-tumoral transcriptional heterogeneity during bone and lung colonization. BMC Biol. 2023, 21, 98. [Google Scholar] [CrossRef]
- Sannino, G.; Marchetto, A.; Kirchner, T.; Grünewald, T.G.P. Epithelial-to-mesenchymal and mesenchymal-to-epithelial transition in mesenchymal tumors: A paradox in sarcomas? Cancer Res. 2017, 77, 4556–4561. [Google Scholar] [CrossRef]
- Gown, A.M.; Vogel, A.M. Monoclonal antibodies to intermediate filament proteins of human cells: Unique and cross-reacting antibodies. J. Cell Biol. 1982, 95, 414–424. [Google Scholar] [CrossRef]
- Huang, X.; Wang, L.; Guo, H.; Zhang, W. Single-cell RNA sequencing reveals SERPINE1-expressing CAFs remodelling tumour microenvironment in recurrent osteosarcoma. Clin. Transl. Med. 2024, 14, e1527. [Google Scholar] [CrossRef]
- Broz, M.T.; Ko, E.Y.; Ishaya, K.; Xiao, J.; De Simone, M.; Hoi, X.P.; Piras, R.; Gala, B.; Tessaro, F.H.G.; Karlstaedt, A.; et al. Metabolic targeting of cancer associated fibroblasts overcomes T-cell exclusion and chemoresistance in soft-tissue sarcomas. Nat. Commun. 2024, 15, 2498. [Google Scholar] [CrossRef]
- Arihiro, K.; Inai, K. Expression of CD31, Met/hepatocyte growth factor receptor and bone morphogenetic protein in bone metastasis of osteosarcoma. Pathol. Int. 2001, 51, 100–106. [Google Scholar] [CrossRef]
- Zhao, J.; Ding, X.; Peng, C.; Tian, X.; Wang, M.; Fu, Y.; Guo, H.; Bai, X.; Zhai, X.; Huang, Q.; et al. Assessment of Ki-67 proliferation index in prognosis prediction in patients with nonmetastatic clear cell renal cell carcinoma and tumor thrombus. Urol. Oncol. Semin. Orig. Investig. 2024, 42, e5–e23. [Google Scholar] [CrossRef]
- Scotlandi, K.; Serra, M.; Manara, M.C.; Maurici, D.; Benini, S.; Nini, G.; Campanacci, M.; Baldini, N. Clinical relevance of Ki-67 expression in bone tumors. Cancer 1995, 75, 806–814. [Google Scholar] [CrossRef]
- Weng, W.; Yu, L.; Li, Z.; Tan, C.; Lv, J.; Lao, I.W.; Hu, W.; Deng, Z.; Liu, Z.; Wang, J.; et al. The immune subtypes and landscape of sarcomas. BMC Immunol. 2022, 23, 46. [Google Scholar] [CrossRef]
- Dufresne, A.; Lesluyes, T.; Ménétrier-Caux, C.; Brahmi, M.; Darbo, E.; Toulmonde, M.; Italiano, A.; Mir, O.; Le Cesne, A.; Le Guellec, S.; et al. Specific immune landscapes and immune checkpoint expressions in histotypes and molecular subtypes of sarcoma. OncoImmunology 2020, 9, 1792036. [Google Scholar] [CrossRef]
- Song, Y.J.; Xu, Y.; Zhu, X.; Fu, J.; Deng, C.; Chen, H.; Xu, H.; Song, G.; Lu, J.; Tang, Q.; et al. Immune Landscape of the Tumor Microenvironment Identifies Prognostic Gene Signature CD4/CD68/CSF1R in Osteosarcoma. Front. Oncol. 2020, 10, 1198. [Google Scholar] [CrossRef]
- Inagaki, Y.; Hookway, E.; Williams, K.A.; Hassan, A.B.; Oppermann, U.; Tanaka, Y.; Soilleux, E.; Athanasou, N.A. Dendritic and mast cell involvement in the inflammatory response to primary malignant bone tumours. Clin. Sarcoma Res. 2016, 6, 13. [Google Scholar] [CrossRef]
