Predicting Absolute Risk of First Relapse in Classical Hodgkin Lymphoma by Incorporating Contemporary Treatment Effects
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
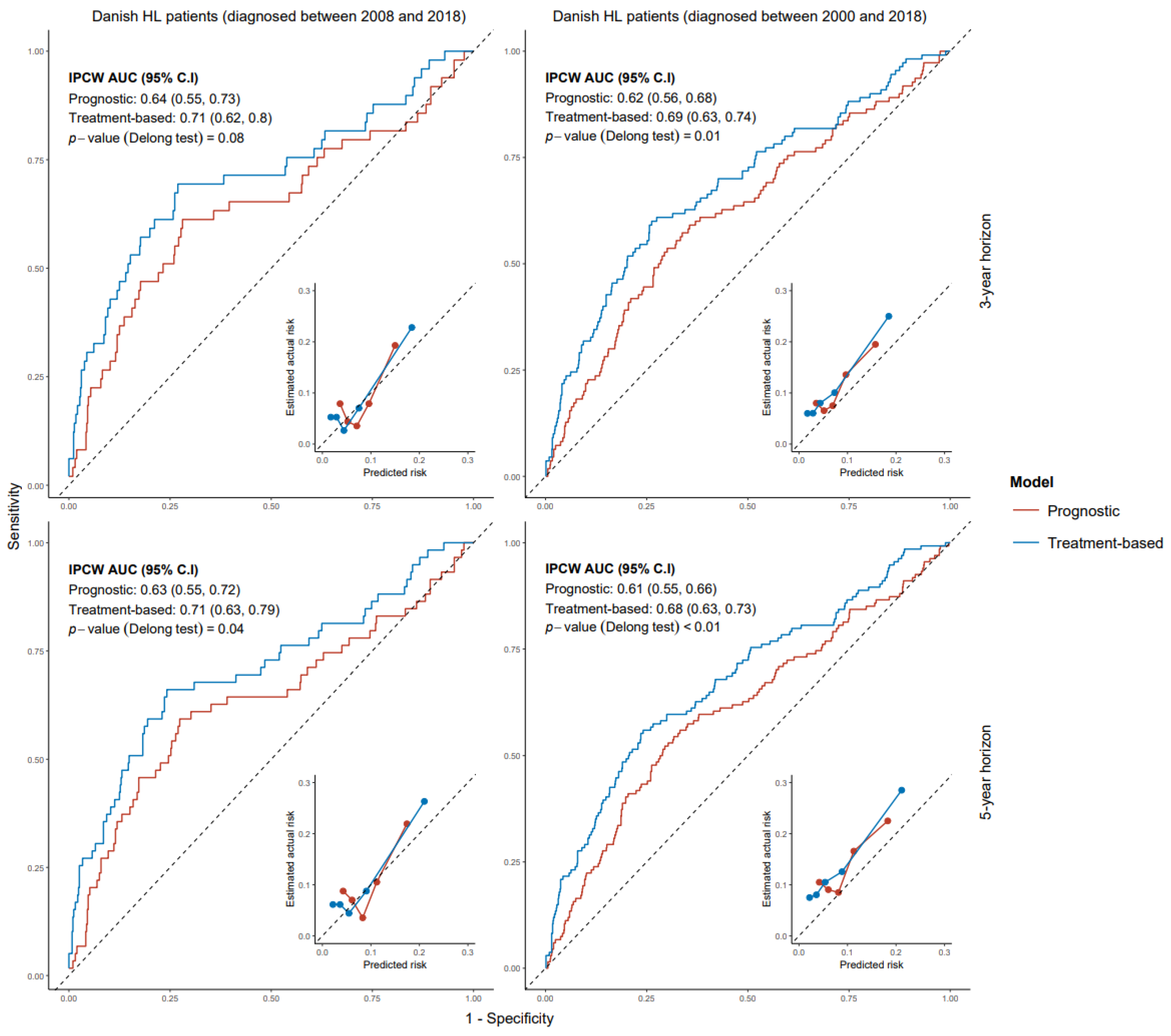
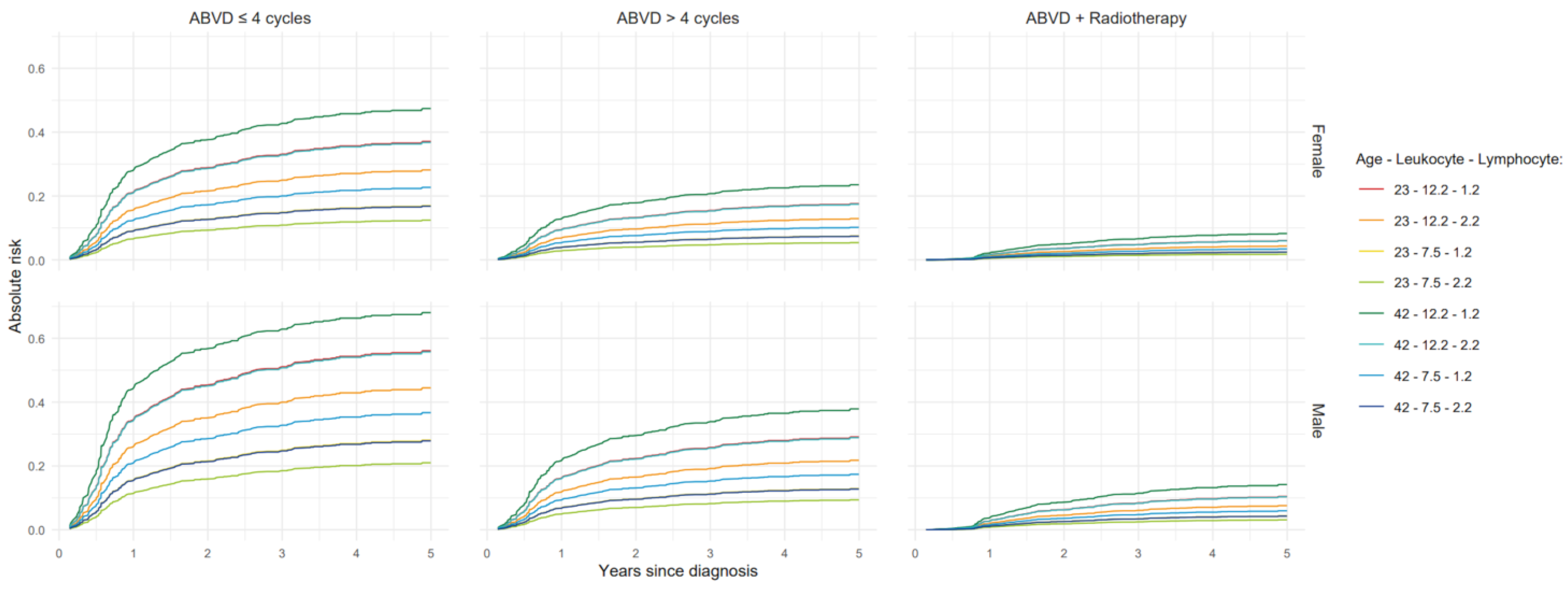
3.1. Models for Progression-Free Survival
3.2. Validation of Models for Progression-Free Survival
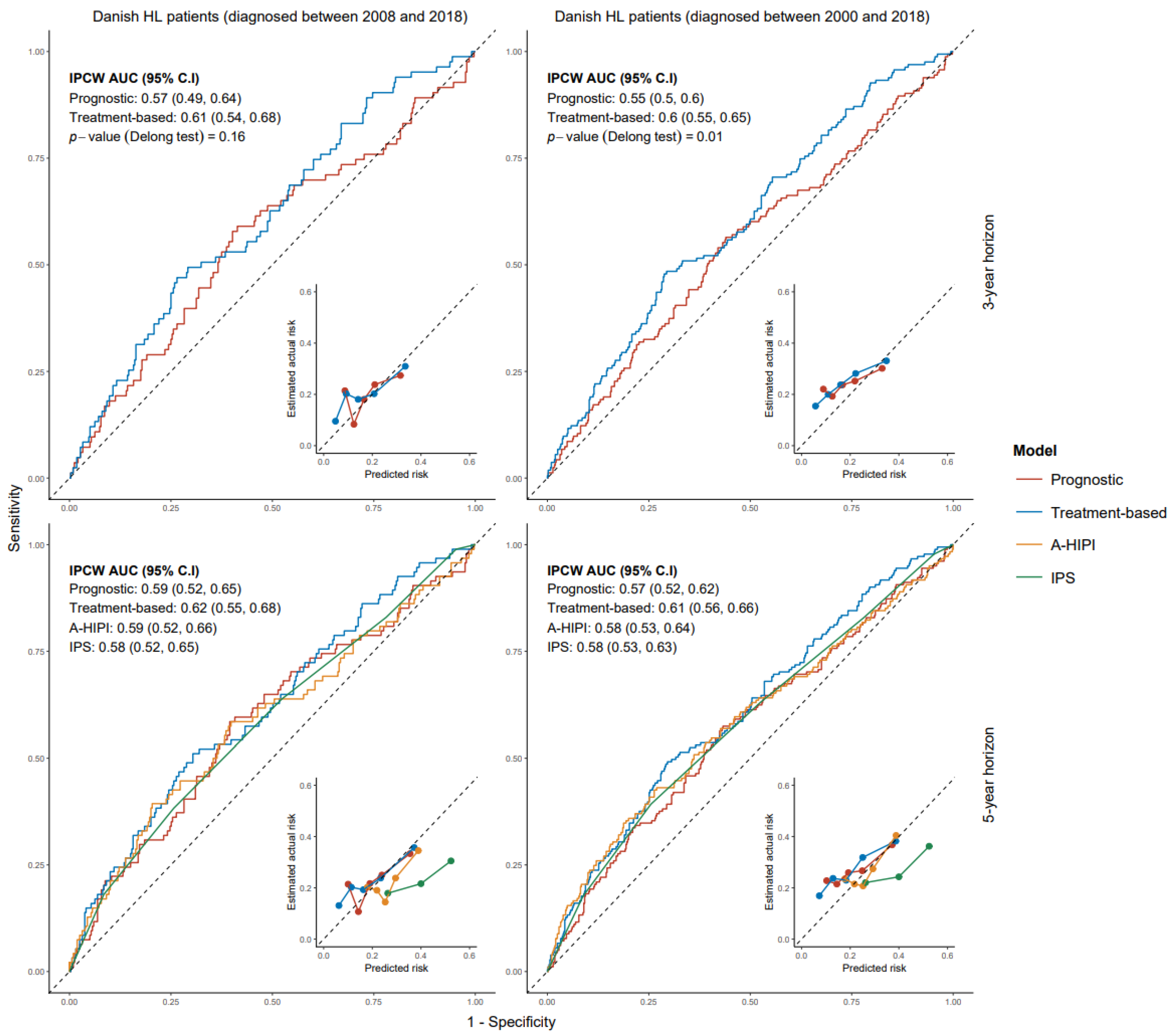
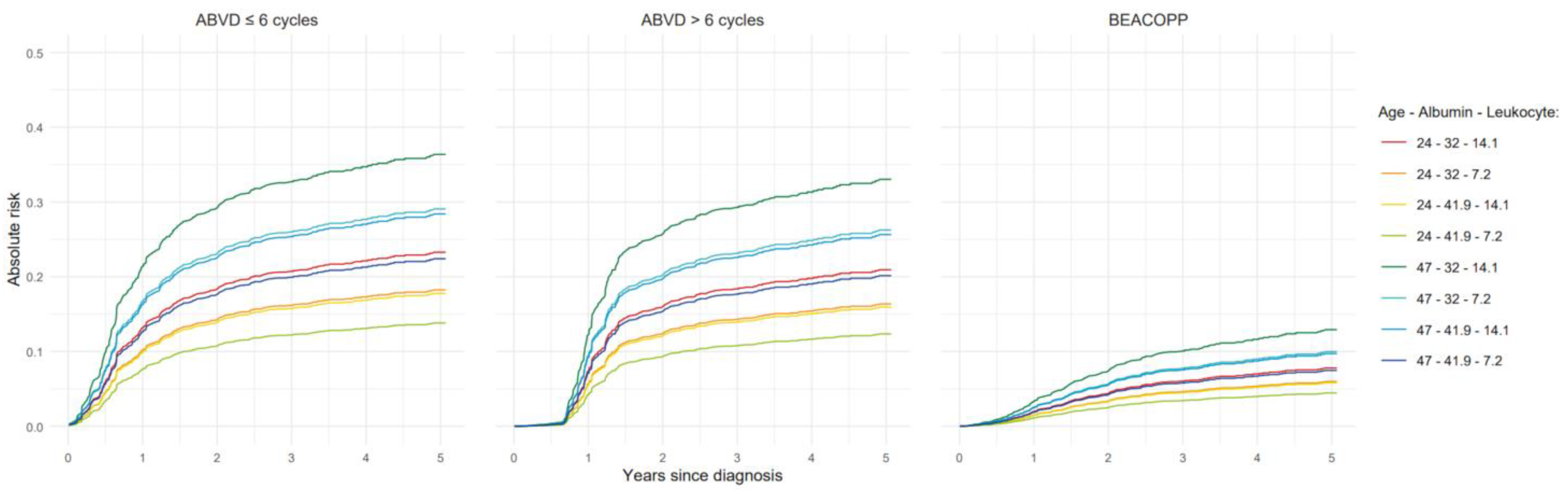
3.3. Models for All-Cause Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HL | Hodgkin Lymphoma |
| GHSG | German Hodgkin Study Group |
| EORTC | European Organization for Research and Treatment of Cancer |
| IPS | International Prognostic Score |
| A-HIPI | Advanced-Stage Hodgkin Lymphoma International Prognostic Index |
| ABVD | Adriamycin (Doxorubicin), Bleomycin, Vinblastine, and Dacarbazine |
| BEACOPP | Bleomycin, Etoposide, Doxorubicin, Cyclophosphamide, Vincristine, Procarbazine, Prednisone |
| PRD | Progression, Relapse or Death |
| PFS | Progression-Free Survival |
| OS | Overall Survival |
| PH | Proportional Hazards |
| LOESS | Locally Estimated Scatterplot Smoothing |
| IPCW | Inverse Probability of Censoring Weighting |
| AUC | Area Under the (ROC) Curve |
| IQR | Inter Quantile Range |
