Role of Radical Prostatectomy in Oligo-Metastatic Hormone-Sensitive Prostate Cancer: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Methodology
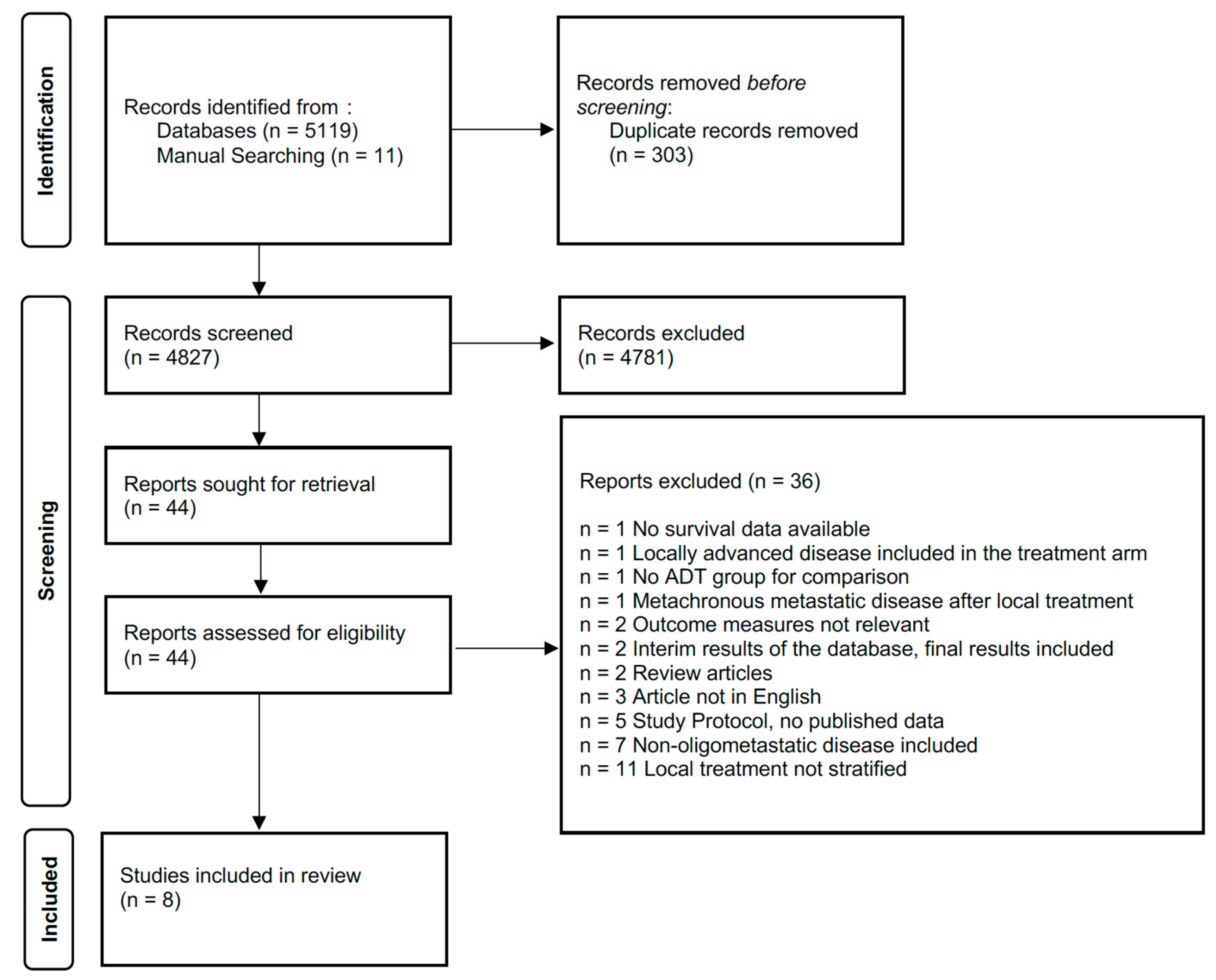
2.1. Search Strategy
2.2. Inclusion Criteria and Data Extraction
2.3. Outcome Measures
2.3.1. Primary Outcomes
- Progression-free survival (PFS)
- Cancer-specific survival (CSS)
- Overall survival (OS)
- Local event Rates/Local event free survival (LEFS)
- Castrate-Resistant prostate cancer-free survival (CRPC-FS)
2.3.2. Secondary Outcomes
- Complication rates
- Functional outcomes
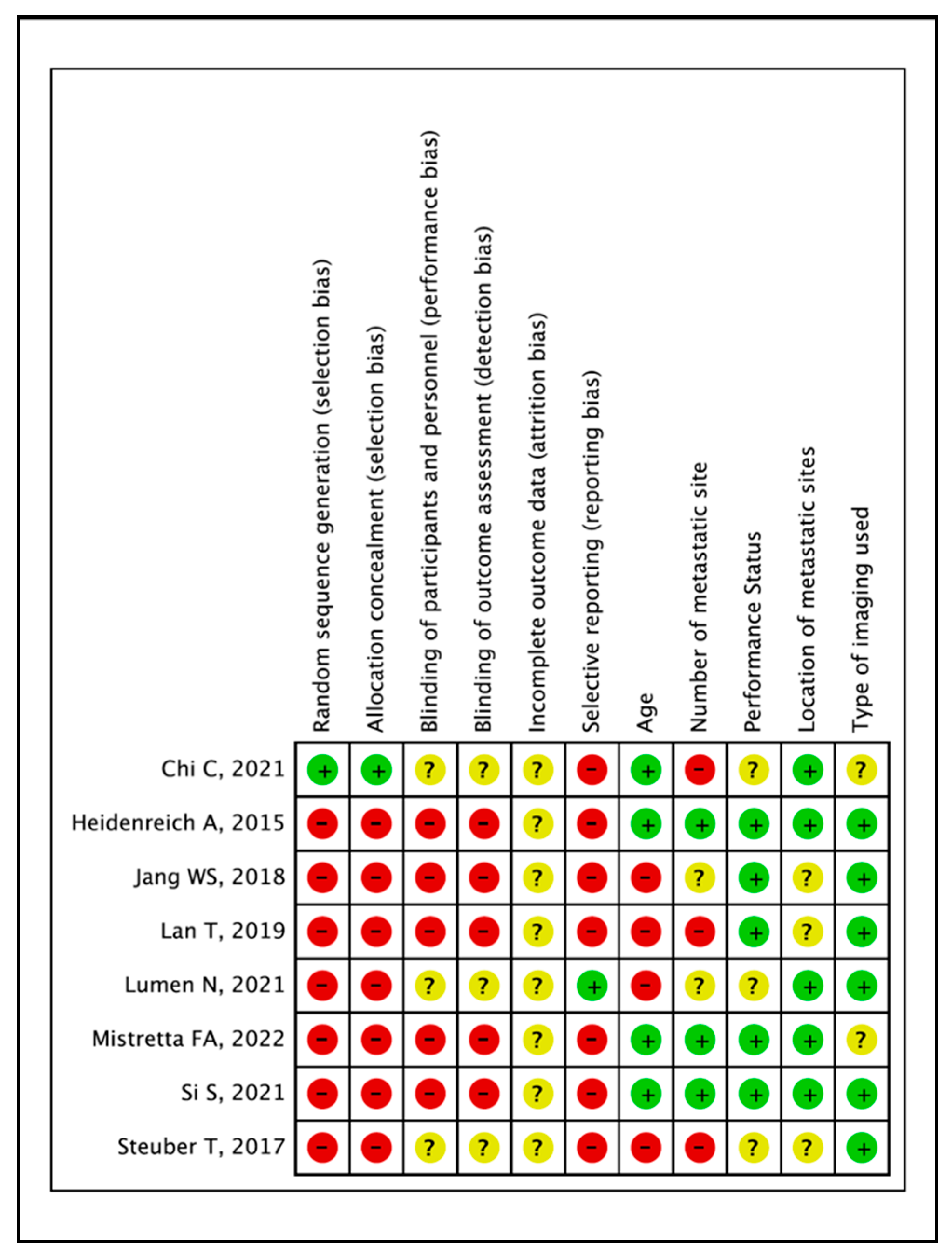
2.4. Assessment of Evidence Quality and Outcome Certainty
- Number of Metastatic Sites
- Location of Metastatic Sites
- Age
- Performance Status
- Type of imaging used to define Metastasis (Molecular imaging vs. Conventional Cross-sectional imaging, e.g., CT/MRI/Bone Scan).
2.5. Data Synthesis
3. Results
3.1. Primary Outcomes
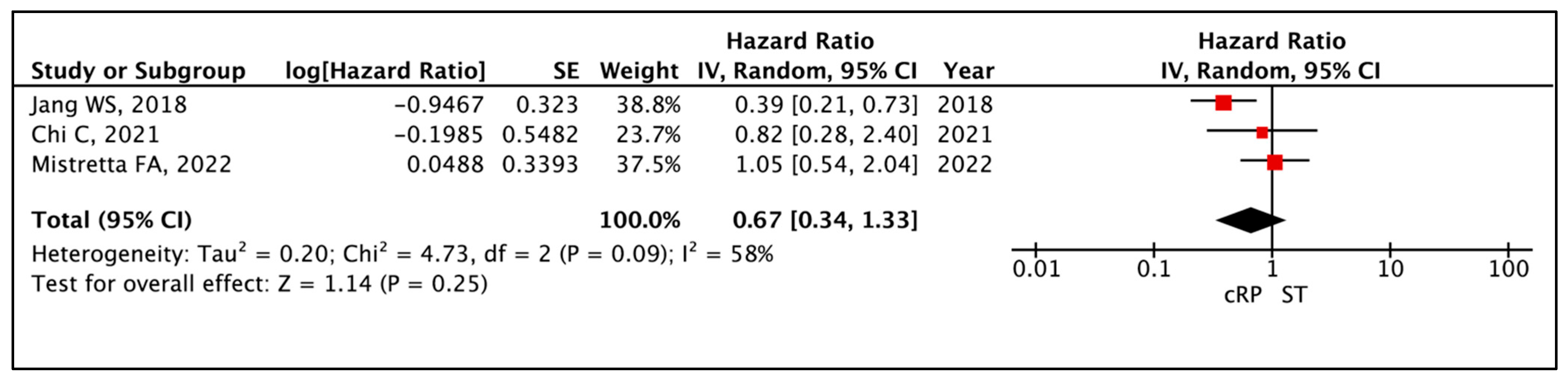
3.1.1. Progression-Free Survival (PFS)
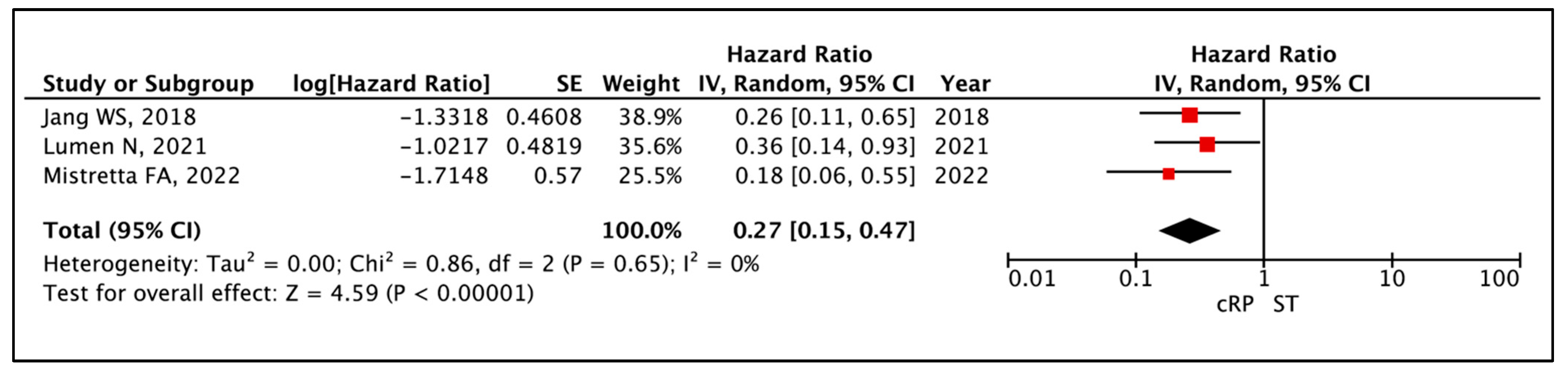
3.1.2. Cancer-Specific Survival (CSS)
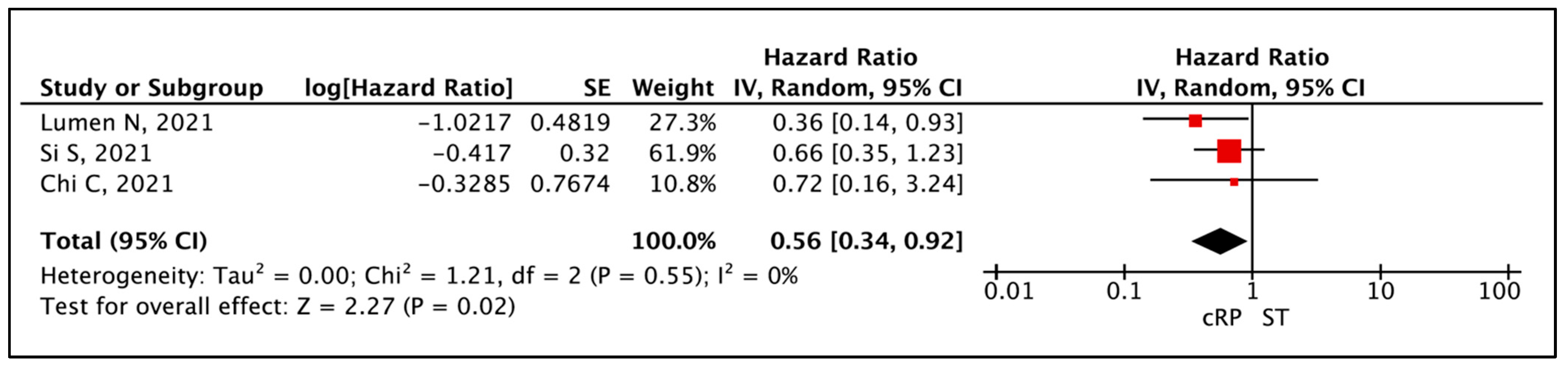
3.1.3. Overall Survival (OS)
3.1.4. Castrate Resistant Prostate Cancer-Free Survival (CRPC-FS)
3.1.5. Local Events
3.2. Secondary Outcomes—Post cRP
3.2.1. Complications
