Clinical Predictors and Recurrence Characteristics Following Radiotherapy for Primary Central Nervous System Lymphoma: A Retrospective Cohort Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Treatment Planning and Administration of Radiotherapy
2.3. Statistical Analysis
3. Results
3.1. Patient, Disease and Radiotherapy Treatment Characteristics
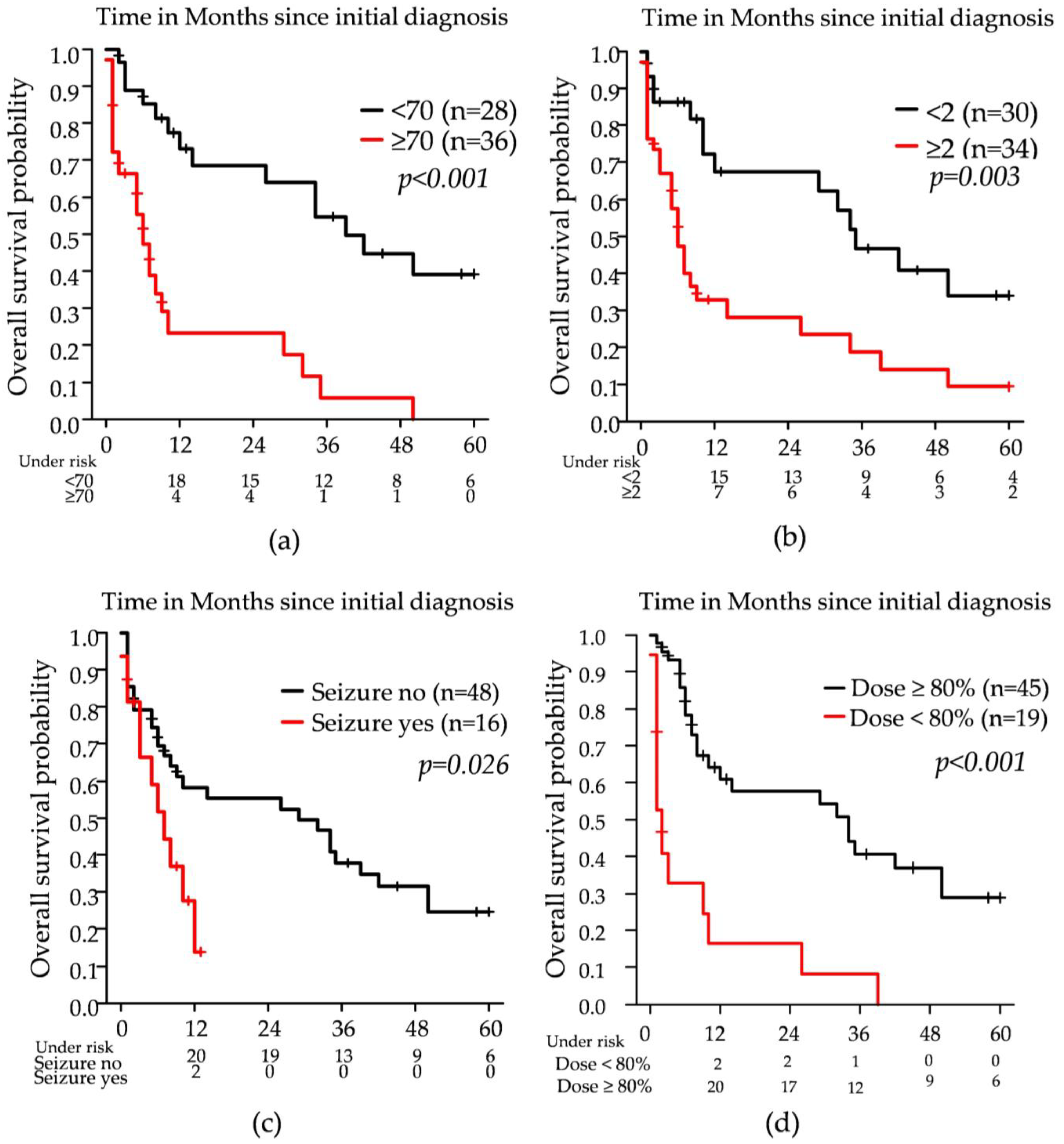
3.2. Survival Parameters and Potential Influencers
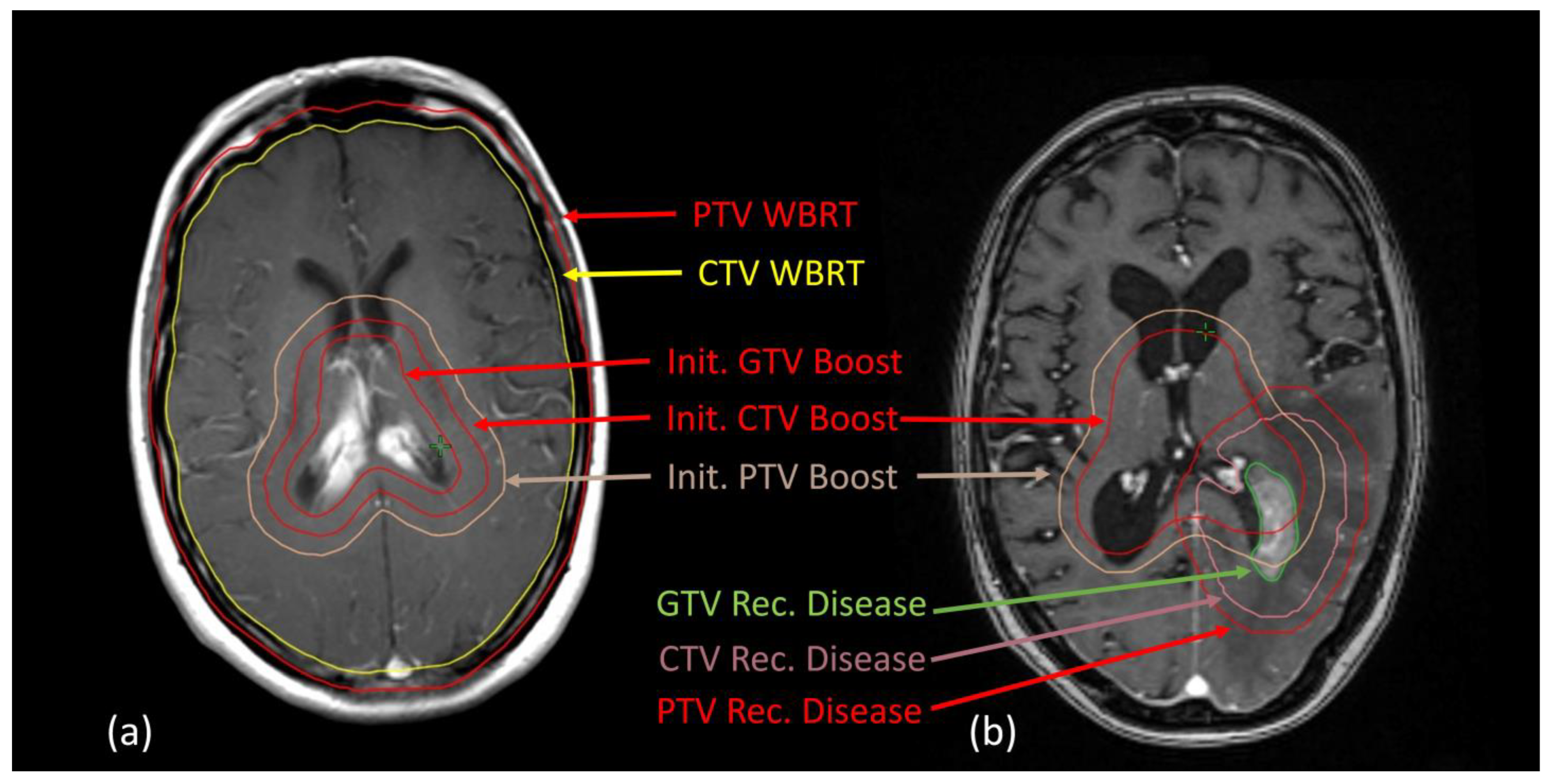
3.3. Local Recurrences
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCI | Charlson Comorbidity Index |
| CI | confidence interval |
| CTV | clinical target volume |
| CTx | Chemotherapy |
| ECOG | Eastern Cooperative Oncology Group |
| GTV | gross tumor volume |
| Gy | Gray |
| HF | helmet field technique |
| IMRT | intensity-modulated radiotherapy |
| ld | Low-dose |
| n.s. | not significant |
| OS | Overall survival |
| PCNSL | Primary central nervous system lymphoma |
| PTV | planning target volume |
| RT | Radiotherapy |
| VMAT | volumetric modulated arc therapy |
| WBRT | whole-brain radiotherapy |
| 3D-cRT | 3dimensional conformal Radiotherapy |
References
- Villano, J.L.; Koshy, M.; Shaikh, H.; Dolecek, T.A.; McCarthy, B.J. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br. J. Cancer 2011, 105, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.G.; Correa, D.D.; Yahalom, J.; Raizer, J.J.; Schiff, D.; Grant, B.; Grimm, S.; Lai, R.K.; Reiner, A.S.; Panageas, K.; et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: Final results and long-term outcome. J. Clin. Oncol. 2013, 31, 3971–3979. [Google Scholar] [CrossRef]
- Schultz, C.; Scott, C.; Sherman, W.; Donahue, B.; Fields, J.; Murray, K.; Fisher, B.; Abrams, R.; Meis-Kindblom, J. Preirradiation chemotherapy with cyclophosphamide, doxorubicin, vincristine, and dexamethasone for primary CNS lymphomas: Initial report of radiation therapy oncology group protocol 88-06. J. Clin. Oncol. 1996, 14, 556–564. [Google Scholar] [CrossRef]
- Abrey, L.E.; Ben-Porat, L.; Panageas, K.S.; Yahalom, J.; Berkey, B.; Curran, W.; Schultz, C.; Leibel, S.; Nelson, D.; Mehta, M.; et al. Primary central nervous system lymphoma: The Memorial Sloan-Kettering Cancer Center prognostic model. J. Clin. Oncol. 2006, 24, 5711–5715. [Google Scholar] [CrossRef] [PubMed]