| CI | Confidence Interval |
| BrECADD | Brentuximab vedotin, Etoposide, Cyclophosphamide, Doxorubicin, Dacarbazine and Dexamethasone |
| ESR | Erythrocyte Sedimentation Rate |
References
- Mohty, R.; Duléry, R.; Bazarbachi, A.H.; Savani, M.; Hamed, R.A.; Bazarbachi, A.; Mohty, M. Latest advances in the management of classical Hodgkin lymphoma: The era of novel therapies. Blood Cancer J. 2021, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Cozen, W.; Steidl, C.; Carbone, A.; Hoppe, R.T.; Flechtner, H.H.; Bartlett, N.L. Hodgkin lymphoma. Nat. Rev. Dis. Primers 2020, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, F.E.; Ng, A.K. Long-term risk of second malignancy and cardiovascular disease after Hodgkin lymphoma treatment. Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Schaapveld, M.; Aleman, B.M.P.; Van Eggermond, A.M.; Janus, C.P.M.; Krol, A.D.G.; Van Der Maazen, R.W.M.; Roesink, J.; Raemaekers, J.M.M.; De Boer, J.P.; Zijlstra, J.M.; et al. Second Cancer Risk Up to 40 Years after Treatment for Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 373, 2499–2511. [Google Scholar] [CrossRef]
- Van Nimwegen, F.A.; Schaapveld, M.; Janus, C.P.M.; Krol, A.D.G.; Petersen, E.J.; Raemaekers, J.M.M.; Kok, W.E.M.; Aleman, B.M.P.; Van Leeuwen, F.E. Cardiovascular disease after Hodgkin lymphoma treatment. JAMA Intern. Med. 2015, 175, 1007. [Google Scholar] [CrossRef]