3.2.2. Functional Outcomes
3.3. Quality of Evidence and Certainty of Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Napoli, G.; Arcangeli, S.; Fionda, B.; Munoz, F.; Tebano, U.; Durante, E.; Tucci, M.; Bortolus, R.; Muraro, M.; Rinaldi, G.; et al. A Systematic Review and a Meta-analysis of Randomized Controlled Trials’ Control Groups in Metastatic Hormone-Sensitive Prostate Cancer (mHSPC). Curr. Oncol. Rep. 2022, 24, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomised controlled phase 3 trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Stevens, D.J.; Sooriakumaran, P. Oligometastatic Prostate Cancer. Curr. Treat. Options Oncol. 2016, 17, 62. [Google Scholar] [CrossRef]
- Psaila, B.; Lyden, D. The metastatic niche: Adapting the foreign soil. Nat. Rev. Cancer 2009, 9, 285–293. [Google Scholar] [CrossRef]
- May, T.; Comeau, R.; Sun, P.; Kotsopoulos, J.; Narod, S.A.; Rosen, B.; Ghatage, P. A Comparison of Survival Outcomes in Advanced Serous Ovarian Cancer Patients Treated With Primary Debulking Surgery Versus Neoadjuvant Chemotherapy. Int. J. Gynecol. Cancer 2017, 27, 668–674. [Google Scholar] [CrossRef]
- Heng, D.Y.; Wells, J.C.; Rini, B.I.; Beuselinck, B.; Lee, J.-L.; Knox, J.J.; Bjarnason, G.A.; Pal, S.K.; Kollmannsberger, C.K.; Yuasa, T.; et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur. Urol. 2014, 66, 704–710. [Google Scholar] [CrossRef]