- Deng, C.; Xu, Y.; Fu, J.; Zhu, X.; Chen, H.; Xu, H.; Wang, G.; Song, Y.; Song, G.; Lu, J.; et al. Reprograming the tumor immunologic microenvironment using neoadjuvant chemotherapy in osteosarcoma. Cancer Sci. 2020, 111, 1899. [Google Scholar] [CrossRef]
- Kishton, R.J.; Sukumar, M.; Restifo, N.P. Metabolic regulation of T cell longevity and function in tumor immunotherapy. Cell Metab. 2017, 26, 94. [Google Scholar] [CrossRef]
- Barnaba, V. T Cell Memory in Infection, Cancer, and Autoimmunity. Front. Immunol. 2022, 12, 811968. [Google Scholar] [CrossRef]
- Lahmar, Q.; Keirsse, J.; Laoui, D.; Movahedi, K.; Van Overmeire, E.; Van Ginderachter, J.A. Tissue-resident versus monocyte-derived macrophages in the tumor microenvironment. Biochim. Biophys. Acta Rev. Cancer 2016, 1865, 23–34. [Google Scholar] [CrossRef]
- Zhou, Q.; Xian, M.; Xiang, S.; Xiang, D.; Shao, X.; Wang, J.; Cao, J.; Yang, X.; Yang, B.; Ying, M.; et al. All-trans retinoic acid prevents osteosarcoma metastasis by inhibiting M2 polarization of tumor-associated macrophages. Cancer Immunol. Res. 2017, 5, 547–559. [Google Scholar] [CrossRef]
- Cersosimo, F.; Lonardi, S.; Bernardini, G.; Telfer, B.; Mandelli, G.E.; Santucci, A.; Vermi, W.; Giurisato, E. Tumor-Associated Macrophages in Osteosarcoma: From Mechanisms to Therapy. Int. J. Mol. Sci. 2020, 21, 5207. [Google Scholar] [CrossRef]
- Gomez-Brouchet, A.; Gilhodes, J.; Van Acker, N.; Brion, R.; Bouvier, C.; Asse-Mat, P.; Gaspar, N.; Aubert, S.; Guinebretiere, J.M.; Marie, B.; et al. Characterization of Macrophages and Osteoclasts in the Osteosarcoma Tumor Microenvironment at Diagnosis: New Perspective for Osteosarcoma Treatment? Cancers 2021, 13, 423. [Google Scholar] [CrossRef]
- Su, Y.; Zhou, Y.; Sun, Y.-J.; Wang, Y.-L.; Yin, J.-Y.; Huang, Y.-J.; Zhang, J.-J.; He, A.-N.; Han, K.; Zhang, H.-Z.; et al. Macrophage-derived CCL18 promotes osteosarcoma proliferation and migration by upregulating the expression of UCA1. J. Mol. Med. 2019, 97, 49–61. [Google Scholar] [CrossRef]
- Taskinen, M.; Karjalainen-Lindsberg, M.L.; Nyman, H.; Eerola, L.M.; Leppä, S. A high tumor-associated macrophage content predicts favorable outcome in follicular lymphoma patients treated with rituximab and cyclophosphamide- doxorubicin-vincristine-prednisone. Clin. Cancer Res. 2007, 13, 5784–5789. [Google Scholar] [CrossRef]
- Shimura, S.; Yang, G.; Ebara, S.; Wheeler, T.M.; Frolov, A.; Thompson, T.C. Reduced infiltration of tumor-associated macrophages in human prostate cancer: Association with cancer progression. Cancer Res. 2000, 60, 5857–5861. [Google Scholar]
- Wang, X.; Ma, T.; Liu, H.; Zhang, S.; Yang, G.; Zhao, Y.; Kong, L.; Gao, R.; Chen, X. Heterogeneous immune landscapes and macrophage dynamics in primary and lung metastatic adenoid cystic carcinoma of the head and neck. Front. Immunol. 2024, 15, 1483887. [Google Scholar] [CrossRef]
- Li, J.; Lin, N.; Zhang, S.; Weng, L.; Chen, C.; Ou, W.; Cao, Y. Characterization of the tumor microenvironment in breast cancer brain metastasis. Heliyon 2024, 10, e34876. [Google Scholar] [CrossRef]