- Hodgson, D.C.; Gilbert, E.S.; Dores, G.M.; Schonfeld, S.J.; Lynch, C.F.; Storm, H.; Hall, P.; Langmark, F.; Pukkala, E.; Andersson, M.; et al. Long-Term solid cancer risk among 5-Year survivors of Hodgkin’s lymphoma. J. Clin. Oncol. 2007, 25, 1489–1497. [Google Scholar] [CrossRef]
- Dores, G.M.; Metayer, C.; Curtis, R.E.; Lynch, C.F.; Clarke, E.A.; Glimelius, B.; Storm, H.; Pukkala, E.; Van Leeuwen, F.E.; Holowaty, E.J.; et al. Second malignant neoplasms among Long-Term survivors of Hodgkin’s Disease: A Population-Based Evaluation over 25 years. J. Clin. Oncol. 2002, 20, 3484–3494. [Google Scholar] [CrossRef]
- Klimm, B.; Goergen, H.; Fuchs, M.; Engert, A.; von Tresckow, B.; Eich, H.-T.; Diehl, V.; André, M.; Eichenauer, D.A.; Borchmann, P. Impact of risk factors on outcomes in early-stage Hodgkin’s lymphoma: An analysis of international staging definitions. Ann. Oncol. 2013, 24, 3070–3076. [Google Scholar] [CrossRef]
- Hasenclever, D.; Diehl, V.; Armitage, J.O.; Assouline, D.; Björkholm, M.; Brusamolino, E.; Canellos, G.P.; Carde, P.; Crowther, D.; Cunningham, D.; et al. A prognostic score for advanced Hodgkin’s disease. N. Engl. J. Med. 1998, 339, 1506–1514. [Google Scholar] [CrossRef]