- Comen, E.; Norton, L.; Massague, J. Clinical implications of cancer self-seeding. Nat. Rev. Clin. Oncol. 2011, 8, 369–377. [Google Scholar] [CrossRef]
- Won, A.C.; Gurney, H.; Marx, G.; De Souza, P.; Patel, M.I. Primary treatment of the prostate improves local palliation in men who ultimately develop castrate-resistant prostate cancer. BJU Int. 2013, 112, E250–E255. [Google Scholar] [CrossRef]
- Burdett, S.; Boevé, L.M.; Ingleby, F.C.; Fisher, D.J.; Rydzewska, L.H.; Vale, C.L.; van Andel, G.; Clarke, N.W.; Hulshof, M.C.; James, N.D.; et al. Prostate Radiotherapy for Metastatic Hormone-sensitive Prostate Cancer: A STOPCAP Systematic Review and Meta-analysis. Eur. Urol. 2019, 76, 115–124. [Google Scholar] [CrossRef]
- Bossi, A.; Foulon, S.; Maldonado, X.; Sargos, P.; MacDermott, R.; Kelly, P.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; et al. Efficacy and safety of prostate radiotherapy in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2024, 404, 2065–2076. [Google Scholar] [CrossRef] [PubMed]
- EAU PCa Guidelines Panel. 2025 EAU—EANM—ESTRO ESUR—ISUP—SIOG Guidelines on Prostate Cancer: European Association of Urology. 2025. Available online: https://uroweb.org/guidelines/prostate-cancer (accessed on 23 June 2025).