- Calimeri, T.; Steffanoni, S.; Gagliardi, F.; Chiara, A.; Ferreri, A.J.M. How we treat primary central nervous system lymphoma. ESMO Open 2021, 6, 100213. [Google Scholar] [CrossRef] [PubMed]
- Houillier, C.; Taillandier, L.; Dureau, S.; Lamy, T.; Laadhari, M.; Chinot, O.; Moluçon-Chabrot, C.; Soubeyran, P.; Gressin, R.; Choquet, S.; et al. Radiotherapy or Autologous Stem-Cell Transplantation for Primary CNS Lymphoma in Patients 60 Years of Age and Younger: Results of the Intergroup ANOCEF-GOELAMS Randomized Phase II PRECIS Study. J. Clin. Oncol. 2019, 37, 823–833. [Google Scholar] [CrossRef]
- Correa, D.D.; Braun, E.; Kryza-Lacombe, M.; Ho, K.-W.; Reiner, A.S.; Panageas, K.S.; Yahalom, J.; Sauter, C.S.; Abrey, L.E.; DeAngelis, L.M.; et al. Longitudinal cognitive assessment in patients with primary CNS lymphoma treated with induction chemotherapy followed by reduced-dose whole-brain radiotherapy or autologous stem cell transplantation. J. Neurooncol. 2019, 144, 553–562. [Google Scholar] [CrossRef]
- Houillier, C.; Soussain, C.; Ghesquières, H.; Soubeyran, P.; Chinot, O.; Taillandier, L.; Lamy, T.; Choquet, S.; Ahle, G.; Damaj, G.; et al. Management and outcome of primary CNS lymphoma in the modern era: An LOC network study. Neurology 2020, 94, e1027–e1039. [Google Scholar] [CrossRef]
- Kaulen, L.D.; Baehring, J.M. Treatment Options for Recurrent Primary CNS Lymphoma. Curr. Treat. Options Oncol. 2022, 23, 1548–1565. [Google Scholar] [CrossRef]
- Mendez, J.S.; Ostrom, Q.T.; Gittleman, H.; Kruchko, C.; DeAngelis, L.M.; Barnholtz-Sloan, J.S.; Grommes, C. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro Oncol. 2018, 20, 687–694. [Google Scholar] [CrossRef]
- Song, J.; Samant, R.; Jay, M.; Chaudry, H.; Fan, X.Y.; MacDonald, D.; Bence-Bruckler, I.; Nair, V. Whole brain radiotherapy improves survival outcomes in primary CNS lymphoma patients ineligible for systemic therapy. Support. Care Cancer 2020, 28, 5363–5369. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, C.; Chiesa, S.; Toppi, L.; Hohaus, S.; Gaudino, S.; D’Alo, F.; Dinapoli, N.; Davide, R.; Zinicola, T.; Bracci, S.; et al. May we routinely spare hippocampal region in primary central nervous system lymphoma during whole brain radiotherapy? Radiat. Oncol. 2023, 18, 161. [Google Scholar] [CrossRef]