- Rodday, A.M.; Parsons, S.K.; Upshaw, J.N.; Huang, I.-C.; Brinkman, T.M.; Loren, A.W.; Bhatt, N.S.; Freyer, D.R.; Flowers, C.R.; Bhatia, S. The Advanced-Stage Hodgkin Lymphoma International Prognostic Index: Development and Validation of a Clinical Prediction Model From the HoLISTIC Consortium. J. Clin. Oncol. 2023, 41, 2076–2086. [Google Scholar] [CrossRef]
- Gerds, T.A.; Kattan, M.W. Medical Risk Prediction Models: With Ties to Machine Learning, 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2021. [Google Scholar]
- Therneau, T.M.; Grambsch, P.M.; Fleming, T.R. Martingale-based residuals for survival models. Biometrika 1990, 77, 147–160. [Google Scholar] [CrossRef]
- van Buuren, S. Flexible Imputation of Missing Data, 2nd ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 2018. [Google Scholar]
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Therneau, T.M.; Crowson, C.; Atkinson, E.J. Using Time-Dependent Covariates and Time-Dependent Coefficients in the Cox Model. Survival (CRAN Package Vignette, Version 3.7-0). 2024. Available online: https://CRAN.R-project.org/web/packages/survival/vignettes/timedep.pdf (accessed on 19 August 2025).