- Heidenreich, A.; Pfister, D.; Porres, D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: Results of a feasibility and case-control study. J. Urol. 2015, 193, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Gratzke, C.; Engel, J.; Stief, C.G. Role of radical prostatectomy in metastatic prostate cancer: Data from the Munich Cancer Registry. Eur. Urol. 2014, 66, 602–603. [Google Scholar] [CrossRef]
- Culp, S.H.; Schellhammer, P.F.; Williams, M.B. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur. Urol. 2014, 65, 1058–1066. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.5 2024 (Updated August 2024). Cochrane, 2024. Available online: https://www.training.cochrane.org/handbook (accessed on 19 January 2025).
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Mistretta, F.A.; Luzzago, S.; Conti, A.; Verri, E.; Marvaso, G.; Ruvolo, C.C.; Catellani, M.; Di Trapani, E.; Cozzi, G.; Bianchi, R.; et al. Oligometastatic Prostate Cancer: A Comparison between Multimodality Treatment vs. Androgen Deprivation Therapy Alone. Cancers 2022, 14, 2313. [Google Scholar] [CrossRef]
- Si, S.; Zheng, B.; Wang, Z.; Niu, Z. Does surgery benefit patients with oligometastatic or metastatic prostate cancer?—A retrospective cohort study and meta-analysis. Prostate 2021, 81, 736–744. [Google Scholar] [CrossRef]
- Lumen, N.; De Bleser, E.; Buelens, S.; Verla, W.; Poelaert, F.; Claeys, W.; Fonteyne, V.; Verbeke, S.; Villeirs, G.; De Man, K.; et al. The Role of Cytoreductive Radical Prostatectomy in the Treatment of Newly Diagnosed Low-volume Metastatic Prostate Cancer. Results from the Local Treatment of Metastatic Prostate Cancer (LoMP) Registry. Eur. Urol. Open Sci. 2021, 29, 68–76. [Google Scholar] [CrossRef]
- Chi, C.; Fan, L.; Dong, B.; Zhu, Y.; Xin, Z.; Pan, J.; Xue, W. Efficacy of Neoadjuvant Chemohormonal Therapy in Oligometastatic Hormone-Sensitive Prostate Cancer: A Prospective, Three-Arm, Comparative Propensity Score Match Analysis. Clin. Genitourin. Cancer 2021, 19, e223–e234. [Google Scholar] [CrossRef]
- Lan, T.; Chen, Y.; Su, Q.; Ye, J. Oncological Outcome of Cytoreductive Radical Prostatectomy in Prostate Cancer Patients With Bone Oligometastases. Urology 2019, 131, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Steuber, T.; Berg, K.D.; Røder, M.A.; Brasso, K.; Iversen, P.; Huland, H.; Tiebel, A.; Schlomm, T.; Haese, A.; Salomon, G.; et al. Does Cytoreductive Prostatectomy Really Have an Impact on Prognosis in Prostate Cancer Patients with Low-volume Bone Metastasis? Results from a Prospective Case-Control Study. Eur. Urol. Focus. 2017, 3, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.S.; Kim, M.S.; Jeong, W.S.; Chang, K.D.; Cho, K.S.; Ham, W.S.; Rha, K.H.; Hong, S.J.; Choi, Y.D. Does robot-assisted radical prostatectomy benefit patients with prostate cancer and bone oligometastases? BJU Int. 2018, 121, 225–231. [Google Scholar] [CrossRef]
- Harris, W.P.; Mostaghel, E.A.; Nelson, P.S.; Montgomery, B. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 2009, 6, 76–85. [Google Scholar] [CrossRef]
- Antwi, S.; Everson, T.M. Prognostic impact of definitive local therapy of the primary tumor in men with metastatic prostate cancer at diagnosis: A population-based, propensity score analysis. Cancer Epidemiol. 2014, 38, 435–441. [Google Scholar] [CrossRef]
- Dai, B.; Zhang, S.; Wan, F.-N.; Wang, H.-K.; Zhang, J.-Y.; Wang, Q.-F.; Kong, Y.-Y.; Ma, X.-J.; Mo, M.; Zhu, Y.; et al. Combination of Androgen Deprivation Therapy with Radical Local Therapy Versus Androgen Deprivation Therapy Alone for Newly Diagnosed Oligometastatic Prostate Cancer: A Phase II Randomized Controlled Trial. Eur. Urol. Oncol. 2022, 5, 519–525. [Google Scholar] [CrossRef]
- Ranasinghe, W.; Chapin, B.F.; Kim, I.Y.; Sooriakumaran, P.; Lawrentschuk, N. The cytoreductive prostatectomy in metastatic prostate cancer: What the individual trials are hoping to answer. BJU Int. 2020, 125, 792–800. [Google Scholar] [CrossRef]