- Foreman, B.E.; Mullikin, T.C.; Floyd, S.R.; Kelsey, C.R.; Patel, M.P.; Peters, K.B.; Kirkpatrick, J.P.; Reitman, Z.J.; Vaios, E.J. Long-term outcomes with reduced-dose whole-brain radiotherapy and a stereotactic radiosurgery boost for primary central nervous system lymphoma. Neurooncol. Adv. 2023, 5, vdad097. [Google Scholar] [CrossRef]
- Shibamoto, Y.; Hayabuchi, N.; Hiratsuka, J.; Tokumaru, S.; Shirato, H.; Sougawa, M.; Oya, N.; Uematsu, Y.; Hiraoka, M. Is whole-brain irradiation necessary for primary central nervous system lymphoma? Patterns of recurrence after partial-brain irradiation. Cancer 2003, 97, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Ney, D.E.; Reiner, A.S.; Panageas, K.S.; Brown, H.S.; DeAngelis, L.M.; Abrey, L.E. Characteristics and outcomes of elderly patients with primary central nervous system lymphoma: The Memorial Sloan-Kettering Cancer Center experience. Cancer 2010, 116, 4605–4612. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, S.; Hamamoto, Y.; Fujii, T.; Ochi, T.; Harada, H.; Ohnishi, T.; Mochizuki, T. Prognosis of primary central nervous system lymphoma treated with radiotherapy alone. Jpn. J. Radiol. 2012, 30, 806–810. [Google Scholar] [CrossRef]
- Aboubakr, O.; Houillier, C.; Alentorn, A.; Choquet, S.; Dupont, S.; Mokhtari, K.; Leclercq, D.; Nichelli, L.; Kas, A.; Rozenblum, L.; et al. Epilepsy in Patients With Primary CNS Lymphoma: Prevalence, Risk Factors, and Prognostic Significance. Neurology 2024, 103, e209748. [Google Scholar] [CrossRef]
- Gross, A.; Ziepert, M.; Scholz, M. KMWin—A convenient tool for graphical presentation of results from Kaplan-Meier survival time analysis. PLoS ONE 2012, 7, e38960. [Google Scholar] [CrossRef]
- Curry, L.D.; Munker, R.; Li, N.; Yan, D.; Pryor, P.; Nozad, S.; Keller, P.; Monohan, G.P.; Iragavarapu, C.; Krem, M.M. Performance status, comorbidities, and cycles of methotrexate exert the greatest influence on outcomes of primary and secondary CNS lymphomas: The Lexington experience. Ann. Hematol. 2023, 102, 141–154. [Google Scholar] [CrossRef]