- Li, H.; Han, Y.; Guo, Q.; Zhang, M.; Cao, X. Cancer-Expanded Myeloid-Derived Suppressor Cells Induce Anergy of NK Cells through Membrane-Bound TGF-β1. J. Immunol. 2009, 182, 240–249. [Google Scholar] [CrossRef]
- Dean, I.; Lee, C.Y.C.; Tuong, Z.K.; Li, Z.; Tibbitt, C.A.; Willis, C.; Gaspal, F.; Kennedy, B.C.; Matei-Rascu, V.; Fiancette, R.; et al. Rapid functional impairment of natural killer cells following tumor entry limits anti-tumor immunity. Nat. Commun. 2024, 15, 683. [Google Scholar] [CrossRef]
- Vahidian, F.; Safarzadeh, E.; Mohammadi, A.; Najjary, S.; Mansoori, B.; Majidi, J.; Babaloo, Z.; Aghanejad, A.; Shadbad, M.A.; Mokhtarzadeh, A.; et al. siRNA-mediated silencing of CD44 delivered by Jet Pei enhanced Doxorubicin chemo sensitivity and altered miRNA expression in human breast cancer cell line (MDA-MB468). Mol. Biol. Rep. 2020, 47, 9541–9551. [Google Scholar] [CrossRef]
- Gao, Y.; Foster, R.; Yang, X.; Feng, Y.; Shen, J.K.; Mankin, H.J.; Hornicek, F.J.; Amiji, M.M.; Duan, Z. Up-regulation of CD44 in the development of metastasis, recurrence and drug resistance of ovarian cancer. Oncotarget 2015, 6, 9313–9326. [Google Scholar] [CrossRef]
- Xu, H.; Tian, Y.; Yuan, X.; Liu, Y.; Wu, H.; Liu, Q.; Wu, G.S.; Wu, K. Enrichment of CD44 in basal-type breast cancer correlates with EMT, cancer stem cell gene profile, and prognosis. OncoTargets Ther. 2016, 9, 431–444. [Google Scholar] [CrossRef]
- Gerardo-Ramírez, M.; Keggenhoff, F.L.; Giam, V.; Becker, D.; Groth, M.; Hartmann, N.; Straub, B.K.; Morrison, H.; Galle, P.R.; Marquardt, J.U.; et al. CD44 Contributes to the Regulation of MDR1 Protein and Doxorubicin Chemoresistance in Osteosarcoma. Int. J. Mol. Sci. 2022, 23, 8616. [Google Scholar] [CrossRef]
- Gao, Y.; Feng, Y.; Shen, J.K.; Lin, M.; Choy, E.; Cote, G.M.; Harmon, D.C.; Mankin, H.J.; Hornicek, F.J.; Duan, Z. CD44 is a direct target of miR-199a-3p and contributes to aggressive progression in osteosarcoma. Sci. Rep. 2015, 5, 11365. [Google Scholar] [CrossRef]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef]
- Lanitis, E.; Dangaj, D.; Irving, M.; Coukos, G. Mechanisms regulating T-cell infiltration and activity in solid tumors. Ann. Oncol. 2017, 28, xii18–xii32. [Google Scholar] [CrossRef]
- Ruffell, B.; Chang-Strachan, D.; Chan, V.; Rosenbusch, A.; Ho, C.M.T.; Pryer, N.; Daniel, D.; Hwang, E.S.; Rugo, H.S.; Coussens, L.M. Macrophage IL-10 Blocks CD8+ T Cell-Dependent Responses to Chemotherapy by Suppressing IL-12 Expression in Intratumoral Dendritic Cells. Cancer Cell 2014, 26, 623–637. [Google Scholar] [CrossRef]
- Laxmanan, S.; Robertson, S.W.; Wang, E.; Lau, J.S.; Briscoe, D.M.; Mukhopadhyay, D. Vascular endothelial growth factor impairs the functional ability of dendritic cells through Id pathways. Biochem. Biophys. Res. Commun. 2005, 334, 193–198. [Google Scholar] [CrossRef]
- Salerno, E.P.; Olson, W.C.; McSkimming, C.; Shea, S.; Slingluff, C.L. T cells in the human metastatic melanoma microenvironment express site-specific homing receptors and retention integrins. Int. J. Cancer J. Int. Du Cancer 2014, 134, 563. [Google Scholar] [CrossRef]