- Sooriakumaran, P.; Wilson, C.; Rombach, I.; Hassanali, N.; Aning, J.; Lamb, A.D.; Cathcart, P.; Eden, C.; Ahmad, I.; Rajan, P.; et al. Feasibility and safety of radical prostatectomy for oligo-metastatic prostate cancer: The Testing Radical prostatectomy in men with prostate cancer and oligo-Metastases to the bone (TRoMbone) trial. BJU Int. 2022, 130, 43–53. [Google Scholar] [CrossRef]
- Saika, T.; Miura, N.; Fukumoto, T.; Yanagihara, Y.; Miyauchi, Y.; Kikugawa, T. Role of robot-assisted radical prostatectomy in locally advanced prostate cancer. Int. J. Urol. 2018, 25, 30–35. [Google Scholar] [CrossRef]
- Graefen, M.; Joniau, S. Radical Prostatectomy or Radiotherapy in Oligometastatic Prostate Cancer: Is It Nearly Time To Call It a Draw? Eur. Urol. Oncol. 2022, 5, 528–529. [Google Scholar] [CrossRef]
- Jones, C.; Gray, S.; Brown, M.; Brown, J.; McCloskey, E.; Rai, B.P.; Clarke, N.; Sachdeva, A. Risk of Fractures and Falls in Men with Advanced or Metastatic Prostate Cancer Receiving Androgen Deprivation Therapy and Treated with Novel Androgen Receptor Signalling Inhibitors: A Systematic Review and Meta-analysis of Randomised Controlled Trials. Eur. Urol. Oncol. 2024, 7, 993–1004. [Google Scholar] [CrossRef]
- Iacovelli, R.; Ciccarese, C.; Bria, E.; Romano, M.; Fantinel, E.; Bimbatti, D.; Muraglia, A.; Porcaro, A.B.; Siracusano, S.; Brunelli, M.; et al. The Cardiovascular Toxicity of Abiraterone and Enzalutamide in Prostate Cancer. Clin. Genitourin. Cancer 2018, 16, e645–e653. [Google Scholar] [CrossRef]
- Nowakowska, M.K.; Ortega, R.M.; Wehner, M.R.; Nead, K.T. Association of Second-generation Antiandrogens With Cognitive and Functional Toxic Effects in Randomized Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2023, 9, 930–937. [Google Scholar] [CrossRef]
- Piombino, C.; Oltrecolli, M.; Tonni, E.; Pirola, M.; Matranga, R.; Baldessari, C.; Pipitone, S.; Dominici, M.; Sabbatini, R.; Vitale, M.G. De Novo Metastatic Prostate Cancer: Are We Moving toward a Personalized Treatment? Cancers 2023, 15, 4945. [Google Scholar] [CrossRef]
- Nickols, N.G.; Tsai, S.; Kane, N.; Tran, S.; Ghayouri, L.; Diaz-Perez, S.; Thein, M.; Anderson-Berman, N.; Eason, J.; Kishan, A.U.; et al. Systemic and Tumor-directed Therapy for Oligometastatic Prostate Cancer: The SOLAR Phase 2 Trial in De Novo Oligometastatic Prostate Cancer. Eur. Urol. 2024, 86, 190–193. [Google Scholar] [CrossRef]
- Impact of Radical Prostatectomy as Primary Treatment in Patients with Prostate Cancer with Limited Bone Metastases (g-RAMPP). NCT02454543 ClinicalTrials.gov [Updated 12 April 2023]. Available online: https://www.clinicaltrials.gov/study/NCT02454543 (accessed on 20 June 2025).
- Morozov, A.; Chuvalov, L.; Taratkin, M.; Enikeev, M.; Rapoport, L.; Singla, N.; Barret, E.; Poddubskaya, E.; Borodina, M.; Salomon, G.; et al. A systematic review of cytoreductive prostatectomy outcomes and complications in oligometastatic disease. Asian J. Urol. 2024, 11, 208–220. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. MetaArXiv 2020. [Google Scholar] [CrossRef] [PubMed]

| Authors | Outcomes Compared | Definition of o-mHSPC | Study Design | Centres | Study Characteristics | Site of Study | Study Period |
|---|---|---|---|---|---|---|---|
| Heidenreich 2015 [13] | cRP vs. ST | ≤3 bony mets, No extra pelvic LN, No bulky (>3 cm pelvic LN) | Retrospective | Single | Case Control—Matched | Germany | Not mentioned |
| Steuber 2017 [24] | cRP vs. ST | 1–3 bony mets, no visceral mets, ≤T3 | Prospective | Multicentre (3) | Case Control | Germany, Denmark | 2008 to 2015 |
| Jang 2018 [25] | cRP vs. ST | ≤5 bony mets, No visceral mets | Retrospective | Single | Observational Cohort | South Korea | 2005 to 2015 |
| Lan 2019 [23] | cRP vs. ST | ≤5 bony mets, No visceral mets | Retrospective | Single | Observational Cohort-Consecutive | Europe | 2005 to 2016 |