- Gerds, T.A.; Ohlendorff, J.; Ozenne, B. riskRegression: Risk Regression Models and Prediction Scores for Survival Analysis with Competing Risks, R package version 2023.12.21; CRAN. [CrossRef]
- van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Herbst, C.; Rehan, F.A.; Brillant, C.; Bohlius, J.; Skoetz, N.; Schulz, H.; Monsef, I.; Specht, L.; Engert, A. Combined modality treatment improves tumor control and overall survival in patients with early stage Hodgkin’s lymphoma: A systematic review. Haematologica 2010, 95, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Fiaccadori, V.; Neven, A.; Fortpied, C.; Aurer, I.; Andre, M.; Federico, M.; Counsell, N.; Phillips, E.H.; Clifton-Hadley, L.; Barrington, S.F.; et al. Relapse patterns in early-PET negative, limited-stage Hodgkin lymphoma (HL) after ABVD with or without radiotherapy—A joint analysis of EORTC/LYSA/FIL H10 and NCRI RAPID trials. Br. J. Haematol. 2023, 200, 731–739. [Google Scholar] [CrossRef] [PubMed]
- André, M.P.E.; Carde, P.; Viviani, S.; Bellei, M.; Fortpied, C.; Hutchings, M.; Gianni, A.M.; Brice, P.; Casasnovas, O.; Gobbi, P.G.; et al. Long-term overall survival and toxicities of ABVD vs. BEACOPP in advanced Hodgkin lymphoma: A pooled analysis of four randomized trials. Cancer Med. 2020, 9, 6565–6575. [Google Scholar] [CrossRef] [PubMed]
- Viviani, S.; Zinzani, P.L.; Rambaldi, A.; Brusamolino, E.; Levis, A.; Bonfante, V.; Vitolo, U.; Pulsoni, A.; Liberati, A.M.; Specchia, G.; et al. ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N. Engl. J. Med. 2011, 365, 203–212. [Google Scholar] [CrossRef]
- Ng, A.K.; van Leeuwen, F.E. Hodgkin Lymphoma: Late Effects of Treatment and Guidelines for Surveillance. Semin. Hematol. 2016, 53, 209–215. [Google Scholar] [CrossRef]
- Borchmann, P.; Haverkamp, H.; Diehl, V.; Cerny, T.; Markova, J.; Ho, A.D.; Eich, H.-T.; Müller-Hermelink, H.K.; Kanz, L.; Greil, R.; et al. Eight cycles of escalated-dose BEACOPP compared with four cycles of escalated-dose BEACOPP followed by four cycles of baseline-dose BEACOPP with or without radiotherapy in patients with advanced-stage Hodgkin’s lymphoma: Final analysis of the HD12 trial of the German Hodgkin Study Group. J. Clin. Oncol. 2011, 29, 4234–4242. [Google Scholar]
- Borchmann, P.; Ferdinandus, J.; Schneider, G.; Moccia, A.; Greil, R.; Hertzberg, M.; Schaub, V.; Hüttmann, A.; Keil, F.; Dierlamm, J.; et al. Assessing the efficacy and tolerability of PET-guided BrECADD versus eBEACOPP in advanced-stage, classical Hodgkin lymphoma (HD21): A randomised, multicentre, parallel, open-label, phase 3 trial. Lancet 2024, 404, 341–352. [Google Scholar] [CrossRef] [PubMed]
- von Tresckow, B.; Plütschow, A.; Fuchs, M.; Klimm, B.; Markova, J.; Lohri, A.; Kral, Z.; Greil, R.; Topp, M.S.; Meissner, J.; et al. Dose-intensification in early unfavorable Hodgkin’s lymphoma: Final analysis of the German Hodgkin Study Group HD14 trial. J. Clin. Oncol. 2012, 30, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Gallamini, A.; Hutchings, M.; Rigacci, L.; Specht, L.; Merli, F.; Hansen, M.; Patti, C.; Loft, A.; Di Raimondo, F.; D’Amore, F.; et al. Early interim 2-[18F]]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: A report from a joint Italian-Danish study. J. Clin. Oncol. 2007, 25, 3746–3752. [Google Scholar] [CrossRef]