- Omuro, A.M.P.; Ben-Porat, L.S.; Panageas, K.S.; Kim, A.K.; Correa, D.D.; Yahalom, J.; DeAngelis, L.M.; Abrey, L.E. Delayed neurotoxicity in primary central nervous system lymphoma. Arch. Neurol. 2005, 62, 1595–1600. [Google Scholar] [CrossRef]
- Morales-Martinez, A.; Lozano-Sanchez, F.; Duran-Peña, A.; Hoang-Xuan, K.; Houillier, C. Primary Central Nervous System Lymphoma in Elderly Patients: Management and Perspectives. Cancers 2021, 13, 3479. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.M.P.; DeAngelis, L.M.; Karrison, T.; Bovi, J.A.; Rosenblum, M.; Corn, B.W.; Correa, D.; Wefel, J.S.; Aneja, S.; Grommes, C.; et al. Randomized phase II study of rituximab, methotrexate (MTX), procarbazine, vincristine, and cytarabine (R-MPV-A) with and without low-dose whole-brain radiotherapy (LD-WBRT) for newly diagnosed primary CNS lymphoma (PCNSL). J. Clin. Oncol. 2020, 38, 2501. [Google Scholar] [CrossRef]
- Tringale, K.R.; Scordo, M.; Yahalom, J.; White, C.; Zhang, Z.; Schefflein, J.; Cederquist, G.; Schaff, L.R.; DeAngelis, L.; Imber, B.S.; et al. Evolving consolidation patterns and outcomes for a large cohort of patients with primary CNS lymphoma. Blood Adv. 2024, 8, 6195–6206. [Google Scholar] [CrossRef] [PubMed]
- Nabors, B.; Portnow, J.; Hattangadi-Gluth, J.; Horbinski, C. NCCN CNS tumor guidelines update for 2023. Neuro Oncol. 2023, 25, 2114–2116. [Google Scholar] [CrossRef]
- Ferreri, A.J.M.; Calimeri, T.; Cwynarski, K.; Dietrich, J.; Grommes, C.; Hoang-Xuan, K.; Hu, L.S.; Illerhaus, G.; Nayak, L.; Ponzoni, M.; et al. Primary central nervous system lymphoma. Nat. Rev. Dis. Primers 2023, 9, 29. [Google Scholar] [CrossRef]
- Ko, M.-K.; Kwak, Y.-K.; Choi, B.-O.; Jeun, S.-S.; Park, J.-S.; Ahn, S.; Song, J.-H. Is reduced-dose whole-brain radiotherapy also feasible in primary CNS lymphoma for curative or salvage purpose? J. Neurooncol. 2023, 165, 321–328. [Google Scholar] [CrossRef]
- Wu, S.Y.; Braunstein, S.E.; Rubenstein, J.L.; Sneed, P.K. Stereotactic Radiosurgery for Primary Central Nervous System Lymphoma. Cureus 2023, 15, e34817. [Google Scholar] [CrossRef]
- Langner-Lemercier, S.; Houillier, C.; Soussain, C.; Ghesquières, H.; Chinot, O.; Taillandier, L.; Soubeyran, P.; Lamy, T.; Morschhauser, F.; Benouaich-Amiel, A.; et al. Primary CNS lymphoma at first relapse/progression: Characteristics, management, and outcome of 256 patients from the French LOC network. Neuro Oncol. 2016, 18, 1297–1303. [Google Scholar] [CrossRef]