- Zheng, Z.; Wieder, T.; Mauerer, B.; Schäfer, L.; Kesselring, R.; Braumüller, H. T Cells in Colorectal Cancer: Unravelling the Function of Different T Cell Subsets in the Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 11673. [Google Scholar] [CrossRef]
- Pesce, E.; Cordiglieri, C.; Bombaci, M.; Eppenberger-Castori, S.; Oliveto, S.; Manara, C.; Crosti, M.; Ercan, C.; Coto, M.; Gobbini, A.; et al. TMEM123 a key player in immune surveillance of colorectal cancer. Front. Immunol. 2023, 14, 1194087. [Google Scholar] [CrossRef]
- Au, Q.; Fang, J.; Juncker-Jensen, A.; Kuo, J.; Leones, E.; Sahafi, F.; Padmanabhan, R.; Hoe, N.; William, J. Characterization of Myeloid-Derived Suppressor Cells and Tumor Associated Macrophages Using MultiOmyxTM Hyperplexed Immunofluorescence Assay in Hodgkin Lymphoma. Blood 2018, 132 (Suppl. 1), 4135. [Google Scholar] [CrossRef]
- Backman, M.; Strell, C.; Lindberg, A.; Mattsson, J.S.M.; Elfving, H.; Brunnström, H.; O’Reilly, A.; Bosic, M.; Gulyas, M.; Isaksson, J.; et al. Spatial immunophenotyping of the tumour microenvironment in non–small cell lung cancer. Eur. J. Cancer 2023, 185, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ji, H.; Niu, X.; Yin, L.; Wang, Y.; Gu, Y.; Wang, J.; Zhou, X.; Zhang, H.; Zhang, Q. Tumor-associated macrophages secrete CC-chemokine ligand 2 and induce tamoxifen resistance by activating PI3K/Akt/mTOR in breast cancer. Cancer Sci. 2020, 111, 47–58. [Google Scholar] [CrossRef]
- Salmiheimo, A.; Mustonen, H.; Vainionpää, S.; Shen, Z.; Kemppainen, E.; Puolakkainen, P.; Seppänen, H. Tumour-associated macrophages activate migration and STAT3 in pancreatic ductal adenocarcinoma cells in co-cultures. Pancreatology 2017, 17, 635–641. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, R.; Shi, S.; Li, Q.; Li, M.; Xiao, M.; Wang, Y.; Yang, Y.; Li, W.; Tang, Y. Circulating IL6 is involved in the infiltration of M2 macrophages and CD8+ T cells. Sci. Rep. 2025, 15, 8681. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Wang, Q.; Zhang, X. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J. Hematol. Oncol. 2017, 10, 36. [Google Scholar] [CrossRef]
- Tatsuno, R.; Ichikawa, J.; Komohara, Y.; Pan, C.; Kawasaki, T.; Enomoto, A.; Aoki, K.; Hayakawa, K.; Iwata, S.; Jubashi, T.; et al. Pivotal role of IL-8 derived from the interaction between osteosarcoma and tumor-associated macrophages in osteosarcoma growth and metastasis via the FAK pathway. Cell Death Dis. 2024, 15, 108. [Google Scholar] [CrossRef]
- Duque, G.A.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Kim, S.U. Cytokine-induced production of macrophage inflammatory protein-1α (MIP- 1α) in cultured human astrocytes. J. Neurosci. Res. 1999, 55, 245–251. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Tsai, H.-C.; Chou, P.-Y.; Wang, S.-W.; Chen, H.-T.; Lin, Y.-M.; Chiang, I.-P.; Chang, T.-M.; Hsu, S.-K.; Chou, M.-C.; et al. CCL3 promotes angiogenesis by dysregulation of miR-374b/ VEGF-A axis in human osteosarcoma cells. Oncotarget 2015, 7, 4310–4325. [Google Scholar] [CrossRef]