- Kishida, M.; Fujisawa, M.; Steidl, C. Molecular biomarkers in classic Hodgkin lymphoma. Semin. Hematol. 2024, 61, 221–228. [Google Scholar] [CrossRef]
- Knecht, H.; Kongruttanachok, N.; Sawan, B.; Brossard, J.; Prévost, S.; Turcotte, E.; Lichtensztejn, Z.; Lichtensztejn, D.; Mai, S. Three-dimensional Telomere Signatures of Hodgkin- and Reed-Sternberg Cells at Diagnosis Identify Patients with Poor Response to Conventional Chemotherapy. Transl. Oncol. 2012, 5, 269–277. [Google Scholar] [CrossRef]
- Rusconi, C.; Barone, A.; Visentin, A.; Bianchi, B.; Zilioli, V.R.; Bernardelli, A.; Iadecola, S.; Olivari, E.; Rossi, F.G.; Gotti, M.; et al. Interim-PET predicts progression-free survival in stage IV Hodgkin lymphoma treated with upfront brentuximab vedotin-AVD. Leuk. Lymphoma 2025, 66, 879–887. [Google Scholar] [CrossRef]
- Herrera, A.F.; LeBlanc, M.; Castellino, S.M.; Li, H.; Rutherford, S.C.; Evens, A.M.; Davison, K.; Punnett, A.; Parsons, S.K.; Ahmed, S.; et al. Nivolumab+AVD in Advanced-Stage Classic Hodgkin’s Lymphoma. N. Engl. J. Med. 2024, 391, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
| Year of Diagnosis: 2008–2010 | Year of Diagnosis: 2014–2018 | Year of Diagnosis: 2008–2018 | ||||
|---|---|---|---|---|---|---|
| Early Stage (N = 503) | Advanced Stage (N = 345) | Early Stage (N = 834) | Advanced Stage (N = 658) | Early Stage (N = 1337) | Advanced Stage (N = 1003) | |
| Sex | ||||||
| female | 261 (51.9%) | 127 (36.8%) | 412 (49.4%) | 233 (35.4%) | 673 (50.3%) | 360 (35.9%) |
| male | 242 (48.1%) | 218 (63.2%) | 422 (50.6%) | 425 (64.6%) | 664 (49.7%) | 643 (64.1%) |
| Age, years | ||||||
| Mean (SD) | 33.4 (12.6) | 36.3 (13.4) | 33.1 (12.0) | 34.9 (13.0) | 33.2 (12.2) | 35.3 (13.1) |
| Median [Min, Max] | 31.0 [15.0, 60.0] | 35.0 [15.0, 60.0] | 31.0 [15.0, 60.0] | 33.0 [15.0, 60.0] | 31.0 [15.0, 60.0] | 34.0 [15.0, 60.0] |
| 15–18 | 41 (8.2%) | 35 (10.1%) | 73 (8.8%) | 63 (9.6%) | 114 (8.5%) | 98 (9.8%) |
| 19–29 | 199 (39.6%) | 91 (26.4%) | 319 (38.2%) | 207 (31.5%) | 518 (38.7%) | 298 (29.7%) |
| 30–39 | 104 (20.7%) | 78 (22.6%) | 190 (22.8%) | 164 (24.9%) | 294 (22.0%) | 242 (24.1%) |
| 40–49 | 77 (15.3%) | 66 (19.1%) | 144 (17.3%) | 99 (15.0%) | 221 (16.5%) | 165 (16.5%) |
| 50–60 | 82 (16.3%) | 75 (21.7%) | 108 (12.9%) | 125 (19.0%) | 190 (14.2%) | 200 (19.9%) |
| Ann arbor stage | ||||||
| I | 86 (17.1%) | - | 138 (16.5%) | - | 224 (16.8%) | - |
| II | 417 (82.9%) | - | 696 (83.5%) | - | 1113 (83.2%) | - |
| III | - | 204 (59.1%) | - | 268 (40.7%) | - | 472 (47.1%) |
| IV | - | 141 (40.9%) | - | 390 (59.3%) | - | 531 (52.9%) |
| Presence of B-symptoms | ||||||
| no | 369 (73.4%) | 126 (36.5%) | 599 (71.8%) | 253 (38.4%) | 968 (72.4%) | 379 (37.8%) |
| yes | 133 (26.4%) | 215 (62.3%) | 216 (25.9%) | 390 (59.3%) | 349 (26.1%) | 605 (60.3%) |
| Missing | 1 (0.2%) | 4 (1.2%) | 19 (2.3%) | 15 (2.3%) | 20 (1.5%) | 19 (1.9%) |
| Extra-nodal disease | ||||||
| no | 489 (97.2%) | 194 (56.2%) | 792 (95.0%) | 240 (36.5%) | 1281 (95.8%) | 434 (43.3%) |
| yes | 14 (2.8%) | 151 (43.8%) | 41 (4.9%) | 418 (63.5%) | 55 (4.1%) | 569 (56.7%) |