| Chi 2021 [22] | cRP vs. ST vs. ADT + Chemo + cRP | <5 metastatic lesions—extra pelvic nodes/bones | Prospective | Single | Propensity score matched | China | 2014 to 2019 |
| Lumen 2021 [21] | cRP vs. RT vs. ST | <4 bony mets, no visceral mets | Prospective | Multicentre (5) | Observational Cohort | Belgium | 2014 to 2020 |
| Si 2021 [20] | cRP vs. ST | ≤5 bony mets, No visceral mets | Retrospective | Single | Observational Cohort | China | 2010 to 2015 |
| Mistretta 2022 [19] | cRP + RT vs. ST | ≤5 bony mets, No visceral mets, locally resectable (T1–T3) | Retrospective | Single | Observational Cohort | Italy | 2010 to 2018 |
| Authors | Groups | cRP (n) | ST (n) | Upfront Rx in cRP Arm | Upfront in ST Arm | Imaging Modality |
|---|---|---|---|---|---|---|
| Heidenreich 2015 [13] | cRP vs. ST | 23 | 38 | Open RP + ePLND + Adjuvant RT if positive margins (66.6 Gy) | ADT only | Conventional imaging only |
| Steuber 2017 [24] | cRP vs. ST | 43 | 40 | RP a,b + ADT | ADT only | Conventional imaging only |
| Jang 2018 [25] | cRP vs. ST | 38 | 41 | RARP + ePLND + ADT | ADT only | Conventional imaging only |
| Lan 2019 [23] | cRP vs. ST | 35 | 76 | RP a + ePLND + ADT | ADT only | Conventional imaging only |
| Chi 2021 [22] | cRP vs. ST | 18 | 18 | RP a + PLND c + ADT | ADT only | Conventional + FDG/PSMA-PET |
| Lumen 2021 [21] | cRP vs. ST | 48 | 35 | RP (Open + RARP) + ePLND ± ST ± SACT (Docetaxel/Abiraterone) | ADT ± SACT (Docetaxel/Abiraterone) | Conventional imaging only |
| Si 2021 [20] | cRP vs. ST | 27 | 57 | RP a + ePLND + ADT + Abiraterone | ADT + Abiraterone | Conventional imaging only |
| Mistretta 2022 [19] | cRP vs. ST | 40 | 34 | RARP + ePLND + ADT ± Adjuvant RT | ADT only | Conventional + Choline PET |
| Author | Age (Years) | p-Value | PSA (ng/mL) | p-Value | Gleason Score Pre-op (n,%) | p-Value | Metastasis Characteristic (n, %) | p-Value | No. of Mets | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Heidenreich 2015 [13] | cRP 61 (42–69) ST 64 (47–83) (Mean, Range) | NR | cRP 135.2 (3.5–150.4) ST 105 (45–195) (Mean, range) | 0.049 | cRP ≤7 = 5 (21.7%) 8 = 7 (30.4%) 9 = 7 (30.4%) 10 = 4 (17.4%) ST ≤7 = 11 (28.9%) 8 = 11 (28.9%) 9 = 8 (21.1%) 10 = 4 (10.5%) | NR | cRP - M1a = 3 (13.1%) - M1b = 23 (100%) ST - M1a = 4 (10.5%) - M1b = 38 (100%) | NR | cRP 2.1 (1–3) ST 2.5 (1–5) (Mean, Range) | NR |
| Steuber 2017 [24] | cRP 70 ST 65 (Median) | <0.01 | cRP 42.5 ST 29 (Median) | 0.02 | cRP ≤7 = 15% 8 = 32.5% 9 = 40% 10 = 12.5% ST ≤7 = 30.2% 8 = 30.2% 9 = 34.9% 10 = 4.7% | 0.22 | NR | NR | NR | NR |
| Jang 2018 [25] | cRP 65 (62–69) ST 71 (67–76) (Median, IQR) | <0.001 | cRP 39.0 (15.0–84.5) ST 50.0 (23.8–162.8) (Median, IQR) | 0.206 | cRP ≤8 = 26 (68.4%) ≥9 = 12 (31.6%) ST ≤8 = 24 (58.5%) ≥9 = 17 (41.5%) | 0.484 | NR | NR | NR | NR |
| Lan 2019 [23] | cRP 67.83 ± 7.19 ST 71.7 ± 7.73 (Mean, SD) | 0.030 | cRP 90.4 ± 152.8 ST 502.9 ± 806.0 (Median, SD) | 0.003 | cRP ≤7 = 27 (77.1%) 8 = 6 (17.1%) 9–10 = 2 (5.7%) ST ≤7 = 27 (35.5%) 8 = 27 (35.5%) 9–10 = 21 (27.6%) | 0.001 | NR | NR | cRP 2.37 ± 1.22 ST 2.93 ± 1.12 (Mean, SD) | 0.019 |
| Chi 2021 [22] | cRP 68.05 ± 8.24 ST 68.22 ± 6.67 (Mean, SD) | 0.94 | cRP 97.43 (124.61) ST 78.25 (58.95) (Median, IQR) | 0.99 | cRP 7 = 6 (33.33%) 8 = 8 (44.44%) 9 = 4 (22.22%) ST 7 = 3 (16.67%) 8 = 9 (50.0%) 9 = 6 (33.33%) | 0.57 | cRP - M1a = 4 (22.2%) - M1b = 16 (88.9%) ST - M1a = 4 (22.2%) - M1b = 15 (83.3%) | p (M1a) = 0.15 p (M1b) = 0.032 | NR | NR |
| Lumen 2021 [21] | cRP 64 (59–72) ST 74 (69–84) (Median, IQR) | <0.001 | cRP 19 (11–42) ST 47 (17–156) (Median, IQR) | 0.008 | cRP ≤7 = 9 (20.9%) 8 = 10 (20.8%) 9–10 = 28 (58.3%) ST ≤7 = 8 (25.1%) 8 = 11 (34.4%) 9–10 = 13 (40.6%) | 0.318 | cRP - M1a = 23 (47.9%) - M1b = 25 (52.1%) ST - M1a = 10 (28.6%) - M1b = 25 (71.4%) | 0.112 | NR | NR |