- Lentzsch, S.; Gries, M.; Janz, M.; Bargou, R.; Dörken, B.; Mapara, M.Y. Macrophage inflammatory protein 1-alpha (MIP-1α) triggers migration and signaling cascades mediating survival and proliferation in multiple myeloma (MM) cells. Blood 2003, 101, 3568–3573. [Google Scholar] [CrossRef]
- Kodama, T.; Koma, Y.-i.; Arai, N.; Kido, A.; Urakawa, N.; Nishio, M.; Shigeoka, M.; Yokozaki, H. CCL3–CCR5 axis contributes to progression of esophageal squamous cell carcinoma by promoting cell migration and invasion via Akt and ERK pathways. Lab. Investig. 2020, 100, 1140–1157. [Google Scholar] [CrossRef]

| Characteristics | N/Total (%) |
|---|---|
| Age (yrs) | |
| <18 | 24/58 (41) |
| ≥18 | 30/58 (52) |
| Unknown | 4/58 (7) |
| Sex | |
| Male | 27/58 (47) |
| Female | 4/58 (6) |
| Unknown | 27/58 (47) |
| Race | |
| Caucasian | 45/58 (78) |
| African American | 6/58 (10) |
| Other | 7/58 (12) |
| Histologic subtype | |
| Osteoblastic | 19/58 (33) |
| Fibroblastic | 18/58 (31) |
| Chondroblastic | 10/58 (17) |
| Telangiectatic | 4/58 (7) |
| Other | 7/58 (12) |
| Primary tumor site | |
| Femur | 27/58 (47) |
| Tibia | 12/58 (21) |
| Other | 19/58 (32) |
| Stage | |
| IIA | 23/58 (40) |
| IIB | 20/58 (34) |
| III or higher | 6/58 (10) |
| Undetermined | 9/58 (16) |
| Metastasis at diagnosis or during follow-up | |
| Yes | 30/58 (52) |
| No | 24/58 (41) |
| Unknown | 4/58 (7) |
| Site of metastasis | |
| Lung | 28/34 (82) |
| Other | 6/34 (18) |
| Disease recurrence after resection | |
| Yes | 30/58 (52) |
| No | 23/58 (40) |
| Unknown | 5/58 (8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyau, B.B.; Wang, J.; Wu, W.; Scull, B.; Major, A.M.; Jin, W.; Cates, J.M.M.; Hicks, J.; Man, T.-K. Multiplex Imaging Mass Cytometry Reveals Prognostic Immunosuppressive Subpopulations and Macrophage-Driven Metastasis in Osteosarcoma. Cancers 2025, 17, 2780. https://doi.org/10.3390/cancers17172780
Gyau BB, Wang J, Wu W, Scull B, Major AM, Jin W, Cates JMM, Hicks J, Man T-K. Multiplex Imaging Mass Cytometry Reveals Prognostic Immunosuppressive Subpopulations and Macrophage-Driven Metastasis in Osteosarcoma. Cancers. 2025; 17(17):2780. https://doi.org/10.3390/cancers17172780
Chicago/Turabian StyleGyau, Benjamin B., Junyan Wang, Weiguo Wu, Brooks Scull, Angela M. Major, Weidong Jin, Justin M. M. Cates, John Hicks, and Tsz-Kwong Man. 2025. "Multiplex Imaging Mass Cytometry Reveals Prognostic Immunosuppressive Subpopulations and Macrophage-Driven Metastasis in Osteosarcoma" Cancers 17, no. 17: 2780. https://doi.org/10.3390/cancers17172780
APA StyleGyau, B. B., Wang, J., Wu, W., Scull, B., Major, A. M., Jin, W., Cates, J. M. M., Hicks, J., & Man, T.-K. (2025). Multiplex Imaging Mass Cytometry Reveals Prognostic Immunosuppressive Subpopulations and Macrophage-Driven Metastasis in Osteosarcoma. Cancers, 17(17), 2780. https://doi.org/10.3390/cancers17172780