| Missing | 0 (0%) | 0 (0%) | 1 (0.1%) | 0 (0%) | 1 (0.1%) | 0 (0%) |
| LDH | ||||||
| normal | 336 (66.8%) | 195 (56.5%) | 661 (79.3%) | 433 (65.8%) | 997 (74.6%) | 628 (62.6%) |
| below/above standard limits | 98 (19.5%) | 94 (27.2%) | 158 (18.9%) | 217 (33.0%) | 256 (19.1%) | 311 (31.0%) |
| Missing | 69 (13.7%) | 56 (16.2%) | 15 (1.8%) | 8 (1.2%) | 84 (6.3%) | 64 (6.4%) |
| Number of involved nodes | ||||||
| 0–4 | 408 (81.1%) | 77 (22.3%) | 655 (78.5%) | 154 (23.4%) | 1063 (79.5%) | 231 (23.0%) |
| 4 | 80 (15.9%) | 248 (71.9%) | 179 (21.5%) | 504 (76.6%) | 259 (19.4%) | 752 (75.0%) |
| Missing | 15 (3.0%) | 20 (5.8%) | 0 (0%) | 0 (0%) | 15 (1.1%) | 20 (2.0%) |
| Hemoglobin, mmol/L | ||||||
| Mean (SD) | 8.18 (1.08) | 7.48 (1.16) | 8.18 (1.14) | 7.49 (1.31) | 8.18 (1.12) | 7.48 (1.26) |
| Median [Min, Max] | 8.20 [4.60, 11.3] | 7.50 [3.90, 10.9] | 8.20 [3.30, 10.9] | 7.60 [1.90, 11.1] | 8.20 [3.30, 11.3] | 7.60 [1.90, 11.1] |
| Missing | 53 (10.5%) | 33 (9.6%) | 37 (4.4%) | 14 (2.1%) | 90 (6.7%) | 47 (4.7%) |
| Albumin, gr/L | ||||||
| Mean (SD) | 40.9 (5.72) | 36.6 (7.01) | 40.5 (5.69) | 36.5 (6.69) | 40.6 (5.70) | 36.5 (6.79) |
| Median [Min, Max] | 41.4 [17.0, 52.0] | 38.0 [7.00, 50.0] | 41.0 [4.40, 53.0] | 37.0 [14.0, 52.9] | 41.0 [4.40, 53.0] | 37.0 [7.00, 52.9] |
| Missing | 142 (28.2%) | 92 (26.7%) | 146 (17.5%) | 78 (11.9%) | 288 (21.5%) | 170 (16.9%) |
| Leukocyte,×109/L | ||||||
| Mean (SD) | 10.3 (4.13) | 10.9 (5.67) | 10.4 (4.16) | 11.3 (5.68) | 10.3 (4.15) | 11.2 (5.68) |
| Median [Min, Max] | 9.50 [0.600, 27.1] | 10.3 [0.500, 39.3] | 9.60 [0.700, 34.3] | 10.7 [0.800, 36.3] | 9.50 [0.600, 34.3] | 10.5 [0.500, 39.3] |
| Missing | 42 (8.4%) | 31 (9.0%) | 33 (4.0%) | 10 (1.5%) | 75 (5.6%) | 41 (4.1%) |
| Lymphocyte,×109/L | ||||||
| Mean (SD) | 2.41 (3.97) | 1.71 (2.10) | 2.09 (2.92) | 1.69 (1.87) | 2.20 (3.31) | 1.70 (1.94) |
| Median [Min, Max] | 1.60 [0.300, 37.9] | 1.40 [0.100, 23.4] | 1.70 [0.200, 37.4] | 1.40 [0.100, 25.4] | 1.60 [0.200, 37.9] | 1.40 [0.100, 25.4] |
| Missing | 135 (26.8%) | 92 (26.7%) | 86 (10.3%) | 42 (6.4%) | 221 (16.5%) | 134 (13.4%) |
| Primary treatment | ||||||
| ABVD ≤ 4 cycles (no RT) | 53 (10.5%) | - | 51 (6.1%) | - | 104 (7.8%) | - |
| ABVD > 4 cycles (no RT) | 60 (11.9%) | - | 133 (15.9%) | - | 193 (14.4%) | - |
| ABVD + RT | 332 (66.0%) | - | 518 (62.1%) | - | 850 (63.6%) | - |
| ABVD ≤ 6 (w/wo RT) | - | 139 (40.3%) | - | 254 (38.6%) | - | 393 (39.2%) |
| ABVD > 6 (w/wo RT) | - | 128 (37.1%) | - | 104 (15.8%) | - | 232 (23.1%) |
| (Escalated) BEACOPP (w/wo RT) | - | 54 (15.7%) | - | 248 (37.7%) | - | 302 (30.1%) |
| other | 58 (11.5%) | 24 (7.0%) | 132 (15.8%) | 52 (7.9%) | 190 (14.2%) | 76 (7.6%) |
| Prognostic Model | Treatment-Based Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Category/Spline Term | Estimate | Std Error | p-Value | PH-p | Estimate | Std Error | p-Value | PH-p |
| Age, years | - | - | - | - | - | 0.017 | 0.007 | 0.017 | 0.552 |
| Sex | female | Reference | Reference | ||||||
| male | 0.460 | 0.178 | 0.009 | 0.188 | 0.579 | 0.181 | 0.001 | 0.294 | |
| Leukocyte, ×/L | −0.204 | 0.049 | <0.001 | 0.612 | −0.207 | 0.049 | <0.001 | 0.592 | |
| −0.015 | 0.037 | 0.243 | 0.002 | 0.036 | 0.310 | ||||