| Si 2021 [20] | cRP 76.67 ± 9.66 ST 76.42 ± 9.69 (Mean, SD) | 0.914 | cRP 28.93 ST 70.83 (Median) | 0.121 | NR | NR | cRP - M1a = 5 (18.5%) - M1b = 100% ST - M1a = 8 (14.0%) - M1b = 100% | p (M1a) = 0.394 p (M1b) = 0.258 | cRP 2.07 ± 0.917 ST 2.18 ± 1.07 | 0.837 |
| Mistretta 2022 [19] | cRP 67 (58–68) ST 64 (60–74) (Median, IQR) | 0.2 | cRP 14 (9–29) ST 87 (35–186) (Median, IQR) | <0.001 | cRP ≤7 = 18 (45%) 8–10 = 22 (55%) ST ≤7 = 9 (26.5%) 8–10 = 23 (67.6%) | 0.1 | cRP - M1a = 16 (40.0%) - M1b = 24 (60.0%) ST - M1a = 7 (20.6%) - M1b = 27 (79.4%) | 0.1 | NR | NR |
| Author | Follow-Up (Months) | Primary Outcomes |
|---|---|---|
| Heidenreich 2015 [13] | cRP vs. ST 40.6 (3–71) vs. 44 (24–96); p = NS (Median, Range) | cRP vs. ST CSS—9 5.6% vs. 84.2%, (p = 0.043) OS—91.3% vs. 78.9, (p = 0.048) Local events—0% vs. 28.9% |
| Steuber 2017 [24] | cRP vs. ST 32.7 (23.5–84.6) vs. 82.2 (37.1–121.2) (Median) | cRP vs. ST OS—p = 0.25 CRPC-FS—p = 0.92 Local events—7% vs. 35% Multivariate analysis * HR (95%CI), p-value CRPC-FS—1.49 (0.58–3.83); p = 0.408 * Multivariate Cox proportional hazard analyses were used to reveal the predictors of survival outcomes using the following covariates: age, PSA, Biopsy Gleason Score, T stage, Number of metastases |
| Jang 2018 [25] | Overall cohort 40 (28–58) (Median, IQR) | cRP vs. ST Median PFS—75 months vs. 28 months, (p = 0.008) Median CSS—Not reached vs. 40 months, (p = 0.002) Local events—7.9% vs. 26.8% Multivariate analysis * HR (95%CI), p-value PFS—0.388 (0.206 0.731); p = 0.003 CSS—0.264 (0.107 0.650); p = 0.004 * Multivariate Cox proportional hazard analyses were used to reveal the predictors of survival outcomes using the following covariates: age, PSA, Biopsy Gleason Score, Charlson’s comorbidity index, T stage, N stage |
| Lan 2019 [23] | cRP vs. ST 35 (22–51) vs. 35 (25–45); p = 0.135 (Median, IQR) | cRP vs. ST Median PFS—32 months vs. 17 months, (p = 0.184) Median CRPC-FS—35 months vs. 21 months, (p = 0.118) Median CSS—Not reached 3-year/5-year CSS—90.8% vs. 87.9%/63.6% vs. 74.9%, (p = 0.773) 3-year/5-year CRPC-FS—42.7% vs. 27%/19% vs. 21% |
| Chi 2021 [22] Propensity Matched (Age, Gleason score, clinical TNM staging and PSA level) | cRP vs. ST 30 vs. 29 (Median) | cRP vs. ST Median PFS—30.6 months vs. 16.1 months, (p = 0.57) Multivariate analysis * HR (95%CI), p-value PFS—0.82 (0.28–2.37); p = 0.70 * Multivariate Cox proportional hazard analyses were used to reveal the predictors of survival outcomes using the following covariates: Age, T stage, N stage, M stage, Gleason Score |
| Lumen 2021 [21] | Overall cohort 32 (16–49) (Median, IQR) | cRP vs. ST 2-yr OS 93 ± 4% vs. 69 ± 9% (HR 0.28, 95% CI 0.11–0.71; p = 0.007) 2-yr CSS 93 ± 4% vs. 75 ± 8% (HR 0.36, 95% CI 0.14–0.94; p = 0.037) 2-yr LEFS 92 ± 4% vs. 60 ± 9% (HR 0.25, 95% CI 0.10–0.64; p = 0.004) Local events—14.6% vs. 37.1% Multivariate analysis * HR (95%CI), p-value OS—0.36, (0.14–0.94); p = 0.037 * Multivariate Cox proportional hazard analyses were used to reveal the predictors of survival outcomes using the following covariates: Age, PSA, Tumour grade, ECOG status, T stage, N stage, M stage, Additional systemic treatment |
| Si 2021 [20] | cRP vs. ST 64.2 (56.4–81.6) vs. 73.0 (56.0–85.4); p = 0.496 | cRP vs. ST Median OS—78.6 months vs. 80.7 months, (p = 0.649) 3-year/5-year OS—96.2% vs. 94.7%/76.0% vs. 74.9% (p = NS) Mean CRPC-FS—91.86 vs. 85.07, (p = 0.183) Univariate analysis * HR (95%CI), p-value OS—0.659 (0.352–1.233); p = 0.192 CRPC-FS—0.587 (0.251–1.376); p = 0.220 * Univariate Cox proportional hazard analyses were used to reveal the predictors of survival outcomes using the following covariates: BMI, Charlson’s co-morbidity index, Smoking, Number of metastases, Gleason score, Pre-op and Post-op PSA |