- Jahnke, K.; Thiel, E.; Martus, P.; Herrlinger, U.; Weller, M.; Fischer, L.; Korfel, A. Relapse of primary central nervous system lymphoma: Clinical features, outcome and prognostic factors. J. Neurooncol. 2006, 80, 159–165. [Google Scholar] [CrossRef]
- Iwabuchi, M.; Shibamoto, Y.; Sugie, C.; Ayakawa, S.; Ogino, H.; Baba, F. Partial-brain radiotherapy for primary central nervous system lymphoma: Multi-institutional experience. J. Radiat. Res. 2016, 57, 164–168. [Google Scholar] [CrossRef]
- Wagner, H.; Ali, A.; Glantz, M.; Blakeley, A. Role of Hippocampal-Avoidance Whole Brain Radiation Therapy (HA-WBRT) in Patients with Primary CNS Lymphoma (PCNSL). Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, E424. [Google Scholar] [CrossRef][Green Version]
- Manickam Gurusamy, V.; Raveendran Divakar, S.; Halsnad Chandramouli, S.; Kunheri, B.; Hussain Al-Abdulla, H.; Shaikh, G.; Chaudary Apsani, R.; Riyaz Poolakundan, M.; Caparrotti, P.; Wafiq Hammoud, R.; et al. The role of radiotherapy in newly diagnosed primary CNS lymphoma: A descriptive review and a pragmatic approach to clinical practice. Clin. Transl. Radiat. Oncol. 2023, 39, 100559. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.M.; Illerhaus, G.; Doorduijn, J.K.; Auer, D.P.; Bromberg, J.E.C.; Calimeri, T.; Cwynarski, K.; Fox, C.P.; Hoang-Xuan, K.; Malaise, D.; et al. Primary central nervous system lymphomas: EHA-ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2024, 35, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Joulié, A.; Houillier, C.; Antoni, D.; Créhange, G.; Jouglar, E.; Colin, P.; Benchalal, M.; Lang, P.; Alfonsi, M.; Hamidou, H.; et al. Brain radiotherapy in patients treated for a newly diagnosed primary central nervous system lymphoma: Professional practice evaluation in 19 French centers. Acta Oncol. 2023, 62, 648–656. [Google Scholar] [CrossRef]
- Palmer, J.D.; Bhamidipati, D.; Shukla, G.; Epperla, N.; Glass, J.; Kim, L.; Shi, W. Outcomes after stereotactic radiosurgery for CNS lymphoma. J. Neurooncol. 2020, 147, 465–476. [Google Scholar] [CrossRef]