| Lymphocyte, ×/L | 0.914 | 0.701 | <0.001 | 0.062 | 1.061 | 0.733 | 0.023 | 0.107 | |
| −0.371 | 0.163 | 0.116 | −0.345 | 0.172 | 0.228 | ||||
| 0.422 | 0.200 | 0.274 | 0.407 | 0.210 | 0.376 | ||||
| Primary Treatment | ABVD ≤ 4 cycles (no RT) | Reference | Reference | ||||||
| ABVD > 4 cycles (no RT) | - | - | - | - | −0.869 | 0.261 | <0.001 | 0.297 | |
| ABVD+RT ≤ 9 months | - | - | - | −3.822 | 0.620 | 0.983 | |||
| ABVD+RT > 9 months | - | - | - | −1.560 | 0.264 | 0.075 | |||
| other | - | - | - | −1.216 | 0.286 | 0.349 | |||
| Global PH p-value: 0.132 | Global PH p-value: 0.061 | ||||||||
| Prognostic Model | Treatment-Based Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Category/Spline Term | Estimate | Std Error | p-Value | PH-p | Estimate | Std Error | p-Value | PH-p |
| Age, years | −0.020 | 0.015 | <0.001 | 0.641 | −0.029 | 0.016 | <0.001 | 0.882 | |
| 0.027 | 0.010 | 0.729 | 0.018 | 0.010 | 0.866 | ||||
| Albumin, gr/L | - | −0.027 | 0.011 | 0.019 | 0.078 | −0.030 | 0.011 | 0.005 | 0.117 |
| Leukocyte, ×/L | 0.095 | 0.039 | <0.001 | 0.391 | 0.077 | 0.039 | <0.001 | 0.387 | |
| 0.127 | 0.029 | 0.714 | 0.120 | 0.029 | 0.801 | ||||
| −0.216 | 0.068 | 0.831 | −0.187 | 0.067 | 0.900 | ||||
| Primary Treatment | ABVD ≤ 6 cycles | Reference | Reference | ||||||
| ABVD > 6 cycles ≤ 8 months | - | - | - | - | −3.341 | 1.010 | <0.001 | 0.148 | |
| ABVD > 6 cycles 8–17 months | - | - | - | 0.690 | 0.247 | 0.685 | |||
| ABVD > 6 cycles > 17 months | - | - | - | −0.029 | 0.296 | 0.841 | |||
| (Escalated) BEACOPP ≤ 8 months | - | - | - | −2.445 | 0.596 | 0.972 | |||
| (Escalated) BEACOPP 8–17 months | - | - | - | −1.220 | 0.420 | 0.772 | |||
| (Escalated) BEACOPP > 17 months | - | - | - | −0.546 | 0.315 | 0.854 | |||
| other | - | - | - | 0.045 | 0.291 | 0.939 | |||
| Global PH p-value: 0.470 | Global PH p-value: 0.932 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roshani, S.; van Leeuwen, F.E.; Rossetti, S.; Hauptmann, M.; Visser, O.; Zijlstra, J.M.; Hutchings, M.; Schaapveld, M.; Aleman, B.M.P. Predicting Absolute Risk of First Relapse in Classical Hodgkin Lymphoma by Incorporating Contemporary Treatment Effects. Cancers 2025, 17, 2760. https://doi.org/10.3390/cancers17172760
Roshani S, van Leeuwen FE, Rossetti S, Hauptmann M, Visser O, Zijlstra JM, Hutchings M, Schaapveld M, Aleman BMP. Predicting Absolute Risk of First Relapse in Classical Hodgkin Lymphoma by Incorporating Contemporary Treatment Effects. Cancers. 2025; 17(17):2760. https://doi.org/10.3390/cancers17172760
Chicago/Turabian StyleRoshani, Shahin, Flora E. van Leeuwen, Sara Rossetti, Michael Hauptmann, Otto Visser, Josée M. Zijlstra, Martin Hutchings, Michael Schaapveld, and Berthe M. P. Aleman. 2025. "Predicting Absolute Risk of First Relapse in Classical Hodgkin Lymphoma by Incorporating Contemporary Treatment Effects" Cancers 17, no. 17: 2760. https://doi.org/10.3390/cancers17172760
APA StyleRoshani, S., van Leeuwen, F. E., Rossetti, S., Hauptmann, M., Visser, O., Zijlstra, J. M., Hutchings, M., Schaapveld, M., & Aleman, B. M. P. (2025). Predicting Absolute Risk of First Relapse in Classical Hodgkin Lymphoma by Incorporating Contemporary Treatment Effects. Cancers, 17(17), 2760. https://doi.org/10.3390/cancers17172760