| Mistretta 2022 [19] | cRP vs. ST 55 vs. 50; p = 0.8 (Median) | cRP vs. ST CSM—5.9% vs. 37.1%, (p = 0.02) Disease Progression—83.1 vs. 62.5%, (p = 0.8) Progression to CRPC—24.0 vs. 62.5%, (p < 0.01) Local events—0% vs. 23.5% Multivariate analysis * HR (95%CI), p-value CRPC-FS—0.40 (0.19–0.84); p = 0.02 PFS—1.19 (0.62–2.28); p = 0.6 CSS—0.18 (0.05–0.56); p = 0.0026 * Multivariate Cox proportional hazard analyses were used to reveal the predictors of survival outcomes using the following covariates: PSA and site of metastases |
| Author | Op Time (mins) | Blood Loss (mL) | Positive Surgical Margins (n, %) | Total Complications (n,%) | CD3+ Complications (n, %) | Hospital Stay | Continence (%, Time) |
|---|---|---|---|---|---|---|---|
| Heidenreich 2015 [13] | 127 (115–145) (Mean, range) | 335 (250–600) (Mean, range) | 4 (14.3%) | 9 (39.1%) | 3 (13.0%) | 7.8 days (6–13) (Mean, range) | 21 (93.3%, Time NR, 0–1 pads/day) |
| Steuber 2017 [24] | NR | NR | 67.4% | NR | NR | NR | NR |
| Jang 2018 [25] | 147 (135–186) (Median, IQR) | 300 (200–500) (Median, IQR) | 30 (78.9%) | 6 (15.7%) | 2 (5.3%) | 5 days (4–7) (Median, IQR) | NR |
| Lan 2019 [23] | NR | NR | 10 (28.6%) | NR | NR | NR | NR |
| Chi 2021 [22] | NR | NR | 8 (47.06%) | 22.71% | NR | 5 (2) Median (IQR) | NR |
| Lumen 2021 [21] | NR | NR | NR | 7 (14.6%) | NR | NR | NR |
| Si 2021 [20] | 165 ± 86.99 (Mean, SD) | 766 ± 361.62 (Mean, SD) | NR | 7 (25.9%) | 0 | 12.78 ± 10.0 (Mean, SD) | 22 (81.5%, Time NR, pad use NR) |
| Mistretta 2022 [19] | NR | NR | NR | NR | NR | NR | NR |
| Cytoreductive Radical Prostatectomy Compared to Standard Treatment for Oligometastatic Prostate Cancer | |||||
|---|---|---|---|---|---|
| Patient or population: Oligometastatic Prostate Cancer Intervention: Cytoreductive Radical Prostatectomy Comparison: Standard Treatment | |||||
| Outcomes | № of participants (studies) Follow-up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
| Risk with Standard Treatment | Risk difference with Cytoreductive Radical Prostatectomy | ||||
| Time to Event Analysis—Progression Free Survival | (3 non-randomised studies) | ⨁◯◯◯ Very low a,b,c | HR 0.67 (0.34 to 1.33) | 0 per 1000 | -- per 1000 (-- to --) |
| Time to Event Analysis—Cancer Specific Survival | (3 non-randomised studies) | ⨁◯◯◯ Very low a,b,c,d | HR 0.27 (0.15 to 0.47) | 0 per 1000 | -- per 1000 (-- to --) |
| Time to Event Analysis—Overall Survival | (3 non-randomised studies) | ⨁◯◯◯ Very low a,b,c,d | HR 0.56 (0.34 to 0.92) | 0 per 1000 | -- per 1000 (-- to --) |
| Time to Event Analysis—Progression to CRPC | (2 non-randomised studies) | ⨁◯◯◯ Very low a,b,c | HR 0.47 (0.26 to 0.83) | 0 per 1000 | -- per 1000 (-- to --) |
| Local Events | 297 (4 non-randomised studies) | ⨁⨁◯◯ Low a,b | RR 0.27 (0.13 to 0.59) | 291 per 1000 | 212 fewer per 1000 (253 fewer to 119 fewer) |
| The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajan, K.; Parmar, K.; Rajamoorthy, S.-I.; Geraghty, R.; Whyte, E.; Rai, B.P. Role of Radical Prostatectomy in Oligo-Metastatic Hormone-Sensitive Prostate Cancer: A Systematic Review and Meta-Analysis. Cancers 2025, 17, 2757. https://doi.org/10.3390/cancers17172757
Rajan K, Parmar K, Rajamoorthy S-I, Geraghty R, Whyte E, Rai BP. Role of Radical Prostatectomy in Oligo-Metastatic Hormone-Sensitive Prostate Cancer: A Systematic Review and Meta-Analysis. Cancers. 2025; 17(17):2757. https://doi.org/10.3390/cancers17172757
Chicago/Turabian StyleRajan, Karthik, Kalpesh Parmar, Shri-Ishvarya Rajamoorthy, Robert Geraghty, Eleanor Whyte, and Bhavan Prasad Rai. 2025. "Role of Radical Prostatectomy in Oligo-Metastatic Hormone-Sensitive Prostate Cancer: A Systematic Review and Meta-Analysis" Cancers 17, no. 17: 2757. https://doi.org/10.3390/cancers17172757
APA StyleRajan, K., Parmar, K., Rajamoorthy, S.-I., Geraghty, R., Whyte, E., & Rai, B. P. (2025). Role of Radical Prostatectomy in Oligo-Metastatic Hormone-Sensitive Prostate Cancer: A Systematic Review and Meta-Analysis. Cancers, 17(17), 2757. https://doi.org/10.3390/cancers17172757