- Seidel, C.; Viehweger, C.; Kortmann, R.-D. Is There an Indication for First Line Radiotherapy in Primary CNS Lymphoma? Cancers 2021, 13, 2580. [Google Scholar] [CrossRef]

| Patients, N (%) | 64 (100%) |
|---|---|
| Age (years), median (min–max) | 71 (31–83) |
| Sex: female:male, N (%) | 42 (65.6):22 (34.4) |
| Body Mass Index, median (min-max) | 26.3 (15.4–35) |
| ECOG Status at Initiation of Radiotherapy: N (%) | |
| 0 | 5 (7.8) |
| 1 | 25 (39.1) |
| 2 | 18 (28.1) |
| 3 | 11 (17.2) |
| 4 | 5 (7.8) |
| Initial disease characteristics N (%) | |
| Ki67% ≥ 90% * | 6 (9.4) |
| Solitary Lesion | 34 (53.1) |
| Initial Seizure | 16 (25) |
| Histopathologically confirmed disease | 62 (96.9) |
| Indication for Radiotherapy | |
| First line RT | 40 (62.5) |
| Unfit for CTx | 32 (50) |
| CTx refused by patient | 1 (1.6) |
| CTx aborted due to toxicity | 5 (7.8) |
| Consolidating RT | 2 (3.1) |
| Second line RT | 24 (37.5) |
| Progressive Disease post CTx | 14 (21.9) |
| Progressive Disease during CTx | 10 (15.6) |
| Radiotherapy Treatment Characteristics | |
| RT completed as intended | 37 (57.8) |
| RT aborted prematurely | 27 (42.2) |
| ≥80% of intended RT dose applied | 45 (70.3) |
| Glucocorticoids during RT | 51 (79.7) |
| Anticonvulsiva during RT | 11 (17.2) |
| Dose, median (min–max) | 45.0 Gy (31.8–59.4) |
| 3DcRT | 61 (95.3) |
| IMRT/VMAT | 3 (4.7) |
| Variable | Hazard Ratio (95% CI) Univariable | p-Value | Hazard Ratio (95% CI) Multivariable | p-Value |
|---|---|---|---|---|
| Age at diagnosis (years) ≥70 (n = 36) vs. <70 (n = 28) | 4.30 (2.13–8.66) | <0.001 | 5.02 (2.23–11.29) | <0.001 |
| Female (n = 42) vs. Male (n = 22) | 0.87(0.45–1.57) | 0.585 | ||
| Initial ECOG: ≥2 (n = 34) vs. <2 (n = 30) | 2.48 (1.30–4.74) | 0.006 | n.s. | |
| CCI: <5 (n = 30) vs. ≥6 (n = 34) | 1.76 (0.93–3.32) | 0.082 | ||
| Smoker (n = 12) vs. non-Smoker (n = 35) | 0.923 (0.43–1.97) | 0.725 | ||
| Systemic therapy: none (n = 31) vs. any (n = 33) | 5.027 (2.47–10.20) | <0.001 | 2.69 (1.29–5.64) | 0.008 |
| Cerebral Lesions: multiple (n = 26) vs. solitary (n = 34) | 1.07 (0.56–2.05) | 0.828 | ||
| KI 67 initially: ≥90 (n = 6) vs. <90 (n = 11) * | 2.12 (0.76–5.90) | 0.150 | ||
| Seizure (n = 16) vs. no seizure (n = 48) | 2.240 (1.05–4.76) | 0.036 | 3.67 (1.68–8.05) | 0.001 |
| RT: incomplete (n = 27) vs. completed as intended (n = 37) | 3.89 (2.01–7.54) | <0.001 | n.s. | |
| RT dose applied as intended: ≥80% (n = 45) vs. <80% (n = 19) | 0.21 (0.11–0.43) | <0.001 | 0.20 (0.09–0.43) | <0.001 |
| Anticonvulsants during RT: yes (n = 11) vs. no (n = 45) | 1.48 (0.71–3.10) | 0.298 | ||
| Corticosteroids during RT: yes (n = 51) vs. no (n = 13) | 1.55 (0.69–3.49) | 0.294 | ||
| Dynamic RT (n = 3) vs. 3DcRT (n = 61) | 0.32 (0.04–2.35) | 0.263 | ||
| Boost: yes (n = 7) vs. no (n = 57) | 0.67 (0.20–2.18) | 0.50 | ||
| RT: Second-line (n = 24) vs. First-line (n = 38) | 0.39 (0.20–0.76) | 0.006 | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bigge, J.C.; Bendrich, S.; Treiber, H.; Aydilek, E.; Brökers, N.; Wulf, G.G.; Zwerenz, C.M.; Anczykowski, M.Z.; Donath, S.; El Shafie, R.A.; et al. Clinical Predictors and Recurrence Characteristics Following Radiotherapy for Primary Central Nervous System Lymphoma: A Retrospective Cohort Study. Cancers 2025, 17, 2176. https://doi.org/10.3390/cancers17132176
Bigge JC, Bendrich S, Treiber H, Aydilek E, Brökers N, Wulf GG, Zwerenz CM, Anczykowski MZ, Donath S, El Shafie RA, et al. Clinical Predictors and Recurrence Characteristics Following Radiotherapy for Primary Central Nervous System Lymphoma: A Retrospective Cohort Study. Cancers. 2025; 17(13):2176. https://doi.org/10.3390/cancers17132176
Chicago/Turabian StyleBigge, Jan Carl, Stephanie Bendrich, Hannes Treiber, Enver Aydilek, Nils Brökers, Gerald Georg Wulf, Carla Marie Zwerenz, Mahalia Zoe Anczykowski, Sandra Donath, Rami A. El Shafie, and et al. 2025. "Clinical Predictors and Recurrence Characteristics Following Radiotherapy for Primary Central Nervous System Lymphoma: A Retrospective Cohort Study" Cancers 17, no. 13: 2176. https://doi.org/10.3390/cancers17132176
APA StyleBigge, J. C., Bendrich, S., Treiber, H., Aydilek, E., Brökers, N., Wulf, G. G., Zwerenz, C. M., Anczykowski, M. Z., Donath, S., El Shafie, R. A., von Diest, L.-A., Oelmann, J. T., Schirmer, M. A., Dröge, L. H., Leu, M., Chapuy, B., Rieken, S., & Guhlich, M. (2025). Clinical Predictors and Recurrence Characteristics Following Radiotherapy for Primary Central Nervous System Lymphoma: A Retrospective Cohort Study. Cancers, 17(13), 2176. https://doi.org/10.3390/cancers17132176









